Abstract
Purpose:
The purpose of this study is to study the demographic profile and pattern of retinopathy of prematurity (ROP) at a tertiary care institute in India.
Methods:
An ambispective study from January 2013 to December 2017. Infants with birth weights (BWs) <1750 g and gestational ages <34 weeks were screened for ROP. Demographic details and ROP severity were recorded.
Results:
Data of 2595 of the 3697 infants screened were analyzed. The number of infants screened and treated for ROP increased from 190 and 29, respectively (2013), to 818 and 132, respectively (2017). The overall incidence of “any ROP” was 32.3%, and severe ROP was 17.7%. Though 39.5% of all infants were outborns (not born in the study center), severe ROP was present in 69.7% of these compared to 18.8% among inborns. Outborns with ROP had a higher mean BW (1308 g) compared to inborns (1202 g) (P < 0.01). ROP Stage 1 was seen in 12%, Stage 2 in 34%, Stage 3 in 13%, Stage 4 in 6%, Stage 5 in 14%, and aggressive posterior ROP (APROP) in 20%. APROP was seen in 16% of infants in 2013, 10% in 2014, 15% in 2015, 22% in 2016, and 28% in 2017. Infants with Stage 4B/Stage5 (15.6% of all ROP) were presented at a mean age of 7.5 months and all had no/delayed screening.
Conclusion:
Incidence of any ROP was 32.3% and was more common in outborns than inborns. The proportion of infants with APROP showed a rising trend over the years. Nearly 15.6% of infants were presented with stage4B/5 ROP due to delayed/absent screening.
Keywords: Demography, incidence, preterm, retinopathy of prematurity, trends
Retinopathy of prematurity (ROP) is a retinal vascular disease that affects premature infants in whom the retinal vasculature is not fully developed at the time of birth. In its more severe forms, it can result in severe visual impairment or blindness. The Global Initiative for the Elimination of Avoidable Blindness[1] targets screening for detection of treatable ROP to reduce the prevalence of childhood blindness. ROP is becoming one of the leading causes of childhood blindness globally.[2] The CRYO-ROP[3] and ETROP[4] studies showed that timely intervention is effective in preventing unfavorable structural outcomes in infants with ROP.
Various studies across the world have shown an incidence of ROP ranging from 9.3 to 37.8% in infants with the mean birth weights (BWs) ranging from 846 to 1301 grams (g) and the gestational ages (GAs) ranging from 27 to 29 weeks.[5,6,7,8,9] The spectrum of ROP predominantly reported includes stage I ROP in 32–60% with few babies progressing to stage V ROP.[5,6,7,8,9] The prevalence of severe ROP has been reported to range from 1.5 to 11.7%.[5,6,7,8,9]
India like other middle income countries is currently facing the third epidemic of ROP.[10] The poor quality of neonatal care, lack of timely screening, and higher rates of premature births have been implicated for the difference in the spectrum of ROP in India compared to the rest of the world.[10] Most studies from India report a higher incidence of ROP (19.3–47.2%) in relatively heavier (mean BWs ranging from 1285 to 1560 g) and more mature (mean GAs ranging from 29.7 to 32.2 weeks) infants.[11,12,13,14,15,16] Few reports also describe severe ROP in infants above 1500 g[17,18] and late presentations with bilateral stage 5 ROP.[19,20]
There is paucity of literature regarding the ROP incidence over time from this part of the subcontinent. Dhingra et al.[21] reported a reduction in the annual incidence of ROP from 49% in 1993 to 26.6% in 2013. Kumar et al.[22] in 2011 reported the 5-year incidence of severe ROP to be 11.9% among infants with mean BW and GA of 1139 g and 29 weeks, respectively. Both studies included only inborn babies of a tertiary care center and therefore did not represent data of the large number of outborn babies that formed the referrals to these centers. There is a paucity of recent data regarding the subject. We aim to provide the demographic profile of ROP over a period of 5 years among preterm infants (both outborn and inborn) screened for ROP from North of India.
Methods
An ambispective study was carried out at our institute for infants screened between 2013 and 2017. Infants meeting the inclusion criteria were recruited prospectively from January 2017 to December 2017. Clinical records of infants screened for ROP between January 2013 and December 2016 were retrospectively reviewed. The study was approved by the Institute Ethics Committee and adhered to the tenets of the Declaration of Helsinki.
