Abstract
The rapid global spread of coronavirus disease 2019 (COVID-19) has promoted concern over human pathogens and their significant threats to public health security. The monitoring and control of human pathogens in public sanitation and health facilities are of great importance. Excessive sludge is an inevitable byproduct of sewage that contains human and animal feces in wastewater treatment plants (WWTPs). It is an important sink of different pollutants and pathogens, and the proper treatment and disposal of sludge are important to minimize potential risks to the environment and public health. However, there is a lack of comprehensive analysis of the diversity, exposure risks, assessment methods and inactivation techniques of pathogenic microorganisms in sludge. Based on this consideration, this review summarizes the control performance of pathogenic microorganisms such as enterovirus, Salmonella spp., and Escherichia coli by different sludge treatment technologies, including composting, anaerobic digestion, aerobic digestion, and microwave irradiation, and the mechanisms of pathogenic microorganism inactivation in sludge treatment processes are discussed. Additionally, this study reviews the diversity, detection methods, and exposure risks of pathogenic microorganisms in sludge. This review advances the quantitative assessment of pathogenic microorganism risks involved in sludge reuse and is practically valuable to optimize the treatment and disposal of sludge for pathogenic microorganism control.
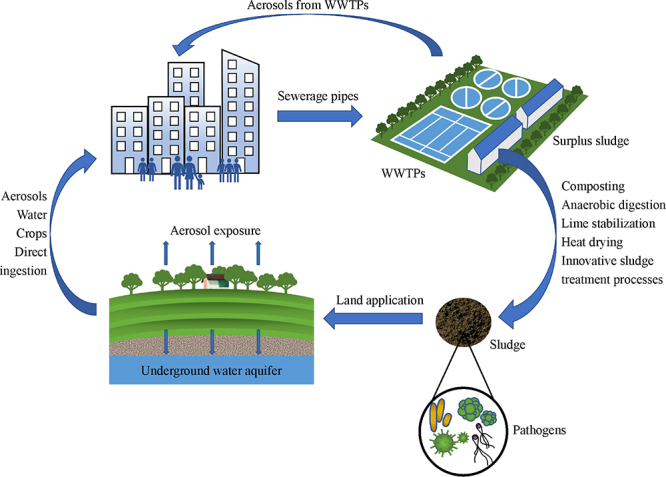
Keywords: Sludge treatment, Pathogenic microorganisms, Inactivation mechanisms, Exposure risks, Land application
Acknowledgements
This study was sponsored by the National Natural Science Foundation of China (Grant Nos. 51925807 and 52091542) and the Excellent Innovation Project of Research Center for Eco-Environmental Sciences (CAS RCEES-EEI-2019-02).
Footnotes
Highlights
• Diversity and detection methods of pathogenic microorganisms in sludge.
• Control performance of sludge treatment processes on pathogenic microorganisms.
• Risk of pathogen exposure in sludge treatment and land application.
References
- Afolabi O O D, Sohail M. Microwaving human faecal sludge as a viable sanitation technology option for treatment and value recovery: A critical review. Journal of Environmental Management. 2017;187:401–415. doi: 10.1016/j.jenvman.2016.10.067. [DOI] [PubMed] [Google Scholar]
- AL-Ghonaiem M I, Ibrahim A S S, Al-Salamah A A. Application of gamma irradiation in treatment of waste activated sludge to obtain class a biosolids. American Journal of Environmental Sciences. 2010;6(6):500–504. doi: 10.3844/ajessp.2010.500.504. [DOI] [Google Scholar]
- Amoah I D, Reddy P, Seidu R, Stenström T A. Concentration of soil-transmitted helminth eggs in sludge from South Africa and Senegal: A probabilistic estimation of infection risks associated with agricultural application. Journal of Environmental Management. 2018;206:1020–1027. doi: 10.1016/j.jenvman.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorós I, Moreno Y, Reyes M, Moreno-Mesonero L, Alonso J L. Prevalence of Cryptosporidium oocysts and Giardia cysts in raw and treated sewage sludges. Environmental Technology. 2016;37(22):2898–2904. doi: 10.1080/09593330.2016.1168486. [DOI] [PubMed] [Google Scholar]
- Armstrong T W, Haas C N. A quantitative microbial risk assessment model for Legionnaires’ disease: Animal model selection and dose-response modeling. Risk Analysis: An Official Publication of the Society for Risk Analysis. 2007;27(6):1581–1596. doi: 10.1111/j.1539-6924.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- Astals S, Venegas C, Peces M, Jofre J, Lucena F, Mata-Alvarez J. Balancing hygienization and anaerobic digestion of raw sewage sludge. Water Research. 2012;46(19):6218–6227. doi: 10.1016/j.watres.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Bakheet B, Prodanovic V, Deletic A, McCarthy D. Effective treatment of greywater via green wall biofiltration and electrochemical disinfection. Water Research. 2020;185:116228. doi: 10.1016/j.watres.2020.116228. [DOI] [PubMed] [Google Scholar]
- Balboa S, Mauricio-Iglesias M, Rodriguez S, Martínez-Lamas L, Vasallo F J, Regueiro B, Lema J M. The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for detection of COVID-19. Science of the Total Environment. 2021;772:145268. doi: 10.1016/j.scitotenv.2021.145268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi M J, Oliaee M A. Impacts of different operational temperatures and organic loads in anaerobic co-digestion of food waste and sewage sludge on the fate of SARS-CoV-2. Process Safety and Environmental Protection. 2021;146:464–472. doi: 10.1016/j.psep.2020.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean C L, Hansen J J, Margolin A B, Balkin H, Batzer G, Widmer G. Class B alkaline stabilization to achieve pathogen inactivation. International Journal of Environmental Research and Public Health. 2007;4(1):53–60. doi: 10.3390/ijerph2007010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito M, Menacho C, Chueca P, Ormad M P, Goñi P. Seeking the reuse of effluents and sludge from conventional wastewater treatment plants: Analysis of the presence of intestinal protozoa and nematode eggs. Journal of Environmental Management. 2020;261:110268. doi: 10.1016/j.jenvman.2020.110268. [DOI] [PubMed] [Google Scholar]
- Bibby K, Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environmental Science & Technology. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm A B, Graham K E, Jennings W C. Can we swim yet? Systematic review, meta-analysis, and risk assessment of aging sewage in surface waters. Environmental Science & Technology. 2018;52(17):9634–9645. doi: 10.1021/acs.est.8b01948. [DOI] [PubMed] [Google Scholar]
- Borrely S I, Cruz A C, Del Mastro N L, Sampa M H O, Somessari E S. Radiation processing of sewage and sludge: A review. Progress in Nuclear Energy. 1998;33(1–2):3–21. doi: 10.1016/S0149-1970(97)87287-3. [DOI] [Google Scholar]
- Brooks J P, McLaughlin M R, Gerba C P, Pepper I L. Land application of manure and Class B biosolids: An occupational and public quantitative microbial risk assessment. Journal of Environmental Quality. 2012;41(6):2009–2023. doi: 10.2134/jeq2011.0430. [DOI] [PubMed] [Google Scholar]
- Brooks J P, Tanner B D, Gerba C P, Haas C N, Pepper I L. Estimation of bioaerosol risk of infection to residents adjacent to a land applied biosolids site using an empirically derived transport model. Journal of Applied Microbiology. 2005;98(2):397–405. doi: 10.1111/j.1365-2672.2004.02484.x. [DOI] [PubMed] [Google Scholar]
- Buzzini A P, Patrizzi L J, Motheo A J, Pires E C. Preliminary evaluation of the electrochemical and chemical coagulation processes in the post-treatment of effluent from an upflow anaerobic sludge blanket (UASB) reactor. Journal of Environmental Management. 2007;85(4):847–857. doi: 10.1016/j.jenvman.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Cai L, Zhang T. Detecting human bacterial pathogens in wastewater treatment plants by a high-throughput shotgun sequencing technique. Environmental Science & Technology. 2013;47(10):5433–5441. doi: 10.1021/es400275r. [DOI] [PubMed] [Google Scholar]
- Calero-Cáceres W, Melgarejo A, Colomer-Lluch M, Stoll C, Lucena F, Jofre J, Muniesa M. Sludge as a potential important source of antibiotic resistance genes in both the bacterial and bacteriophage fractions. Environmental Science & Technology. 2014;48(13):7602–7611. doi: 10.1021/es501851s. [DOI] [PubMed] [Google Scholar]
