Abstract
Microorganisms of the genus Abiotrophia, members of the oral flora, are known as important causes of bacterial endocarditis. In this study, we report two individual cases of acute vitreous infection caused by Abiotrophia adiacens and Abiotrophia defectiva approximately a week after cataract extraction. Abiotrophia isolates were recovered by cultivation of vitreous humor on chocolate agar and identified via conventional and API 20 Strep identification systems. An 83-year-old male patient (A) and an 80-year-old female patient (B) demonstrated almost identical symptoms of infectious endophthalmitis manifested as hypopyon and opaque media. The vision of both patients was reduced to detection of hand motion in the left and the right eyes, respectively. An emergency pars plana core vitrectomy was performed, and intraocular antibiotics were administered to each patient, who presented 8 months apart in two different institutions. Patients A and B were treated with an intravitreal injection of vancomycin-amikacin and vancomycin-ceftazidime, respectively, which resulted in complete recovery.
Species of the genus Abiotrophia, formerly known as nutritionally variant streptococci (NVS), and members of the oral and upper respiratory flora have been known to be important causes of bacterial endocarditis and bacteremia and may responsible for some cases of culture-negative endocarditis (3, 9, 21, 23). They usually appear as small satellite colonies around colonies of associated bacterial species. The growth of these nonhemolytic or alpha-hemolytic satelliting colonies is usually supported by many gram-positive and gram-negative bacteria. The microscopic morphology of the organisms is medium dependent because they vary from typical gram-positive streptococci to gram-variable enlarged pleomorphic coccobacilli (2). Pyridoxal hydrochloride at a concentration of 0.001% and l-cysteine at a concentration of 0.01% are the necessary medium constituents for the growth of these NVS (25). In general, their growth in unsupplemented tryptic soy agar with 5% sheep blood or chocolate agar is either absent or variable, respectively (19, 22). Sulfhydryl-containing compounds added to basal media can negate the nutritional defect and support the growth of Abiotrophia spp. Additionally, successful cultivation of Abiotrophia spp. in thioglycolate or thiol broth has been reported (7, 10, 23).
Some investigators have reported a close resemblance between Abiotrophia spp. and viridans streptococci, based on the biochemical characteristics of Abiotrophia spp. determined with pyridoxal-supplemented media (4, 7, 23). Further evidence suggested a close relationship to Streptococcus mitis. In 1989, Bouvet and coworkers proposed two new species for these organisms, including Streptococcus defectivus and Streptococcus adjacens, which could be differentiated biochemically (3). Subsequently, the 16S rRNA sequences of the type strains of these organisms classified them in the new genus Abiotrophia as A. defectiva and A. adiacens, respectively (17). A newly described species, Abiotrophia elegans, which requires l-cysteine hydrochloride growth factor instead of pyridoxal hydrochloride, has been reported as a possible cause of culture-negative endocarditis (26). In addition to being an important cause of bacterial endocarditis, this group of organisms have also been implicated in cirrhosis, pancreatic abscess, otitis media, wound infections, conjunctivitis, and keratitis (1, 2, 4, 16, 18).
In this study, we report two cases of acute vitreous infection caused by Abiotrophia spp. approximately 1 week after cataract extraction. These cases appear to be the first reports of endophthalmitis caused by Abiotrophia spp. in the medical literature.
Case reports. (i) Patient A.
An 83-year-old man who had had a cataract extracted from the left eye 5 days earlier was admitted to the hospital with the diagnosis of endophthalmitis. On examination, he was noted to have a reduced visual response to hand motion, hypopyon, a severe anterior chamber reaction, and vitreous infection. His past medical history consisted of hypertension, asthma, and prostate carcinoma. On admission, a pars plana vitrectomy was performed with intravitreal injection of amikacin and vancomycin, and, postoperatively, the patient was placed on topical cefazolin and tobramycin, as well as oral steroids. A sample of his vitreous fluid was submitted for microbiological analysis. Initially, the patient showed a slight improvement, but due to complete regression, vitrectomy and intravitreal administration of antibiotics were repeated 5 days after surgery. Subsequently, his endophthalmitis resolved completely without further complications, and the patient was discharged on a regimen of topical atropine, tobramycin, and cefazolin.
(ii) Patient B.