Inclusion criteria for the study were in accordance with the screening guidelines given by the National Neonatology Forum of India[23], i.e. all infants born before 34 weeks GA and/or BW less than 1750 g. Those with GA between 34 and 36 weeks or BW between 1750 and 2000 g screened for ROP in view of the presence of additional risk factors (as advised by the treating neonatologist) were also enrolled. Those with a history of ROP treatment done elsewhere, incomplete clinical records, and follow up of less than 3 months were excluded. Infants born in the study institute were termed inborn and those born elsewhere and referred to the study hospital for ROP screening/treatment were termed as outborn.
The following information was recorded – BW, GA, gender, age at presentation, and inborn/outborn status. For inborns, initial examination was done not later than 4 weeks of birth or at discharge, whichever was earlier. For outborns – screening was done at presentation. Pupils were dilated using a fixed dose combination of 0.4% tropicamide and 2.5% phenylephrine. Screening was done using a binocular indirect ophthalmoscope and a +20D lens. All examinations were performed by one of two ROP experts (MRD and DK). Repeat examination was done as per the ETROP guidelines.[4]
Examination was continued till treatable stage of ROP was reached or spontaneous regression and/or complete vascularization of retina was observed. Details of ROP at presentation were documented in terms of the type (Staged ROP or aggressive posterior ROP (APROP)), stage (1,2,3,4A,4B,5), zone (I, II, III), and presence or absence of plus disease as per the ICROP classification.[24] ROP was finally classified depending upon the maximum stage reached. Severe ROP included Type 1 ROP,[24] APROP, and Stage 4A ROP, as well as those infants with ROP who had an unfavorable presentation (Stage 4B/Stage 5/major ROP sequelae such as falciform fold). Nonsevere ROP referred to Type 2 ROP. Treatment details including type and number of laser sittings, anti-VEGF injections or surgery (if any) were noted. Outcome assessed at 3 months was classified as either favorable or unfavorable. Unfavorable outcome was defined as progression to Stage 4B, Stage 5, falciform fold, or central media opacity precluding retinal examination.
Statistical analysis
The data were analyzed through IBM Statistical Package for Social Sciences Software, Version 25. Nominal and categorical variables were described as proportions. Continuous variables were described as mean and standard deviation. As the data were large in size, parametric tests were used irrespective of skewness of data as per Central Limit Theorem. To see association among categorical variables, Chi-Square test was used. To ascertain significant differences in variables, a parametric test in the form of Student's t-test was used. P value of less than 0.05 was considered as statistically significant.
Results
A total of 3697 infants were screened during the entire study period. Of these, 349 were excluded due to incomplete records, 148 due to primary treatment done elsewhere, and 605 due to lack of follow up. Data of 2595 infants were analyzed. The mean BW of infants screened was 1451 ± 405 g (range 560–3600 g). The mean period of gestation was 31.3 ± 2.8 weeks (range 20–41 weeks). The mean age at presentation was 7.3 ± 8.6 weeks (range 1–156 weeks). The mean duration of follow up was 12.4 ± 2.0 weeks (range 12–44 weeks). Thirty-eight percent of infants with ROP were from the neighboring state of Punjab, followed by 33% from Haryana, 13% from Himachal Pradesh, 8% from Chandigarh, 4% from Jammu and Kashmir, and 2% from Rajasthan, whereas infants from other states (Madhya Pradesh, Maharashtra, New Delhi, Uttarakhand, Uttar Pradesh) constituted 2% of diseased infants.
