Abstract
Purpose:
The aim of this study was to describe causes of severe visual impairment and blindness (SVI/BL), and assess the mental health and quality of life of children in schools for the blind in North-East India in two phases.
Methods:
A total of 515 children were examined in 17 schools for the blind in the first phase of study across eight states in North-East India, 6 in Assam, 2 each in Meghalaya, Manipur, Mizoram, and Tripura, 1 each in Arunachal Pradesh, Nagaland, and Sikkim. WHO/PBL eye examination record was used to document findings. In the second phase of study, mental health and quality of life were objectively measured using depression anxiety stress scales (DASS) and low-vision quality of life (LVQOL) questionnaires in 442 children.
Results:
Approximately 3.1% of children had SVI and 71.84% of children were blind. Anatomical sites of SVI/BL were the whole globe in 44.85%, cornea in 17.66%, and retina in 11.65% of children. The underlying cause of visual loss was undetermined in 55% of children. Hereditary pattern was observed in 1.35% of cases. Approximately 74.94% of children were either blind or severely visually impaired since birth. DASS score revealed that 56.56% of children manifested some levels of anxiety and stress while 85.52% had some reduction in quality of life.
Conclusion:
A large significant number of these children suffered from potentially preventable and/or treatable cause of SVI/BL. Though nonvisual factors such as physical and mental health were strong predictors of quality of life, this study proves that visual impairment also plays a considerable role in one's quality of life in a population with low vision.
Keywords: Mental health, ocular morbidity, pediatric ophthalmology, quality of life, school for the blind
An estimate for global blindness is 37 million, of which 1.5 million are children and almost three-quarters of them live in developing countries.[1] The prevalence of blindness in children ranges from approximately 0.3/1000 children in affluent regions to 1.5/1000 within the poorest communities.[2] World Health Organization (WHO) defines blindness as corrected visual acuity in the better eye of < 10/200 and severe visual impairment as corrected visual acuity in the better eye of <20/200 but equal to or better than 10/200. The Indian definition has recently been modified to be in tune with that of WHO.[3,4] But despite low prevalence, childhood blindness has been given priority in VISION 2020 program due to its high magnitude particularly prevailing in developing countries and the high number of blind years resulting from the same.[5,6] The problems faced by the child, the burden on their families, society and the impact on the nation have to be considered as most of the blindness in childhood is avoidable, preventable, and treatable if detected at an early age.
Population-based data on the causes of childhood blindness are difficult to find in developing countries as registers of the blind do not exist, and very large sample sizes would be required for formal cross-sectional surveys. Low vision affects many areas of quality of life including daily functioning and mental health.[7] Mental health is technically a facet of good quality of life, as are daily activities. A quantitative measurement will be more apt than subjectively asking the low vision patient of their feelings on mental health, quality of life and well-being and tools such as Depression Anxiety Stress Scales (DASS) and Low vision quality of life questionnaires (LVQOL) assess the same.[8,9]
Considering all these factors a cross-sectional survey of all the children studying in the Schools for the Blind in eight states of North-East India was undertaken. The aim of the study was: To analyze the various causes of blindness and severe visual impairment in children residing in Schools for the Blind in Northeastern India. We also assessed the mental health of these children using the DASS and LVQOL questionnaires.
Methods
North-East India, which comprises eight states, namely, Assam, Meghalaya, Manipur, Mizoram, Arunachal Pradesh, Nagaland, Tripura, and Sikkim,[10] has a population of 45.7 million people (National Population Census 2011) and has 17 Schools for the Blind. These schools were identified with the help of blindness control societies of each state. This was a descriptive, interventional, cross-sectional study of all the students studying in the 17 Schools for the Blind of the eight states of North-East India. The study was conducted after clearance from the institutional research and ethics committee. The required permission for the screening of the children was obtained from the principal/headmaster of each school after briefing them about the aims and objectives of the study. The school authorities were also requested to inform the parents of the children about screening.