- Capizzi-Banas S, Deloge M, Remy M, Schwartzbrod J. Liming as an advanced treatment for sludge sanitisation: Helminth eggs elimination: Ascaris eggs as model. Water Research. 2004;38(14–15):3251–3258. doi: 10.1016/j.watres.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Carraturo F, Del Giudice C, Morelli M, Cerullo V, Libralato G, Galdiero E, Guida M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environmental Pollution. 2020;265:115010. doi: 10.1016/j.envpol.2020.115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J L, Ortiz R, Steele T W J, Stuckey D C. Toxicants inhibiting anaerobic digestion: A review. Biotechnology Advances. 2014;32(8):1523–1534. doi: 10.1016/j.biotechadv.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Chen Y H, Yan C, Yang Y F, Ma J X. Quantitative microbial risk assessment and sensitivity analysis for workers exposed to pathogenic bacterial bioaerosols under various aeration modes in two wastewater treatment plants. Science of the Total Environment. 2021;755:142615. doi: 10.1016/j.scitotenv.2020.142615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetochine A S, Brusseau M L, Gerba C P, Pepper I L. Leaching of phage from Class B biosolids and potential transport through soil. Applied and Environmental Microbiology. 2006;72(1):665–671. doi: 10.1128/AEM.72.1.665-671.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheunbarn T, Pagilla K R. Anaerobic thermophilic/mesophilic dual-stage sludge treatment. Journal of Environmental Engineering. 2000;126(9):796–801. doi: 10.1061/(ASCE)0733-9372(2000)126:9(796). [DOI] [Google Scholar]
- Chmielewski A G, Han B. Electron Beam Technology for Environmental Pollution Control. Topics in Current Chemistry (Cham) 2016;374(5):68. doi: 10.1007/s41061-016-0069-4. [DOI] [PubMed] [Google Scholar]
- Claesson M J, Wang Q, O’Sullivan O, Greene-Diniz R, Cole J R, Ross R P, O’Toole P W. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Research. 2010;38(22):e200. doi: 10.1093/nar/gkq873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho N M G, Droste R L, Kennedy K J. Evaluation of continuous mesophilic, thermophilic and temperature phased anaerobic digestion of microwaved activated sludge. Water Research. 2011;45(9):2822–2834. doi: 10.1016/j.watres.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Cosgun S, Semerci N. Combined and individual applications of ozonation and microwave treatment for waste activated sludge solubilization and nutrient release. Journal of Environmental Management. 2019;241:76–83. doi: 10.1016/j.jenvman.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Cui X, Quicksall A N, Blake A B, Talley J W. Electrochemical disinfection of Escherichia coli in the presence and absence of primary sludge particulates. Water Research. 2013;47(13):4383–4390. doi: 10.1016/j.watres.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Dai X, Li X, Yang W, Dai L, Dong B. Virus in sewage sludge from wastewater treatment plant: Occurrence and potential risk during sludge treatment and disposal. Water & Wastewater Engineering. 2020;46(3):60–73. [Google Scholar]
- Dauknys R, Mažeikienė A, Paliulis D. Effect of ultrasound and high voltage disintegration on sludge digestion process. Journal of Environmental Management. 2020;270:110833. doi: 10.1016/j.jenvman.2020.110833. [DOI] [PubMed] [Google Scholar]
- Deng W, Su Y, Yu W. Theoretical calculation of heat transfer coefficient when sludge drying in a nara-type paddle dryer using different heat carriers. Procedia Environmental Sciences. 2013;18:709–715. doi: 10.1016/j.proenv.2013.04.096. [DOI] [Google Scholar]
- Diehl D L, LaPara T M. Effect of temperature on the fate of genes encoding tetracycline resistance and the integrase of class 1 integrons within anaerobic and aerobic digesters treating municipal wastewater solids. Environmental Science & Technology. 2010;44(23):9128–9133. doi: 10.1021/es102765a. [DOI] [PubMed] [Google Scholar]
- Drogui P, Bureau M A, Mercier G, Blais J F. Effectiveness of electrooxidation process for stabilizing and conditioning of urban and industrial wastewater sludge. Water Environment Research: A Research Publication of the Water Environment Federation. 2013;85(1):35–43. doi: 10.2175/106143012X13415215907257. [DOI] [PubMed] [Google Scholar]
- Eamens G J, Waldron A M, Nicholls P J. Survival of pathogenic and indicator bacteria in biosolids applied to agricultural land. Australian Journal of Soil Research. 2006;44(7):647–659. doi: 10.1071/SR06015. [DOI] [Google Scholar]
- Eisenberg J N S, Moore K, Soller J A, Eisenberg D, Colford J M., Jr Microbial risk assessment framework for exposure to amended sludge projects. Environmental Health Perspectives. 2008;116(6):727–733. doi: 10.1289/ehp.10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J G, Taylor-Edmonds L, Andrews R C. Quantitative microbial risk assessments for drinking water facilities: Evaluation of a range of treatment strategies. Environmental Science. Water Research & Technology. 2019;5(11):1943–1955. doi: 10.1039/C9EW00348G. [DOI] [Google Scholar]
- Elmerdahl Olsen J, Errebo Larsen H. Bacterial decimation times in anaerobic digestions of animal slurries. Biological Wastes. 1987;21(3):153–168. doi: 10.1016/0269-7483(87)90121-2. [DOI] [Google Scholar]
- Elving J, Ottoson J R, Vinnerås B, Albihn A. Growth potential of faecal bacteria in simulated psychrophilic/mesophilic zones during composting of organic waste. Journal of Applied Microbiology. 2010;108(6):1974–1981. doi: 10.1111/j.1365-2672.2009.04593.x. [DOI] [PubMed] [Google Scholar]
- Espinosa M F, Sancho A N, Mendoza L M, Mota C R, Verbyla M E. Systematic review and meta-analysis of time-temperature pathogen inactivation. International Journal of Hygiene and Environmental Health. 2020;230:113595. doi: 10.1016/j.ijheh.2020.113595. [DOI] [PubMed] [Google Scholar]
- Ferreira L C, Castro-Alférez M, Nahim-Granados S, Polo-López M I, Lucas M S, Li Puma G, Fernández-Ibáñez P. Inactivation of water pathogens with solar photo-activated persulfate oxidation. Chemical Engineering Journal. 2020;381:122275. doi: 10.1016/j.cej.2019.122275. [DOI] [Google Scholar]
- Fidjeland J, Nordin A, Pecson B M, Nelson K L, Vinnerås B. Modeling the inactivation of ascaris eggs as a function of ammonia concentration and temperature. Water Research. 2015;83:153–160. doi: 10.1016/j.watres.2015.06.030. [DOI] [PubMed] [Google Scholar]
- Foladori P, Cutrupi F, Segata N, Manara S, Pinto F, Malpei F, Bruni L, La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: What do we know? A review. Science of the Total Environment. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font R, Gomez-Rico M F, Fullana A. Skin effect in the heat and mass transfer model for sewage sludge drying. Separation and Purification Technology. 2011;77(1):146–161. doi: 10.1016/j.seppur.2010.12.001. [DOI] [Google Scholar]
- Forbis-Stokes A A, O’Meara P F, Mugo W, Simiyu G M, Deshusses M A. On-Site Fecal Sludge Treatment with the AnaerobicDigestion Pasteurization Latrine. Environmental Engineering Science. 2016;33(11):898–906. doi: 10.1089/ees.2016.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster-Carneiro T, Riau V, Pérez M. Mesophilic anaerobic digestion of sewage sludge to obtain class B biosolids: Microbiological methods development. Biomass and Bioenergy. 2010;34(12):1805–1812. doi: 10.1016/j.biombioe.2010.07.010. [DOI] [Google Scholar]
