Abstract
The coronavirus disease 2019 (COVID-19) pandemic has caused large-scale economic and social losses and worldwide deaths. Although most COVID-19 patients have initially complained of respiratory insufficiency, the presence of neuropsychiatric manifestations is also reported frequently, ranging from headache, hyposmia/anosmia, and neuromuscular dysfunction to stroke, seizure, encephalopathy, altered mental status, and psychiatric disorders, both in the acute phase and in the long term. These neuropsychiatric complications have emerged as a potential indicator of worsened clinical outcomes and poor prognosis, thus contributing to mortality in COVID-19 patients. Their etiology remains largely unclear and probably involves multiple neuroinvasive pathways. Here, we summarize recent animal and human studies for neurotrophic properties of severe acute respiratory syndrome coronavirus (SARS-CoV-2) and elucidate potential neuropathogenic mechanisms involved in the viral invasion of the central nervous system as a cause for brain damage and neurological impairments. We then discuss the potential therapeutic strategy for intervening and preventing neuropsychiatric complications associated with SARS-CoV-2 infection. Time-series monitoring of clinical–neurochemical–radiological progress of neuropsychiatric and neuroimmune complications need implementation in individuals exposed to SARS-CoV-2. The development of a screening, intervention, and therapeutic framework to prevent and reduce neuropsychiatric sequela is urgently needed and crucial for the short- and long-term recovery of COVID-19 patients.
Subject terms: Psychiatric disorders, Pathogenesis
Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection causes coronavirus disease 2019 (COVID-19). SARS-CoV-2 is a single-stranded positive-sense RNA virus, belongs to the beta coronavirus genus, and is genetically and structurally like SARS-CoV. Phylogenetic analysis of the complete viral genome revealed that SARS-CoV-2 was a close relative to SARS-CoV, with 89.1% nucleotide similarity and 79% genetic similarity [1, 2]. This virus has spread rapidly worldwide, having devastating consequences on healthcare systems, society, and economies.
Up to date, this virus has infected millions and affected billions of lives in more than 200 countries. The mortality rate varies dramatically from country to country, but deaths are age-dependent. Deaths of individuals 60 years of age and older account for more than 80% of all deaths in the United States and the United Kingdom [3, 4]. The elderly are more susceptible to COVID-19 and have a higher risk of morbidity and mortality than the general population. Many factors, including frailty, comorbidities (e.g., hypertension, diabetes, cardiovascular disease, and chronic respiratory disease), and compromised immune function may contribute to worse health outcomes and a high mortality rate. Cardiovascular disease, diabetes, hypertension, or other comorbidities, preexisting microvascular pathology may further facilitate the neuroinvasion of the coronavirus and contribute to the development of neuropsychiatric symptoms and neuropathology associated with the viral infection [5]. The presence of chronic neurological comorbidity is an independent predictor of all-cause mortality in hospitalized COVID-19 patients [6]. A cohort study of multiple sclerosis patients with COVID-19 identified age, neurological disability, and obesity as the independent risk factors associated with COVID-19 severity [7].
While commonly manifested as fever and cough, atypical neuropsychiatric symptoms are also frequently reported in COVID-19 patients and include delirium, confusion, and neurocognitive disorders. These neuropsychiatric symptoms further hinder and delay diagnosis and treatment and have short- and long-term impacts on population health [8]. Several studies report risk or prognostic factors that are associated with a fatal outcome in patients hospitalized with COVID-19 and include medical comorbidities, dyspnea, time from disease onset to hospitalization, high procalcitonin levels, and lymphocytopenia [9, 10]. Mechanistically, coronavirus can invade the central nervous system (CNS) through blood vessels and neuronal retrograde pathways, thereby causing brain injury and dysfunction of the cardiorespiratory center in the brainstem, manifested as neurological symptoms and respiratory failure in infected animals and patients [11–13]. Conditional bursting pacemaker neurons in the brainstem region are crucial for the generation of respiratory rhythm [14], and SARS-CoV-2 may affect these respiratory control mechanisms, leading to indirect respiratory dysfunction in addition to primary pulmonary injury [15].
Historically, two similar human coronaviruses, SARS-CoV and the Middle East respiratory syndrome (MERS)-CoV, have caused severe acute respiratory syndrome outbreaks in 2003 and 2012. During these two pandemics, neuropsychiatric complications included narcolepsy, seizures, encephalitis, encephalopathy, Guillain–Barrè syndrome (GBS), and neuromuscular and psychiatric disorders [16–19]. These neuropsychiatric sequelae were closely associated with morbidity and risk of mortality. Emerging evidence suggests that SARS-CoV-2 presents an analogous neurotropic property and its infection can result in acute and long-term neuropsychiatric consequences. Neurotropic RNA coronaviruses could disrupt the blood–brain barrier (BBB), invade the CNS, and affect neuroimmune interactions through macrophages, microglia, or astrocytes [20, 21]. Clinical trials reported that patients with COVID-19-related pneumonia exhibited neurological disorders (e.g., stroke, encephalopathy, encephalitis, delirium, and GBS) and psychiatric disorders (e.g., depression, anxiety, insomnia, and post-traumatic stress disorder) [22–25]. Moreover, in some cases, neurological or psychiatric complications may precede or present without typical respiratory manifestations [26]. A recent meta-analysis reported that psychiatric and neurological disorders increased the susceptibility to COVID-19, illness severity, and mortality [27].
Here, we provide a comprehensive overview of the prevalence and presentation of neuropsychiatric manifestations of COVID-19 in patients based on epidemiological studies, clinical reports, and neuroimaging findings and discuss potential neuroinvasive mechanisms and pathways that contribute to these neuropathological changes and CNS dysfunction after SARS-CoV-2 infection. We also discuss possible and promising interventions that prevent and reduce these neuropsychiatric complications.
The presence and prevalence of neuropsychiatric manifestations in COVID-19 patients
Neurological and psychiatric complications of COVID-19 are increasingly reported, but most are individual cases or case series. Headache, anosmia, and myalgia are most commonly reported in patients infected with SARS-CoV-2. SARS-CoV-2 infection can attack the CNS and induce spine demyelinating lesions [28], which could further lead to neuropsychiatric symptoms affecting cognitive, affective, behavioral, and perceptual domains. These neuropsychiatric symptoms, including cerebrovascular, psychiatric, and neuromuscular disorders, frequently occur in elderly patients and individuals with multiple comorbidities or severe infection. Both SARS and MERS are associated with delirium, depression, anxiety, memory impairment, and insomnia during the acute phase. Depression, insomnia, anxiety, memory impairment, and sleep disorders are frequently reported during the post-illness phase [23]. A significant proportion of patients with COVID-19 develop delirium, agitation, altered consciousness, and other neuropsychiatric symptoms, including encephalopathy, encephalitis, depression, anxiety, and post-traumatic stress disorder [23].
A UK-wide surveillance study of acute neurological and psychiatric complications in 153 COVID-19 patients demonstrated that cerebrovascular events (62%) and altered mental status (31%, including encephalopathy, encephalitis, and psychiatric disorders, were reported, often occurring in younger patients [29]. An observational series of 58 COVID-19 patients in Strasbourg, France reported encephalopathy, prominent agitation and confusion, corticospinal tract signs, and acute ischemic strokes [30]. A tertiary-care hospital at Karachi, Pakistan reported on 350 patients with COVID-19 describing headache (6%), vertigo (3.4%), numbness/paresthesia (3.1%), impaired consciousness (2%), hyposmia/anosmia (1.4%), and encephalitis (0.9%) [31]. A retrospective, observational case series of 214 patients in Wuhan, China found that 78 patients (36.4%) had neurologic manifestations, including acute cerebrovascular diseases, impaired consciousness, and skeletal muscle injury [32]. Analysis of data from 86 critically ill COVID-19 patients at the intensive care unit (ICU) of Tongji Hospital, Wuhan, China showed that 26 patients (30.2%) presented with neurological symptoms including delirium, stroke, cerebrovascular, and neuromuscular diseases [33]. A retrospective multicenter cohort study of 917 patients in three regions in China demonstrated that new-onset critical neurologic events, mainly impaired consciousness and stroke, occurred in 3.5% of the total population and in 9.4% of severe or critical patients [34]. A prospective multicenter observational study in New York City showed that 13.5% (606/4491) hospitalized COVID-19 patients developed a new neurological disorder including encephalopathy (309/606, 51%), strokes (84/606, 14%), seizures (74/606, 12%), and hypoxic/ischemic brain injury (65/606, 11%), and these disorders led to higher rates of in-hospital mortality and lower rates of discharge home [35]. A survey of physician-reported neurological symptoms in COVID-19 patients from Italy showed that 87.3% of practitioners reported neurological symptoms, mainly mild and nonspecific manifestations such as headache, myalgia, and loss of smell [36].
GBS and myelitis are also reported in COVID-19 patients, indicating a post-infective autoimmune reaction in peripheral nerves [37]. COVID-19 patients frequently presented with stroke and subsequent mortality when complicated by older age, comorbidities, and severe respiratory symptoms [38]. A meta-analysis of 58,104 COVID-19 patients revealed a 0.46% hemorrhagic stroke rate and a 1.11% ischemic stroke rate, with mortality rates of 44.7% for hemorrhagic and 36.2% for ischemic stroke [39]. Evidence from tissue histology, neuroimaging, and clinic symptoms revealed that 1.4% of COVID-19 patients (23/1683) developed cerebral ischemia, intracerebral hemorrhage, or encephalopathy [40]. A possible pathophysiology for this cerebrovascular damage may be BBB dysfunction and subsequent cytokine release induced by SARS-CoV-2 infection of the brain itself [41].