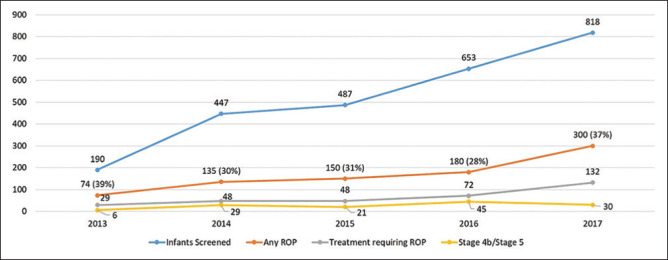
Overall incidence of any ROP was 32.3% (1678 eyes of 839 infants). There was an increase in the number of infants screened each year, those with ROP, as well as those requiring ROP treatment across the duration of the study [Fig. 1]. Stage 1 ROP was seen in 204 eyes (12.2%); Stage 2 in 566 eyes (33.7%); Stage 3 in 216 eyes (12.9%); Stage 4 in 106 eyes (6.3%); Stage 5 in 226 eyes (13.5%); and APROP in 342 eyes (20.1%), while 18 eyes (1.1%) presented with features of spontaneously regressed ROP. Spontaneous regression occurred in 758 eyes (45.2%), and 658 eyes (39.2%) required treatment, whereas unfavorable and late presentation with stage 4B or stage 5 was seen in 262 eyes of 131 infants (15.6%). Overall, severe ROP was seen in 920 eyes (17.7%). ROP was present in zone 2 in 1304 eyes (92.1%), zone 1 in 102 eyes (7.2%), and zone 3 in 10 eyes (0.7%). A rise in the proportion of infants with APROP versus staged ROP was seen across 5 years. [Fig. 2].
Figure 1.
It shows the distribution of infants screened, infants with any ROP, infants with treatment requiring ROP, and Stage 4B/Stage 5 ROP over 5 years (values are in number of infants; values in brackets are in percentage)
Figure 2.

It shows an annual proportion of eyes with Stage vs APROP over 5 years
Of the 658 eyes that were provided treatment, 650 (98.8%) received laser photocoagulation (610 with 532 nm double-frequency Nd-Yag laser, 40 with 810 nm diode laser), 6 eyes (0.9%) required a primary surgery (lens sparing vitrectomy), and 2 eyes (0.3%) were managed only with intravitreal anti-VEGF injections. Additional intervention was required in 144 eyes (22.0%) of which 64 received supplement laser, 38 underwent surgery, 24 received anti-VEGF injections, 8 required supplement laser and surgery, 6 required supplement laser and anti-VEGF injections, and 4 required anti-VEGF injections and surgery. Despite treatment, 108 eyes (16.4%) progressed to unfavorable outcome. The differences in the demographic profile of infants with ROP as well as their outcomes are illustrated in [Table 1]. The incidence of any ROP was 33.1% among females and 31.8% among males; the difference of which was not statistically significant (P = 0.5). Severe ROP was however significantly higher in males (59.5%) than among females (47.9%) (P < 0.01). There was no sex predilection for outcomes of treatment (P = 0.53). Among all infants with ROP, 150 of all 839 ROP (17.8%) babies had a BW of more than 1500 g, 78 of whom (9.7%) had severe ROP. Two hundred fifty nine of ROP babies (31%) had a GA of more than 30 weeks, and 90 of whom (10.7%) had a GA of more than 32 weeks. Among all infants screened, 39.5% were outborns. The overall mean BW of inborns was comparable to outborns (1447 ± 386 g vs 1457 ± 433 g, P = 0.57). Incidence of any ROP was 15.6% among inborns, while it was 58.6% among outborns. Inborns with ROP had predominantly staged disease in 93.5% and APROP in only 6.5%, while outborns had staged disease in 73.9% and APROP in 26.1%. Among infants with any ROP, severe ROP was present in 18.8% of inborns compared to 69.7% of outborns, and this difference was statistically significant (P < 0.01). Inborns with ROP had a lower BW as compared to outborns (1202 ± 298 g vs 1308 ± 359 g, P < 0.01), while both groups had similar periods of gestation (29.7 ± 2.9 weeks vs 29.3 ± 2.8 weeks, P = 0.08). Among infants with severe ROP, inborns had a lower BW than outborns (1102 ± 330 g vs 1285 ± 347 g, P < 0.01) with a comparable period of gestation (28.6 ± 1.8 weeks vs 29.1 ± 2.8 weeks, P = 0.1). Unfavorable outcome post treatment was seen only among outborns.
Table 1.