- Gale P. Using event trees to quantify pathogen levels on root crops from land application of treated sewage sludge. Journal of Applied Microbiology. 2003;94(1):35–47. doi: 10.1046/j.1365-2672.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- Gale P. Land application of treated sewage sludge: Quantifying pathogen risks from consumption of crops. Journal of Applied Microbiology. 2005;98(2):380–396. doi: 10.1111/j.1365-2672.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- Gantzer C, Gaspard P, Galvez L, Huyard A, Dumouthier N, Schwartzbrod J. Monitoring of bacterial and parasitological contamination during various treatment of sludge. Water Research. 2001;35(16):3763–3770. doi: 10.1016/S0043-1354(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Gaviria-Figueroa A, Preisner E C, Hoque S, Feigley C E, Norman R S. Emission and dispersal of antibiotic resistance genes through bioaerosols generated during the treatment of municipal sewage. Science of the Total Environment. 2019;686:402–412. doi: 10.1016/j.scitotenv.2019.05.454. [DOI] [PubMed] [Google Scholar]
- Ge D, Dong Y, Zhang W, Yuan H, Zhu N. A novel Fe2+/persulfate/tannic acid process with strengthened efficacy on enhancing waste activated sludge dewaterability and mechanism insight. Science of the Total Environment. 2020;733:139146. doi: 10.1016/j.scitotenv.2020.139146. [DOI] [PubMed] [Google Scholar]
- Gil A, Siles J A, Martín M A, Chica A F, Estévez-Pastor F S, Toro-Baptista E. Effect of microwave pretreatment on semi-continuous anaerobic digestion of sewage sludge. Renewable Energy. 2018;115:917–925. doi: 10.1016/j.renene.2017.07.112. [DOI] [Google Scholar]
- Goberna M, Simón P, Hernández M T, García C. Prokaryotic communities and potential pathogens in sewage sludge: Response to wastewaster origin, loading rate and treatment technology. Science of the Total Environment. 2018;615:360–368. doi: 10.1016/j.scitotenv.2017.09.240. [DOI] [PubMed] [Google Scholar]
- Gomes L A, Santos A F, Pinheiro C T, Góis J C, Quina M J. Screening of waste materials as adjuvants for drying sewage sludge based on environmental, technical and economic criteria. Journal of Cleaner Production. 2020;259:120927. doi: 10.1016/j.jclepro.2020.120927. [DOI] [Google Scholar]
- Graff J, Ticehurst J, Flehmig B. Detection of hepatitis A virus in sewage sludge by antigen capture polymerase chain reaction. Applied and Environmental Microbiology. 1993;59(10):3165–3170. doi: 10.1128/aem.59.10.3165-3170.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grübel K, Suschka J. Hybrid alkali-hydrodynamic disintegration of waste-activated sludge before two-stage anaerobic digestion process. Environmental Science and Pollution Research International. 2015;22(10):7258–7270. doi: 10.1007/s11356-014-3705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guajardo-Leiva S, Chnaiderman J, Gaggero A, Díez B. Metagenomic Insights into the sewage RNA virosphere of a large city. Viruses. 2020;12(9):1050. doi: 10.3390/v12091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait S, Tare V. Optimizing vermistabilization of waste activated sludge using vermicompost as bulking material. Waste Management (New York, N.Y.) 2011;31(3):502–511. doi: 10.1016/j.wasman.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Hamilton K A, Haas C N. Critical review of mathematical approaches for quantitative microbial risk assessment (QMRA) of Legionella in engineered water systems: research gaps and a new framework. Environmental Science. Water Research & Technology. 2016;2(4):599–613. doi: 10.1039/C6EW00023A. [DOI] [Google Scholar]
- Hamilton K A, Hamilton M T, Johnson W, Jjemba P, Bukhari Z, LeChevallier M, Haas C N, Gurian P L. Risk-based critical concentrations of Legionella pneumophila for indoor residential water uses. Environmental Science & Technology. 2019;53(8):4528–4541. doi: 10.1021/acs.est.8b03000. [DOI] [PubMed] [Google Scholar]
- Han Y P, Li L, Wang Y, Ma J W, Li P Y, Han C, Liu J X. Composition, dispersion, and health risks of bioaerosols in waste-water treatment plants: A review. Frontiers of Environmental Science & Engineering. 2021;15(3):16. [Google Scholar]
- Hansen J J, Warden P S, Margolin A B. Inactivation of adenovirus type 5, rotavirus Wa and male specific coliphage (MS2) in biosolids by lime stabilization. International Journal of Environmental Research and Public Health. 2007;4(1):61–67. doi: 10.3390/ijerph2007010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S M, Park J K, Lee Y O. Mechanisms of microwave irradiation involved in the destruction of fecal coliforms from biosolids. Water Research. 2004;38(6):1615–1625. doi: 10.1016/j.watres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Hong S M, Park J K, Teeradej N, Lee Y O, Cho Y K, Park C H. Pretreatment of sludge with microwaves for pathogen destruction and improved anaerobic digestion performance. Water Environment Research: A Research Publication of the Water Environment Federation. 2006;78(1):76–83. doi: 10.2175/106143005X84549. [DOI] [PubMed] [Google Scholar]
- Hu S, Zhao W, Hu J, Liu B, Wang D, Zhu Q, Yang J, Hou H. Integration of electrochemical and calcium hypochlorite oxidation for simultaneous sludge deep dewatering, stabilization and phosphorus fixation. Science of the Total Environment. 2021;750:141408. doi: 10.1016/j.scitotenv.2020.141408. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wang F, Lv G, Chi Y. Enhancing the biogas production of sludge anaerobic digestion by a combination of zero-valent iron foil and persulfate. Energy & Fuels. 2019;33(8):7436–7442. doi: 10.1021/acs.energyfuels.9b01475. [DOI] [Google Scholar]
- Huang J, Elektorowicz M, Oleszkiewicz J A. Dewatering and disinfection of aerobic and anaerobic sludge using an electrokinetic (EK) system. Water Science and Technology: A Journal of the International Association on Water Pollution Research. 2008;57(2):231–236. doi: 10.2166/wst.2008.009. [DOI] [PubMed] [Google Scholar]
- Huang K, Mao Y, Zhao F, Zhang X X, Ju F, Ye L, Wang Y, Li B, Ren H, Zhang T. Free-living bacteria and potential bacterial pathogens in sewage treatment plants. Applied Microbiology and Biotechnology. 2018;102(5):2455–2464. doi: 10.1007/s00253-018-8796-9. [DOI] [PubMed] [Google Scholar]
- Huang K, Xia H, Zhang Y, Li J, Cui G, Li F, Bai W, Jiang Y, Wu N. Elimination of antibiotic resistance genes and human pathogenic bacteria by earthworms during vermicomposting of dewatered sludge by metagenomic analysis. Bioresource Technology. 2020;297:122451. doi: 10.1016/j.biortech.2019.122451. [DOI] [PubMed] [Google Scholar]
- Inglis G D, McAllister T A, Larney F J, Topp E. Prolonged survival of Campylobacter species in bovine manure compost. Applied and Environmental Microbiology. 2010;76(4):1110–1119. doi: 10.1128/AEM.01902-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranpour R, Cox H H J. Recurrence of fecal coliforms and Salmonella species in biosolids following thermophilic anaerobic digestion. Water Environment Research: A Research Publication of the Water Environment Federation. 2006;78(9):1005–1012. doi: 10.2175/106143006X143911. [DOI] [PubMed] [Google Scholar]
- Iranpour R, Cox H H J. Evaluation of thermophilic anaerobic digestion processes for full-scale Class A biosolids disinfection at Hyperion Treatment Plant. Biotechnology and Bioengineering. 2007;97(1):19–39. doi: 10.1002/bit.21176. [DOI] [PubMed] [Google Scholar]
- Jafari M, Botte G G. Electrochemical treatment of sewage sludge and pathogen inactivation. Journal of Applied Electrochemistry. 2021;51(1):119–130. doi: 10.1007/s10800-020-01481-6. [DOI] [Google Scholar]
- Jahne M A, Brinkman N E, Keely S P, Zimmerman B D, Wheaton E A, Garland J L. Droplet digital PCR quantification of norovirus and adenovirus in decentralized wastewater and graywater collections: Implications for onsite reuse. Water Research. 2020;169:115213. doi: 10.1016/j.watres.2019.115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebri S, Hmaied F, Lucena F, Saavedra M E, Yahya M, Hamdi M. A comparison of two extraction methods for the detection of Enteroviruses in raw sludge. Journal of Virological Methods. 2014;200:1–5. doi: 10.1016/j.jviromet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Jin B, Niu J, Zhang J, Niu J, Zhou P, Dai J, Li N, Tao H, Ma Z, Zhang Z. Response of extracellular polymeric substances and enzymatic activity to salinity for the waste activated sludge anaerobic fermentation process. Bioprocess and Biosystems Engineering. 2020;43(4):737–745. doi: 10.1007/s00449-019-02253-z. [DOI] [PubMed] [Google Scholar]