During the COVID-19 pandemic, psychiatric disorders such as depression, anxiety, post-traumatic stress disorder, and insomnia have also been frequently reported in COVID-19 patients, vulnerable populations, healthcare workers, and even the general population [23, 42–48]. Poor sleep is associated with worsened clinical outcomes in hospitalized patients with COVID-19, and long-term sustainable improvements in sleep quality are needed for this subpopulation [49, 50]. The prevalence and severity of these psychiatric symptoms, attention deficits, and hyperactivity symptoms increased the risk for problematic internet use during the COVID-19 pandemic [51]. Moreover, a case series reported that critically ill COVID-19 patients with multiple acute bilateral ischemic lesions exhibited alterations of mental status but no neurological deficits [52]. A group of experts convened by the UK Academy of Medical Sciences and the mental health research charity also advocate monitoring and evaluating brain function, and mental health issues such as anxiety, depression, insomnia, self-harm, and suicide in COVID-19 patients and related vulnerable populations [53]. A Nationwide Cohort Study also revealed that hospitalization with infection increased the risk of death by suicides in prospective and dose–response relationships [54]. Another cohort study demonstrated that a history of schizophrenia spectrum disorder was significantly associated with an increased risk for mortality among COVID-19 patients [55]. However, it is difficult to distinguish whether this high prevalence is due to direct SARS-CoV-2 infection or the adverse psychological effects of other social and environmental factors such as social distancing and quarantine, self-isolation, changes in sleep and lifestyle behaviors, fear of death, and economic burden [56].
Overall, neurologic and neuropsychiatric manifestations, such as alterations of mental status and stroke, are common among hospitalized COVID-19 patients and could be predictors of disease severity and mortality [57, 58]. A retrospective cohort study of 236,379 COVID-19 survivors revealed that the incidence of neurological or psychiatric morbidity (e.g., intracranial hemorrhage, ischemic stroke, dementia, and anxiety disorder) was 33.6% in the 6 months follow-up, indicating the neuropsychiatric sequela was long-lasting [59]. An international cohort study found that neurological symptoms did not recover in COVID-19 patients at 7-month follow-up [60]. Clinicians should seriously consider these neurological and neuropsychiatric symptoms to avoid delayed diagnosis or misdiagnosis and to reduce the risk of death.
Neuroimaging and neurochemical findings of brain dysfunction in COVID-19 patients
Early brain imaging and neurochemical examinations in COVID-19 patients with neuropsychiatric symptoms are critical and important for timely interventions that can improve clinical outcomes. Moreover, advanced neuroimaging and evaluation of biomarkers are critical means in the clinical evaluation and diagnosis of brain injury and neurological disorders. We summarize recent findings on neuroimaging, electroencephalography (EEG), and neurochemical biomarkers that reflect CNS dysfunction in COVID-19 patients, especially those with neuropsychiatric manifestations (Table 1).
Table 1.
Neuroimaging and neurochemical findings of brain dysfunction in COVID-19 patients, especially those with neuropsychiatric manifestations.
| Study | Region | Participants | Sample size | Study design | Symptoms | Detection method | Main findings |
|---|---|---|---|---|---|---|---|
| Neuroimaging | |||||||
| Coolen et al., 2020 [68] | Belgium | COVID-19 decedents | 19 | Case series | Headache, agitation, confusion, disorientation, seizures | MRI | Subcortical microbleeds and macrobleeds, cortico/subcortical edematous changes, deep white matter changes |
| Delorme et al., 2020 [64] | France | COVID-19-related encephalopathy | 4 | Case series | Various degrees of cognitive impairment with predominantly frontal lobe impairment | MRI, FDG-PET/CT | Frontal hypometabolism, cerebellar hypermetabolism |
| Freeman et al., 2020 [62] | USA | Inpatients with COVID-19 | 59 | Case series | Not specified | MRI | Six (10.2%) had MRI findings suspicious of COVID-19-related disseminated leukoencephalopathy |
| Jensen et al., 2021 [70] | UK | Patients with fatal COVID-19 | 2 | Case series | Not specified | Coronial autopsy | Cerebral cortical infarction, brainstem encephalitis |
| Kremer et al., 2020 [66] | France | Severe COVID-19 with neurologic manifestations | 37 | Cohort | Alterations of consciousness (27), pathological wakefulness after sedation (15), confusion (12), agitation (7) | MRI | Signal abnormalities in the medial temporal lobe, non-confluent multifocal white matter hyperintense lesions on FLAIR and diffusion with variable enhancement, associated with hemorrhagic lesions and extensive and isolated white matter microhemorrhage |
| Lu et al., 2020 [65] | China | COVID-19 patients | 60 | Prospective cohort | Mood changes (25), fatigue (16), headache (15), vision changes (13), myalgia (9), mobility impairment (7), memory loss (8), taste loss (4), limb numbness (4), tremor (4). loss of smell (2), hearing loss (1) | MRI | Recovered COVID-19 patients were more likely to have enlarged olfactory cortices, hippocampi, insulas, Heschl’s gyrus, Rolandic operculum, and cingulate gyrus and a general decline of mean diffusivity, axial diffusivity, and radial diffusivity, accompanied by an increase in white matter fractional anisotropy |
| Paterson et al., 2020 [188] | UK | COVID-19 patients | 43 | Case series | Encephalopathy, neuroinflammatory syndrome, stroke, transverse myelitis | MRI, CSF | SARS-CoV-2 infection was associated with a wide spectrum of neurological syndromes that affect the whole neuraxis |
| Poyiadji et al., 2020 [67] | USA | COVID-19-associated encephalopathy | 1 | Case report | Cough, fever, altered mental status | CT, MRI | Hemorrhagic rim enhancing lesions within bilateral thalami, medial temporal lobes, and subinsular regions |
| Sharifi-Razavi et al., 2020 [63] | Iran | Case of 79-year-old man | 1 | Case report | Fever and cough, acute loss of consciousness, rapid heart rate, rapid breathing | CT | Massive intracerebral hemorrhage in the right hemisphere, accompanied by intraventricular and subarachnoid hemorrhage |
| Solomon et al., 2020 [69] | USA | COVID-19 patients who died in hospital | 18 | Case series | Myalgia (3), headache (2), decreased taste (1) | CT, autopsy | Acute hypoxic injury in cerebrum and cerebellum in all patients, with loss of neurons in the cerebral cortex, hippocampus, and cerebellar Purkinje cell layer but no thrombi or vasculitis |
| Zanin et al., 2020 [28] | Italy | COVID-19 patient | 1 | Case report | Seizures, anosmia, ageusia | MRI | Brain MRI revealed alterations of periventricular white matter, with normal chemical and physical CSF examination |
| EEG | |||||||
| Canham et al., 2020 [73] | UK | Cases of COVID-19 admitted to intensive care unit | 10 | Case series | Delirium with visual hallucinosis and drowsiness, unresponsive, drowsy | EEG | EEG features in severe COVID-19 indicated generalized symmetrical slowing |
| Chen et al., 2020 [74] | USA | Critically ill patients | 5 | Case series | All patients had encephalopathy and three also had seizure-like movements | EEG | EEG showed nonspecific markers of encephalopathy, including diffuse slowing and generalized rhythmic delta activity |
| De Stefano et al., 2020 [76] | Switzerland | COVID-19 patient undergoing mechanical ventilation | 1 | Case report | Altered mental status | EEG | Cerebral microbleeds, focal dysfunction |
| Louis et al., 2020 [75] | USA | COVID-19 patients | 22 | Retrospective cohort | Intracerebral hemorrhage (1), acute ischemic stroke (1), imaging concerning for possible ischemia (2) | EEG | Various epileptiform abnormalities on EEG, a higher proportion of patients with electrographic seizures |
| Vespignani et al., 2020 [72] | France | Severe COVID-19-infected patients | 5 | Case series | Face and eye myoclonus, delayed awakening, poor arousal, confusion, lethargy | EEG | High-amplitude frontal monomorphic delta waves without epileptic activity, indicating potential CNS injury |
| Neurochemical biomarkers | |||||||
| Alexopoulos et al., 2020 [85] | Greece | COVID-19 patients with signs of encephalopathy | 8 | Case series | Pulmonary imaging of diffuse infiltrates and ground-glass opacities, with renal failure in four patients, hepatic failure in one patient, and agitated confusion in one patient | Serum, CSF | High-titer anti-SARS-CoV-2 antibodies in both serum and CSF, with 14-3-3-protein positivity in CSF in four patients |
| Ameres et al., 2020 [78] | Germany | Healthcare workers (28 positive for COVID-19, 72 negative for COVID-19) | 100 | Prospective cohort | Mild-to-moderate symptoms, no or only minor neurological symptoms, including anosmia and headache | Serum | Mild-to-moderate COVID-19 was associated with higher serum NfL levels |
| Domingues et al., 2020 [87] | Brazil | 42-year-old patient | 1 | Case report | Mild respiratory symptoms, neurological manifestations | CSF | SARS-CoV-2 RNA detected in CSF |
| Edén et al., 2021 [82] | Sweden | COVID-19 patients with neurologic abnormalities | 6 | Case series | All had respiratory symptoms with hypoxemia and encephalopathy | CSF | Marked elevations of two soluble inflammatory biomarkers in all patients, abnormal CSF NfL in two patients |
| Espíndola et al., 2020 [90] | Brazil | Patients with COVID-19 | 8 | Case series | Meningoencephalitis (1), encephalitis (1), facial palsy (2), delirium (2), intracranial hypertension (1), new daily persistent headache (1) | CSF | Undetectable or extremely low levels of SARS-CoV-2 RNA in CSF |
| Helms et al., 2020 [30] | France | Patients with COVID-19 and ARDS | 58 | Case series | Agitation (40), corticospinal tract signs (39), dysexecutive syndrome (14) | CSF, MRI | Negative RT-PCR for SARS-CoV-2 in CSF |
| Kanberg et al., 2020 [77] | Sweden | Patients with confirmed COVID-19 | 47 | Cross-sectional | Symptoms of confusion (4), single seizure episode (1) | Plasma | Higher plasma concentrations of NfL and glial fibrillary acidic protein in severe COVID-19 patients |
| Kanberg et al., 2021 [80] | Sweden | Patients with confirmed COVID-19 | 100 | Cohort | Headache (41%) and dysgeusia (11%), myalgia, hyposmia, dysgeusia, altered cognition | Plasma | Post-COVID-19 neurological sequelae not accompanied by ongoing CNS injury |
| Moriguchi et al., 2020 [86] | Japan | Meningitis associated with SARS-CoV-2 | 1 | Case report | Convulsions accompanied by unconsciousness, fatigue, fever | CSF, serum | specific SARS-CoV-2 RNA detected in CSF |
| Oussalah et al., 2020 [81] | France | Patients with severe COVID-19 | 162 | Longitudinal cohort | Not specified | Blood, urine | C-reactive protein and urea nitrogen associated with risk of COVID-19-related acute respiratory failure and death |
| Paterson et al., 2021 [83] | USA | COVID-19 patients | 152 | Prospective cohort | Not specified | Serum, CSF | High concentrations of NfL in CSF among patients with CNS inflammation |
| Prudencio et al., 2021 [79] | USA | Patients hospitalized with COVID-19 | 142 | Cohort | Not specified | Serum | Higher NfL in serum in patients with COVID-19 compared with healthy controls, higher serum NfL levels associated with worse clinical outcomes |
| Ye et al., 2020 [88] | China | COVID-19 case presented as encephalitis | 1 | Case report | Fever, shortness of breath, myalgia, confusion, meningeal irritation signs, extensor plantar response | CSF | Negative test for SARS-CoV-2 in CSF |
MRI magnetic resonance imaging, FDG-PET/CT [18F]fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography, CSF cerebrospinal fluid, CT computed tomography, EEG electroencephalogram, CNS, central nervous system, NfL neurofilament light-chain protein.