Comparison of birth weight, gestational age, and age at presentation among infants with/without ROP, severe/nonsevere ROP, and those with favorable/unfavorable outcomes
| Mean Birth Weight (in grams) | Mean Period of Gestation (weeks) | Mean Age at Presentation (weeks) | |
|---|---|---|---|
| ROP | 1277±345 | 29.5±2.6 | 10.2±13.7 |
| No ROP | 1534±408 | 32.2±2.6 | 5.9±4.0 |
| P | <0.01 | <0.01 | <0.01 |
| Severe ROP | 1267±349 | 29±2.4 | 13.0±17.3 |
| Nonsevere ROP | 1290±334 | 30±2.3 | 6.8±5.2 |
| P | 0.3 | <0.01 | <0.01 |
| Favorable outcome | 1253±359 | 29±2.3 | 5.9±2.4 |
| Unfavorable outcome | 1198±275 | 28.6±2.6 | 7.3±4.5 |
| P | 0.39 | 0.3 | <0.01 |
Among infants with a diagnosis of APROP (342 eyes), mean BW was 1280 ± 364 g, mean GA was 29.2 ± 3.2 weeks, and majority were outborns (90.6%). The period of gestation between the inborns and outborns with APROP was comparable (mean 28.9 ± 2.0 weeks vs 29.3 ± 2.3 weeks, P = 0.65), while BWs were significantly higher among outborns with APROP than inborns (1134 ± 409 g vs 1295 ± 343 g, P < 0.01).
One hundred thirty-one infants (262 eyes) presented late with unfavorable disease at presentation (stage 4b/stage 5), which constituted 15.6% of all ROP babies. Their mean BW was 1326 ± 348 g, and mean GA was 29.0 ± 2.6 weeks. These babies presented late at a mean age of 7.5 months and had delayed or no screening for ROP.
Discussion
In India and other similar middle-income countries, ROP has been reported to be the “third epidemic”.[10] This has been attributed to: 1) Higher rates of premature births, 2) compromised neonatal care, and 3) lack of screening and treatment programs.
Previous studies from India report a high incidence of ROP and severe ROP in heavier BW babies.[11,12,13,14,15,16,22] Long-term studies document a much lower incidence of ROP in the western world as compared to the Indian scenario. Gerull et al.[8] reported a 9.3% incidence of ROP across 10 years in Switzerland, with the incidence of severe ROP being 1.8%. Painter et al.[9] evaluating the 20-year epidemiology of ROP in England from 1990 to 2010 reported an incidence of ROP ranging from 1.28% in 1990 to 12.5% in 2010. Ludwig et al.[25] reported an increasing incidence of ROP from 14.7% in 2000 to 19.88% in 2012 in the United States of America. Thomas et al.[26] reported an incidence of severe ROP to be 12.7% across 8 years in Canada.
The present study was carried out over a 5-year period between 2013 and 2017 in a single tertiary care institute in North India. We report an overall incidence of ROP of 32.3% among all “at risk” infants screened (ranging between 28 and 39% across the years [Fig. 1], with severe ROP seen among 17.7% infants. The demographics of the present study compared to studies across the country and the globe are presented in [Table 2]. In Taiwan, studies report an incidence of ROP of 37.8%, which is slightly higher than our study.[6] Most of the studies from India are of short duration[11,12,13,14,15,16] and report incidences of ROP, which are higher than the western world. Limited information is available on trends over time. Kumar et al.[22] report an incidence of 11.9% across 5 years, but they had a limited cohort of infants who were only inborn.
Table 2.