- Ju F, Lau F, Zhang T. Linking Microbial Community, Environmental Variables, and Methanogenesis in Anaerobic Biogas Digesters of Chemically Enhanced Primary Treatment Sludge. Environmental Science & Technology. 2017;51(7):3982–3992. doi: 10.1021/acs.est.6b06344. [DOI] [PubMed] [Google Scholar]
- Ju F, Li B, Ma L, Wang Y, Huang D, Zhang T. Antibiotic resistance genes and human bacterial pathogens: Co-occurrence, removal, and enrichment in municipal sewage sludge digesters. Water Research. 2016;91:1–10. doi: 10.1016/j.watres.2015.11.071. [DOI] [PubMed] [Google Scholar]
- Jyoti A, Vajpayee P, Singh G, Patel C B, Gupta K C, Shanker R. Identification of environmental reservoirs of nontyphoidal salmonellosis: Aptamer-assisted bioconcentration and subsequent detection of salmonella typhimurium by quantitative polymerase chain reaction. Environmental Science & Technology. 2011;45(20):8996–9002. doi: 10.1021/es2018994. [DOI] [PubMed] [Google Scholar]
- Kabrick R M, Jewell W J. Fate of pathogens in thermophilic aerobic sludge digestion. Water Research. 1982;16(6):1051–1060. doi: 10.1016/0043-1354(82)90041-0. [DOI] [Google Scholar]
- Kearney T E, Larkin M J, Levett P N. The effect of slurry storage and anaerobic digestion on survival of pathogenic bacteria. Journal of Applied Bacteriology. 1993;74(1):86–93. doi: 10.1111/j.1365-2672.1993.tb03000.x. [DOI] [PubMed] [Google Scholar]
- Kelessidis A, Stasinakis A S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Management (New York, N.Y.) 2012;32(6):1186–1195. doi: 10.1016/j.wasman.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Kennedy N A, Walker A W, Berry S H, Duncan S H, Farquarson F M, Louis P, Thomson J M, Satsangi J, Flint H J, Parkhill J, Lees C W, Hold G L, the UK IBD Genetics Consortium The impact of different DNA extraction kits and laboratories upon the assessment of human gut microbiota composition by 16S rRNA gene sequencing. PLoS One. 2014;9(2):e88982. doi: 10.1371/journal.pone.0088982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwairakpam M, Bhargava R. Vermitechnology for sewage sludge recycling. Journal of Hazardous Materials. 2009;161(2–3):948–954. doi: 10.1016/j.jhazmat.2008.04.088. [DOI] [PubMed] [Google Scholar]
- Kim T H, Lee M, Park C. Gamma ray irradiation for sludge solubilization and biological nitrogen removal. Radiation Physics and Chemistry. 2011;80(12):1386–1390. doi: 10.1016/j.radphyschem.2011.06.011. [DOI] [Google Scholar]
- Kong F E, Deighton M A, Thurbon N A, Smith S R, Rouch D A. Cryptosporidium parvum decay during air drying and stockpiling of mesophilic anaerobically digested sewage sludge in a simulation experiment and oocyst counts in sludge collected from operational treatment lagoons in Victoria, Australia. Journal of Water and Health. 2018;16(3):435–448. doi: 10.2166/wh.2018.018. [DOI] [PubMed] [Google Scholar]
- Lagier J C, Armougom F, Million M, Hugon P, Pagnier I, Robert C, Bittar F, Fournous G, Gimenez G, Maraninchi M, Trape J F, Koonin E V, La Scola B, Raoult D. Microbial culturomics: paradigm shift in the human gut microbiome study. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012;18(12):1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- Lammerding A M. Modeling and risk assessment for Salmonella in meat and poultry. Journal of AOAC International. 2006;89(2):543–552. doi: 10.1093/jaoac/89.2.543. [DOI] [PubMed] [Google Scholar]
- Lau C H F, Li B, Zhang T, Tien Y C, Scott A, Murray R, Sabourin L, Lapen D R, Duenk P, Topp E. Impact of pre-application treatment on municipal sludge composition, soil dynamics of antibiotic resistance genes, and abundance of antibiotic-resistance genes on vegetables at harvest. Science of the Total Environment. 2017;587–588:214–222. doi: 10.1016/j.scitotenv.2017.02.123. [DOI] [PubMed] [Google Scholar]
- Lei Q, Zheng J, Ma J, Wang X, Wu Z, Wang Z. Simultaneous solid-liquid separation and wastewater disinfection using an electrochemical dynamic membrane filtration system. Environmental Research. 2020;180:108861. doi: 10.1016/j.envres.2019.108861. [DOI] [PubMed] [Google Scholar]
- Levantesi C, Beimfohr C, Blanch A R, Carducci A, Gianico A, Lucena F, Tomei M C, Mininni G. Hygienization performances of innovative sludge treatment solutions to assure safe land spreading. Environmental Science and Pollution Research International. 2015;22(10):7237–7247. doi: 10.1007/s11356-014-3572-6. [DOI] [PubMed] [Google Scholar]
- Lewis D L, Gattie D K. Pathogen risks from applying sewage sludge to land. Environmental Science & Technology. 2002;36(13):286A–293A. doi: 10.1021/es0223426. [DOI] [PubMed] [Google Scholar]
- Li B, Ju F, Cai L, Zhang T. Profile and fate of bacterial pathogens in sewage treatment plants revealed by high-throughput metagenomic approach. Environmental Science & Technology. 2015;49(17):10492–10502. doi: 10.1021/acs.est.5b02345. [DOI] [PubMed] [Google Scholar]
- Li D C, Gao J F, Zhang S J, Gao Y Q, Sun L X. Emergence and spread patterns of antibiotic resistance genes during two different aerobic granular sludge cultivation processes. Environment International. 2020;137:105540. doi: 10.1016/j.envint.2020.105540. [DOI] [PubMed] [Google Scholar]
- Li J, Zhou L T, Zhang X Y, Xu C J, Dong L M, Yao M S. Bioaerosol emissions and detection of airborne antibiotic resistance genes from a wastewater treatment plant. Atmospheric Environment. 2016;124:404–412. doi: 10.1016/j.atmosenv.2015.06.030. [DOI] [Google Scholar]
- Li M, Yang Y, Lu Y, Zhang D, Liu Y, Cui X, Yang L, Liu R, Liu J, Li G, Qu J. Natural host-environmental media-human: A new potential pathway of COVID-19 outbreak. Engineering (Beijing, China) 2020;6(10):1085–1098. doi: 10.1016/j.eng.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li Y, Hong L. A novel self-assembling DNA nano chip for rapid detection of human Papillomavirus genes. PLoS One. 2016;11(10):e0162975. doi: 10.1371/journal.pone.0162975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Lu X, Rensing C, Friman V P, Geisen S, Chen Z, Yu Z, Wei Z, Zhou S, Zhu Y. Hyperthermophilic composting accelerates the removal of antibiotic resistance genes and mobile genetic elements in sewage sludge. Environmental Science & Technology. 2018;52(1):266–276. doi: 10.1021/acs.est.7b04483. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhu N, Li L Y. The one-stage autothermal thermophilic aerobic digestion for sewage sludge treatment. Chemical Engineering Journal. 2011;174(2–3):564–570. doi: 10.1016/j.cej.2011.09.043. [DOI] [Google Scholar]
- Lizasoain A, Tort L F L, García M, Gillman L, Alberti A, Leite J P G, Miagostovich M P, Pou S A, Cagiao A, Razsap A, Huertas J, Berois M, Victoria M, Colina R. Human enteric viruses in a wastewater treatment plant: Evaluation of activated sludge combined with UV disinfection process reveals different removal performances for viruses with different features. Letters in Applied Microbiology. 2018;66(3):215–221. doi: 10.1111/lam.12839. [DOI] [PubMed] [Google Scholar]
- Lloret E, Pastor L, Martínez-Medina A, Blaya J, Pascual J A. Evaluation of the removal of pathogens included in the Proposal for a European Directive on spreading of sludge on land during autothermal thermophilic aerobic digestion (ATAD) Chemical Engineering Journal. 2012;198–199:171–179. doi: 10.1016/j.cej.2012.05.068. [DOI] [Google Scholar]