Diagnostic MRI/PET/CT findings
Many severe COVID-19 patients who are admitted to an ICU risk infection spread when transported to imaging suites. In response to this risk, clinicians should consider a recently developed novel portable, low-field magnetic resonance imaging (MRI) device to evaluate neurological injury such as stroke and hemorrhage at the bedside of critically ill ICU patients [61]. Brain MRI examinations revealed uncommon but important findings of disseminated leukoencephalopathy in COVID-19 patients with neurologic symptoms [62]. Brain computed tomography (CT) scan in a patient with SARS-CoV-2 infection revealed a massive intracerebral hemorrhage in the right hemisphere, accompanied by intraventricular and subarachnoid hemorrhage [63]. Brain [18F]fluoro-2-deoxy-d-glucose-positron emission tomography/CT imaging in four cases of COVID-19-related encephalopathy showed consistent frontal hypometabolism and cerebellar hypermetabolism [64]. An MRI-based 3-month follow-up study showed that 55% of COVID-19 patients who presented with neurological symptoms had microstructural and functional brain integrity disruption during this recovery stage [65]. Besides ischemic infarction, signal abnormalities in the medial temporal lobe, non-confluent multifocal white matter hyperintense lesions, and extensive and isolated white matter microhemorrhages are frequently found in severe COVID-19 patients with neurological symptoms [66]. A COVID-19 patient presenting with altered mental status showed acute necrotizing encephalopathy on CT and MRI [67].
Early postmortem brain MRI in COVID-19 non-survivors have demonstrated white matter changes, brain hemorrhages, and encephalopathy without brainstem changes, which all may result from BBB impairment [68]. Neuropathology in 18 COVID-19 non-survivors uncovered acute hypoxic injury in the cerebrum and cerebellum, with neuronal loss in the cerebral cortex, hippocampus, and cerebellar Purkinje cell layer, but no encephalitis [69]. Coronial autopsies of two fatal COVID-19 patients showed cerebral cortical infarction and brainstem encephalitis, but found no SARS-CoV-2 RNA in these postmortem brain tissues using RNAscope in situ hybridization and reverse transcription-polymerase chain reaction (RT-PCR) [70]. These neuropathological findings may be related to hyperinflammatory and hypercoagulable status induced by SARS-CoV-2 infection.
Diagnostic EEG findings
EEG has shown no reliable findings related to COVID-19 [71]. EEG examinations in five severe COVID-19 patients showed high-amplitude frontal monomorphic delta waves without epileptic activity, indicating potential CNS injury [72]. Two case series reported generalized symmetrical slowing as predominant EEG features of encephalopathy in patients with severe COVID-19, and EEG monitoring is very important in this population to identify status epilepticus and thus guide timely anti-seizure interventions [73, 74]. Continuous EEG was also recommended to discover asymptomatic seizures or to identify status epilepticus in COVID-19 patients [75]. EEG-MRI examinations have revealed cerebral microbleeds and focal dysfunction in critical illness COVID-19 patients with acute neurological complications after ruling out nonconvulsive status epilepticus [76].
Diagnostic neurochemical findings
Two plasma biomarkers of CNS injury, neurofilament light-chain protein (NfL, a marker of neuroaxonal injury) and glial fibrillary acidic protein (GFAP, a marker of astrocytic activation/injury), were increased in severe COVID-19 patients [77]. Another study in healthcare workers demonstrated that mild-to-moderate COVID-19 was associated with increased serum NfL levels, indicating the potential neurodestructive capability of SARS-CoV-2 [78]. Higher serum NfL concentrations were associated with worse clinical outcomes, such as mechanical ventilation and ICU admission, in hospitalized COVID-19 patients [79]. A longitudinal study showed that elevated NfL and GFAP concentrations normalized at 6-month follow-up, regardless of prior disease severity or persisting neurological symptoms, in all COVID-19 patients [80]. A longitudinal cohort study with time-series design to estimate the spectrum of biochemical dataset suggests two other biochemical markers, C-reactive protein (CRP) and urea nitrogen, are associated with the risk of COVID-19-related acute respiratory failure and death [81]. A case series showed that levels of biomarkers of inflammation in cerebrospinal fluid (CSF), including neopterin and β2-microglobulin, increased in six COVID-19 patients with neurological symptoms, including encephalopathies, suspected meningitis, and dysgeusia [82]. A large prospective biomarker study showed that serum NfL levels increased across hospitalized COVID-19 patients despite neurological manifestations, whereas CSF NfL levels increased specifically in patients with CNS inflammation, including encephalitis and acute disseminated encephalomyelitis, but not in patients with encephalopathy or GBS [83]. Moreover, a previous study evaluated nine antiphospholipid antibodies and found that anti-phosphatidylserine/prothrombin IgG was associated with COVID-associated neurological manifestations, specifically acute disseminated encephalomyelitis [84]. This neurochemical evidence supports the presence of neuroinflammation and microvascular and CNS injury in the acute phase of the disease.
Enzyme-linked immunosorbent assay (ELISA) revealed that high-titer anti-SARS-CoV-2 antibodies are detectable in both serum and CSF of patients with encephalopathy and comatose. These patients showed a breakdown of BBB integrity and neurodegeneration as indicated by finding albumin and 14-3-3-protein in the CSF [85]. In addition, SARS-CoV-2 RNA detection in the CSF could provide direct evidence to support the neurotropism theory. Recently, the first case of positive SARS-CoV-2 RT-PCR results in the CSF of COVID-19 patients with meningitis/encephalitis was reported [86], supporting neuroinvasion of the CNS after SARS-CoV-2 infection. SARS‑COV‑2 RNA was also detected in the CSF sampling of a patient with neurological manifestations of demyelinating disease, although the respiratory symptoms were mild [87]. Other studies using RT-PCR assays of CSF samples from COVID-19 patients with neurological damage have been negative for SARS-CoV-2 [28, 30, 76, 88], which may be due to low sensitivity or diagnostic complications of the method (i.e., false-negative results), CSF clearance, low CSF virus titer, and delayed sampling [89, 90].
Overall, the neuroimaging (MRI/CT/PET) and neurochemical (RT-PCR assays/ELISA) methods could help to identify candidate biomarkers to access the effects of SARS-CoV-2 infection on the CNS function and brain inflammation status [53]. Combined with clinical symptoms, self-reporting, and behavioral testing of emotional and cognitive domains, comprehensive diagnosis of disease and implicating proper interventions could be achieved.
The potential mechanisms underlying the invasion of SARS-CoV-2 into the nervous system
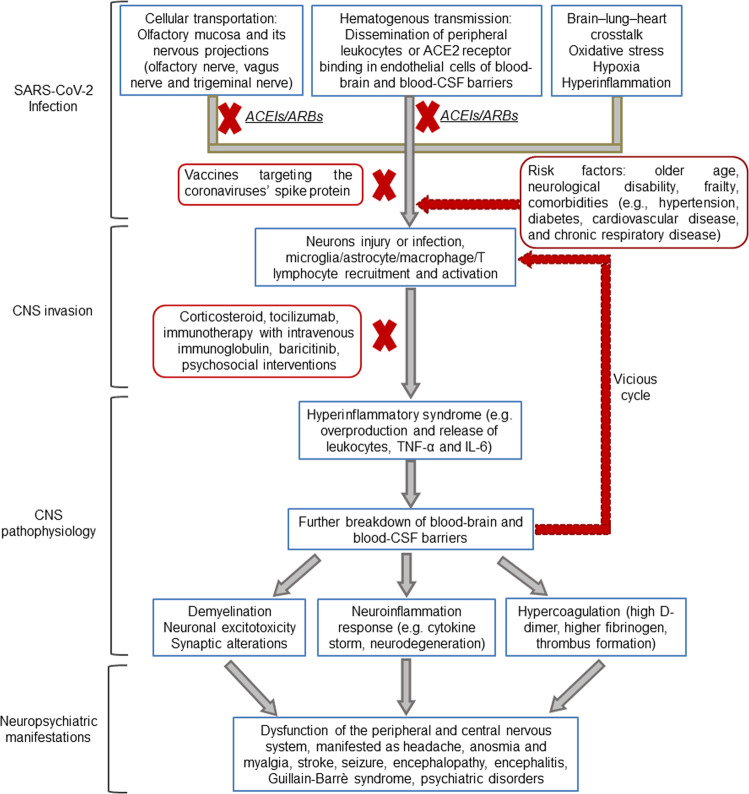
Growing and convincing evidence supports the neurotropism of SARS-CoV-2 (Table 2), similar to other coronaviruses. Quantitative data for tropism, replication kinetics, and cell damage revealed that SARS-CoV-2 modestly replicated in neuronal cells, highlighting the potential that this virus can cause neuropsychiatric manifestations in COVID-19 patients [91]. This virus could affect the CNS and cause brain damage and neuropsychiatric alterations through several pathways (Fig. 1).
Table 2.
