Abstract
Purpose:
We aim to compare the incidence of corneal epithelial defects after laser for retinopathy of prematurity (ROP) with and without the use of postoperative erythromycin ointment.
Methods:
In this retrospective observational cohort study, a total of 100 infants (200 eyes) consecutively treated with laser for ROP between 2012 and 2018. The primary outcome was presence or absence of corneal epithelial defect using fluorescein on bedside examination within the first week following laser for ROP. Additional data assessed included: the use of postoperative prophylactic erythromycin ointment for 1 week, postoperative day on which examination using fluorescein occurred, presence of corneal opacity, gender, birth weight, and gestation age. The presence or absence of postoperative corneal epithelial defects was compared between eyes receiving postoperative erythromycin ointment or not using a Fisher's exact test.
Results:
Postoperative corneal epithelial defects were more common in eyes which did not receive postoperative erythromycin (7 of 40 eyes; 17.5%), compared to eyes which did receive erythromycin (1 of 160 eyes; 0.6%; P < 0.0001). Postoperative bedside examinations with fluorescein were performed within 2 days of surgery on 136 of 200 of eyes (68%). Corneal opacities were noted in 3 of 200 eyes (1.5%).
Conclusion:
We observed less corneal epithelial defects in eyes which received postoperative erythromycin ointment for 1 week after laser for ROP than in those which did not. While multiple variables may influence the presence or absence of postoperative corneal epithelial defects following laser for ROP, consideration for postoperative lubricating ointment following laser for ROP seems reasonable.
Keywords: Corneal epithelial defects, postoperative lubrication, retinopathy of prematurity
Retinopathy of prematurity (ROP) is a potential cause of blindness in premature infants.[1] Regular ophthalmic screening examinations are required to detect treatment-warranted ROP.[2] Pan-retinal photocoagulation laser eye surgery to the avascular retina is a standard treatment for ROP.[3,4,5] While generally safe and effective, adverse outcomes have been reported after laser treatments for ROP, including corneal epithelial defects and opacities.[6,7] While risks of treatment for ROP have been described, methods to decrease these risks have not been firmly established.[7,8,9,10,11,12] Our goal is to compare the incidence of corneal epithelial defects after laser for ROP with and without the use of postoperative erythromycin ointment.
Methods
This retrospective cohort study was approved by our Institutional Review Board at Albany Medical College (Protocol Number: 5540) and adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. A retrospective chart review of consecutive infants treated for ROP from April 2012 to September 2018 was conducted. All patients were screened regularly in accordance with guidelines from the American Academy of Pediatrics and were found to have treatment-warranted ROP.[2,13,14,15,16] All pan-retinal photocoagulation laser surgeries were performed by a single attending physician in the operating room under general anesthesia. Frequent ocular lubrication was used on the corneal surface throughout each laser procedure. No corneal epithelial defects or opacities were apparent immediately following the laser. Occasionally, additional surgical interventions were performed under the same anesthesia as the laser for ROP. In these cases, the ophthalmologist taped the eyelids closed during the nonocular procedure to ensure complete lid closure. Yes, approval was obtained from the ethics committee on August 24, 2019.
All infants in this study received a penlight examination using fluorescein stain and a cobalt blue filter from an indirect ophthalmoscope within 1 week postoperatively from laser. In many cases, the postoperative examination occurred within 48 h of laser. For all postoperative examinations, a penlight examination was performed first to note the presence or absence of corneal opacity prior to the application of fluorescein stain. For infants receiving an examination before postoperative day 6, an additional examination was performed on postoperative day 6. Exclusion criteria included: Lack of documented penlight examination with fluorescein within 1 week postlaser and treatment with intravitreal injection of bevacizumab only.
