Abstract
Purpose:
The aim of this study was to describe the effectiveness of goniotomy for childhood glaucoma in Indian eyes.
Methods:
Consecutive patients with pediatric glaucoma who underwent goniotomy between July 2017 and June 2020, in a single center in Northwest India were prospectively analyzed. Goniotomy was done as a primary procedure or a re-do surgery of the untreated angle in failed filtering surgery. Success was defined as intraocular pressure (IOP) ≦18 mm Hg with or without 2 topical medications.
Results:
A total of 172 eyes of 126 children underwent goniotomy during this period (37.9% of all pediatric glaucoma surgeries). Goniotomy comprised 132 of 211 (62.5%) primary pediatric glaucoma surgeries and 40 of 243 (16.5%) re-do surgeries. 145, 112, and 54 eyes had a six months, 1-year and 2-year follow-up, respectively. At 1 year, success rates in Primary Congenital Glaucoma (PCG) were 79.7% for primary surgery and 68.4% for re-do surgery. For non-PCG eyes, the success rate was 62% at 1 year. Among PCG subgroups, infantile and newborn glaucoma had 87.5% and 57.1% success rates, respectively. On logistic regression analysis, lower baseline IOP and lesser axial length at presentation were significantly predictive of successful outcomes (P = 0.03 and P = 0.02, respectively). At 1 year, in the primary surgery group, 50% had good vision (better than logMAR 0.5), 28.9% had moderate (better than LOGMAR 1.0) and 20% had severe visual impairment. There were no significant intraoperative or post-operative complications.
Conclusion:
Goniotomy appears to be an effective surgery for childhood glaucoma in Indian eyes. Being minimally invasive, it obviates the need for conjunctival and scleral dissection and antifibrotic agents.
Keywords: Ab interno glaucoma surgery, angle surgery, childhood glaucoma, goniotomy, secondary goniotomy
Glaucoma in children is a potentially blinding disease that poses challenges in both diagnosis and management. Blindness due to glaucoma may occur even with appropriate treatment.[1,2] The Childhood Glaucoma Research Network (CGRN)[3] has put in place a universally accepted system of classification of childhood glaucoma. Patients with isolated trabeculodysgenesis are deemed to have “primary congenital glaucoma (PCG)” “Secondary non-acquired glaucoma” is an umbrella term for glaucomas associated with ocular or systemic abnormalities in addition to the trabecular dysgenesis.
Pediatric glaucoma is primarily a surgical disease. The unfavorable efficacy and lack of long-term safety profile have resulted in a limited use of medical treatment.[4] Over the years, it has been widely debated which primary surgical method is to be preferred in patients with PCG. Angle surgery which mainly includes goniotomy and traditional trabeculotomy with a Harm's trabeculotome has remained a major part of the surgical management of childhood glaucoma, with similar success reported between these 2 techniques (especially if repeated), ranging from 47% to 90%.[4,5,6,7,8] The choice between these 2 approaches is mainly based on surgeon preference and corneal clarity. Goniotomy scores over other surgical procedures owing to its efficacy and low rate of complications. The conjunctiva is not violated, Mitomycin C is not used, and if angle surgery fails, more surgical choices are available afterwards compared to if a trabeculectomy or a combined trabeculotomy with trabeculectomy was the initial surgery.
In comparison to the high success rates of goniotomy in the Western population, it has been widely believed that in South East Asian and the Middle East where the age of onset is earlier and more cases are familial, the poor corneal clarity precludes goniotomy.[9,10] However there is no literature available of goniotomy in either Indian eyes or Middle Eastern eyes.
We run a large pediatric glaucoma service in our Institution and do come across many children with PCG who have reasonably clear corneas which would be amenable to a goniotomy procedure. We started goniotomy in our Institution in July 2017 and found it to be a useful surgical procedure in Indian eyes with PCG also (Unpublished data). We also used goniotomy as a re-operation procedure to treat the residual angle of failed trabeculectomy procedures in children in whom the cornea was clear enough for the procedure.
We present a prospective evaluation of goniotomy done for all kinds of congenital glaucoma, both as a primary and as a re-operation procedure. We believe this is the first paper describing results of goniotomy in Indian eyes.
Methods
In this prospective clinical cohort study carried out in a single center in Northwest India, consecutive children with glaucoma requiring surgical lowering of IOP who presented in the 3-year period between 1st July 2017 and 30th June 2020, and underwent goniotomy, were prospectively recruited and analyzed. The study adhered by the tenets of the Declaration of Helsinki, and the Institutional Ethics Committee gave ethical clearance for the study (vide INT/IEC/866). Informed consent was taken from parents/legal guardians of all patients, for participation in the study and for using pictures of the children for academic purposes only.
All infants underwent a comprehensive history and a preliminary ocular examination with torchlight. Those with any two of the following features underwent a detailed examination under anesthesia (EUA): A large-sized eye, cloudy cornea, or a cup-disc ratio >0.3 if visible or cupping determined on ocular B-scan ultrasonography. Epiphora and photophobia were considered corroborating factors.