Evidence supporting the direct neurotropic effects of SARS-CoV-2.
| Models | Targets | Main findings | Methods | Immunostaining (colocalization with SARS-CoV-2 spike protein or nucleoprotein) | References |
|---|---|---|---|---|---|
| hPSC-derived neural progenitor cells, neurospheres, and brain organoids | Cortical neurons and neural progenitor cells | Human neural progenitor cells and neurospheres are permissive to SARS-CoV-2 infection and supported productive virus replication; SARS-CoV-2 can directly infect cortical neurons and neural progenitor cells in brain organoids | qRT-PCR; electron microscopy; immunofluorescence | Neuronal cell marker TUJ1, and proliferation neural progenitor cell marker NESTIN | Zhang et al., 2020 [96] |
| hPSC-derived monolayer brain cells, region-specific brain organoids, and choroid plexus organoids | Neurons, astrocytes, choroid plexus epithelial cells; cortical, hippocampal, hypothalamic, and midbrain regions | SARS-CoV-2 sparsely infects human neurons and astrocytes, but robustly infects choroid plexus epithelial cells; SARS-CoV-2 increases cell death in choroid plexus organoids | qRT-PCR; immunofluorescence; single-cell RNA-seq | Neuronal marker doublecortin, astrocyte marker GFAP, choroid plexus marker transthyretin | Jacob et al., 2020 [99] |
| hPSC-derived brain organoids | Choroid plexus epithelial cells | SARS-CoV-2 spike pseudovirus and live virus infects choroid plexus epithelial cells, but not neurons or glia; SARS-CoV-2 damages the choroid plexus epithelium and disrupts the blood–CSF barrier | qRT-PCR; immunofluorescence; single-cell RNA-seq | Choroid plexus marker transthyretin | Pellegrini et al., 2020 [101] |
| hPSC-derived neuroprogenitor cells and BrainSphere | Neuronal cell body and neurite structures | SARS-CoV-2 infects neuronal cell body and neurite structures with the replication of the virus | qRT-PCR; immunofluorescence | Neuronal marker MAP2 | Bullen et al., 2020 [105] |
| hPSC-derived brain organoids; mice overexpressing human ACE2; brain autopsy samples of deceased COVID-19 patients | Neurons; forebrain cortex, cerebellum, dentate gyrus, the globus pallidus | SARS-CoV-2 infects human brain organoids accompanying metabolic changes and induces cell death; SARS-CoV-2 neuroinvasion in vivo in mice; the presence of SARS-CoV-2 in cortical neurons of autopsy samples | qRT-PCR; electron microscopy; immunofluorescence; single-cell RNA-seq | Neuronal marker MAP2, vascular endothelium marker CD31, and podocalyxin | Song et al., 2021 [106] |
| hPSC-derived cortical neurons and brain organoids | Cortical neuron | SARS-CoV-2 preferably targets cortical neurons of brain organoids and causes aberrant Tau localization and neuronal death | qRT-PCR; immunofluorescence; ELISA | Neuronal cell marker TUJ1 | Ramani et al., 2020 [108] |
| hPSC-derived cells and organoids | Dopaminergic neurons | Dopaminergic neurons, but not cortical neurons or microglia, were permissive to SARS-CoV-2 infection | qRT-PCR; immunofluorescence; single-cell RNA-seq | Dopaminergic neuronal marker FOXA2 and MAP2, cortical neuronal marker beta III-tubulin, microglial marker Iba1 | Yang et al., 2020 [110] |
| Human embryonic stem cell-derived monolayer cortical neurons and dorsal forebrain organoids | Cortical neuron | SARS-CoV-2 pseudovirus infects only 10% of cortical neurons | Immunofluorescence | Neuronal cell marker TUJ1 | Yi et al., 2020 [107] |
| hPSC-derived neurons, astrocytes, and brain organoids | Neurons and astrocytes | SARS-CoV-2 infects hiPSC-derived neurons, astrocytes, and brain organoids; an increased rate of SARS-CoV-2 infection in ApoE4 neurons and astrocytes | qRT-PCR; immunofluorescence | Neuronal cell marker TUJ1 and MAP2; astrocyte marker SOX9 and GFAP | Wang et al., 2021 [97] |
| hPSC-derived neural progenitor cells, neurons, astrocytes, and cerebral organoids | Neurons and astrocytes | SARS-CoV-2 infects human cerebral organoids; SARS-CoV-2 infects neurons more efficiently than neural progenitor cells and astrocytes | qRT-PCR; immunofluorescence | Neuronal marker MAP2 and β-tubulin-III; astrocyte marker GFAP | Tiwari et al., 2021 [109] |
| hPSC-derived cortical organoids | Astrocytes | SARS-CoV-2 infects astrocytes in both primary human cortical tissue and human stem cell-derived cortical organoids | Immunofluorescence | Astrocyte marker GFAP; neuronal marker NeuN | Andrews et al., 2021 [111] |
| hPSC-derived cortical organoid and pericyte containing cortical organoids | Pericyte-like cells and astrocytes | SARS-CoV-2 infects pericyte-like cells and astrocytes | qRT-PCR, immunofluorescence, single-cell RNA-seq | Pericyte markers (NG2 and PDGFR-β) | Wang et al., 2021 [102] |
| hPSC-derived neural cultures | Neurons | SARS-CoV-2 does not replicate and only infects a minority of individual mature neurons but induces the production of type III interferons and interleukin-8 | Immunofluorescence | Neuronal marker (MAP2), astrocyte marker (GFAP), neural progenitor cell marker (SOX2) | Bauer et al., 2021 [103] |
| Human embryonic stem cell-derived cortical organoids | Neurons, astrocytes, choroid plexus cells | SARS-CoV-2 only minimally infected neurons, but preferentially infected astrocytes and choroid plexus cells | Immunofluorescence | Neuronal markers (DCX and MAP2), choroid plexus marker (5-HT2C), astrocytic marker (GFAP and ALDH1L1) | McMahon et al., 2021 [100] |
| Deer mouse model of SARS-CoV-2 infection | Neurons and microglia | The presence of SARS-CoV-2 in neurons and microglia of the afferent nerves (trigeminal nerve) and in the glomerular layer of the olfactory bulb | Immunofluorescence; ELISA | Neuronal marker MAP2, microglial marker Iba1 | Fagre et al., 2021 [131] |
| Rhesus monkey model of SARS-CoV-2 infection | Neurons, astrocytes, microglia, olfactory bulb, olfactory trigone, entorhinal area, hippocampus, thalamus, medulla oblongata, frontal lobe, occipital lobe | Intracranial inoculation with SARS-CoV-2 caused the distribution of viral antigen nucleoprotein in neurons, astrocytes, and microglial cells in multiple brain regions | qRT-PCR, immunofluorescence, single-cell RNA-seq | Neuronal marker (NeuN), astrocytic marker (GFAP_, microglial marker (Iba1) | Jiao et al., 2021 [132] |
| Brain autopsy samples of deceased COVID-19 patients | Neurons and cerebral vascular endothelial cells; medulla oblongata and cerebellum | the presence of SARS-CoV-2 RNA and protein in medulla oblongata and cerebellum; the presence of SARS-CoV-2 protein in the olfactory mucosa and its nervous tissues; the presence of SARS-CoV S protein in the endothelial cells of small brain vessels | qRT-PCR; electron microscopy; immunofluorescence | Neuronal marker TUJ1, NF200, and OMP | Meinhardt et al., 2020 [133] |
| Brain autopsy samples of deceased COVID-19 patients | Medulla oblongata and cranial nerves | The presence of SARS-CoV-2 viral proteins in cranial nerves originating from the lower brainstem and in isolated cells of the medulla oblongata | qRT-PCR; immunohistochemistry | — | Matschke et al., 2020 [134] |
hPSC human pluripotent stem cells, CSF cerebrospinal fluid, TuJ1 class III β-tubulin, GFAP glial fibrillary acidic protein, MAP2 microtubule-associated protein 2, Iba1 ionized calcium-binding adaptor molecule 1, NF200 neurofilament 200, OMP olfactory membrane protein.
Fig. 1.
Neuropsychiatric manifestations, possible mechanisms of neurological impairments after SARS-CoV-2 infection, and potential therapeutic interventions.
Neurotropism of SARS-CoV-2 using human pluripotent stem cell technology and brain organoids
Human pluripotent stem cell (hPSC) technology has been successfully used to study viral infections of the CNS such as the Zika virus, and have immensely implicated to identify the specificity of infected cell types and brain organoids, model neurodevelopmental or neurodegenerative disorders, elucidate disease progress and mechanisms, and develop potential therapeutic agents [92–94]. Using these models including human neural progenitor cells, neurospheres, monolayer brain cells, and three-dimensional (3D) region-specific brain organoids, the emerging experimental evidence has demonstrated that SARS-CoV-2 can invade the human CNS and infect various cell types (Table 2) [95–98].
SARS-CoV-2 was found to sparsely infect human neurons, but robustly infect choroid plexus epithelial cells and increase cell death, indicating that SARS-CoV-2 may invade the CNS by acting on the blood–CSF barrier in the choroid plexus [99, 100]. SARS-CoV-2 spike pseudovirus and live virus specifically infected choroid plexus epithelial cells, but not neurons or glia, and disrupted the blood–CSF barrier function in human iPSC-derived brain organoids [101]. By integrating pericyte-like cells into hPSC-derived cortical organoids, Wang et al. found that pericyte-like cells were extensively infected by SARS-CoV-2 and facilitated viral spread to astrocytes [102]. Several studies showed that SARS-CoV-2 does not replicate in hiPSC-derived neural cultures, arguing that most brain damage that is caused by SARS-CoV-2 infection is attributable to local immune responses rather than robust viral replication in the CNS [103, 104]. However, other studies using human brain organoids found the replication of SARS-CoV-2 in the neuronal cell body and neurite structures, accompanying metabolic changes [105–107]. Furthermore, SARS-CoV-2 preferably targets cortical neurons of 3D human brain organoids, where it has altered Tau distribution, and phosphorylation, which increased expression of neurodegeneration genes and induced neuronal death, providing further insights into the neuropathogenesis of SARS-CoV-2 infection [108, 109]. Yang et al. found that the SARS-CoV-2 pseudo-entry virus and SARS-CoV-2 virus infects dopaminergic neurons, but not cortical neurons or microglia [110]. Andrews et al. found that SARS-CoV-2 infects astrocytes in both primary human cortical tissue and human stem cell-derived cortical organoids [111]. Although 2D or 3D in vitro experimental models could not perfectly recapitulate the complex clinical symptoms and multifactorial cellular effects in COVID-19 patients, the development of mature and complex brain organoids comprising neurons, astrocytes, microglia, vasculature, and choroid plexus provides promising means to explore the neuropathogenesis of SARS-CoV-2 infection.