Every patient in the study received a single dose of erythromycin ophthalmic ointment (Bausch and Lomb) immediately following laser. Erythromycin ointment was selected as it is highly viscous, readily available, preservative-free, FDA-approved for use in neonates and offers excellent lubrication for the cornea. Infants in the first 2 years of the study period did not receive any further erythromycin ointment. In contrast, infants in the final 6 years of the study period typically received prophylactic erythromycin ointment each eye between every 3 and 6 h for 1 week following laser. In addition to erythromycin, all patients in each group received prednisolone acetate 1% 4 times a day and atropine 1% each eye for 1 week. The primary outcome measure was the presence of any corneal epithelial defect after laser for ROP, as noted using a penlight, fluorescein staining, and cobalt blue filter. Data were also collected on presence of any corneal opacity after laser for ROP, postoperative day (POD) of penlight exam, birth weight, gender and gestational age. Outcome measures were compared using the two-tailed Fisher exact test or a t-test.
Results
A total of 112 charts were reviewed. Twelve total patients were excluded, nine who were treated only with bevacizumab and three with inadequate documentation of fluorescein staining on initial post-operative examination. A total of 100 patients (200 eyes; 58 males; 42 females) were included in the study. A total of 68 of 100 infants (68%) received a bedside post-operative examination with fluorescein with two days of laser surgery, while 32 of 100 infants (32%) received a post-op examination between POD 3 and 6. [Table 1] The first postoperative examination occurred on a mean of postoperative day 2.4.
Table 1.
Number of patients seen at each postoperative follow-up day
| Postoperative day | Number of patients |
|---|---|
| 1 | 54 |
| 2 | 14 |
| 3 | 5 |
| 4 | 6 |
| 5-6 | 21 |
Infants who did not received prophylactic lubricating ointment after laser were more likely to develop corneal epithelial defects, (7 of 40 eyes; 17.5%) than eyes which did receive prophylactic erythromycin ointment after laser (1 of 160 eyes; 0.6%; P < 0.0001). [Fig. 1, Table 2] In total, 8 of 200 treated eyes (4%) in 5 of 100 patients (5%) developed a corneal epithelial defect after laser. Three patients had both eyes affected; two patients had only one eye affected.
Figure 1.
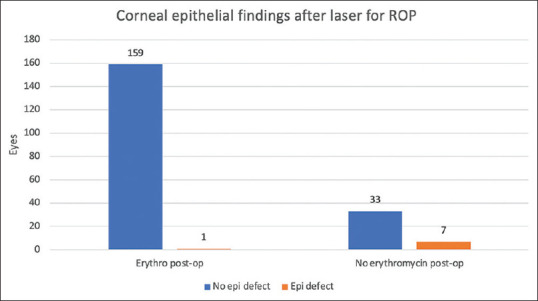
Number of eyes with corneal epithelial defect compared to use of erythromycin ointment
Table 2.
Comparison of primary outcome with and without use of erythromycin postoperatively
| Erythromycin postop (n=160) | No erythromycin postop (n=40) | Total eyes (n=200) | |
|---|---|---|---|
| No corneal epithelial defect postop (eyes) | 159 | 33 | 192 |
| Corneal epithelial defect postop (eyes) | 1 (0.6%) | 7 (17.5%) | 8 (4%) |
P<0.0001
Corneal opacities were noted in 3 of 200 eyes (1.5%). There was no significant difference in the rate of corneal opacities noted in patients not receiving postoperative erythromycin ointment (2 of 40; 5%) compared to those receiving postoperative erythromycin (1 of 160; 0.6%; P = 0.1025). The corneal opacities resolved in two of three cases by 2 weeks. There was no difference between patients who did and did not develop postoperative epithelial defects in regard to gender (five males: Zero female with epithelial defects, compared to 53 male: 42 female without epithelial defects; P = 0.0722), gestational age (mean 24.9 vs. 25.6 weeks; P = 0.4601), birth weight (mean 727.0 vs. 753.9 gm; P = 0.7721, or day of postop examination (mean 3 vs. 2.4 days; P = 0.4970) [Table 3].
Table 3.