Examination under anesthesia
A detailed examination was done under anesthesia using Sevoflurane. Intraocular Pressure (IOP) and Central corneal thickness ((CCT) was measured using a Perkins tonometer (Haag-Streit, Koeniz, Switzerland) and an ultrasonic pachymeter (SP-100, Tomey Corporation, Nagoya, Japan), respectively. Corneal diameters were measured using Castroviejo's calipers. The corneal clarity was graded objectively by a four-stage system suggested by Gupta et al.,[11] with modification.[12] Goniosocpy was done with the Ahmed Direct goniolens (Ocular Instruments Inc., WA, USA), and angles were visualized using the operating microscope in eyes with a corneal clarity of Grade 2 or better. Angle details were classified as Type 1 or Type 2, as classified by Sampaolesi.[13] The axial length was measured using A-Scan ultrasonography (AL-100, Tomey Corporation, Nagoya, Japan). If the corneal haze did not allow fundus evaluation, optic disc cupping was evaluated on B-scan ultrasonography (HiScan touch unit, Optikon, Roma, Italy). Any excavation in the optic nerve head was noted. Previous studies have indicated that detection of an optic disc cup on ultrasound denotes cupping of greater than 0.6.[14,15]
A clinical diagnosis of glaucoma was made if two of the following criteria were present: IOP >18 mmHg on repeated testing or when IOP <18 in the presence of corneal changes such as Haab's striae, limbal stretching or corneal edema; Optic cup-disc asymmetry of ≥0.2, focal rim thinning or optic-disc cupping on USG in eyes where the posterior segment was not visualized due to the corneal haze,; Corneal changes/findings: Haab's striae or corneal diameter >11 mm in a newborn, >12 mm in child up to 1 year and >13 mm at any age; and axial length more than that expected for age. Any increase in axial length beyond that expected for the normal growth of the eye for age even if the IOP was not high, was considered suspicious. Since the axial length grows rapidly within the first 6 months, no set criteria were defined. The axial length was routinely plotted against a published axial length growth curve in normal children and any increase from the normative data was taken as increased.
Children had to have a corneal clarity of at least Grade 2 to be eligible for goniotomy. We did not do epithelial scraping in any infant with a hazy cornea. Any eye with a corneal clarity precluding visualization of the angle underwent a combined trabedulotomy with trabeculectomy. We feel that the risk of infection in our population is too high to take that risk. Patients who had no prior glaucoma surgery were considered to be in the primary surgery group and those with previous trabeculotomy-trabeculectomy surgery with cornea clear enough for a goniotomy were deemed to have a Re-do goniotomy.
Surgical procedure
The surgery was performed from the temporal side, treating the nasal angle as much as possible. A Swan Jacob lens was placed on the cornea, after lubricating the surface with sodium hyaluronate, and the angle was brought in to clear view. Pilocarpine 2% was injected into the anterior chamber to constrict the pupil. Goniotomy was performed using a microvitreoretinal (MVR) blade or a 24-gauge needle mounted on a syringe loaded with a dispersive viscoelastic (Hydroxy propyl methyl cellulose 2%). Successful goniotomy was indicated by the anteriorly inserted iris falling back with formation of a cleft in the angle [Fig. 1]. The incision was closed with a single 10-0 nylon suture.
Figure 1.
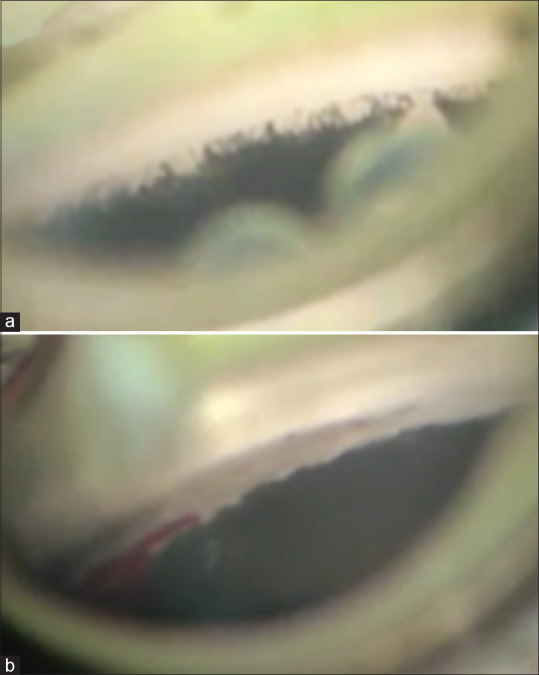
(a) Intraoperative view through the Swan Jacob lens of the untreated angle; (b) Intraoperative appearance of the same angle after goniotomy. Note the large cleft in the angle and the iris that has fallen back
Post-operatively, the mother was instructed to keep the child's head tilted towards the side of the surgery to ensure any hyphema would be directed away from the raw incised area. All infants were treated with topical Betamethasone sodium phosphate 0.1% 2-hourly, Tobramycin 0.3% drops q.i.d., and Pilocarpine 2% drops t.i.d for 4 weeks. Betamethasone drops were then tapered weekly and stopped.
Follow-up
After surgery the patients underwent an examination-under-anesthesia (EUA) at six weeks to three months, six months, and then as and when required. Children with a visual axis clear enough to permit refraction were tested for refractive errors. Visual status was assessed using the Cardiff-Acuity-Test and expressed in the LOGMAR scale for near vision. The visual status was determined as per the international Council of Ophthalmology (ICO)[16] criteria as Good (LOGMAR 0-0.5), moderate impairment (LOGMAR 0.6-1.0), severe impairment (LOGMAR 1.1-1.8) and blind (<1.8).