SARS-CoV-2 receptor ACE2
An analysis based on decades-long structural studies of SARS coronavirus revealed that SARS-CoV-2 may utilize angiotensin-converting enzyme-2 (ACE2) as its host receptor, consistent with its capacity for human cell infection and human-to-human transmission [112]. ACE2 is the SARS-CoV-2 docking receptor, and transmembrane protease serine-2 (TMPRSS2) is the main enzyme for proteolysis of the viral spike protein [113]. Both the receptor and enzyme are abundantly expressed in multiple cell types within various organs such as oral and nasal mucosa, olfactory region, lung, heart, esophagus, kidney, bladder, ileum, vasculature, and brain, which is consistent with the multiorgan impairments and tissue damage in COVID-19 patients [22, 114–117]. Meta-analysis of single-cell RNA-seq datasets for putative SARS-CoV-2 targets revealed that ACE2 acts in concert with TMPRSS2 or cathepsin L to promote its cellular entry in specific cell subsets across tissues [118, 119]. They also identified ACE2 as a human species-specific interferon (IFN)-stimulated gene. A recent study resolved the crystal structure of SARS-CoV-2 and demonstrated that this virus interacts with human ACE2 via the C-terminal domain of the spike protein and it displays a stronger affinity for receptor binding than SARS-CoV does [120].
It has been reported that glial cells and neurons express ACE2 receptors, and SARS-CoV can invade the brain primarily via the olfactory bulb and cause neuronal death in mice [121–123]. Autopsy examinations of the patients with acute SARS-CoV illness have also demonstrated the presence of the virus in the brain or CSF, as indicated by electron microscopy, immunohistochemistry, and real-time RT-PCR testing [121, 124]. In addition, immunohistochemistry showed heterogeneous expression of the SARS-CoV-2 receptor ACE2 in the human upper and lower respiratory tract, which may be related to the susceptibility and/or severe disease development to COVID-19 [125]. Besides in the lung, ACE2 and TMPRSS2 are also highly expressed in the human peripheral and CNS including enteric neurons of the small and large intestine, choroid plexus epithelial cells, excitatory and inhibitory neurons, astrocytes, and oligodendrocytes [101, 126, 127].
Cellular transportation via olfactory mucosa and its nervous projections
SARS-CoV-2 could infect the CNS via retrograde or anterograde axonal transport, neuron-to-neuron or neuron-to-nonneuronal propagation, or through actions on the olfactory, vagus, and trigeminal nerves. Bulk and single-cell RNA sequencing in both humans and mouse revealed that ACE2 and coronavirus cell entry-related genes are expressed in respiratory epithelium and olfactory epithelium, but not in olfactory sensory neurons or olfactory bulb neurons, indicating that SARS-CoV-2 may infect nonneuronal cell types in the olfactory bulb and thus lead to anosmia in COVID-19 patients [128].
Similar to other coronaviruses, SARS-CoV-2 can attack the olfactory bulb or peripheral nerves such as the trigeminal nerve, which connect the brainstem with different organs of the respiratory tract and then affect the CNS during or after infection [129]. Previous studies in mice showed that the neurotropic influenza virus could invade the CNS from the respiratory mucosa and the vagus nerve directly [130]. SARS-CoV-2 was directly injected into deer mice, and immunohistochemistry examinations showed the presence of SARS-CoV-2 in trigeminal ganglionic neurons, neurons, and microglia of the afferent nerves, and the glomerular layer of the olfactory bulb, indicating that this virus may enter into the brain via the gustatory-olfactory-trigeminal pathway [131]. SARS-CoV-2 was also shown to invade the CNS in rhesus monkeys primarily via the olfactory bulb, subsequently spreading to multiple brain regions, including the hippocampus, thalamus, and medulla oblongata, and causing neuroinflammation and local pathological changes [132]. Autopsies from COVID-19 patients detected the presence of SARS-CoV-2 RNA and protein in olfactory mucosal, cerebellum, and cortical neurons [106, 133]. Moreover, analysis of autopsy material from 33 deceased COVID-19 patients revealed colocalization of SARS-CoV spike protein with various neuronal markers in olfactory mucosa, indicating that SARS-CoV-2 can invade the CNS via crossing the olfactory mucosal–neural interface [133]. A postmortem case series also revealed the presence of SARS-CoV-2 viral proteins in cranial nerves originating from the lower brainstem and in isolated cells of the medulla oblongata [134]. In addition, the enteric nervous system and its vagal afferents to the CNS could be alternative targets for SARS-CoV-2 neuroinvasion, considering the large amount of ACE2 receptor expression and the prominent gastrointestinal symptoms in COVID-19 patients [135].
Hematogenous dissemination via blood–brain and blood–CSF barriers
The coronavirus could diffuse into the CNS through the dissemination of peripherally infected immune cells including monocytes, neutrophils, and T cells, or via binding to the ACE2 receptors in endothelial cells of the BBB or the blood–CSF barrier in the choroid plexus. Single-nucleus transcriptomes from postmortem choroid plexus samples from COVID-19 patients revealed the dysfunction of choroid plexus barrier cells and peripheral T cell infiltration [136]. Emerging evidence indicates that SARS-CoV-2 could infect CNS cells, especially the brain microvascular endothelial cells, thus damaging the BBB and blood–CSF integrity and causing widespread neuroinflammation, which ultimately contributes to the neuropsychiatric complications in COVID-19 patients [101, 137]. In addition, ACE2 is a vasoconstrictor and exerts pro-inflammatory function [138], and SARS-CoV-2 may act on ACE2 in the brain, leading to arterial wall rupture and intracranial bleeding in COVID-19 patients [63]. The systemic inflammation induced by SARS-CoV-2 infection is also likely to increase the barrier permeability, thus promoting peripheral infected granulocytes, systemically released inflammatory cytokines, and possibly the coronavirus itself invade into the CNS.
Hypoxia, hyperinflammation, and neuroimmune deficits
Growing evidence indicates that SARS-CoV-2 can induce hypoxic conditions caused by lung injury and modulate innate and adaptive immune responses in the host, thus further promoting SARS-CoV-2 neuroinvasion from the periphery to the brain [139]. A case series of radiographic and clinical neurologic presentations showed that hypoxemia secondary to COVID-19-related acute respiratory distress syndrome plays a critical role in hypoxia neuronal injury and neurocognitive impairment [140]. Dysregulation of the brain–lung–heart interactions could also cause hypoxic–ischemic brain damage, hyperinflammation, and procoagulative states, which would further contribute to the neurological manifestations of SARS-CoV-2 infection [141]. In addition to fighting against the coronavirus infection, the immune system is activated and CD4+ T cells produce granulocyte–macrophage colony-stimulating factor, which further promotes the overproduction and release of leukocytes and pro-inflammatory cytokines, especially interleukin-6 (IL-6), causing overactivation of the complement system and coagulation cascades, and ultimately contributing to a vicious cycle of the cytokine storm and adverse clinical outcomes [142–144]. Astrocytes and microglia, the resident immune cells in the brain, also play a critical role in SARS-CoV-2 infection, neuroinflammation, and CNS injury [145]. After a neurotropic coronavirus infection, microglia is required for debris clearance and the initiation of remyelination [146], which may be correlated with spinal demyelinating lesions in COVID-19 patients.
COVID-19-related neuropsychiatric symptoms such as encephalopathy, stroke, depression, and post-traumatic stress disorder are associated with cytokine release syndrome, BBB dysfunction (as indicated by hyper-albumin-orrachia and increased astroglial protein S100B levels), neuroinflammatory, neurochemical changes, and immune-mediated mechanisms [147, 148]. Immune analysis on a cohort of 50 COVID-19 patients with various disease severities revealed impaired type I IFN activity, characterized by no IFN-β and low IFN-α production. These low IFN levels were associated with a persistent viral load in the blood and an exacerbated inflammatory response (overproduction and release of tumor necrosis factor-α [TNF-α] and IL-6), which may be a hallmark of severe COVID-19 [149]. Moreover, IL-6 and TNF-α levels have been shown to predict disease severity, clinical progression, and death in hospitalized COVID-19 patients [150, 151]. A case report demonstrated a marked increase of several inflammatory cytokines and chemokines in the CSF of a COVID-19 patient [152]. Single-cell sequencing analysis of CSF immune cells from COVID-19 patients with neurological manifestations revealed expansion of dedifferentiated monocytes, exhausted CD4+ T cells, and reduced IFN response [153]. Autopsies of COVID-19 patients revealed astrocytosis, axonal damage, BBB leakage, and alterations of CD8+ T cell–microglia crosstalk in the CNS [154]. These findings indicate that CNS inflammation and immune-mediated mechanisms may contribute to COVID-19-related long-lasting neurological sequelae.
Hyperinflammatory syndrome in COVID-19 manifested as fever, macrophage activation, hematological dysfunction, hepatic injury, coagulopathy, and cytokinemia, is closely associated with worse respiratory symptoms and in-hospital mortality [155]. Severe COVID-19 patients also mostly exerted viral infection-induced hyperinflammatory state, mainly characterized by sustained increases in TNF-α, IL-6, and IL-1 levels, and disrupted monocyte and dendritic cell phenotypes, which could ultimately contribute to poor prognosis and mortality [151, 156]. A meta-analysis revealed that increased neutrophil-to-lymphocyte ratios and decreased lymphocyte-to-CRP ratios, which are two markers of systemic inflammation, were found in severe COVID-19 patients and may indicate a poor prognosis [157]. Synergism of TNF-α and IFN-γ triggered lethal cytokine shock syndromes and treatment with their neutralizing antibodies prevented SARS-CoV-2 infection-induced mortality in mice [158]. Cross-reactive antibodies to SARS-CoV-2 may also contribute to COVID-19-related disease pathology and the persistence of neurological symptoms even in recovered patients [159]. Sex differences in immune responses also appear to make males more susceptible to SARS-CoV-2 infection, and worse clinical outcomes that are associated with poor T cell-mediated immunity in male COVID-19 patients [160, 161].