- Lloret E, Pastor L, Pradas P, Pascual J A. Semi full-scale thermophilic anaerobic digestion (TAnD) for advanced treatment of sewage sludge: Stabilization process and pathogen reduction. Chemical Engineering Journal. 2013;232:42–50. doi: 10.1016/j.cej.2013.07.062. [DOI] [Google Scholar]
- López A, Rodríguez-Chueca J, Mosteo R, Gómez J, Ormad M P. Microbiological quality of sewage sludge after digestion treatment: A pilot scale case of study. Journal of Cleaner Production. 2020;254:120101. doi: 10.1016/j.jclepro.2020.120101. [DOI] [Google Scholar]
- Lu F, Hu T Y, Wei S Y, Shao L M, He P J. Bioaerosolization behavior along sewage sludge biostabilization. Frontiers of Environmental Science & Engineering. 2021;15(3):45. doi: 10.1007/s11783-020-1339-5. [DOI] [Google Scholar]
- Lu X, Zhang X X, Wang Z, Huang K, Wang Y, Liang W, Tan Y, Liu B, Tang J. Bacterial pathogens and community composition in advanced sewage treatment systems revealed by metagenomics analysis based on high-throughput sequencing. PLoS One. 2015;10(5):e0125549. doi: 10.1371/journal.pone.0125549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung A J, Lin C M, Kim J M, Marshall M R, Nordstedt R, Thompson N P, Wei C I. Destruction of Escherichia coli O157:H7 and Salmonella enteritidis in cow manure composting. Journal of Food Protection. 2001;64(9):1309–1314. doi: 10.4315/0362-028X-64.9.1309. [DOI] [PubMed] [Google Scholar]
- Luukkonen T, Prokkola H, Pehkonen S O. Peracetic acid for conditioning of municipal wastewater sludge: Hygienization, odor control, and fertilizing properties. Waste Management (New York, N. Y.) 2020;102:371–379. doi: 10.1016/j.wasman.2019.11.004. [DOI] [PubMed] [Google Scholar]
- Lv B, Xing M, Yang J. Exploring the effects of earthworms on bacterial profiles during vermicomposting process of sewage sludge and cattle dung with high-throughput sequencing. Environmental Science and Pollution Research International. 2018;25(13):12528–12537. doi: 10.1007/s11356-018-1520-6. [DOI] [PubMed] [Google Scholar]
- Magri M E, Fidjeland J, Jönsson H, Albihn A, Vinnerås B. Inactivation of adenovirus, reovirus and bacteriophages in fecal sludge by pH and ammonia. Science of the Total Environment. 2015;520:213–221. doi: 10.1016/j.scitotenv.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Major N, Schierstaedt J, Jechalke S, Nesme J, Ban S G, Černe M, Sørensen S J, Ban D, Schikora A. Composted Sewage Sludge Influences the Microbiome and Persistence of Human Pathogens in Soil. Microorganisms. 2020;8(7):1020. doi: 10.3390/microorganisms8071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandilara G, Mavridou A, Lambiri M, Vatopoulos A, Rigas F. The use of bacteriophages for monitoring the microbiological quality of sewage sludge. Environmental Technology. 2006;27(4):367–375. doi: 10.1080/09593332708618657. [DOI] [PubMed] [Google Scholar]
- Maqbool T, Cho J, Hur J. Improved dewaterability of anaerobically digested sludge and compositional changes in extracellular polymeric substances by indigenous persulfate activation. Science of the Total Environment. 2019;674:96–104. doi: 10.1016/j.scitotenv.2019.04.115. [DOI] [PubMed] [Google Scholar]
- Martín-Díaz J, Lucena F, Blanch A R, Jofre J. Review: Indicator bacteriophages in sludge, biosolids, sediments and soils. Environmental Research. 2020;182:109133. doi: 10.1016/j.envres.2020.109133. [DOI] [PubMed] [Google Scholar]
- Mawioo P M, Garcia H A, Hooijmans C M, Velkushanova K, Simonič M, Mijatović I, Brdjanovic D. A pilot-scale microwave technology for sludge sanitization and drying. Science of the Total Environment. 2017;601–602:1437–1448. doi: 10.1016/j.scitotenv.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta C M, Palni U, Franke-Whittle I H, Sharma A K. Compost: its role, mechanism and impact on reducing soil-borne plant diseases. Waste Management (New York, N.Y.) 2014;34(3):607–622. doi: 10.1016/j.wasman.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Mignotte-Cadiergues B, Gantzer C, Schwartzbrod L. Evaluation of bacteriophages during the treatment of sludge. Water Science and Technology: A Journal of the International Association on Water Pollution Research. 2002;46(10):189–194. doi: 10.2166/wst.2002.0327. [DOI] [PubMed] [Google Scholar]
- Millner P D, Powers K E, Enkiri N K, Burge W D. Microbially mediated growth suppression and death of salmonella in composted sewage sludge. Microbial Ecology. 1987;14(3):255–265. doi: 10.1007/BF02012945. [DOI] [PubMed] [Google Scholar]
- Min Jang H, Choi S, Shin J, Kan E, Mo Kim Y. Additional reduction of antibiotic resistance genes and human bacterial pathogens via thermophilic aerobic digestion of anaerobically digested sludge. Bioresource Technology. 2019;273:259–268. doi: 10.1016/j.biortech.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Mocé-Llivina L, Muniesa M, Pimenta-Vale H, Lucena F, Jofre J. Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Applied and Environmental Microbiology. 2003;69(3):1452–1456. doi: 10.1128/AEM.69.3.1452-1456.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moletta-Denat M, Bru-Adan V, Delgenes J P, Hamelin J, Wéry N, Godon J J. Selective microbial aerosolization in biogas demonstrated by quantitative PCR. Bioresource Technology. 2010;101(19):7252–7257. doi: 10.1016/j.biortech.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Mondal T, Rouch D A, Thurbon N, Smith S R, Deighton M A. Factors affecting decay of Salmonella Birkenhead and coliphage MS2 during mesophilic anaerobic digestion and air drying of sewage sludge. Journal of Water and Health. 2015;13(2):459–472. doi: 10.2166/wh.2014.313. [DOI] [PubMed] [Google Scholar]
- Monpoeho S, Maul A, Bonnin C, Patria L, Ranarijaona S, Billaudel S, Ferré V. Clearance of human-pathogenic viruses from sludge: study of four stabilization processes by real-time reverse transcription-PCR and cell culture. Applied and Environmental Microbiology. 2004;70(9):5434–5440. doi: 10.1128/AEM.70.9.5434-5440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy F, Aira M, Domínguez J. Reduction of total coliform numbers during vermicomposting is caused by short-term direct effects of earthworms on microorganisms and depends on the dose of application of pig slurry. Science of the Total Environment. 2009;407(20):5411–5416. doi: 10.1016/j.scitotenv.2009.06.048. [DOI] [PubMed] [Google Scholar]
- Mosquera-Losada M R, Rigueiro-Rodríguez A, Ferreiro-Domínguez N. Residual effects of lime and sewage sludge inputs on soil fertility and tree and pasture production in a Pinus radiata D. Don silvopastoral system established in a very acidic soil. Agriculture, Ecosystems & Environment. 2012;161:165–173. doi: 10.1016/j.agee.2012.08.001. [DOI] [Google Scholar]
- Naidoo D, Appleton C C, Archer C E, Foutch G L. The inactivation of Ascaris suum eggs by short exposure to high temperatures. Journal of Water, Sanitation, and Hygiene for Development: a Journal of the International Water Association. 2019;9(1):19–27. doi: 10.2166/washdev.2018.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab Daneshmand T, Beton R, Hill R J, Gehr R, Frigon D. Inactivation mechanisms of bacterial pathogen indicators during electro-dewatering of activated sludge biosolids. Water Research. 2012;46(13):3999–4008. doi: 10.1016/j.watres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Navarro I, Jiménez B, Lucario S, Cifuentes E. Application of Helminth ova infection dose curve to estimate the risks associated with biosolid application on soil. Journal of Water and Health. 2009;7(1):31–44. doi: 10.2166/wh.2009.113. [DOI] [PubMed] [Google Scholar]