Patient B, an 80-year-old diabetic female, was admitted with an acute infectious endophthalmitis of the right eye. She had undergone cataract extraction and implant surgery 6 days prior to admission. She was afflicted with mild discomfort in the eye and near loss of vision at the level of hand motion. Her examination revealed a grossly purulent anterior chamber fluid and opaque media. Due to the rapid development of a rather fulminant endophthalmitis, with the possibility of blindness, the patient immediately underwent surgery, which included an anterior chamber paracentesis for therapeutic purposes as well as for anterior chamber cleaning. A subtotal vitrectomy was followed by an intraocular and subconjunctival injection of vancomycin and ceftazidime. The purulent vitreous fluid specimen was submitted to the laboratory for microbiological evaluation. Due to her relatively severe corneal edema and a poorly dilated pupil, a complete vitrectomy was not possible. Postoperatively, the patient recovered with no complications and was discharged the next day on Tylenol, Diamox acetazolamide (Lederle), and ciprofloxacin.
Microbiology.
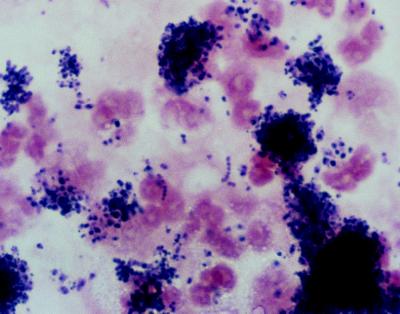
The purulent vitreous aspirates collected during surgery were inoculated onto brucella agar; chocolate agar; 5% sheep blood agar; MacConkey agar; Sabouraud’s dextrose agar; or brain heart infusion agar supplemented with 5% sheep blood, gentamicin, and chloramphenicol or inoculated into vitamin-K1 hemin-enriched thioglycolate broth (Becton Dickinson Microbiology Systems, Cockeysville, Md.). The inoculated media were incubated anaerobically (brucella agar) and aerobically in 5% CO2 at 35°C for bacterial isolation and at 25°C for fungal isolation. Smears were prepared from the aspirates for Gram staining and KOH analysis. Gram-stained smears in both cases were remarkable for many neutrophils and aggregations of small gram-positive cocci in chains and pairs (Fig. 1). After 48 h of incubation, primary cultures on chocolate agar grew small pinpoint colonies, which, upon Gram staining, showed gram-variable pleomorphic coccobacilli. Because growth did not occur on any other media, a nutritionally variant Abiotrophia strain was suspected, and the isolates were tested for satellitism around Staphylococcus aureus, showing alpha-hemolytic colonies adjacent to an S. aureus streak. Satellite growth also occurred around a pyridoxal disk superimposed onto a lawn of the isolates on blood agar. The Abiotrophia isolates did not grow in unsupplemented tryptic soy broth (TSB). However, they grew abundantly (turbidity of about a no. 2 McFarland standard) when inoculated into TSB supplemented with 20 μg of pyridoxal hydrochloride per ml, as well as in TSB containing 200 μg of l-alanine and l-cysteine per ml (Sigma, St. Louis, Mo.). By using the API 20 Strep identification system (bio-Merieux Vitek, Inc., Hazelwood, Mo.) inoculated with a 24-h growth from blood agar supplemented with 20 μg of pyridoxal hydrochloride per ml, isolates A and B were identified as A. adiacens and A. defectiva, respectively.
FIG. 1.
Direct Gram stain of vitreous aspirate from patient A. The micrograph is remarkable for the many polymorphonuclear neutrophils and aggregations of gram-positive cocci in pairs and chains.