The study was done in two phases. In the first phase, the children were assessed based on the “World Health Organization (WHO)/Prevention of Blindness (PBL) Examination Record for Children with Blindness and Low Vision Coding Instruction” form.[11] The form is accompanied by instructions for use, definitions, and methods of classification. For each child, the cause of visual loss was recorded using the anatomical and etiological classification provided in the form. A team comprising of an Ophthalmologist and optometrists from our tertiary care center examined the children in their respective school premises. Binocular visual acuity, and when possible, uniocular visual acuity were measured using Snellen charts (Tumbling E). The children who did not co-operate with the ‘E’ chart were assessed for the ability to fix and follow the light. Figure charts equivalent to N were used to assess near vision. The visual status of children was recorded using WHO categories.[3] The optometrist assessed the refraction and low vision of all children who were able to perform the test of functional vision. The assessment of functional vision was performed by the child's ability to walk around between two chairs or similar objects 1-m apart with both eyes open and wearing spectacle correction. Recognition of faces at a distance of 3-meters and ability to see the print at ½-meter were assessed. Refraction with cycloplegia was carried out when required. Visual fields were assessed by confrontation. Anterior segment examination was performed using a handheld slit-lamp or flashlight. The posterior segment examination was performed by indirect ophthalmoscopy after mydriasis.
In the second phase, the actual state of mental health and quality of life of the children in the Schools for the Blind were objectively measured using DASS and LVQOL questionnaires. The children were divided into those above the age of 10 and those below the age of 10 for analysis. This survey was conducted by Optometrists familiar with the language of each state so that the children could understand the questions and respond comfortably.[8,9] The DASS-21 is a 21-item scale providing an overall assessment of general psychological distress as well as three domains: depressive mood, anxiety, and perceptions of stress.[12]
The depression, anxiety, and stress categories were further subdivided into mild, moderate, severe, and very severe depending on the questionnaire scores. Participants completed 21, 4-point Likert items (0–3). Scores are calculated by summing items.[8] Higher scores indicate elevated distress. The DASS-21 has sound psychometric properties including acceptable internal reliability and validity.[12] Based on DASS-21 norms, a total score of 32 is believed to represent clinically elevated levels of general psychological distress, while a score of 10–12 on the depressive mood domain is believed to represent probable depression, and a score of 8 on the anxiety domain is believed to represent probable anxiety disorder.[12]
LVQOL assessment questionnaire was used to assess the quality of life. The score ranges from 0 (no quality of life) to 125 (best achievable quality of life).[9] The LVQOL questionnaire is divided into four subscales, three of which are concerned with functional vision [(1) distance vision, mobility, and lighting; (2) adjustment to the vision loss; (3) reading and fine work; and (4) other activities of daily living]. Although the authors suggest a total score for this questionnaire, each scale can be scored separately if researchers are interested in certain aspects of quality of life.[9] These instruments were chosen to assess the quality of life of the children in Schools for the Blind as it included 3 subscales that are concerned with functional vision.
Statistical analysis
Descriptive measures such as mean with standard deviation (SD) and/or median with interquartile range (IQR) were presented for all continuous variables whereas frequencies and percentages were presented for all categorical variables. IBM SPSS Statistics version 21.0 was used for analysis and P < 0.05% was considered as statistically significant.
Results
A total of 515 children were enrolled in this study. Using the WHO reporting form, 370 (71.84%) students were classified as being blind and 16 (3.1%) had severe visual impairment (SVI) [Fig. 1]. To explore possible trends in the major causes of visual impairment and blindness over time, the data were analyzed after dividing the children into two age groups below 16 (<16) and above 16 (>16) years. There were 285 males and 230 females. There was a significant association between the age group and the visual acuity of the children with a P value < 0.0001. Our study showed that <16 years were more affected with visual impairment than individuals above 16 years of age [Table 1]. The most common anatomical sites of SVI and blindness were whole globe (n = 231, 44.85%), cornea (n = 91, 17.66%), retina (n = 60, 11.65%), optic nerve (n = 51, 9.9%), lens (n = 48, 9.3%), uvea (n = 32, 6.21%) and others (2, 0.38%). On one to one comparison of all the causes of visual impairment, cornea vs optic nerve, retina vs lens, and retina vs optic nerve, no statistical significance was observed. Whereas there was a statistically significant difference between, whole globe vs cornea (P = 0.014), whole globe vs retina (P = 0.00001), whole globe vs lens (P = 0.00001), whole globe vs optic nerve (P = 0.00085), cornea vs retina (P = 0.018), cornea vs lens (P = 0.0005) and lens vs optic nerve (P = 0.014). Whole globe includes Phthisis bulbi, anophthalmos, Microphthalmos, Buphthalmos, Glaucoma, removed, disorganized or others like proptosis.