- Orzi V, Scaglia B, Lonati S, Riva C, Boccasile G, Alborali G L, Adani F. The role of biological processes in reducing both odor impact and pathogen content during mesophilic anaerobic digestion. Science of the Total Environment. 2015;526:116–126. doi: 10.1016/j.scitotenv.2015.04.038. [DOI] [PubMed] [Google Scholar]
- Paez-Rubio T, Ramarui A, Sommer J, Xin H, Anderson J, Peccia J. Emission rates and characterization of aerosols produced during the spreading of dewatered class B biosolids. Environmental Science & Technology. 2007;41(10):3537–3544. doi: 10.1021/es061786p. [DOI] [PubMed] [Google Scholar]
- Pandey P K, Soupir M L. Escherichia coli inactivation kinetics in anaerobic digestion of dairy manure under moderate, mesophilic and thermophilic temperatures. AMB Express. 2011;1(1):18. doi: 10.1186/2191-0855-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecson B M, Barrios J A, Jiménez B E, Nelson K L. The effects of temperature, pH, and ammonia concentration on the inactivation of Ascaris eggs in sewage sludge. Water Research. 2007;41(13):2893–2902. doi: 10.1016/j.watres.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Pietronave S, Fracchia L, Rinaldi M, Martinotti M G. Influence of biotic and abiotic factors on human pathogens in a finished compost. Water Research. 2004;38(8):1963–1970. doi: 10.1016/j.watres.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Plachá I, Venglovský J, Maková Z, Martinéz J. The elimination of Salmonella typhimurium in sewage sludge by aerobic mesophilic stabilization and lime hydrated stabilization. Bioresource Technology. 2008;99(10):4269–4274. doi: 10.1016/j.biortech.2007.08.056. [DOI] [PubMed] [Google Scholar]
- Prado T, Gaspar A M C, Miagostovich M P. Detection of enteric viruses in activated sludge by feasible concentration methods. Brazilian Journal of Microbiology. 2014;45(1):343–349. doi: 10.1590/S1517-83822014000100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard D L, Penney N, McLaughlin M J, Rigby H, Schwarz K. Land application of sewage sludge (biosolids) in Australia: Risks to the environment and food crops. Water Science and Technology: A Journal of the International Association on Water Pollution Research. 2010;62(1):48–57. doi: 10.2166/wst.2010.274. [DOI] [PubMed] [Google Scholar]
- Quince C, Walker A W, Simpson J T, Loman N J, Segata N. Shotgun metagenomics, from sampling to analysis. Nature Biotechnology. 2017;35(9):833–844. doi: 10.1038/nbt.3935. [DOI] [PubMed] [Google Scholar]
- Rahube T O, Marti R, Scott A, Tien Y C, Murray R, Sabourin L, Zhang Y, Duenk P, Lapen D R, Topp E. Impact of fertilizing with raw or anaerobically digested sewage sludge on the abundance of antibiotic-resistant coliforms, antibiotic resistance genes, and pathogenic bacteria in soil and on vegetables at harvest. Applied and Environmental Microbiology. 2014;80(22):6898–6907. doi: 10.1128/AEM.02389-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Massé D I, Singh G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresource Technology. 2013;143:632–641. doi: 10.1016/j.biortech.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Ranković B, Sagatova A, Vujcic I, Masic S, Veljovic D, Pavicevic V, Kamberovic Z. Utilization of gamma and e-beam irradiation in the treatment of waste sludge from a drinking water treatment plant. Radiation Physics and Chemistry. 2020;177:109177. doi: 10.1016/j.radphyschem.2020.109174. [DOI] [Google Scholar]
- Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy M C, Michael I, Fatta-Kassinos D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Science of the Total Environment. 2013;447:345–360. doi: 10.1016/j.scitotenv.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Robledo-Mahón, Gómez-Silván C, Andersen G L, Calvo C, Aranda E. Assessment of bacterial and fungal communities in a full-scale thermophilic sewage sludge composting pile under a semi-permeable cover. Bioresource Technology. 2020;298:122550. doi: 10.1016/j.biortech.2019.122550. [DOI] [PubMed] [Google Scholar]
- Robledo-Mahón T, Silva-Castro G A, Kuhar U, Jamnikar-Ciglenečki U, Barlič-Maganja D, Aranda E, Calvo C. Effect of composting under semipermeable film on the sewage sludge virome. Microbial Ecology. 2019;78(4):895–903. doi: 10.1007/s00248-019-01365-z. [DOI] [PubMed] [Google Scholar]
- Romdhana M H, Lecomte D, Ladevie B, Sablayrolles C. Monitoring of pathogenic microorganisms contamination during heat drying process of sewage sludge. Process Safety and Environmental Protection. 2009;87(6):377–386. doi: 10.1016/j.psep.2009.08.003. [DOI] [Google Scholar]
- Rumky J, Visigalli S, Turolla A, Gelmi E, Necibi C, Gronchi P, Sillanpää M, Canziani R. Electro-dewatering treatment of sludge: Assessment of the influence on relevant indicators for disposal in agriculture. Journal of Environmental Management. 2020;268:110689. doi: 10.1016/j.jenvman.2020.110689. [DOI] [PubMed] [Google Scholar]
- Sado K, Ayusawa D, Enomoto A, Suganuma T, Oshimura M, Sato K, Koyama H. Identification of a mutated DNA ligase IV gene in the X-ray-hypersensitive mutant SX10 of mouse FM3A cells. Journal of Biological Chemistry. 2001;276(13):9742–9748. doi: 10.1074/jbc.M010530200. [DOI] [PubMed] [Google Scholar]
- Sahlström L. A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresource Technology. 2003;87(2):161–166. doi: 10.1016/S0960-8524(02)00168-2. [DOI] [PubMed] [Google Scholar]
- Sano D, Fukushi K, Yoshida Y, Omura T. Detection of enteric viruses in municipal sewage sludge by a combination of the enzymatic virus elution method and RT-PCR. Water Research. 2003;37(14):3490–3498. doi: 10.1016/S0043-1354(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Santos A F, Santos C P, Matos A M, Cardoso O, Quina M J. Effect of thermal drying and chemical treatments with wastes on microbiological contamination indicators in sewage sludge. Microorganisms. 2020;8(3):376. doi: 10.3390/microorganisms8030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser O, Huyard A, Cartnick K, Yañez A, Catalán V, Do Quang Z. Bioaerosol in composting facilities: Occupational health risk assessment. Water Environment Research: A Research Publication of the Water Environment Federation. 2009;81(9):866–877. doi: 10.2175/106143009X407258. [DOI] [PubMed] [Google Scholar]
- Schlosser O, Robert S, Debeaupuis C, Huyard A. Inhalable dust as a marker of exposure to airborne biological agents in composting facilities. Waste Management (New York, N.Y.) 2018;81:78–87. doi: 10.1016/j.wasman.2018.09.051. [DOI] [PubMed] [Google Scholar]
- Schöniger-Hekele M, Petermann D, Weber B, Müller C. Tropheryma whipplei in the environment: survey of sewage plant influxes and sewage plant workers. Applied and Environmental Microbiology. 2007;73(6):2033–2035. doi: 10.1128/AEM.02335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Chandra T S. Do earthworms affect dynamics of functional response and genetic structure of microbial community in a lab-scale composting system. Bioresource Technology. 2009;100(2):804–811. doi: 10.1016/j.biortech.2008.07.047. [DOI] [PubMed] [Google Scholar]
- Shannon K E, Lee D Y, Trevors J T, Beaudette L A. Application of real-time quantitative PCR for the detection of selected bacterial pathogens during municipal wastewater treatment. Science of the Total Environment. 2007;382(1):121–129. doi: 10.1016/j.scitotenv.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Sidhu J, Gibbs R A, Ho G E, Unkovich I. The role of indigenous microorganisms in suppression of Salmonella regrowth in composted biosolids. Water Research. 2001;35(4):913–920. doi: 10.1016/S0043-1354(00)00352-3. [DOI] [PubMed] [Google Scholar]