Patients completing a minimum follow-up of 1 year were analyzed.
Primary outcome measures
Primary outcomes were measured in terms of IOP control and requirement of additional glaucoma surgery. Previous reports of childhood glaucoma have taken ≤21 mm Hg as success criteria. However, since the normal IOP in an infant is less than that of adults[17] we used a more stringent criteria of ≤18 mm Hg to define success rates. Outcomes were defined as complete success, qualified success, and failure, as follows: Complete success: when IOP was ≤18 mm Hg without drugs; Qualified success: when IOP was ≤18 mm Hg with up to two topical drugs; Failure: when IOP was >18 mm Hg on two topical drugs, there was a need for systemic drugs for IOP control or requirement of re-surgery.
Children were considered for re-surgery if any of the following were present on the post-operative evaluations
IOP >18 on 2 topical drugs
Progressive increase in corneal diameter or optic disc cupping on 2 consecutive visits even in the presence of “target” IOP.
Any increase in axial length beyond that expected for the normal growth of the eye for age even if the IOP was not high. The axial length was routinely plotted against a published axial length growth curve in normal children.[18] The change in the growth slope following surgery was recorded and visually inspected to see whether it lay between 95% confidence intervals or grew at a pace greater than expected, or parallel to the normal growth curve.
A second goniotomy was not considered as an additional surgery.
Secondary outcome measures
The secondary outcome measures were determined in terms of additional procedures required and complications. Primary goniotomy and goniotomy done as a re-do surgery were analyzed separately. We stratified patients undergoing primary goniotomy based upon the age of surgery. We analyzed success rates of infants operated prior to two months, between two-twenty four months and more than twenty-four months of age.
Statistical methods
IBM-SPSS® software version 21.0 was used for statistical analysis. Since the two eyes of the patients were clinically dissimilar (Correlation coefficient -0.018; P = 0.887), they were considered as two separate entities. Baseline characteristics associated with successful outcome was analyzed using logistic regression analysis. Outcomes between sub-groups (PCG vs Non-PCG, primary vs secondary goniotomy) were compared. Kaplan Meier survival plots were calculated for PCG and non-PCG groups.
Results
1140 new children with glaucoma presented to our service in the 3-year period. Of a total of 454 pediatric glaucoma surgeries done, 172 eyes of 126 children underwent goniotomy (37.9% of all surgeries). Goniotomy comprised 132 of 211 (62.5%) primary surgeries and 40/243 (16.5%) of re-do surgeries. 87 children were male (69.2%). In the primary surgery group, 39 of the 93 children underwent bilateral goniotomy. Of these 132 eyes, 97 were neonatal or infantile PCG, 13 were late-onset PCG, and 23 were non-PCG eyes. The non-PCG eyes comprised 9 eyes of Sturge Weber Syndrome, 7 Axenfeld Reiger anomaly, 3 Juvenile open angle glaucoma, 2 post cataract surgery glaucoma and one eye each was steroid induced glaucoma and uveitic glaucoma. The numbers were too small to analyze each individually, so they were analyzed as a Non-PCG group as a whole. 7 of the 33 children in the re-operation group underwent bilateral goniotomy. 30 of the 40 re-do surgeries were in PCG eyes.
Baseline characteristics
Children with PCG (other than late-onset) were significantly younger than those with non-PCG (P < 0.001), [Table 1]. The corneal diameter in PCG eyes was significantly greater, and the cornea was significantly more cloudy than non -PCG eyes (P = 0.025 and P = 0.004, respectively). There was no difference in the IOP and CCT between any patient groups.
Table 1.
Baseline Characteristics of children undergoing goniotomy
| Diagnosis | Age at presentation Mean±SD (months) | IOP (Mean±SD) (mm Hg) | Axial Length (Mean±SD) (mm) | Corneal Diameter (Mean±SD) (mm) | Corneal thickness (Mean±SD) (microns) | Antiglaucoma Medications (Mean±SD) |
|---|---|---|---|---|---|---|
| Primary Surgery | ||||||
| Neonatal onset PCG* -(n=10; 5 patients) | 0.6±0.2 | 22.4±7.2 | 18.7±2.2 | 10.8±1.1 | 647±137.3 | 1.0±0.4 |
| Infantile onset PCG -(n=84; 48 patients) | 7.4±4.7 | 23.0±9.1 | 22.0±1.9 | 12.9±1.1 | 611±91.9 | 1.2±0.6 |
| Late-onset PCG -(n=15; 8 patients) | 86.6±42.1 | 24.0±8.9 | 26.6±3.1 | 13.1±1.1 | 577±71.0 | 2.0±1.1 |
| Non-PCG (n=23; 32 patients) | 24.3±43.8 | 23.1±8.7 | 22.8±2.8 | 12.7±1.1 | 610±94.0 | 1.34±0.8 |
| Total (n=132 eyes; 93 patients) | 24.3±43.8 | 23.1±8.7 | 22.8±2.8 | 12.7±1.1 | 610±94.0 | 1.34±0.8 |
| Re-Do Surgery | ||||||
| PCG (n=30 eyes; 23 patients) | 21.6±33.4 | 26.2±6.5 | 24.2±2.3 | 13.9±1.42 | 590±86 | 2.3±1.3 |
| Non-PCG (n=10 eyes; 10 patients | 70.7±84.2 | 27.2±10.5 | 23.56±3.8 | 12.3±1.3 | 575±77.8 | 2.9±1.4 |
| Total (n=40 eyes; 33 patients) | 33.9±53.4 | 26.4±7.6 | 24.0±2.5 | 13.5±1.34 | 586±78.8 | 2.45±1.4 |
Follow-up
145, 112, and 54 eyes had a six months, 1-year and 2-year follow-up, respectively [Fig. 2]. The mean IOP reduction was greater, and antiglaucoma medication requirement was significantly less for primary goniotomy compared to re-do surgery. For children undergoing primary surgery, the corneal clarity grade significantly improved in both groups (from 1.1 ± 0.8 to 0.4 ± 0.6 in PCG eyes and 0.5 ± 0.7 to 0.08 ± 0.3 in the non-PCG eyes) at 1-year follow-up. In the re-operation group, the IOP and mean anti-glaucoma medications required were significantly greater in the non-PCG group compared to the PCG group at all time points (P = 0.001). There was no difference in the improvement in corneal clarity grade between the two patient groups (Mean 1.02 to 0.6 in PCG eyes and 1.05 to 0.3 in the non-PCG group [P = 0.079]).