Table comparing demographic profile of ROP studies across the world
| Author | Duration | Place | Percentage of ROP | Mean Birth Weight (in grams) | Mean Gestational Age (in weeks) | Percentage with Severe ROP |
|---|---|---|---|---|---|---|
| Iu, 2017[5] | 1 year | Hong Kong | 16.9% | 846 | 27 | 3.4% |
| Li, 2013[6] | 10 years | Taiwan | 37.8% | 940 | 27 | 11.7% |
| Fortesfilho, 2009[7] | 5 years | Brazil | 25.5% | 1050 | 29 | 5.8% |
| Thomas, 2015[26] | 8 years | Canada | NA | 771 | 25.4 | 12.7% |
| Hungi, 2011[12] | 1.5 years | South India | 41.5% | 1555 | 32.2 | 10.2% |
| Charan, 1995[13] | 1 year | North India | 47.3% | 1285 | 31.5 | 24.4% |
| Ahuja, 2018[15] | 1.5 year | South India | 32.6% | 1285 | 29.71 | 4.3% |
| Vasavada, 2018[16] | 1.5 year | Western India | 19.3% | 1560 | 30.3 | 10.3% |
| Kumar, 2011[22] | 5 years | North India | 11.9% | NA | NA | 4.7% |
| Present study | 5 years | North India | 32.3% | 1277 | 29.5 | 17.7% |
In the present study, the mean BW of children with ROP was 1277 g, which was higher than studies from Hong Kong,[5] Taiwan[6], and Brazil.[7] The BW in Indian studies varies from 1285 to 1560 g[11,12,13,14,15,16] due to variations in study period, design, and number of infants. These were, however, higher than the BW of the western world indicating that we see ROP in heavier babies. Mean BW in the present study was comparable to others reported from India.
The mean GA in the present study was 29.5 weeks, which was higher than that of the rest of the world (ranging from 25.4 to 29 weeks).[5,6,7,26] Other Indian studies report a mean GA ranging from 29.7 to 32.2 weeks[11,12,13,14,15,16], which was comparable to the present study.
The Indian guidelines for screening of ROP released in 2010 advocated screening of heavier babies with an older period of gestation as compared to the guidelines of the United States (>1500 g BW and >30 weeks GA)[27] and the United Kingdom (>1500 g BW and >32 weeks GA).[28] In the present study, up to 31% babies with ROP would have likely been missed if western guidelines were used for screening in India.
A recent change in the Indian guidelines[29] warrants the screening of all infants with a BW of less than 2000 g and a GA of less than 34 weeks to be done within the first 4 weeks of birth, with an earlier screening of more premature (<28 weeks) or lighter babies (<1200 g) which is to be done within the first 2–3 weeks of life.
We report a higher percentage of APROP as compared to staged ROP in comparison to previously reported literature. Majority of disease was in Zone II, which was comparable to other Indian literature. Comparison of Stage wise and Zone wise distribution of ROP with other published literature is given in [Table 3].
Table 3.
Comparison of stage and zone wise distribution of ROP among different studies
| Author | Place | Stage I | Stage II | Stage III | Stage IV | Stage V | APROP |
|---|---|---|---|---|---|---|---|
| Iu, 2017[5] | Hong Kong | 60% | 20% | 20% | 0 | 0 | 0 |
| Li, 2013[6] | Taiwan | 32.1% | 18.9% | 42.6% | 5.7% | 0.6% | 0 |
| Fortesfilho, 2009[7] | Brazil | 44.23% | 32.69% | 21.15% | 0.96% | 0.96% | 0 |
| Gerull, 2017[8] | Switzerland | 49.3% | 31.1% | 18.9% | 0.2% | 0.5% | 0 |
| Hungi, 2011[12] | South India | 28.6% | 56% | 2.2% | 0 | 0 | 13.2% |
| Charan, 1995[13] | North India | 35.9% | 37.2% | 24.4% | 2.5% | 0 | 0 |
| Present Study | North India | 12.2% | 33.8% | 12.9% | 6.3% | 13.5% | 20.4%a |
|
| |||||||
| Zone I | Zone II | Zone III | |||||
|
| |||||||
| Hungi, 2011[12] | 15.4% | 31.9% | 52.7% | ||||
| Kumar, 2011[22] | 1.2% | 76.2% | 22.6% | ||||
| Present study | 7.2% | 92.1% | 0.7% | ||||
While the overall percentage of ROP in the present study ranges between 28 and 39% between years 2013 and 2017 [Fig. 1], there was a rise in the proportion of APROP during the study duration [Fig. 2]. There was also an increase in the number and proportion of children presenting late with an unfavorable presentation (Stage 4B/Stage 5 ROP) in whom treatment has variable outcomes. Outborns showed a significant higher incidence of any ROP and severe ROP as compared to inborns. While a referral bias can be a cause of this difference, the observation of worse disease in heavier outborns as compared to inborns reflects the possibility of variation in neonatal care across different neonatal centers in India. By continuously improving the quality of neonatal care, expanding ROP screening services, and increasing awareness about the risk factors, we hope this difference in demography of inborn and outborn infants with ROP is reduced.