Figure 1.
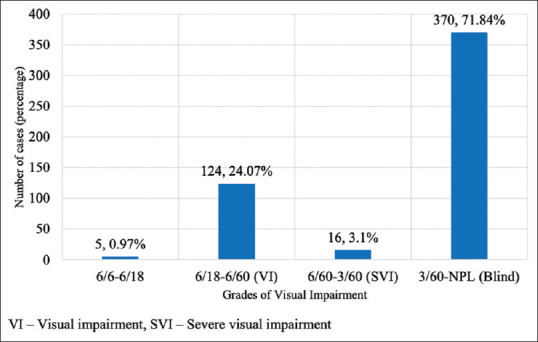
Distribution of cases based on WHO visual impairment grading
Table 1.
Distribution of cases based on cause of visual impairment
| Age (in years) | Causes | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Whole Globe | Cornea | Retina | Lens | Optic Nerve | Uvea | |
| <16 | 188 | 72 | 47 | 40 | 44 | 28 |
| >16 | 43 | 19 | 13 | 8 | 7 | 4 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Total cases (%) | 231, (44.85%) | 91, (17.66%) | 60, (11.65%) | 48, (9.32%) | 51, (9.9%) | 32, (6.21%) |
The highest number of children examined were from the state of Assam with 195 (37.86%), followed by Meghalaya with 92 (17.86%), Tripura 73 (14.17%), Manipur 53 (10.29%), Mizoram 43 (8.34%), Sikkim 30 (5.82%), Nagaland 18 (3.49%) and Arunachal Pradesh with 11 (2.13%) children. No statistically significant association was found between the category of visual impairment of male and female.
The etiological cause of visual impairment was undetermined in 55% (n = 278) of cases. In 27.37% (n = 141) though it was hereditary, the cause could not be specified and only in 1.35% (n = 7) the hereditary pattern could be specified. Other causes were intrauterine 12.40% (n = 64), perinatal 0.7% (n = 4) and postnatal/childhood 4% (n = 21).
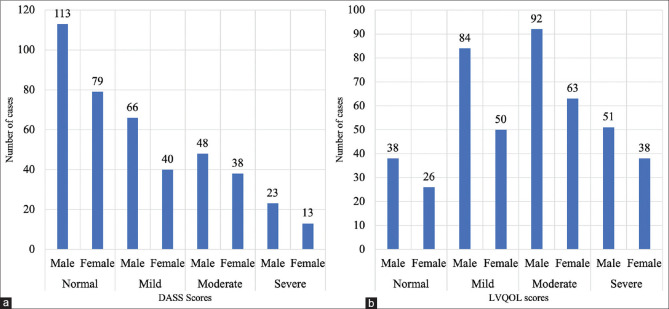
The second phase for assessment of the mental health which was after 6 months from the first survey saw in a drop in a considerable number of children as they had not returned from their vacations. In fact, one school comprising of 18 children was shut down. Hence only 442 children could be assessed using DASS and LVQOL questionnaires. There were 265 males (59.95%) and 177 females (40.05%) with 64 (14.48%) children below 10 years of age. The analysis of the DASS score showed that 192 (43.44%) children belonged to the normal category, and 250 (56.56%) had some levels of anxiety and stress. Fig. 2a shows the DASS scores along with demographic data. Only one component could be examined as children could not differentiate between the groups. The analysis of the LVQOL scores revealed that 378 (85.52%) of the children had some reduction in the quality of life which included 56 (12.67%) from below 10 years. Only 64 (14.48%) of the children examined had normal scores. Fig. 2b shows the LVQOL scores with demographic data.