Comparison of secondary outcomes
| Total (n=100) | Control (No epithelial defect) n=95 | Cases (Epithelial defect) n=5 | P | |
|---|---|---|---|---|
| Gender (Male:Female) | 58:42 | 53:42 | 5:0 | 0.0722 |
| Gestational age mean (wks.) | 25.5 | 25.6 | 24.9 | 0.4601 |
| Birth weight Mean (gm.) | 752.6 | 753.9 | 727.0 | 0.7721 |
| Postop follow-up exam, mean (days) | 2.4 | 2.4 | 3 | 0.4970 |
Discussion
Corneal epithelial defects and opacities following laser for ROP are rare but potentially serious complications. Modi et al. noted corneal epithelial defects in 10 of 110 eyes (9.1%) treated for ROP, with progression to ulcerative keratitis in 4 of 98 (4.08%) of eyes treated with laser.[6] The authors emphasized the potential of corneal epithelial defects after laser to develop into ulcerative keratitis.[6] Potential corneal scarring is particularly important in neonates given the potential for deprivation amblyopia. Accordingly, it is appropriate to take reasonable steps to reduce the risk of corneal epithelial defects after laser for ROP. In the Early Treatment for Retinopathy of Prematurity (ET-ROP) study, corneal opacities were noted after laser in 1.5% of earlier-treated eyes and 2.6% of conventionally treated eyes; however, these findings were noted 2 years after laser.[7] Data on corneal epithelial defects within the first week after laser were not available from the ET-ROP study however, so it is not clear if the corneal opacities at 2 years were related to epithelial defects within the first week following laser.[7]
In our study, patients receiving erythromycin ointment following laser treatment showed fewer corneal epithelial defects than those who did not receive postoperative erythromycin. Erythromycin ointment has the benefit of being both preservative-free and highly viscous. We are unable to determine to what extent the reduction of postoperative corneal epithelial defects after laser was due to the mechanical lubrication or the antibiotic component of erythromycin. It is possible that equally good results would be observed using a preservative-free lubricating ointment without antibiotic. Further, we found that gender, gestational age, and birth weight were not associated with the development of corneal epithelial defects.
The mechanism of corneal epithelial defects in infants following laser for ROP is not entirely clear from our data. As no corneal epithelial defects were noted immediately following laser, epithelial defects that developed likely did so sometime after laser but within the first week. Multiple factors may contribute to corneal epithelial defects after laser for ROP. Eyelid edema and chemosis were noted in some infants after laser treatment. Both could be a result of mechanical insult from scleral depression or systemic predisposition from low protein or permeable endothelial cells. Regardless of etiology, it is plausible that eyelid edema and chemosis could cause mechanical irritation of the overlying corneal epithelium and contribute to epithelial defects after laser, respectively. In our study, we did not quantify either the eyelid edema or chemosis postoperatively in a rigorous enough manner to correlate it to epithelial defects. Neonates, particularly premature neonates, have been noted to have basal and reflex tear deficiency, which may increase the risk of corneal epithelial defects after laser.[17,18] Eyelid speculums used during laser create corneal exposure. Frequent lubrication should be used throughout the laser procedure. Balanced saline solution, or another ocular lubricant without preservative, may be ideal to lubricate the corneal surface without introducing the risk of preservative-induced keratopathy. Reduced operating time may also be helpful to minimize corneal exposure. When nonocular procedures are performed under the same anesthesia as laser for ROP, eyelids should be securely taped closed during the nonocular procedures.
Some patients remain partially sedated after laser. Postoperative sedation may decrease the infant's natural ability to protect the ocular surface through blinking and eyelid closure. Direct heat in the incubator after laser treatment may also contribute to drying of the corneal surface. Indirect heat may presumably offer less risk of drying the corneal surface. Some mechanical ventilatory modalities may create air movement, which may contribute to drying of the ocular surface. There are a variety of other factors that may contribute to corneal epithelial defects and opacities after laser for ROP, including neuropathy and poor limbal stem cell production or function.
Our study had multiple limitations. It is possible that confounding variables other than erythromycin may have influenced the development of epithelial defects. Preoperative ocular surface status was not generally noted. Operative time and laser power varied somewhat from patient to patient. Some patients received only laser for ROP, while others received other nonophthalmic procedures during the same anesthesia. Not all infants received postoperative examination on the same day. It is possible that variable follow-up intervals allowed for the development and healing corneal epithelial defects before a given postoperative exam. Conversely, a strength of our study was that 68% of patients received a postoperative examination within 48 h of laser and that all postoperative examinations involved fluorescein staining of the cornea.