Antibiotic susceptibility testing of the Abiotrophia strains was performed with gram-positive MIC type 6 dried MicroScan panels (Dade International, Inc., West Sacramento, Calif.). The diluent of Prompt Inoculation bottles (Dade International, Inc.) was supplemented with 20 μg of pyridoxal hydrochloride per ml prior to inoculation with the Abiotrophia strains, by replacement of 0.3 ml of the diluent with the same volume of 2-mg/ml filter-sterilized solution of pyridoxal hydrochloride and used according to the manufacturer’s recommendations. The control organisms S. aureus ATCC 29213 and Enterococcus faecalis ATCC 29212, inoculated into pyridoxal-containing diluent and unsupplemented diluent, rendered identical MIC results in both diluents for the antibiotics represented on gram-positive MIC type 6 dried MicroScan panels. A. adiacens was susceptible to penicillin, rifampin, vancomycin, tetracycline, and erythromycin (MICs of 0.03, ≤1, ≤2, ≤2, and ≤0.25 μg/ml, respectively) and resistant to clindamycin and gentamicin (MICs of 1 and 4 μg/ml, respectively). The antibiotic susceptibility results for the A. defectiva strain were rather different, because it was susceptible to penicillin, rifampin, vancomycin, clindamycin, and gentamicin (MICs of 0.06, ≤1, ≤2, ≤0.25, and ≤1, respectively) and resistant to tetracycline and erythromycin (MICs of 128 and 4 μg/ml, respectively).
Discussion.
Despite several published reports of Abiotrophia spp. conjunctivitis and keratitis in humans as well as corneal ulcers in horses, these microorganisms have not been reported as a cause of endophthalmitis. In this report, we document two cases of acute vitreous infections by A. adiacens and A. defectiva following cataract extraction in two elderly patients. Acute postoperative endophthalmitis is usually caused by ocular surface microflora, with the majority of cases caused by Staphylococcus species (20). This premise is supported by the genetic similarity among bacteria recovered from intraocular fluid, the ocular surface, and the nasal cavity of patients with acute postoperative endophthalmitis (27). Postoperative endophthalmitis, due to ocular surface microflora, is usually facilitated by preoperative risk factors, such as prosthesis in the eye, contact lens wear, contaminated eye drops, preexisting conjunctivitis, tear drainage obstruction, blepharitis, and dacryocystitis (20). In addition, chronic immunosuppressive therapy and diabetes mellitus are known to be significant risk factors for postoperative endophthalmitis (15). Under these circumstances, the inocula of ocular surface microflora may become markedly elevated or may be replaced by more virulent organisms. Entry of these organisms into the vitreous chamber can then be facilitated following cataract extraction by mechanical manipulation of the wound (29).
Viridans streptococci, including Abiotrophia spp., the predominant oral, pharyngeal, and occasional ocular surface microflora (19), have a tendency to colonize and infect abnormal cardiac valves and are responsible for 5 to 6% of bacterial endocarditis (23, 28). Additionally, Abiotrophia spp. have been implicated in neonatal conjunctivitis (1), infectious crystalline keratopathy (19), and equine microbial keratitis (11). The frequent colonization and infection of cornea and cardiac valves with Abiotrophia spp. may suggest their marked tendency to localize to avascular collagenous tissue. The acute purulent inflammation of vitreous fluid caused by Abiotrophia strains within 5 to 7 days post-cataract extraction in these patients supports the hypothesis that vitreous fluid may contain the required concentration of pyridoxal or other growth factors, including l-cysteine and l-alanine, that can offset the pyridoxal requirement.
The incidence of acute postoperative endophthalmitis following cataract extraction in conjunction with intraocular lens implantation has been reported to be between 0.07 and 0.12% (13, 15). Endophthalmitis is normally diagnosed by culture of vitreous humor and biopsies on the appropriate microbiological media. Additionally, some facilities, including ours, may directly inoculate blood culture bottles with vitreous fluid, which has been shown to be as effective as direct medium inoculation (14). Despite adherence to the diagnostic protocols mentioned above, Forster et al. have reported positive cultures of vitreous specimens in only 58% of the patients with clinically suspected infectious postoperative endophthalmitis (9). Their explanations for such a low recovery rate included the paucity of organisms, possible delay in transportation, and improper processing of specimens in the microbiology laboratory. We believe that a reasonable explanation for the lack of previous reports of intraocular infections with Abiotrophia spp. is the variable efficacy of microbiological media to support the growth and hence isolation and correct recognition of these nutritionally exacting microorganisms from ocular specimens (24, 30). If a Gram stain of vitreous fluid indicates gram-positive cocci in chains or pairs, the addition of pyridoxal-containing medium or cross-inoculation of the inoculated blood agar plate with S. aureus may increase detection of Abiotrophia isolates. Additionally, because the growth rate of Abiotrophia spp. is slower than that of viridans streptococci, prolonged incubation in an atmosphere containing 5 to 10% CO2 is routinely recommended. In both of our patients, the presence of many neutrophils in vitreous fluid, in conjunction with gram-positive cocci, was a clear indication of acute infection, which was treated immediately by intravitreous injection of vancomycin-amikacin and vancomycin-ceftazidime.