Figure 2.
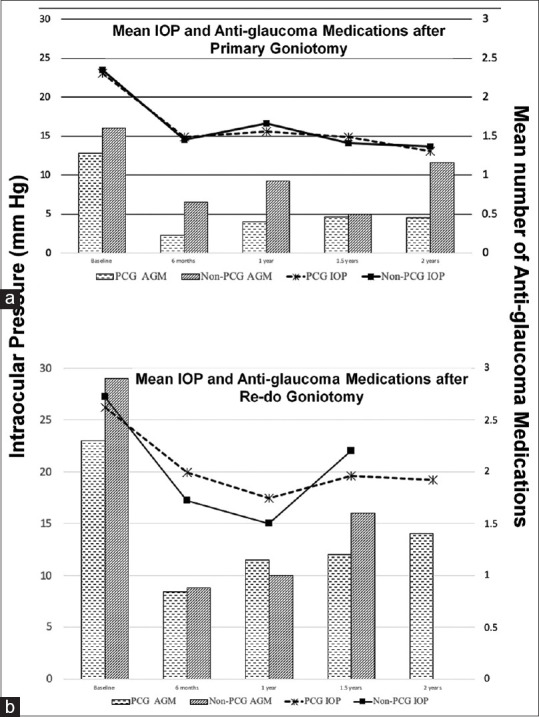
(a) Intraocular pressure (IOP) trend (line graph) and requirement of antiglaucoma medications (Bar graphs) after Primary goniotomy; (b)Intraocular pressure trend (line graph) and requirement of antiglaucoma medications (Bar graphs) after goniotomy done as a Re-do procedure
Success rates
Success rates are shown in Table 2. Five eyes (3 in the Primary surgery group and 2 in the re-do goniotomy group) underwent 2 goniotomies. This did not alter the success rates significantly. At 1 year, the overall success rates for primary surgery was 77% (79.6% for PCG; 61.5% for non-PCG; P = 0.34). The mean time to failure in PCG and non-PCG was 10.2 ± 6.4 months and 7.0 ± 2.4 months, respectively. For the re-operation group, the overall success was 60% (68.4% for PCG and 33.3% for non-PCG eyes; P = 0.16). At 2 years, the overall success in eyes undergoing primary surgery remained at 75.7% (74.2% for PCG and 83% for non-PCG eyes), but in those undergoing re-do surgery, 58.8% of eyes had failed.
Table 2.
Success rates of primary and re-do goniotomy at 1 and 2 years
| 1 year (n=112) | 2 years (n=54) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| PCG* (n=74) | Non-PCG (n=13) | Total (n=87) | PCG* (n=31) | Non-PCG (n=6) | Total (n=37) | |
| Primary Surgery | ||||||
| Complete Success | 49 (66.3%) | 7 (53.8%) | 56 (64.4%) | 21 (67.7%) | 5 (83.3%) | 26 (70.3%) |
| Qualified Success | 10 (13.3%) | 1 (7.7%) | 11 (12.6%) | 2 (6.5%) | 0 | 2 (5.4%) |
| Failure | 15 (20.3%) | 5 (38.5%) | 20 (23%) | 8 (25.8%) | 1 (16.7%) | 9 (24.3%) |
|
| ||||||
| PCG* (n=19) | Non-PCG (n=6) | Total (n=25) | PCG* (n=16) | Non-PCG (n=1) | Total (n=17) | |
|
| ||||||
| Re-do Surgery | ||||||
| Complete Success | 6 (31.6%) | 2 (33.3%) | 8 (32%) | 4 (25%) | 0 | 4 (23.5%) |
| Qualified Success | 7 (36.8%) | 0 | 7 (28%) | 3 (18.8%) | 0 | 3 (17.6%) |
| Failure | 6 (31.6%) | 4 (66.7%) | 10 (40%) | 9 (56.3%) | 1 | 10 (58.8%) |
*PCG: Primary Congenital Glaucoma
The outcome was also analyzed according to the age at surgery [Table 3]. The success rate in infantile-onset PCG was 87.5% with one goniotomy. Late onset glaucoma had least success rates (55.6%). In the non-PCG group, there were no neonatal onset eyes, and the 11 infants between 2-24 months were controlled with goniotomy. Fig. 3 depicts clinical pictures of primary and re-do surgery.