Central and peripheral immunological responses to SARS-CoV-2 infection are distinct. Immune analysis of mononuclear cells from COVID-19 patients with neurological symptoms revealed unique B cell responses that markedly differed between the CSF and peripheral blood [162]. Furthermore, a mouse model of SARS-CoV-2 infection found that antibody responses can occur separately in the brain without being evident in the systemic circulation. Specifically, a case report showed COVID-19-related meningitis without respiratory manifestations and negative oropharyngeal/nasopharyngeal RT-PCRs, but the CSF RT-PCR assay was positive [163]. A case report also showed a patient with serious neurological damage and mental abnormalities without respiratory symptoms, but with strong IgM and IgG antibodies against SARS-CoV-2 in the CSF. This patient also had negative RT-PCR results in both nasopharyngeal swabs and CSF [164].
COVID-19 appears to affect children more mildly than adults, which may be due to the reduced ACE2 level in children’s respiratory tract, their protective T cell and T-helper 2 (Th2) immunity, and their decreased inflammatory responses [165]. However, a multisystem inflammatory syndrome in children (MIS-C) is fatal and worrisome and has been increasingly reported and noticed [166]. A case report of a 4-year-old COVID-19 child presenting with MIS-C and prominent neurologic symptoms, and described how a cytokine storm and decreased BDNF level may contribute to his neurocognitive dysfunction [167]. The increased concentrations of CRP and IL-6 are strongly associated with severe outcomes in COVID patients with MIS-C [168]. A population-based longitudinal study revealed that higher levels of the systemic inflammatory marker IL-6 at age 9 years were more likely to develop depression and psychosis at age 18 years [169]. Mechanically, maternal inflammation affects multiple steps of cortical GABAergic interneuron development, thus contributing to the cognitive impairment of the affected offspring [170]. Furthermore, early-life mental health has been associated with decreased levels of fibrinogen and CRP, two indicators of inflammation and cardiovascular disease, and increased risk for all-cause mortality in later life [171].
Overall, the SARS-CoV-2 may directly invade the CNS via cellular transportation and hematogenous dissemination by acting on ACE2 receptors in endothelial cells of BBB or the blood–CSF barrier, and indirectly damage the brain via hypoxia, hyperinflammation, and neuroimmune system. This leads to the recruitment and activation of immune cells including resident microglia and astrocytes, and peripheral leukocytes and macrophages, which increases the production and release of inflammatory cytokines and chemokines, further induces the breakdown of BBB and blood–CSF barriers, and causes neuroinflammation, demyelination, neuronal excitotoxicity, and synaptic plasticity deficits in the brain, forming a vicious cycle of the cytokine storm and neuronal injury (Fig. 2). The dysfunction of the peripheral and CNS could ultimately contribute to the neurologic and neuropsychiatric manifestations in COVID-19 patients.
Fig. 2. Schematic illustration of proposed consequences of SARS-CoV-2 neuroinvasion.
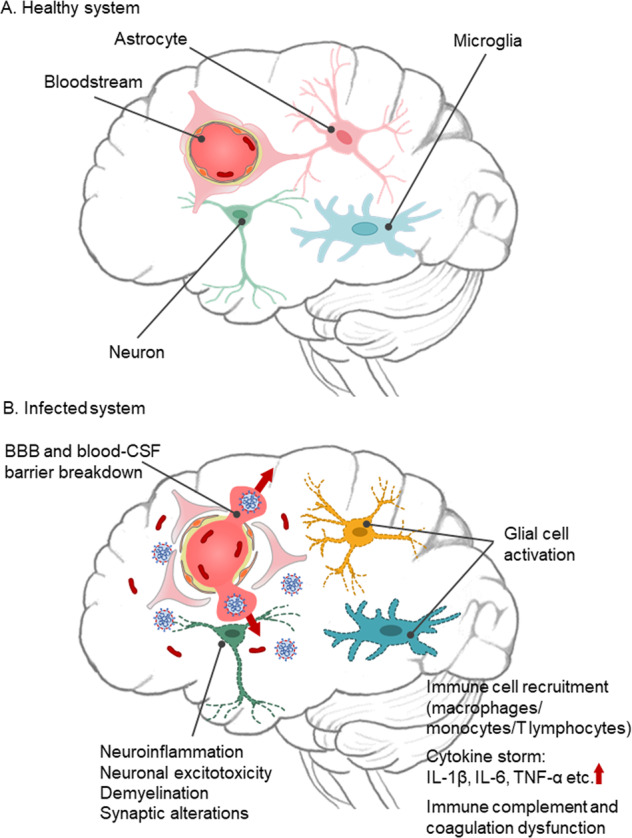
A In healthy systems, the blood-rain barrier (BBB) prevents most of the macromolecules and neurotoxins in the bloodstream from entering the brain tissues, which is achieved through the components of the neurovascular unit (e.g., endothelial cells, astrocytes). B Growing evidence suggests that SARS-CoV-2 may invade brain tissue. The virus may enter the bloodstream through multiple pathways and infect the neurovascular cells, causing the breakdown of the BBB and blood–cerebrospinal fluid (CSF) barrier. Their breakdown will further trigger the inflammatory responses, including the recruitment of macrophage and T lymphocytes, activation of astrocytes and microglia, cause a vicious cycle of the cytokine storm (production and release of IL-1β, IL-6, TNF-α, etc.), and disrupt immune complement and coagulation cascades. The infection may also cause immune-mediated demyelination, neuronal excitotoxicity, and dysfunction of synaptic plasticity, which ultimately contributes to worsened neurological and neuropsychiatric symptoms in COVID-19 patients.
Strategies for treatment of SARS-CoV-2-related neuropsychiatric complications
As the neurologic and neuropsychiatric manifestations are commonly reported in COVID-19 patients, especially in severe conditions, there is an urgent need to develop effective strategies and therapeutic frameworks to prevent and reduce them in the clinic (Table 3).
Table 3.
Treatment of COVID-19-associated neuropsychiatric complications.
| Study | Region | Participants | Sample size | Study design | Symptoms | Interventions (n patients) | Clinical outcomes | Main findings |
|---|---|---|---|---|---|---|---|---|
| ACEIs/ARBs | ||||||||
| De Spiegeleer et al., 2020 [176] | Belgium | COVID-19-diagnosed residents | 154 | Retrospective cohort | Not specified | ACEIs/ARBs (30), statin (31) | ACEIs/ARBs (6 serious, 24 nonserious), statin (6 serious, 25 nonserious) | Statin treatment associated with beneficial effects on COVID-19-related clinical symptoms, statin treatment combined with ACEI or ARB associated with less severe clinical outcomes |
| Luzzi et al., 2020 [179] | Italy | COVID-19 patients affected by stroke | 6 | Case series | Not specified | ACEIs/ARBs | Recovery | Unclear whether and how pharmacomodulation of the renin–angiotensin system affected the clinical course of COVID-19 patients who were affected by stroke |
| Matsuzawa et al., 2020 [175] | Japan | COVID-19 patients (39 with hypertension) | 151 | Retrospective cohort | Mental confusion related to pneumonia (14) | ACEIs/ARBs (21), non-ACEIs/ARBs (18) | In-hospital death (14), mechanical ventilation (14), oxygen therapy (58) | ACEIs/ARBs could be beneficial for the prevention of confusion in COVID-19 patients with hypertension |
| Meng et al., 2020 [173] | China | COVID-19 patients with hypertension | 42 | Retrospective cohort | Not specified | ACEIs/ARBs (17), non-ACEIs/ARBs (25) | ACEIs/ARBs (4 severe, 0 died), non-ACEIs/ARBs (12 severe, 1 died) | Renin–angiotensin system inhibitors improve clinical outcomes of COVID-19 patients with hypertension |
| Zhang et al., 2020 [174] | China | Patients with hypertension and COVID-19 | 1128 | Retrospective cohort | Non-ACEI/ARB group had a higher prevalence of fever, dyspnea, and bilateral lung lesion | ACEIs/ARBs (188), non-ACEIs/ARBs (940) | 99 deaths out of 1128 patients, risk of 28-day all-cause mortality was significantly lower in ACEI/ARB group | Among hospitalized patients with COVID-19 and coexisting hypertension, inpatient use of ACEI/ARB associated with a lower risk of all-cause mortality compared with ACEI/ARB nonusers |
| Intravenous immunoglobulin | ||||||||
| Khaja et al., 2020 [196] | USA | COVID-19 with Guillain–Barré syndrome | 1 | Case report | Bilateral facial weakness, unable to raise eyebrows, unable to close eyes, unable to smile, loss of taste sensation | Intravenous immunoglobulin | Discharged on day 12 | Infection with SARS-CoV-2 resulted in acute neurological involvement, leading to loss of taste, Guillain–Barré syndrome, and facial weakness |
| Manganotti et al., 2021 [195] | Italy | COVID-19 patients with drug-resistant status epilepticus | 2 | Case series | Convulsive status epilepticus, severe respiratory failure symptoms | Intravenous immunoglobulin (0.4 g/kg) | Discharged from hospital | Support the use of Intravenous immunoglobulin therapy in new-onset refractory status epilepticus COVID-19 patients with suspected autoimmune encephalitis to prevent negative outcomes |
| Muccioli et al., 2021 [194] | Italy | COVID-19 patients | 5 | Case series | Encephalopathy, confusion, delirium, agitation, akinetic mutism, apraxia, and pyramidal, extrapyramidal, and frontal release signs | Intravenous immunoglobulin (0.4 g/kg) | Complete electroclinical recovery | Intravenous immunoglobulin may be a safe and effective treatment for COVID-19-associated encephalopathy |
| Tocilizumab | ||||||||
| Dastan et al., 2020 [189] | Iran | Severe or critical SARS-CoV-2 patients | 42 | Noncontrolled trial | Receiving oxygen via face mask (31), receiving oxygen via nasal cannula (11) | Tocilizumab (20 severe, 22 critical) | Severe (19 survivors, 1 non-survivor), critical (16 survivors, 6 non-survivors) | Tocilizumab may be promising for patients with severe or critical SARS-CoV-2 infection if promptly initiated during the severe stage |
| Muccioli et al., 2020 [192] | Italy | COVID-19-related encephalopathy | 1 | Case report | Expressive aphasia, inattentiveness, agitation, marked confusion | Tocilizumab | Discharged asymptomatic | Neuropsychiatric symptoms resolved following treatment with tocilizumab, future studies should further investigate the role of tocilizumab in treating COVID-19-related encephalopathy |
| Talluri et al., 2021 [193] | USA | COVID-19 patients with encephalopathy | 1 | Case report | Fever, cough, exertional dyspnea, hypoactive delirium | Tocilizumab | Died on day 27 | Clinicians should consider posterior reversible encephalopathy syndrome in differential diagnoses when managing COVID-19 patients with encephalopathy, especially those who receive immunotherapy (e.g., tocilizumab) |
ACEIs/ARBs angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
ACEIs/ARBs
Previous studies showed that ACE2 expression level was correlated with susceptibility to SARS-CoV infection and the development of neuropsychiatric symptoms [172]. Angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) are commonly used in the treatment of cardiovascular diseases such as hypertension and coronary heart disease. Comorbidity with hypertension has mostly been reported among patients who died of COVID-19. Many of these patients used renin–angiotensin system inhibitors, such as ACEIs and ARBs, which increase ACE2 receptor expression at the cell membrane in experimental animals, and theoretically this larger number of ACE2 receptors enhance viral entry and lead to a higher risk of SARS-CoV-2 infection [116, 138]. However, little current human evidence supports this assertion. Recent clinical evidence indicates that the use of these drugs improves clinical outcomes by reducing the occurrence of new-onset or worsening mental confusion (e.g., mild disorientation or hallucinations) and decreases the risk of all-cause mortality in COVID-19 patients with hypertension [173–175]. A retrospective multicenter cohort study reported that statin treatment in combination with an ACEI or ARB had beneficial, albeit nonsignificant, effects on serious clinical outcomes of COVID-19 in older adults who lived in nursing homes [176]. Society and practice guidelines recommend continued therapy for patients who have previously prescribed these drugs for another indication [177]. ACEIs/ARBs should not be discontinued for COVID-9 infected patients as their protective role in heart injuries and lung damage caused by the infection. However, it is not recommended to initiate ACEIs/ARBs because there has been no definitive evidence that they benefit COVID- 19 patients’ survival [138, 178]. Randomized clinical trials are needed to further clarify the safety and efficacy of ACEIs/ARBs in COVID-19 patients with neuropsychiatric complications such as stroke [179].