Conclusion
This large study involving 2595 infants screened between 2013 and 2017 in the setting of a tertiary care hospital in India showed 32.3% overall incidence of ROP and 17.7% severe ROP. The incidence of ROP was more common in outborns than inborns. The proportion of infants with APROP showed a rising trend over the years. Presentation with advanced ROP due to delayed/absent screening continues to be a problem.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Thylefors B. A global initiative for the elimination of avoidable blindness. Am J Ophthalmol. 1998;125:90–3. doi: 10.1016/s0002-9394(99)80239-6. [DOI] [PubMed] [Google Scholar]
- 2.Wheatley CM, Dickinson JL, Mackey DA, Craig JE, Sale MM. Retinopathy of prematurity: Recent advances in our understanding. Arch Dis Child Fetal Neonatal Ed. 2002;87:78–82. doi: 10.1136/fn.87.2.F78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Multicenter trial of cryotherapy for retinopathy of prematurity: Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1988;106:471–9. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 4.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, et al. Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010;128:663–71. doi: 10.1001/archophthalmol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iu LP, Lai CH, Fan MC, Wong IY, Lai JS. Screening for retinopathy of prematurity and treatment outcome in a tertiary hospital in Hong Kong. Hong Kong Med J. 2017;23:41–7. doi: 10.12809/hkmj154811. [DOI] [PubMed] [Google Scholar]
- 6.Li ML, Hsu SM, Chang YS, Shih MH, Lin YC, Lin CH, et al. Retinopathy of prematurity in southern Taiwan: A 10-year tertiary medical center study. J Formos Med Assoc. 2013;112:445–53. doi: 10.1016/j.jfma.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Fortesfilho JB, Eckert GU, Valiatti FB, da Costa MC, Bonomo PP, Procianoy RS. Prevalence of retinopathy of prematurity: An institutional cross-sectional study of preterm infants in Brazil. Rev Panam Salud Publica. 2009;26:216–20. doi: 10.1590/s1020-49892009000900005. [DOI] [PubMed] [Google Scholar]
- 8.Gerull R, Brauer V, Bassler D, Laubscher B, Pfister RE, Nelle M, et al. Incidence of retinopathy of prematurity (ROP) and ROP treatment in Switzerland 2006-2015: A population-based analysis. Arch Dis Child Fetal Neonatal Ed. 2018;103:337–42. doi: 10.1136/archdischild-2017-313574. [DOI] [PubMed] [Google Scholar]
- 9.Painter SL, Wilkinson AR, Desai P, Goldacre MJ, Patel CK. Incidence and treatment of retinopathy of prematurity in England between 1990 and 2011: Database study. Br J Ophthalmol. 2015;99:807–11. doi: 10.1136/bjophthalmol-2014-305561. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: Implications for screening programs. Pediatrics. 2005;115:518–25. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 11.Jalali S, Anand R, Kumar H, Dogra MR, Azad R, Gopal L. Programme planning and screening strategy in retinopathy of prematurity. Indian J Ophthalmol. 2003;51:89–99. [PubMed] [Google Scholar]
- 12.Hungi B, Vinekar A, Datti N, Kariyappa P, Braganza S, Chinnaiah S, et al. Retinopathy of Prematurity in a rural Neonatal Intensive Care Unit in South India--A prospective study. Indian J Pediatr. 2012;79:911–5. doi: 10.1007/s12098-012-0707-y. [DOI] [PubMed] [Google Scholar]
- 13.Charan R, Dogra MR, Gupta A, Narang A. The incidence of retinopathy of prematurity in a neonatal care unit. Indian J Ophthalmol. 1995;43:123–6. [PubMed] [Google Scholar]
- 14.Murthy KR, Murthy PR, Shah DA, Nandan MR, S NH, Benakappa N. Comparison of profile of retinopathy of prematurity in semiurban/rural and urban NICUs in Karnataka, India. Br J Ophthalmol. 2013;97:687–9. doi: 10.1136/bjophthalmol-2012-302801. [DOI] [PubMed] [Google Scholar]