- Simmons F J, Xagoraraki I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Research. 2011;45(12):3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Soobhany N, Mohee R, Garg V K. Inactivation of bacterial pathogenic load in compost against vermicompost of organic solid waste aiming to achieve sanitation goals: A review. Waste Management (New York, N.Y.) 2017;64:51–62. doi: 10.1016/j.wasman.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Soueidan H, Schmitt L A, Candresse T, Nikolski M. Finding and identifying the viral needle in the metagenomic haystack: trends and challenges. Frontiers in Microbiology. 2015;5:739. doi: 10.3389/fmicb.2014.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J Q, Wei B, Ou-Yang W Y, Huang F Y, Zhao Y, Xu H J, Zhu Y G. Antibiotic resistome and its association with bacterial communities during sewage sludge composting. Environmental Science & Technology. 2015;49(12):7356–7363. doi: 10.1021/acs.est.5b01012. [DOI] [PubMed] [Google Scholar]
- Sutherland K P, Porter J W, Turner J W, Thomas B J, Looney E E, Luna T P, Meyers M K, Futch J C, Lipp E K. Human sewage identified as likely source of white pox disease of the threatened Caribbean elkhorn coral, Acropora palmata. Environmental Microbiology. 2010;12(5):1122–1131. doi: 10.1111/j.1462-2920.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- Swati A, Hait S. A Comprehensive Review of the Fate of Pathogens during Vermicomposting of Organic Wastes. Journal of Environmental Quality. 2018;47(1):16–29. doi: 10.2134/jeq2017.07.0265. [DOI] [PubMed] [Google Scholar]
- Tanner B D, Brooks J P, Gerba C P, Haas C N, Josephson K L, Pepper I L. Estimated occupational risk from bioaerosols generated during land application of class B biosolids. Journal of Environmental Quality. 2008;37(6):2311–2321. doi: 10.2134/jeq2007.0193. [DOI] [PubMed] [Google Scholar]
- Tong J, Fang P, Zhang J, Wei Y, Su Y, Zhang Y. Microbial community evolution and fate of antibiotic resistance genes during sludge treatment in two full-scale anaerobic digestion plants with thermal hydrolysis pretreatment. Bioresource Technology. 2019;288:121575. doi: 10.1016/j.biortech.2019.121575. [DOI] [PubMed] [Google Scholar]
- Tong M, Liu F, Dong Q, Ma Z, Liu W. Magnetic Fe3O4-deposited flower-like MoS2 nanocomposites for the Fenton-like Escherichia coli disinfection and diclofenac degradation. Journal of Hazardous Materials. 2020;385:121604. doi: 10.1016/j.jhazmat.2019.121604. [DOI] [PubMed] [Google Scholar]
- Traub F, Spillmann S K, Wyler R. Method for determining virus inactivation during sludge treatment processes. Applied and Environmental Microbiology. 1986;52(3):498–503. doi: 10.1128/aem.52.3.498-503.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsifli S, Tsoukalas D S. Water Safety Plans and HACCP implementation in water utilities around the world: benefits, drawbacks and critical success factors. Environmental Science and Pollution Research International. 2021;28(15):18837–18849. doi: 10.1007/s11356-019-07312-2. [DOI] [PubMed] [Google Scholar]
- Uma R, Adish Kumar S, Kaliappan S, Yeom I, Rajesh Banu J. Impacts of microwave pretreatments on the semi-continuous anaerobic digestion of dairy waste activated sludge. Waste Management (New York, N.Y.) 2013;33(5):1119–1127. doi: 10.1016/j.wasman.2013.01.016. [DOI] [PubMed] [Google Scholar]
- USEPA . Land application of sewage sludge: A guide for land appliers on the requirements of the federal standard for the use or disposal of sewage sludge, 40 CFR Part 503. Washington, DC: USEPA; 1994. [Google Scholar]
- Valderrama C, Granados R, Cortina J L. Stabilisation of dewatered domestic sewage sludge by lime addition as raw material for the cement industry: Understanding process and reactor performance. Chemical Engineering Journal. 2013;232:458–467. doi: 10.1016/j.cej.2013.07.104. [DOI] [Google Scholar]
- Van Abel N, Schoen M E, Kissel J C, Meschke J S. Comparison of risk predicted by multiple norovirus dose-response models and implications for quantitative microbial risk Assessment. Risk Analysis: An Official Publication of the Society for Risk Analysis. 2017;37(2):245–264. doi: 10.1111/risa.12616. [DOI] [PubMed] [Google Scholar]
- Venieri D, Karapa A, Panagiotopoulou M, Gounaki I. Application of activated persulfate for the inactivation of fecal bacterial indicators in water. Journal of Environmental Management. 2020;261:110223. doi: 10.1016/j.jenvman.2020.110223. [DOI] [PubMed] [Google Scholar]
- Viau E, Bibby K, Paez-Rubio T, Peccia J. Toward a consensus view on the infectious risks associated with land application of sewage sludge. Environmental Science & Technology. 2011;45(13):5459–5469. doi: 10.1021/es200566f. [DOI] [PubMed] [Google Scholar]
- Viau E, Peccia J. Survey of wastewater indicators and human pathogen genomes in biosolids produced by Class A and Class B stabilization treatments. Applied and Environmental Microbiology. 2009;75(1):164–174. doi: 10.1128/AEM.01331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A W, Martin J C, Scott P, Parkhill J, Flint H J, Scott K P. 16S rRNA gene-based profiling of the human infant gut microbiota is strongly influenced by sample processing and PCR primer choice. Microbiome. 2015;3(1):26. doi: 10.1186/s40168-015-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang J. Application of radiation technology to sewage sludge processing: A review. Journal of Hazardous Materials. 2007;143(1–2):2–7. doi: 10.1016/j.jhazmat.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Wang M, Chen H, Liu S, Xiao L. Removal of pathogen and antibiotic resistance genes from waste activated sludge by different pre-treatment approaches. Science of the Total Environment. 2021;763:143014. doi: 10.1016/j.scitotenv.2020.143014. [DOI] [PubMed] [Google Scholar]
- Wang M L, Li R Y, Zhao Q. Distribution and removal of antibiotic resistance genes during anaerobic sludge digestion with alkaline, thermal hydrolysis and ultrasonic pretreatments. Frontiers of Environmental Science & Engineering. 2019;13(3):43. doi: 10.1007/s11783-019-1127-2. [DOI] [Google Scholar]
- Wang W, Wang H, Li G, Wong P K, An T. Visible light activation of persulfate by magnetic hydrochar for bacterial inactivation: Efficiency, recyclability and mechanisms. Water Research. 2020;176:115746. doi: 10.1016/j.watres.2020.115746. [DOI] [PubMed] [Google Scholar]
- Ward R L, Ashley C S. Heat inactivation of enteric viruses in dewatered wastewater sludge. Applied and Environmental Microbiology. 1978;36(6):898–905. doi: 10.1128/aem.36.6.898-905.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sano D, Omura T. Risk evaluation for pathogenic bacteria and viruses in sewage sludge compost. Water Science and Technology: A Journal of the International Association on Water Pollution Research. 2002;46(11–12):325–330. doi: 10.2166/wst.2002.0757. [DOI] [PubMed] [Google Scholar]
- Watcharasukarn M, Kaparaju P, Steyer J P, Krogfelt K A, Angelidaki I. Screening Escherichia coli, Enterococcus faecalis, and Clostridium perfringens as indicator organisms in evaluating pathogen-reducing capacity in biogas plants. Microbial Ecology. 2009;58(2):221–230. doi: 10.1007/s00248-009-9497-9. [DOI] [PubMed] [Google Scholar]
- Wéry N, Lhoutellier C, Ducray F, Delgenès J P, Godon J J. Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Research. 2008;42(1–2):53–62. doi: 10.1016/j.watres.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Wichuk K M, Mccartney D. A review of the effectiveness of current time-temperature regulations on pathogen inactivation during composting. Journal of Environmental Engineering and Science. 2007;6(5):573–586. doi: 10.1139/S07-011. [DOI] [Google Scholar]