Anti-inflammatory drugs and interventions
Early adaptive immune responses, such as the recruitment of multiple immune cells and the concomitant production of immunoglobulin antibodies, may predict better clinical outcomes in COVID-19 patients [180]. During aging, immune system function declines, and adaptive immunity fails to develop, which can significantly weaken the defense response to SARS-CoV-2 infection in the elderly and worsen clinical outcomes. Because of the risk for a cytokine storm syndrome in a subgroup of patients with severe COVID-19, immunosuppression is recommended for COVID-19 treatment [156]. Immunosuppressive agents are also widely used to combat several disorders of both the central and peripheral nervous systems such as multiple sclerosis. Corticosteroids were commonly used during the outbreaks of SARS-CoV and MERS-CoV, especially for patients with critical conditions [181, 182]. Corticosteroids inhibit systemic inflammation, but also suppress immune activity and prevent viral clearance. Some clinical evidence suggests that corticosteroid treatment had no beneficial effects on SARS-CoV-2-induced lung injury or mortality [183–185]. Conversely, a meta-analysis revealed that low-dose corticosteroid therapy appeared to reduce all-cause mortality in critically ill patients with COVID-19 [186, 187]. Corticosteroids were also reported to exert potential beneficial effects on COVID-19-associated neurological disorders, including encephalitis and acute disseminated encephalomyelitis [188].
IL-6 is prominent in the cytokine storm that underlies COVID-19-related acute respiratory distress syndrome and brain injury. Increasing evidence supports the safety and efficiency of the IL-6 receptor blocker tocilizumab for treating patients with severe or critical COVID-19, especially those with cytokine release syndrome [189–191]. Case reports described that tocilizumab exerted potential beneficial effects on COVID-19-related neuropsychiatric symptoms, including encephalopathy presenting with aphasia and posterior reversible encephalopathy syndrome [192, 193]. Case reports and case series showed that immunotherapy with intravenous immunoglobulin was safe and effective for the treatment of COVID-19-associated neurological disorders, including encephalopathy, GBS, and new-onset refractory status epilepticus [194–196]. Janus kinase inhibitor baricitinib has been shown to reduce macrophage and neutrophil recruitment and suppress inflammation in a rhesus macaque model of SARS-CoV-2 infection [197]. Baricitinib treatment could affect both inflammation and cellular viral endocytosis and entry into cells, thus it could be a potential treatment for COVID-19 [198]. Thiamine, a vitamin and dietary supplement, was recently reported to attenuate the Th17 cell-mediated IL-17 pro-inflammatory cytokine storm related to COVID-19, and is a promising repurposed drug for treating neurological symptoms in COVID-19 patients [199]. Meta-analysis of randomized clinical trials showed that psychosocial interventions are associated with enhanced immune system function for at least 6 months following treatment cessation, as indexed by decreases in levels of pro-inflammatory cytokines or markers (e.g., IL-6, CRP), and by increases in immune cell counts (e.g., CD56, CD4) [200]. Psychosocial interventions, including cognitive behavioral therapy, psychological first aid, and a community-based psychosocial arts program, were reported to have beneficial effects on psychological and psychiatric complications in COVID-19 patients [201].
Other approaches
Large-scale compound repurposing of known antiviral drugs identified several small molecules, such as the PIKfyve kinase inhibitor and the cysteine protease inhibitors, as candidate therapeutic drugs for COVID-19 [202]. Antimalarials such as chloroquine and hydroxychloroquine were found to counter neuroinflammation and have promising beneficial effects on COVID-19-related neurological/neurovascular complications [203], although randomized clinical trials showed that hydroxychloroquine had no efficacy to prevent transmission of SARS-CoV-2 among hospital-based healthcare workers [204], and was associated with increased mortality in COVID-19 patients [205]. In addition, potential neuropsychiatric side effects of chloroquine and hydroxychloroquine suggest avoiding them as COVID-19 treatments, and if these agents are used, evaluation of patients’ psychiatric presentations such as psychosis, mood disorders, and suicide risk should be implemented [206–208]. A vaccine BNT162b1 targeting the receptor-binding domain of the SARS-CoV-2 spike protein elicited robust T cell and strong human antibody responses, indicating promising therapeutic and prevention potential against COVID-19 [209]. A pilot randomized controlled study intends to evaluate the effects of low-intensity transcranial direct current stimulation on dyspnea relief in COVID-19 patients requiring mechanical ventilation in ICU [210]. In addition, intranasal delivery is proposed in the management of neurological and neuropsychiatric manifestations of COVID-19 as it could overcome the BBB impediment and enter the CNS, with minimal adverse effects [211].
Conclusions and perspectives
Neurological and neuropsychiatric symptoms are common in COVID-19 patients. Emerging neuroimaging and neurochemical evidence indicate neuroimmune dysfunction and brain injury in severe COVID-19 patients, especially those with neuropsychiatric manifestations. These neuropsychiatric complications are recognized as critical contributors to morbidity and mortality during the COVID-19 pandemic and have become major public health challenges. SARS-CoV-2 infection can directly invade the CNS through the blood circulation and neuronal pathways, and indirectly affect the innate and adaptive immune system and cause neuroinflammation. Both of these disruptions to immune functioning and neuroinflammation ultimately lead to brain lesions and accelerate the progression and worsening of the clinical outcomes of neuropsychiatric disorders.
Immun-suppressive therapies, vaccines targeting the coronaviruses’ spike protein, and pharmacological agents that improve endothelial integrity, reduce hypercoagulation status or targeting the host’s ACE2 receptor, to which the virus binds, could prevent neuropsychiatric complications such as stroke and benefit an individual’s mental health such as poststroke depression in COVID-19 patients (Fig. 1). However, to date, no pharmacologic guidelines or standard protocols exist for the management of neuropsychiatric symptoms in COVID-19 patients. Integrated multilayered and multidisciplinary research including epidemiology, pharmacology, imaging, translational experimental studies, and randomized clinical trials are urgently needed to develop safe and effective interventions to reduce the short- and long-term neuropsychiatric complications in hospitalized or recovered patients with COVID-19.
Some neurological and neuropsychiatric manifestations may be due to systemic involvement rather than direct CNS infection. Thus, it should be made with caution when lacking solid evidence for neuroinvasion in COVID-19 patients with neurological symptoms such as the absence of viral detection in CSF samples or abnormalities in brain imaging. Further experimental animal studies, prospective or retrospective observational studies, randomized clinical trials, autopsies, and CSF analysis are urgently needed to elaborate the direct or indirect effects of SARS-CoV-2 infection on different cell types in the CNS, spinal cord, and peripheral nervous system, clarify the neuroinvasive pathways of this devastating virus and confirm the causative roles of inflammation or immune system dysfunction, hypoxic–ischemic brain injury, and hypercoagulability in disease pathology. These clinical–epidemiological laboratory and biological mechanism investigations will ultimately guide the clinic to prioritize and individualize therapeutic protocols based on the disease severity, neuropsychiatric presentations, and predominant organ involvement. Awareness and management of these neuropsychiatric complications are critical to improve the prognosis and reduce mortalities of critically ill COVID-19 patients.
Search strategy and selection criteria
References for this review were identified by searching PubMed for articles on COVID-19 from database inception to August 2021, without language restrictions. The following search terms were used: “COVID-19”, “SARS-CoV-2”, “neurological”, “psychiatric”, “neuropsychiatric”, “neurotropism”, “neuroinvasion”, “nervous system”, “treatment”, and “intervention”. Additional articles were identified by searching the references of relevant articles. The final list of included articles was generated based on relevance and originality with regard to the topics covered in this review.