Figure 2.
(a) Distribution of cases based on DASS scores (b) Distribution of cases based on LVQOL scores
Discussion
Even though the blindness in children is relatively uncommon, severe visual loss in this age group can affect their education, development and employment prospects. In European countries, the recorded prevalence of SVI/BL varies from 0.1 to 0.41 per 1000 children.[13,14] There are several blind school studies from India. The causes of blindness vary from region to region over time and are shown in Table 2.[13,14,15,16,17,18,19,20,21] In our study the most common cause of childhood blindness was whole globe abnormalities (44.85%) comparable to studies by Titiyal et al. and Bhalerao et al.[17,21] The second most common cause of blindness was corneal diseases (17.66%) comparable to many other studies.[17,21] A changing trend is noticed over the past few years in the major causes of blindness with a change from corneal blindness to whole globe anomalies.[18] The decline in corneal causes is probably due to better vitamin A intake and measles vaccination coverage. Congenital anomalies and posterior segment pathologies were the most common causes of childhood blindness in recent years.[22]
Table 2.
Causes of childhood blindness across various regions over time
| Authors | Year | Population | State/s, UT | Major cause of blindness | Second Major cause |
|---|---|---|---|---|---|
| Rahi JS et al. (13) | 1999 | 1318 | 9 states across India | Cornea (26.4%) | WG (20.7%) |
| Hornby SJ et al. (16) | 2000 | 291 | Andhra Pradesh | Retina (31.1%) | Cornea (24.3%) |
| Titiyal JS et al. (17) | 2003 | 703 | Delhi | WG (27.4%) | Cornea (21.7%) |
| Gogate P et al. (18) | 2007 | 1778 | Maharashtra | CA (41.3%) | Cornea (22.2%) |
| Bhattacharjee H et al. (19) | 2008 | 258 | 4 North East States | Cornea (36.7%) | CA (36.1%) |
| Krishnaiah S et al. (20) | 2012 | 113 | Andhra Pradesh | CA (41.4%) | Retina (18.9%) |
| Bhalerao SA et al. (21) | 2015 | 90 | Allahabad | WG (54.4%) | Cornea (24.5%) |
| Prakash MV et al. (22) | 2017 | 302 | Tamil Nadu (Chennai) | Optic Nerve (25.8%) | Retina (18.2%) |
| Present Study | 2019 | 515 | 8 North East States | WG (44.85%) | Cornea (17.66%) |
WG: Whole globe; CA: Congenital anomalies
In our study, there was a positive family history in 59 patients (11.45%) with a definite hereditary cause in only about 1.35% of patients. The huge numbers patients of undetermined etiology (about 55%) reflects the limited investigation facility in this part of the country. Among all these children there were 24 (4.66%) who had other general disabilities in addition to their visual impairment. In our study, a total of 370 students (71.84%) were classified as blind (3/60-NPL) and 16 as SVI (6/60-3/60) (2.91%). Though visual rehabilitation in children could be difficult, all efforts to achieve the best attainable vision are highly desirable and, in our study, 124 children (24.07%) benefitted from Low vision aids. This speaks volumes of how a child's residual visual potential could be used to the optimum. Congenital cataract is the leading cause of surgically correctable blindness in most developing countries.[23] 3.8% (20 children) were referred for cataract surgery to the tertiary eye care institute. In our study more than 45.04% of children had developed defective vision early in their life which resulted in dense amblyopia. We also noted that 5 children with relatively good vision between 6/6 to 6/18 (0.97%) landed in blind schools which could have been avoided if proper and meticulous screening before admission into schools was practiced. This emphasizes the need for community programs to screen for ocular morbidity in children in schools, preschools as well as in pediatric clinics to reduce avoidable blindness. Multidisciplinary collaboration will be required over the long-term with comprehensive service delivery that should encompass health promotion, specific preventive measures, optical, medical, surgical services as well as low vision care, special education, and rehabilitation.[16]