Table 3.
Outcome in patients undergoing primary surgery stratified according to age at surgery
| Age of surgery | PCG (n=91) | Non PCG (n=20) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Success | Failure | Total | Success | Failure | Total | |
| Neonatal onset (Birth to 2 months) | 8 (57.1%) | 6 (42.9%) | 14 | 0 | ||
| Infantile onset (24 months) | 56 (87.5%) | 8 (12.5%) | 64 | 11 | 0 | 11 |
| Late onset (> 2 Years) | 7 (55.6%) | 6 (46.2%) | 13 | 2 (22.2%) | 7 (77.8%) | 9 |
| TOTAL | 71 (78%) | 20 (22%) | 91 | 13 (65%) | 7 (35%) | 20 |
Figure 3.
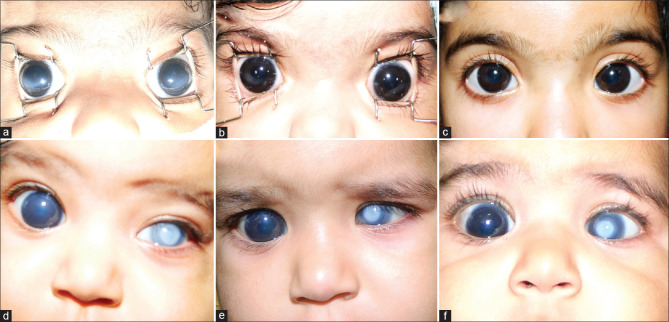
(a) Preoperative picture of a baby with neonatal onset glaucoma. Note the bilateral corneal haze and large corneas. (b) Postoperative appearance of the same baby 9 months after surgery. Note the significantly clear cornea. (c) Postoperative appearance of same baby at 2 years of follow-up. (d) Pre-operative picture of a baby with primary congenital glaucoma (PCG) in the right eye and Peter's anomaly with glaucoma in the left eye. She underwent combined trabeculotomy-with-trabeculectomy in both eyes. (e) The cornea cleared moderately in the right eye, but the IOP was 20.0 mm Hg on antiglaucoma medication. She needed another surgery and underwent goniotomy of the untreated angle in the right eye. (f) Post-operative appearance of the same baby 6 months after surgery. Note the significantly clearer cornea in the right eye and peripheral clear cornea in the left eye
Baseline characteristics predictive of success
In the primary surgery group, success rates were significantly negatively correlated to IOP (P = 0.03) and axial length (P = 0.02) at presentation. The median time to failure was 6 months. 13.6% of PCG failed compared to 26% of Non-PCG. The mean time to failure in the two groups was 10.3 ± 6.4 and 7.0 ± 2.4 months, respectively, but the difference was not statistically significant (P = 0.08). Kaplan Meier plots are depicted in Fig. 4.
Figure 4.
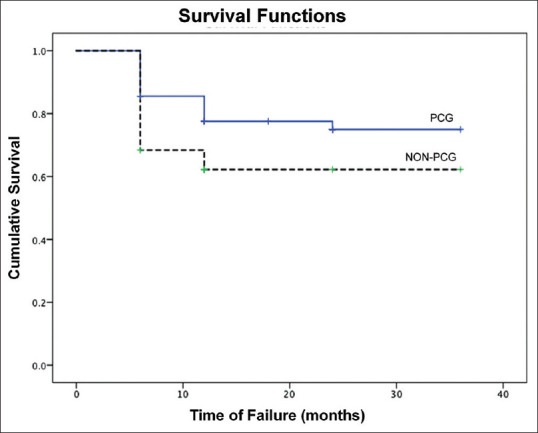
Kaplan–Meier survival analysis plots of Primary congenital glaucoma (PCG) and non-PCG eyes
Additional surgery
At 1 year follow-up 70/87 (80.5%) of eyes undergoing primary goniotomy required no additional glaucoma surgery. Table 4 depicts eyes requiring additional surgery. Additional surgery after 1 year follow-up is also included. 17 of 25 (68%) eyes of patients undergoing Goniotomy as a re-do procedure required no additional surgery. The outcome in PCG eyes were significantly better than non-PCG (83.8% versus 61.5%; P = 0.012). The most commonly performed additional glaucoma procedure was Trabeculectomy with Mitomycin C (MMC).
Table 4.