The antimicrobial susceptibilities of Abiotrophia spp. have been shown to vary, depending on the medium used for susceptibility testing (5, 12). According to Cooksey and Swenson, however, the MICs for Abiotrophia spp. examined by pyridoxal-supplemented agar dilution and broth microdilution methods were comparable (6). Notably, these pyridoxal hydrochloride-supplemented MicroScan gram-positive panels, used in our laboratory to test the antimicrobial susceptibility of Abiotrophia isolates for the past 2 years, have been found to be practical and useful.
REFERENCES
- 1.Barrios H, Bump C M. Conjunctivitis caused by a nutritionally variant streptococcus. J Clin Microbiol. 1986;23:379–380. doi: 10.1128/jcm.23.2.379-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottone E J, Thomas C A, Lindquist D, Janda J M. Difficulties encountered in identification of a nutritionally deficient streptococcus on the basis of its failure to revert to streptococcal morphology. J Clin Microbiol. 1995;33:1022–1024. doi: 10.1128/jcm.33.4.1022-1024.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvet A, Grimont F, Grimont P A D. Streptococcus defectivus sp. nov. and Streptococcus adjacens sp. nov., nutritionally variant streptococci from human clinical specimens. Int J Syst Bacteriol. 1989;39:290–294. doi: 10.1099/00207713-41-4-483. [DOI] [PubMed] [Google Scholar]
- 4.Carey R B, Gross K C, Roberts R B. Vitamin B6-dependent Streptococcus mitior (mitis) isolated from patients with systemic infections. J Infect Dis. 1975;131:722–726. doi: 10.1093/infdis/131.6.722. [DOI] [PubMed] [Google Scholar]
- 5.Clark R B, Gordon R E, Bottone E J, Reitano M. Morphological aberrations of nutritionally deficient streptococci: association with pyridoxal (vitamin B6) concentration and potential role in antibiotic resistance. Infect Immun. 1983;42:414–417. doi: 10.1128/iai.42.1.414-417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooksey R C, Swenson J M. In vitro antimicrobial inhibition patterns of nutritionally variant streptococci. Antimicrob Agents Chemother. 1979;16:514–518. doi: 10.1128/aac.16.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooksey R C, Thompson F S, Facklam R R. Physiological characterization of nutritionally variant streptococci. J Clin Microbiol. 1979;10:326–330. doi: 10.1128/jcm.10.3.326-330.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn H W, Pflugfelder S C, Culbertson W W, Davis J L. Recognition, treatment, and prevention of endophthalmitis. Semin Ophthalmol. 1989;4:69–83. [Google Scholar]
- 9.Forster R K, Abbott R L, Gelender H. Management of infectious endophthalmitis. Ophthalmology. 1980;87:313–319. doi: 10.1016/s0161-6420(80)35241-x. [DOI] [PubMed] [Google Scholar]
- 10.George R H. The isolation of symbiotic streptococci. J Med Microbiol. 1974;7:77–83. doi: 10.1099/00222615-7-1-77. [DOI] [PubMed] [Google Scholar]
- 11.Gross K C, Houghton M P, Roberts R B. Evaluation of blood culture media for isolation of pyridoxal-dependent Streptococcus mitior (mitis) J Clin Microbiol. 1981;14:266–272. doi: 10.1128/jcm.14.3.266-272.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins R, Biberstein E L, Jang S S. Nutritionally variant streptococci from corneal ulcers in horses. J Clin Microbiol. 1984;20:1130–1134. doi: 10.1128/jcm.20.6.1130-1134.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloway Y, Dankert J. Penicillin tolerance in nutritionally variant streptococci. Antimicrob Agents Chemother. 1982;22:1073–1075. doi: 10.1128/aac.22.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javitt J C, Vitale S, Canner J K, Street D A, Krakauer H, McBeam A M, Sommer A. National outcomes of cataract extraction. Endophthalmitis following inpatient surgery. Arch Ophthalmol. 1991;109:1085–1089. doi: 10.1001/archopht.1991.01080080045025. [DOI] [PubMed] [Google Scholar]