- Wong J W C, Selvam A. Reduction of indicator and pathogenic microorganisms in pig manure through fly ash and lime addition during alkaline stabilization. Journal of Hazardous Materials. 2009;169(1–3):882–889. doi: 10.1016/j.jhazmat.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Wong K, Onan B M, Xagoraraki I. Quantification of enteric viruses, pathogen indicators, and Salmonella bacteria in Class B anaerobically digested biosolids by culture and molecular methods. Applied and Environmental Microbiology. 2010;76(19):6441–6448. doi: 10.1128/AEM.02685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Jiang Y, Ke G, Liu Y. Effect of gamma-ray irradiation on the dewaterability of waste activated sludge. Radiation Physics and Chemistry. 2017;130:164–170. doi: 10.1016/j.radphyschem.2016.08.011. [DOI] [Google Scholar]
- Xu P, Zhang C, Mou X, Wang X C. Bioaerosol in a typical municipal wastewater treatment plant: concentration, size distribution, and health risk assessment. Water Science and Technology: A Journal of the International Association on Water Pollution Research. 2020;82(8):1547–1559. doi: 10.2166/wst.2020.416. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang R N, Zhao X Y. Emission characteristics of bioaerosol and quantitative microbiological risk assessment for equipping individuals with various personal protective equipment in a WWTP. Chemosphere. 2021;265:129117. doi: 10.1016/j.chemosphere.2020.129117. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhang G, Wang H. Current state of sludge production, management, treatment and disposal in China. Water Research. 2015;78:60–73. doi: 10.1016/j.watres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Yang K, Li L, Wang Y, Xue S, Han Y, Liu J. Airborne bacteria in a wastewater treatment plant: Emission characterization, source analysis and health risk assessment. Water Research. 2019;149:596–606. doi: 10.1016/j.watres.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Yang W, Cai C, Dai X. The potential exposure and transmission risk of SARS-CoV-2 through sludge treatment and disposal. Resources, Conservation and Recycling. 2020;162:105043. doi: 10.1016/j.resconrec.2020.105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Zhang T. Pathogenic bacteria in sewage treatment plants as revealed by 454 pyrosequencing. Environmental Science & Technology. 2011;45(17):7173–7179. doi: 10.1021/es201045e. [DOI] [PubMed] [Google Scholar]
- Yenigün O, Demirel B. Ammonia inhibition in anaerobic digestion: A review. Process Biochemistry. 2013;48(5–6):901–911. doi: 10.1016/j.procbio.2013.04.012. [DOI] [Google Scholar]
- Yin F B, Li Z F, Saino M, Dong H M. Performance of alkaline pretreatment on pathogens inactivation and sludge solubilization. International Journal of Agricultural and Biological Engineering. 2017;10(2):216–223. [Google Scholar]
- Yin Z, Hoffmann M, Jiang S. Sludge disinfection using electrical thermal treatment: The role of ohmic heating. Science of the Total Environment. 2018;615:262–271. doi: 10.1016/j.scitotenv.2017.09.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Zheng G D, Wang X D, Wang M, Chen T B. Biodegradation of triclosan and triclocarban in sewage sludge during composting under three ventilation strategies. Frontiers of Environmental Science & Engineering. 2019;13(3):41. doi: 10.1007/s11783-019-1125-4. [DOI] [Google Scholar]
- Yu D T, He J Z, Zhang L M, Han L L. Viral metagenomics analysis and eight novel viral genomes identified from the Dushanzi mud Volcanic soil in Xinjiang, China. Journal of Soils and Sediments. 2019;19(1):81–90. doi: 10.1007/s11368-018-2045-9. [DOI] [Google Scholar]
- Yu J, Xiao K, Yang J, Yu W, Pei K, Zhu Y, Wang J, Liang S, Hu J, Hou H, Liu B. Enhanced sludge dewaterability and pathogen inactivation by synergistic effects of zero-valent iron and ozonation. ACS Sustainable Chemistry & Engineering. 2019;7(1):324–331. doi: 10.1021/acssuschemeng.8b03605. [DOI] [Google Scholar]
- Yu R, Zhang S W, Chen Z K, Li C Y. Isolation and application of predatory Bdellovibrio-and-like organisms for municipal waste sludge biolysis and dewaterability enhancement. Frontiers of Environmental Science & Engineering. 2017;11(1):10. doi: 10.1007/s11783-017-0900-3. [DOI] [Google Scholar]
- Yu W, Wen Q, Yang J, Xiao K, Zhu Y, Tao S, Lv Y, Liang S, Fan W, Zhu S, Liu B, Hou H, Hu J. Unraveling oxidation behaviors for intracellular and extracellular from different oxidants (HOCl vs. H2O2) catalyzed by ferrous iron in waste activated sludge dewatering. Water Research. 2019;148:60–69. doi: 10.1016/j.watres.2018.10.033. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chan W I, Liao P H, Lo K V. Disinfection and solubilization of sewage sludge using the microwave enhanced advanced oxidation process. Journal of Hazardous Materials. 2010;181(1–3):1143–1147. doi: 10.1016/j.jhazmat.2010.05.134. [DOI] [PubMed] [Google Scholar]
- Zaleski K J, Josephson K L, Gerba C P, Pepper I L. Potential regrowth and recolonization of salmonellae and indicators in biosolids and biosolid-amended soil. Applied and Environmental Microbiology. 2005;71(7):3701–3708. doi: 10.1128/AEM.71.7.3701-3708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneti R N, Girardi V, Spilki F R, Mena K, Westphalen A P C, da Costa Colares E R, Pozzebon A G, Etchepare R G. Quantitative microbial risk assessment of SARS-CoV-2 for workers in wastewater treatment plants. Science of the Total Environment. 2021;754:142163. doi: 10.1016/j.scitotenv.2020.142163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Zan F, Hao T, Biswal B K, Lin S, van Loosdrecht M C M, Chen G. Electrochemical pretreatment for stabilization of waste activated sludge: Simultaneously enhancing dewaterability, inactivating pathogens and mitigating hydrogen sulfide. Water Research. 2019;166:115035. doi: 10.1016/j.watres.2019.115035. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xu L, Zhang Y F, Jiang M C. The transformation of PAHs in the sewage sludge incineration treatment. Frontiers of Environmental Science & Engineering. 2016;10(2):336–340. doi: 10.1007/s11783-014-0766-6. [DOI] [Google Scholar]
- Zhang H, Zhang Z, Song J, Cai L, Yu Y, Fang H. Foam shares antibiotic resistomes and bacterial pathogens with activated sludge in wastewater treatment plants. Journal of Hazardous Materials. 2021;408:124855. doi: 10.1016/j.jhazmat.2020.124855. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen Y, Ma C, Liu L, Pan J, Li B, Wu X, Wang Q. Improving heavy metals removal, dewaterability and pathogen removal of waste activated sludge using enhanced chemical leaching. Journal of Cleaner Production. 2020;271:122512. doi: 10.1016/j.jclepro.2020.122512. [DOI] [Google Scholar]
- Zhang T, Zhang M, Zhang X, Fang H H. Tetracycline resistance genes and tetracycline resistant lactose-fermenting Enterobacteriaceae in activated sludge of sewage treatment plants. Environmental Science & Technology. 2009;43(10):3455–3460. doi: 10.1021/es803309m. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Liu Y. Is anaerobic digestion a reliable barrier for deactivation of pathogens in biosludge. Science of the Total Environment. 2019;668:893–902. doi: 10.1016/j.scitotenv.2019.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimek Z. Economical evaluation of radiation processing with high-intensity X-rays. Nukleonika. 2020;65(3):167–172. doi: 10.2478/nuka-2020-0027. [DOI] [Google Scholar]
- Zou X, Tang G, Zhao X, Huang Y, Chen T, Lei M, Chen W, Yang L, Zhu W, Zhuang L, Yang J, Feng Z, Wang D, Wang D, Shu Y. Simultaneous virus identification and characterization of severe unexplained pneumonia cases using a metagenomics sequencing technique. Science China. Life Sciences. 2017;60(3):279–286. doi: 10.1007/s11427-016-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