Additional surgery required after Goniotomy
| Additional Surgery required by 1 year | Primary Goniotomy | Re-do Goniotomy | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| PCG* (n=74) | Non-PCG (n=13) | Total (n=87) | PCG (n=19) | Non-PCG (n=6) | Total (n=25) | |
| No Additional surgery | 62 (83.8%) | 8 (61.5%) | 70 (80.5%) | 14 (73.7%) | 3 (50%) | 17 (68%) |
| Trabeculectomy with MMC** | 11 (14.9%) | 3 (23.1%) | 14 (16.7%) | 1 (5.3%) | 0 | 1 (4.0%) |
| CTT# | 0 | 1 (7.1%) | 1 (1.1%) | 0 | 0 | 0 |
| GDD$ | 0 | 1 (7.7%) | 1 (1.1%) | 2 (10.6%) | 2 (33.3%) | 4 (16%) |
| DLCP& | 1 (1.4%) | 0 | 1 (1.1%) | 2 (10.6%) | 1 (16.7%) | 3 (12%) |
| Total requiring additional surgery | 12 (16.2%) | 5 (38.5%) | 17 (19.5%) | 5 (26.3%) | 3 (50%) | 8 (32%) |
*PCG: Primary Congenital Glaucoma; MMC**: Mitomycin C; CTT#: Combined Trabeculotomy with Trabeculectomy; GDD$: Glaucoma Drainage Device; DLCP&: Diode laser cyclophotocoagulation
Complications
There were no sight-threatening complications in any patient. The most common complication were varying grades of hyphema in the post-operative period, all of which resolved by one week. 2 eyes of the initial patients developed a cataract, most likely iatrogenic, and underwent phacoemulsification with IOL implantation subsequently.
Visual outcome
In children undergoing primary surgery, visual acuity assessment was available in 90 of the 112 eyes of patients who completed 1-year follow-up and is shown in Table 5. The mean refractive error in PCG and non-PCG eyes was -3.57 ± 4.7 and -2.82 ± 5.03, respectively. 50% had good vision (better than LOGMAR 0.5), 28.9% had moderate visual impairment (better than LOGMAR 1.0). 20% had severe visual impairment or were blind. There was no significant differences observed between the PCG and non-PCG groups (P = 0.68).
Table 5.
Visual outcomes 1 year after primary goniotomy surgery
| Visual Outcomes (LOGMAR) | PCG (n=76) | Non-PCG (n=14) | Total (n=90) |
|---|---|---|---|
| Good Vision (0-0.5) | 36 (47.4%) | 9 (64.3%) | 45 (50%) |
| Moderate impairment (0.6-1.0) | 23 (30.3%) | 3 (21.4%) | 26 (28.9%) |
| Severe impairment (1.1-1.8) | 11 (14.5%) | 1 (7.1%) | 12 (13.3%) |
| Blind (worse than 1.8) | 6 (7.9%) | 1 (7.1%) | 7 (7.8%) |
| Total | 76 | 14 | 90 |
Discussion
Childhood glaucoma is a treatable cause of blindness provided it is recognized, diagnosed and treated in time.[19,20] It was considered a universally blinding disease until Barkan first described the technique of goniotomy in 1938.[5] Goniotomy reduces IOP by incising the abnormal trabecular meshwork, allowing access of aqueous to the Schlemm's canal and outflow channels. The best results have been reported with PCG where there is an isolated trabeulodysgenesis.[21]
A study from India in 1983 reported that 80% of congenital glaucoma cases presented with hazy cornea.[22] Following Mandal et al.’s[23,24] reported success with combined trabeculectomy with trabeculotomy (CTT), his procedure has largely been the most commonly done surgery across the spectrum of childhood glaucoma in India. There have been no reports of the outcome of goniotomy in Indian eyes. Though it is believed that goniotomy is not possible in the vast majority of children with congenital glaucoma in India, owing to severe corneal haze,[9] our experience was different. We were able to successfully complete a goniotomy procedure in 132 of the 211 primary pediatric glaucoma surgeries (62.6%) in the 3 years period of the study. Our results were similar to those reported in Western literature.
The expected outcome of goniosurgery for primary congenital glaucoma has a reported success rate of over 80%.[25] Mendicino et al.[26] studied their goniotomy outcome over 9 years in PCG eyes undergoing primary surgery. They reported an 89% success rate at 1 year, which dropped to 57.5% at the last follow-up (mean follow-up 9 years). Shaffer[21] analyzed 287 operated eyes and reported that one or two goniotomies cured 94% of patients diagnosed with glaucoma between 1 month and 24 months of age. This success rate dropped to 30% when glaucoma was present at birth or after the age of 2 years. However, the success criteria adopted was IOP <21 mm Hg at 6 months post-operatively. Broughton and Parks[27] reported an overall 88% success rate (with a mean follow-up of 5 years) after one or more goniotomies in their 20-year experience with 50 eyes of patients who underwent goniotomy.
Most papers have indicated that the best results of goniotomy appear to be in infantile PCG. Newborn glaucoma[21,27,28] and glaucoma secondary to ocular anomalies[29] appear to have a less optimal surgical outcome. Patients with newborn glaucoma have been reported to have a lower success rate with goniotomy.[16,24,25,27] Hassanein et al.[30] found that a positive consanguinity of the parents and surgery before the end of the first month are the major predictors of failure of goniotomy. Berger et al.[8] in a retrospective analysis reported qualified success in goniotomy was 37%. They attributed the low success rate to the inclusion of previously treated eyes and secondary glaucomas known to be associated with a lower success rate compared to PCG.