- 15.Joondeph B C, Flynn H W, Miller D, Joondephet H C. A new culture method for infectious endophthalmitis. Arch Ophthalmol. 1989;107:1334–1337. doi: 10.1001/archopht.1989.01070020404044. [DOI] [PubMed] [Google Scholar]
- 16.Kattan H M, Flynn H W, Pflugfelder S C, Robertson C, Forsteret R K. Nosocomial endophthalmitis survey. Current incidence of infection after intraocular surgery. Ophthalmology. 1991;98:227–238. [PubMed] [Google Scholar]
- 17.Kawamura Y, Hou X-G, Sultana F, Liu S, Yamamoto H, Ezaki T. Transfer of Streptococcus adjacens and Streptococcus defectivus to Abiotrophia gen. nov. as Abiotrophia adiacens comb. nov. and Abiotrophia defectiva comb. nov., respectively. Int J Syst Bacteriol. 1995;45:798–803. doi: 10.1099/00207713-45-4-798. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy L R, Bottone E J. Bacteremia and endocarditis caused by satelliting streptococci. Am J Clin Pathol. 1974;61:585–591. doi: 10.1093/ajcp/61.5.585. [DOI] [PubMed] [Google Scholar]
- 19.Ormerod L D, Ruoff K L, Meisler D M, Wasson P J, Kintner J C, Dunn S P, Lass J H, van de Rijn I. Infectious crystalline keratopathy: role of nutritionally variant streptococci and other bacterial factors. Ophthalmology. 1991;98:159–169. doi: 10.1016/s0161-6420(91)32321-2. [DOI] [PubMed] [Google Scholar]
- 20.Peterson C E, Cook J L, Burke J P. Media-dependent subculture of nutritionally variant streptococci. Am J Clin Pathol. 1981;75:634–636. doi: 10.1093/ajcp/75.4.634. [DOI] [PubMed] [Google Scholar]
- 21.Pflugfelder S C, Flynn H W., Jr . Infectious endophthalmitis. In: Barza M, Baum J, editors. Infectious disease of North America, ocular infections. W. B. Philadelphia, Pa: Saunders, Co.; 1992. pp. 859–873. [PubMed] [Google Scholar]
- 22.Pompei R, Caredda E, Piras V, Serra C, Pintus I. Production of bacteriolytic activity in the oral cavity by nutritionally variant streptococci. J Clin Microbiol. 1990;28:1623–1627. doi: 10.1128/jcm.28.7.1623-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimer L G, Reller L B. Growth of nutritionally variant streptococci on common laboratory and 10 commercial blood culture media. J Clin Microbiol. 1981;14:329–332. doi: 10.1128/jcm.14.3.329-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts R B, Krieger A G, Schiller N L, Gross K C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979;1:955–965. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- 25.Roberts R B, Sidlak M J. Satellite streptococci: a major cause of “negative” blood cultures in bacterial endocarditis? JAMA. 1979;241:2293–2294. doi: 10.1001/jama.241.21.2293. [DOI] [PubMed] [Google Scholar]
- 26.Roggenkamp A, Abele-Horn M, Trebesius K, Tretter U, Autenrieth I B, Heesemann J. Abiotrophia elegans sp. nov., a possible pathogen in patients with culture-negative endocarditis. J Clin Microbiol. 1998;36:100–104. doi: 10.1128/jcm.36.1.100-104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruoff K L. Nutritionally variant streptococci. Clin Microbiol Rev. 1991;4:184–190. doi: 10.1128/cmr.4.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speaker M G, Milch F A, Shah M K, Eisner W, Kreiswirth B N. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639–649. doi: 10.1016/s0161-6420(91)32239-5. [DOI] [PubMed] [Google Scholar]
- 29.Stein D S, Nelson K E. Endocarditis due to nutritionally deficient streptococci: therapeutic dilemma. Rev Infect Dis. 1987;9:908–916. doi: 10.1093/clinids/9.5.908. [DOI] [PubMed] [Google Scholar]
- 30.Townshend L, Slomovic A, Hunter W. Infectious crystalline keratopathy. Can J Ophthalmol. 1989;24:325–326. [PubMed] [Google Scholar]