- 15.Ahuja AA, V Reddy YC, Adenuga OO, Kewlani D, Ravindran M, Ramakrishnan R. Risk factors for retinopathy of prematurity in a district in South India: A prospective cohort study. Oman J Ophthalmol. 2018;11:33–7. doi: 10.4103/ojo.OJO_97_2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasavada D, Sengupta S, Prajapati VK, Patel S. Incidence and risk factors of retinopathy of prematurity in Western India-Report from A Regional Institute of Ophthalmology. Nepal J Ophthalmol. 2017;9:112–1120. doi: 10.3126/nepjoph.v9i2.19254. [DOI] [PubMed] [Google Scholar]
- 17.Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed. 2012;97:371–5. doi: 10.1136/fetalneonatal-2011-301121. [DOI] [PubMed] [Google Scholar]
- 18.Sanghi G, Dogra MR, Katoch D, Gupta A. Aggressive posterior retinopathy of prematurity in infants≥1500 g birth weight. Indian J Ophthalmol. 2014;62:254–7. doi: 10.4103/0301-4738.128639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanghi G, Dogra MR, Katoch D, Gupta A. Demographic profile of infants with stage 5 retinopathy of prematurity in North India: Implications for screening. Ophthalmic Epidemiol. 2011;18:72–4. doi: 10.3109/09286586.2010.551575. [DOI] [PubMed] [Google Scholar]
- 20.Azad R, Chandra P, Gangwe A, Kumar V. Lack of screening underlies most stage-5 retinopathy of prematurity among cases presenting to a tertiary eye center in India. Indian Pediatr. 2016;53:103–6. [PubMed] [Google Scholar]
- 21.Dhingra D, Katoch D, Dutta S, Samanta R, Aggarwal K, Dogra MR. Change in the incidence and severity of retinopathy of prematurity (ROP) in a neonatal intensive care unit in northern India after 20 years: Comparison of two similar prospective cohort studies. Ophthalmic Epidemiol. 2019;26:169–74. doi: 10.1080/09286586.2018.1562082. [DOI] [PubMed] [Google Scholar]
- 22.Kumar P, Sankar MJ, Deorari A, Azad R, Chandra P, Agarwal R, et al. Risk factors for severe retinopathy of prematurity in preterm low birth weight neonates. Indian J Pediatr. 2011;78:812–6. doi: 10.1007/s12098-011-0363-7. [DOI] [PubMed] [Google Scholar]
- 23.Pejawar R, Vinekar A, Bilagi A. National Neonatology Foundation's Evidence-based Clinical Practise Guidelines (2010) Retinopathy of Prematurity, NNF India, New Delhi. 2010. [Last accessed on 2021 Feb 20]. pp. 253–62. Available from: https://www.ontop-in.org/ontop-pen/Week-12-13/ROP%20NNF%20Guidelines%20.pdf .
- 24.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig CA, Chen TA, Hernandez-Boussard T, Moshfeghi AA, Moshfeghi DM. The epidemiology of retinopathy of prematurity in the United States. Ophthalmic Surg Lasers Imaging Retina. 2017;48:553–62. doi: 10.3928/23258160-20170630-06. [DOI] [PubMed] [Google Scholar]
- 26.Thomas K, Shah PS, Canning R, Harrison A, Lee SK, Dow KE. Retinopathy of prematurity: Risk factors and variability in Canadian neonatal intensive care units. J Neonatal Perinatal Med. 2015;8:207–14. doi: 10.3233/NPM-15814128. [DOI] [PubMed] [Google Scholar]
- 27.Fierson WM. American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson AR, Haines L, Head K, Fielder AR. UK retinopathy of prematurity guideline. Early Hum Dev. 2008;84:71–4. doi: 10.1016/j.earlhumdev.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Ministry of Health and Family Welfare. Guidelines for Universal Eye Screening in Newborns Including Retinopathy of Prematurity. [Last accessed on 2020 Oct 25]. Available from: http://www.nhm.gov.in/images/pdf/programmes/RBSK/Resource_Documents/Revised_ROP_Guidelines-Web_Optimized.pdf .