Our success rate in infantile-onset PCG was 87.5% with one goniotomy. Neonatal-onset and late onset PCG had lesser success rates (57.1% and 55.6%, respectively), but still better than reported in literature. Our overall success rates in secondary pediatric glaucomas (Non-PCG) and re-do surgeries were reasonably good at 70% and 65%, respectively. Our success rates must also be viewed keeping in mind our success criteria, which was more stringent than those of reported studies. Previous studies have kept 21.0 mm Hg as their success criteria.[21,25,26] We defined an IOP limit of 18 mm Hg and allowed only 2 topical medications before considering the goniotomy a failure. For a meaningful comparison with published literature, we should have compared our results keeping the same criteria. However, since the IOP in our infants were not allowed to reach 21.0 mm Hg, that comparison was not possible.
Currently, angle surgery is considered in terms of 360 degree surgery (360 degree trabeculotomy by either ab-interno or ab-externo approaches) Since we usually treat 150-180 degrees nasally, with one goniotomy, and the second goniotomy simply treats the untreated temporal angle, we considered that as one surgery (since the whole angle was not completed in one sitting). Only five of our patients underwent a second goniotomy, and it did not change our analysis significantly. We concede that we may have tried a second procedure in more eyes, but we were new to the technique and were not sure whether a second goniotomy procedure would work. We felt it safe to go ahead with the more familiar procedure trabeculectomy that we had been doing for many years. In the present time, our second procedure of choice is goniotomy of the temporal angle and early results seem encouraging. Catalano et al.[31] evaluated seven infants with bilateral PCG, in whom one eye underwent a single goniotomy, and the other eye underwent two separate goniotomies. The authors found no significant difference between both eyes and did not recommend routinely using this approach as the initial surgical procedure.
Visual outcomes in early onset childhood glaucoma are reported to be less favorable. The decreased vision is reportedly caused by breaks in Descemet's membrane (Haabs’ stirae) in the visual axis and amblyopia due to anisometropia and astigmatism. 53% of PCG eyes in Shaffer et al.’s[21] series and 52.5% in Mendicino’s[26] series achieved good vision (>20/50) and 25% and 15% had poor visual outcome (<20/200), respectively. Our overall results were similar (50% good vision, 28.5% moderate impairment and 21% severe impairment, respectively).
Our preliminary results are encouraging. Though the follow-up is short, in the 37 infants who completed a 2-year follow-up after primary surgery, the overall success was still 75.8%, and the mean IOP was maintained below 15 mm Hg in all patients. Our median time to failure was 6 months, which prompts us to think that the 2-year results may endure.
Conclusion
Our study showed that goniotomy could be successfully offered as initial surgery in more than half of the cases of childhood glaucoma. Goniotomy in Indian eyes appears to have favorable outcomes in both newborn and infantile types of primary congenital glaucoma when performed as a primary surgery. Its advantages of no conjunctival dissection, no anti metabolite use and an excellent safety profile should prompt us to consider this in our armamentarium of surgeries for congenital glaucoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kargi SH, Koc F, Biglan AW, Davis JS. Visual acuity in children with glaucoma. Ophthalmology. 2006;113:229–38. doi: 10.1016/j.ophtha.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Allingham RR, Damji KF, Freedman S, Mori SE, Shafranov G, editors , editors. Congenital glaucoma. Shield's Textbook of Glaucoma. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 235–52. [Google Scholar]
- 3.Beck AD, Chang TCP, Freedman SF, Weinreb RN, et al. Childhood Glaucoma: Consensus Series 9. Amsterdam: Kugler; 2013. “Definition, Classification, Differential Diagnosis.”. [Google Scholar]
- 4.Sacchi M, Lizzio RAU, Villani E, Monsellato G, Lucentini S, Cremonesi E, et al. Medical management of pediatric glaucoma: Lessons learned from randomized clinical trials. Graefes Arch Clin Exp Ophthalmol. 2020;258:1579–86. doi: 10.1007/s00417-020-04767-9. [DOI] [PubMed] [Google Scholar]
- 5.Barkan O. Goniotomy for congenital glaucoma; urgent need for early diagnosis and operation. J Am Med Assoc. 1947;133:526–33. doi: 10.1001/jama.1947.02880080018005. [DOI] [PubMed] [Google Scholar]
- 6.Akimoto M, Tanihara H, Negi A, Nagata M. Surgical results of trabeculotomy ab externo for developmental glaucoma. Arch Ophthalmol. 1994;112:1540–4. doi: 10.1001/archopht.1994.01090240046024. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DR. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology. 1983;90:805–6. doi: 10.1016/s0161-6420(83)34484-5. [DOI] [PubMed] [Google Scholar]