In our study for the assessment of mental health and quality of life the children were divided into those above the age of 10 years and those below the age of 10 years because children above the age of 10 years develop the ability to express their feelings clearly. Blindness and low vision can reduce the quality of life though non-visual factors such as physical and mental health are thought to affect the quality of the life. But the Low vision quality of life questionnaires from our study highlights that almost 85.52% of the children had some form of reduced quality of life. This is a pointer that blindness and low vision reduces one's quality of life. In our study, 74.94% children were either blind (3/60-NPL) or severely visually impaired (6/60-3/60) since birth. DASS scores were normal in (n = 192) 43.4%. and mild in (n = 106) 23.98%. suggesting that blind or visually impaired since birth do not suffer from depression and stress and learn to live or adjust to their disability. It was also found that the scores were same for all the 3 components depression, anxiety and stress and the children could not categorically differentiate between the three groups. This correlates with a study conducted by Jeff et al. which states that only one component should be extracted, indicating that the test does not differentiate depression, anxiety, and stress in children and adolescents.[24]
A better scoring tool to detect depression, anxiety and stress levels need to be designed as it does not seem to be valid for children. Our study emphasizes the fact that visual impairment should be treated as an issue of major concern and efforts should be made to include quality of life assessment in care protocol for the visually impaired and blind. This will help in effective rehabilitation of the visually impaired, maximizing the use of residual vision and helping them to enjoy life and perform daily tasks to the optimum. Blind schools should be in a position not only to give education but also to improve the overall quality of life of these children.
Conclusion
The pattern of childhood blindness in North-eastern states reveals an alarming incidence of whole globe anomalies (44.85%) and an overall 33.5% blind from potentially preventable/or treatable conditions. Recognition of the impact of the visual impairment on the mental health and quality of life is critical to the development of a holistic plan for the visual rehabilitation and management of these children. Nonvisual factors, such as physical and mental health, were found to be stronger predictors of quality of life in people, but the fact that 85.52% had some form of reduced quality of life researchers need to be aware that when measuring quality of life in a population with low vision that even vision related quality of life is strongly influenced by non-visual variables.
Financial support and sponsorship
We would like to thank Lions Club Guwahati Care for their support.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are thankful to Sri Kanchi Sankara Health and Educational Foundation, Mr. Kashyap Talukdar, Mr. Tilak Das, Ms. Ibtesam Zaman, Ms. Thangjam Bembem and Mr. Syed Sagir Rahman for their role and support in the study.
References
- 1.Blindness and Deafness Unit and International Agency for the Prevention of Blindness. Preventing blindness in children: Report of a WHO/IAPB scientific meeting, Hyderabad, India, 13.17 April 1999. World Health Organization. [Last accessed on 2020 May 01]. Available from: https://apps.who.int/iris/handle/10665/66663 .
- 2.Rahi JS, Dezateux C British Congenital Cataract Interest Group. Measuring and interpreting the incidence of congenital ocular anomalies: Lessons from a national study of congenital cataract in the UK. Invest Ophthalmol Vis Sci. 2001;42:1444–8. [PubMed] [Google Scholar]
- 3.Blindness and vision impairment. World Health Organization. [Last accessed on 2020 May 01]. Available from: https://www.who.int/news.room/fact.sheets/detail/blindness-and-visual-impairment .