Conclusion
Laser photocoagulation in infants with ROP remains an important treatment modality with a long record of safety and efficacy. Our data suggests that postoperative corneal epithelial defects are observed less frequently after laser for ROP when erythromycin ointment is used for 1 week postoperatively than when erythromycin is not used postoperatively. Our results may prompt further study regarding lubricating the corneal surface following laser for ROP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–57. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fierson WM. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 3.Wallace DK, Wu KY. Current and future trends in treatment of severe retinopathy of prematurity. Clin Perinatol. 2013;40:297–310. doi: 10.1016/j.clp.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Fierson WM. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142:e20183061. doi: 10.1542/peds.2018-3061. [DOI] [PubMed] [Google Scholar]
- 5.Good WV Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233. [PMC free article] [PubMed] [Google Scholar]
- 6.Modi KK, Chu DS, Wagner RS, Guo S, Zarbin MA, Bhagat N. Infectious ulcerative keratitis following retinopathy of prematurity treatment. J Pediatr Ophthalmol Strabismus. 2015;52:221–5. doi: 10.3928/01913913-20150602-01. [DOI] [PubMed] [Google Scholar]
- 7.Good WV Early Treatment for Retinopathy of Prematurity Cooperative Group. The early treatment for retinopathy of prematurity study: Structural findings at age 2 years. Br J Ophthalmol. 2006;90:1378–82. doi: 10.1136/bjo.2006.098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibowitz HM, Luzzio AJ. Laser-induced cataract: Clinical observations. Arch Ophthalmol. 1970;83:608–12. doi: 10.1001/archopht.1970.00990030608016. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro A, Tso MOM, Goldberg MF. Argon laser-Induced cataract: A clinicopathologic study. Arch Ophthalmol. 1984;102:579–83. doi: 10.1001/archopht.1984.01040030457026. [DOI] [PubMed] [Google Scholar]
- 10.Fallaha N, Lynn MJ, Aaberg TM, Lambert SR. Clinical outcome of confluent laser photoablation for retinopathy of prematurity. J AAPOS. 2002;6:81–5. doi: 10.1067/mpa.2002.121452. [DOI] [PubMed] [Google Scholar]
- 11.Ali SF, Edmond JC, Suelflow JR, Coats DK, Yen KG. Band keratopathy in children previously treated with diode laser for type 1 retinopathy of prematurity. J AAPOS. 2019;23:232–4. doi: 10.1016/j.jaapos.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darlow BA, Gilbert C. Retinopathy of prematurity - A world update. Semin Perinatol. 2019;43:315–6. doi: 10.1053/j.semperi.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds JD, Hardy RJ, Kennedy KA, Spencer R, van Heuven WA, Fielder AR. Lack of efficacy of light reduction in preventing retinopathy of prematurity. Light reduction in retinopathy of prematurity (LIGHT-ROP) cooperative group. N Engl J Med. 1998;338:1572–6. doi: 10.1056/NEJM199805283382202. [DOI] [PubMed] [Google Scholar]
- 15.Ho SF, Mathew MR, Wykes W, Lavy T, Marshall T. Retinopathy of prematurity: An optimum screening strategy. J AAPOS. 2005;9:584–8. doi: 10.1016/j.jaapos.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds JD, Dobson V, Quinn GE, Fielder AR, Palmer EA, Saunders RA, et al. Evidence-based screening criteria for retinopathy of prematurity: Natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol. 2002;120:1470–6. doi: 10.1001/archopht.120.11.1470. [DOI] [PubMed] [Google Scholar]
- 17.Isenberg SJ, Apt L, McCarty J, Cooper LL, Lim L, Del Signore M. Development of tearing in preterm and term neonates. Arch Ophthalmol. 1998;116:773–6. doi: 10.1001/archopht.116.6.773. [DOI] [PubMed] [Google Scholar]
- 18.Akar Y, Cira A, Apaydin C, Erman MA, Yilmaz A. The effect of prematurity on tear production. Curr Eye Res. 2004;28:145–51. doi: 10.1076/ceyr.28.2.145.26235. [DOI] [PubMed] [Google Scholar]