- 8.Berger O, Mohamed-Noriega J, Low S, Daniel MC, Petchyim S, Papadopoulos M, et al. From conventional angle surgery to 360-degree trabeculotomy in pediatric glaucoma. Am J Ophthalmol. 2020;219:77–86. doi: 10.1016/j.ajo.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Mandal AK, Chakrabarti D. Update on congenital glaucoma. Indian J Ophthalmol. 2011;59(Suppl 1):S148–57. doi: 10.4103/0301-4738.73683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandal AK, Gothwal VK, Nutheti R. Surgical outcome of primary developmental glaucoma: A single surgeon's long-term experience from a tertiary eye care centre in India. Eye (Lond) 2007;21:764–74. doi: 10.1038/sj.eye.6702324. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N, Kalaivani M, Tandon R. Comparison of prognostic value of Roper hall and Dua classification systems in acute ocular burns. Br J Ophthalmol. 2011;95:194–8. doi: 10.1136/bjo.2009.173724. [DOI] [PubMed] [Google Scholar]
- 12.Choi H, Phillips C, Oh JY, Stock EM, Kim DK, Won JK, et al. Comprehensive modeling of corneal alkali injury in the rat eye. Curr Eye Res. 2017;42:1348–57. doi: 10.1080/02713683.2017.1317817. [DOI] [PubMed] [Google Scholar]
- 13.Sampaolesi R, Zarate J, Sampaolesi JR. Pathological chamber angle in congenital glaucoma and its implications in indications for surgery. The Glaucomas Volume 1 Pediatric Glaucomas. Springer-Verlag Berlin Heidelberg. 2009:107–10. [Google Scholar]
- 14.Winder S, Atta H. Ultrasonography of the optic disc cup in discs of various sizes. Eye. 1996;10:732–6. doi: 10.1038/eye.1996.170. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JS, Stone RD, Hetherington J, Jr, Bullock J. Glaucomatous cupping of the optic disk by ultrasonography. Am J Ophthalmol. 1976;82:24–6. doi: 10.1016/0002-9394(76)90659-0. [DOI] [PubMed] [Google Scholar]
- 16.Colenbrander A : Aspects of vision loss–visual functions and functional vision. Visual Impairment Res. 2002;5:115–36. [Google Scholar]
- 17.Fayed MA, Chen TC. Pediatric intraocular pressure measurements: Tonometers, central corneal thickness, and anesthesia. Surv Ophthalmol. 2019;64:810–25. doi: 10.1016/j.survophthal.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Sampaolesi R, Caruso R. Ocular echometry in the diagnosis of congenital glaucoma. Arch Ophthalmol. 1982;100:574–7. doi: 10.1001/archopht.1982.01030030576003. [DOI] [PubMed] [Google Scholar]
- 19.Foster A, Gilbert C. Epidemiology of childhood blindness. Eye. 1992;6:173–6. doi: 10.1038/eye.1992.34. [DOI] [PubMed] [Google Scholar]
- 20.Preventing blindness in children: report of WHO/IAPB scientific meeting. Geneva: WHO; 2000. (WHO/PBL/00.77.) [Google Scholar]
- 21.Shaffer RN. Prognosis of goniotomy in primary infantile glaucoma (trabeculodysgenesis) Trans Am Ophthalmol Soc. 1982;80:321–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal HC, Sood NN, Kalra BR. Clinical presentation of congenital glaucoma. Indian J Ophthalmol. 1983;31:619–22. [PubMed] [Google Scholar]
- 23.Mandal AK, Naduvilath TJ, Jayagandan A. Surgical results of combined trabeculotomy-trabeculectomy for developmental glaucoma. Ophthalmology. 1998;105:974–82. doi: 10.1016/S0161-6420(98)96022-5. [DOI] [PubMed] [Google Scholar]
- 24.Mandal AK, Gothwal VK, Bagga H, Nutheti R, Mansoori T. Outcome of surgery on infants younger than 1 month with congenital glaucoma. Ophthalmology. 2003;110:1909–15. doi: 10.1016/S0161-6420(03)00671-7. [DOI] [PubMed] [Google Scholar]
- 25.de Luise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma) Surv Ophthalmol. 1983;28:1–19. doi: 10.1016/0039-6257(83)90174-1. [DOI] [PubMed] [Google Scholar]
- 26.Mendicino ME, Lynch MG, Drack A, Beck AD, Harbin T, Pollard Z, et al. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. J AAPOS. 2000;4:205–10. doi: 10.1067/mpa.2000.106201. [DOI] [PubMed] [Google Scholar]
- 27.Broughton WL, Parks MM. An analysis of treatment of congenital glaucoma by goniotomy. Am J Ophthalmol. 1981;91:566–72. doi: 10.1016/0002-9394(81)90054-4. [DOI] [PubMed] [Google Scholar]
- 28.Walton DS, Katsavounidou G. Newborn primary congenital glaucoma: 2005 update. J Pediatr Ophthalmol Strabismus. 2005;46:333–41. doi: 10.3928/01913913-20051101-01. [DOI] [PubMed] [Google Scholar]
- 29.Al-Hazmi A, Awad A, Zwaan J, Al-Mesfer SA, Al-Jadaan I, Al-Mohammed A. Correlation betweensurgical success rate and severity of congenital glaucoma. Br J Ophthalmol. 2005;89:449–53. doi: 10.1136/bjo.2004.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassanein DH, Awadein A, Elhilali H. Factors associated with early and late failure after goniotomy for primary pediatric glaucoma. Eur J Ophthalmol. 2020;30:162–7. doi: 10.1177/1120672118805872. [DOI] [PubMed] [Google Scholar]
- 31.Catalano RA, King RA, Calhoun JH, Sargent RA. One versustwo simultaneous goniotomies as the initial surgical procedure for primary infantile glaucoma. J Pediatr Ophthalmol Strabismus. 1989;26:9–13. doi: 10.3928/0191-3913-19890101-04. [DOI] [PubMed] [Google Scholar]