- 4.Vashist P, Senjam SS, Gupta V, Gupta N, Kumar A. Definition of blindness under National Programme for Control of Blindness: Do we need to revise it? Indian J Ophthalmol. 2017;65:92–6. doi: 10.4103/ijo.IJO_869_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020--The right to sight. Bull World Health Organ. 2001;79:227–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Senjam SS, Foster A, Bascaran C, Vashist P, Gupta V. Assistive technology for students with visual disability in schools for the blind in Delhi. Disabil Rehabil Assist Technol. 2020;15:663–9. doi: 10.1080/17483107.2019.1604829. [DOI] [PubMed] [Google Scholar]
- 7.Choi SU, Chun YS, Lee JK, Kim JT, Jeong JH, Moon NJ. Comparison of vision-related quality of life and mental health between congenital and acquired low-vision patients. Eye. 2019;33:1540–6. doi: 10.1038/s41433-019-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2. Sydney: School of Psychology, University of NSW; 1995. [Google Scholar]
- 9.Wolffsohn JS, Cochrane AL. Design of the low vision quality-of-life questionnaire (LVQOL) and measuring the outcome of low-vision rehabilitation. Am J Ophthalmol. 2000;130:793–802. doi: 10.1016/s0002-9394(00)00610-3. [DOI] [PubMed] [Google Scholar]
- 10.Northeast India. Wikipedia. [Last accessed on 2020 May 01]. Available from: https://en.wikipedia.org/w/index.php?title=Northeast_India&oldid=957802159 .
- 11.Coding instructions for the WHO/PBL eye examination record (version III). 1998. World Health Organization. Available from: https://apps.who.int/iris/handle/10665/67896 .
- 12.Henry JD, Crawford JR. The short-form version of the depression anxiety stress scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44:227–39. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 13.Rahi JS, Gilbert CE, Foster A, Minassian D. Measuring the burden of childhood blindness. Br J Ophthalmol. 1999;83:387–8. doi: 10.1136/bjo.83.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster A, Gilbert C. Epidemiology of childhood blindness. Eye (Lond) 1992;6:173–6. doi: 10.1038/eye.1992.34. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg T, Flage T, Hansen E, Riise R, Rudanko SL, Viggosson G, et al. Incidence of registered visual impairment in the Nordic child population. Br J Ophthalmol. 1996;80:49–53. doi: 10.1136/bjo.80.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornby SJ, Adolph S, Gothwal VK, Gilbert CE, Dandona L, Foster A. Evaluation of children in six blind schools of Andhra Pradesh. Indian J Ophthalmol. 2000;48:195–200. [PubMed] [Google Scholar]
- 17.Titiyal JS, Pal N, Murthy GV, Gupta SK, Tandon R, Vajpayee RB, et al. Causes and temporal trends of blindness and severe visual impairment in children in schools for the blind in North India. Br J Ophthalmol. 2003;87:941–5. doi: 10.1136/bjo.87.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gogate P, Deshpande M, Sudrik S, Taras S, Kishore H, Gilbert C. Changing pattern of childhood blindness in Maharashtra, India. Br J Ophthalmol. 2007;91:8–12. doi: 10.1136/bjo.2006.094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharjee H, Das K, Borah RR, Guha K, Gogate P, Purukayastha S, et al. Causes of childhood blindness in the northeastern states of India. Indian J Ophthalmol. 2008;56:495–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnaiah S, Subba Rao B, Lakshmi Narasamma K, Amit G. A survey of severe visual impairment in children attending schools for the blind in a coastal district of Andhra Pradesh in South India. Eye. 2012;26:1065–70. doi: 10.1038/eye.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhalerao SA, Tandon M, Singh S, Dwivedi S, Kumar S, Rana J. Visual impairment and blindness among the students of blind schools in Allahabad and its vicinity: A causal assessment. Indian J Ophthalmol. 2015;63:254–8. doi: 10.4103/0301-4738.156930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prakash MV, Sivakumar S, Dayal A, Chitra A, Subramaniam S. Ocular morbidity patterns among children in schools for the blind in Chennai. Indian J Ophthalmol. 2017;65:733–7. doi: 10.4103/ijo.IJO_294_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gogate P, Muhit M. Blindness and cataract in children in developing countries. Community Eye Health. 2009;22:4–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Patrick J, Dyck M, Bramston P. Depression anxiety stress scale: Is it valid for children and adolescents? J Clin Psychol. 2010;66:996–1007. doi: 10.1002/jclp.20696. [DOI] [PubMed] [Google Scholar]