Acknowledgements
This work was supported in part by the National Key Research and Development Program of China (nos. 2019YFC0118604 and 2021YFC0863700), the National Natural Science Foundation of China (nos. 32071058, 82171514 and 81821092), and Special Research Fund of PKUHSC for Prevention and Control of COVID-19 and the Fundamental Research Funds for the Central Universities (no. BMU2020HKYZX008).
Author contributions
Y.H. wrote the first draft of the manuscript. K.Y. prepared the figures. Y.H., Z.W., W.-J.L., and Z.-A.L. searched for the references and related articles based on the different topics of this review. Y.H. and Lin Liu prepared the first draft of the tables. L.S., W.Y., J.-L.Y., J.-L.L., J.S., Z.-C.L., G.-H.W., and T.K. revised the manuscript. Y.-P.B. and Lin Lu supervised this review and revised the manuscript. All authors contributed to the article and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan-Ping Bao, Email: baoyp@bjmu.edu.cn.
Lin Lu, Email: linlu@bjmu.edu.cn.
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–9. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of V. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–44. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynn JR. Protecting workers aged 60-69 years from COVID-19. Lancet Infect Dis. 2020;20:1123. doi: 10.1016/S1473-3099(20)30311-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finelli L, Gupta V, Petigara T, Yu K, Bauer KA, Puzniak LA. Mortality among US patients hospitalized with SARS-CoV-2 infection in 2020. JAMA Netw Open. 2021;4:e216556. doi: 10.1001/jamanetworkopen.2021.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLean MA, Kamintsky L, Leck ED, Friedman A. The potential role of microvascular pathology in the neurological manifestations of coronavirus infection. Fluids Barriers CNS. 2020;17:55. doi: 10.1186/s12987-020-00216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Azorín D, Martínez-Pías E, Trigo J, Hernández-Pérez I, Valle-Peñacoba G, Talavera B, et al. Neurological comorbidity is a predictor of death in COVID-19 disease: a cohort study on 576 patients. Front Neurol. 2020;11:781. doi: 10.3389/fneur.2020.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louapre C, Collongues N, Stankoff B, Giannesini C, Papeix C, Bensa C, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77:1079–88. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–83. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A. 2020;75:1788–95. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–45. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–5. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–27. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–9. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra R, Florez-Perdomo WA, Vasquez HE, Moscote-Salazar LR, Agrawal A. SARS-CoV 2 and the pathobiology of the respiratory control mechanisms in the brainstem. J Formos Med Assoc. 2020;120:767–8. doi: 10.1016/j.jfma.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Zhuang J, Stone WS, Zhang L, Zhao Z, Wang Z, et al. Symptoms and occurrences of narcolepsy: a retrospective study of 162 patients during a 10-year period in Eastern China. Sleep Med. 2014;15:607–13. doi: 10.1016/j.sleep.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Tsai LK, Hsieh ST, Chao CC, Chen YC, Lin YH, Chang SC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61:1669–73. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 18.Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, et al. Neurological complications during treatment of Middle East Respiratory Syndrome. J Clin Neurol. 2017;13:227–33. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam MH, Wing YK, Yu MW, Leung CM, Ma RC, Kong AP, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–7. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 20.Al-Obaidi MMJ, Bahadoran A, Wang SM, Manikam R, Raju CS, Sekaran SD. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018;62:16–27. doi: 10.4149/av_2018_102. [DOI] [PubMed] [Google Scholar]
- 21.Soung A, Klein RS. Viral encephalitis and neurologic diseases: focus on astrocytes. Trends Mol Med. 2018;24:950–62. doi: 10.1016/j.molmed.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–32. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–27. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behzad S, Aghaghazvini L, Radmard AR, Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: rRadiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–15. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed A, Qureshi AS, Mohamed SA. Neurological manifestations of COVID-19 in absence of respiratory symptoms or fever. Cureus. 2021;13:e13887. doi: 10.7759/cureus.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Ni SY, Yan W, Lu QD, Zhao YM, Xu YY, et al. Mental and neurological disorders and risk of COVID-19 susceptibility, illness severity and mortality: a systematic review, meta-analysis and call for action. EClinicalMedicine. 2021;40:101111. [DOI] [PMC free article] [PubMed]
- 28.Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162:1491–4. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varatharaj A, Thomas N, Ellul MA, Davies N, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–82. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–70. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iltaf S, Sr., Fatima M, Salman S, Sr., Salam JU, Abbas S. Frequency of neurological presentations of coronavirus disease in patients presenting to a tertiary care hospital during the 2019 coronavirus disease pandemic. Cureus. 2020;12:e9846. doi: 10.7759/cureus.9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol. 2020;77:683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan S, Xiao M, Han F, Xia P, Bai X, Chen H, et al. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front Neurol. 2020;11:806. doi: 10.3389/fneur.2020.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong W, Mu J, Guo J, Lu L, Liu D, Luo J, et al. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95:e1479–e1487. doi: 10.1212/WNL.0000000000010034. [DOI] [PubMed] [Google Scholar]
- 35.Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P, et al. A prospective study of neurologic disorders in hospitalized COVID-19 patients in New York city. Neurology. 2020;96:e575–e586. doi: 10.1212/WNL.0000000000010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campiglio L, Priori A. Neurological symptoms in acute COVID-19 infected patients: a survey among Italian physicians. PLoS ONE. 2020;15:e0238159. doi: 10.1371/journal.pone.0238159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuccio FG, Barra M, Claudio G, Claudio S. A rare case of acute motor axonal neuropathy and myelitis related to SARS-CoV-2 infection. J Neurol. 2020;268:2327–30. doi: 10.1007/s00415-020-10219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridman S, Bres Bullrich M, Jimenez-Ruiz A, Costantini P, Shah P, Just C, et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. 2020;95:e3373–e3385. doi: 10.1212/WNL.0000000000010851. [DOI] [PubMed] [Google Scholar]
- 39.Syahrul S, Maliga HA, Ilmawan M, Fahriani M, Mamada SS, Fajar JK, et al. Hemorrhagic and ischemic stroke in patients with coronavirus disease 2019: incidence, risk factors, and pathogenesis - a systematic review and meta-analysis. F1000Res. 2021;10:34. doi: 10.12688/f1000research.42308.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martín Ó, Barrena C, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143:3089–103. doi: 10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franceschi AM, Ahmed O, Giliberto L, Castillo M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. Am J Neuroradiol. 2020;41:1173–6. doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–7. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob Health. 2020;16:57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi L, Lu ZA, Que JY, Huang XL, Liu L, Ran MS, et al. Prevalence of and risk factors associated with mental health symptoms among the general population in China during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2014053. doi: 10.1001/jamanetworkopen.2020.14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Que J, Shi L, Deng J, Liu J, Zhang L, Wu S, et al. Psychological impact of the COVID-19 pandemic on healthcare workers: a cross-sectional study in China. Gen Psychiatr. 2020;33:e100259. doi: 10.1136/gpsych-2020-100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bao Y, Sun Y, Meng S, Shi J, Lu L. 2019-nCoV epidemic: address mental health care to empower society. Lancet. 2020;395:e37–e38. doi: 10.1016/S0140-6736(20)30309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan K, Gong YM, Liu L, Sun YK, Tian SS, Wang YJ, et al. Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: a meta-analysis and systematic review. Mol Psychiatry. 2021;1–17. [DOI] [PMC free article] [PubMed]
- 48.Shi L, Que JY, Lu ZA, Gong YM, Liu L, Wang YH, et al. Prevalence and correlates of suicidal ideation among the general population in China during the COVID-19 pandemic. Eur Psychiatry. 2021;64:e18. doi: 10.1192/j.eurpsy.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Ding H, Zhang Y, Zhang B, Guo Y, Cheung T, et al. Prevalence of poor psychiatric status and sleep quality among frontline healthcare workers during and after the COVID-19 outbreak: a longitudinal study. Transl Psychiatry. 2021;11:223. doi: 10.1038/s41398-020-01190-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Xu D, Xie B, Zhang Y, Huang H, Liu H, et al. Poor-sleep is associated with slow recovery from lymphopenia and an increased need for ICU care in hospitalized patients with COVID-19: a retrospective cohort study. Brain Behav Immun. 2020;88:50–58. doi: 10.1016/j.bbi.2020.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y, Jiang Z, Guo S, Wu P, Lu Q, Xu Y, et al. Association of symptoms of attention deficit and hyperactivity with problematic internet use among university students in Wuhan, China during the COVID-19 pandemic. J Affect Disord. 2021;286:220–7. doi: 10.1016/j.jad.2021.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Díaz-Pérez C, Ramos C, López-Cruz A, Muñoz Olmedo J, Lázaro González J, De Vega-Ríos E, et al. Acutely altered mental status as the main clinical presentation of multiple strokes in critically ill patients with COVID-19. Neurol Sci. 2020;41:2681–4. doi: 10.1007/s10072-020-04679-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmes EA, O'Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–60. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lund-Sørensen H, Benros ME, Madsen T, Sørensen HJ, Eaton WW, Postolache TT, et al. A nationwide cohort study of the association between hospitalization with infection and risk of death by suicide. JAMA Psychiatry. 2016;73:912–9. doi: 10.1001/jamapsychiatry.2016.1594. [DOI] [PubMed] [Google Scholar]
- 55.Nemani K, Li C, Olfson M, Blessing EM, Razavian N, Chen J, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78:380–6. doi: 10.1001/jamapsychiatry.2020.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Shi L, Que J, Lu Q, Liu L, Lu Z, et al. The impact of quarantine on mental health status among general population in China during the COVID-19 pandemic. Mol Psychiatry. 2021;1–10. [DOI] [PMC free article] [PubMed]
- 57.Amanat M, Rezaei N, Roozbeh M, Shojaei M, Tafakhori A, Zoghi A, et al. Neurological manifestations as the predictors of severity and mortality in hospitalized individuals with COVID-19: a multicenter prospective clinical study. BMC Neurol. 2021;21:116. doi: 10.1186/s12883-021-02152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eskandar EN, Altschul DJ, de la Garza Ramos R, Cezayirli P, Unda SR, Benton J, et al. Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology. 2021;96:e1527–e1538. doi: 10.1212/WNL.0000000000011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–27. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;01019. [DOI] [PMC free article] [PubMed]
- 61.Sheth KN, Mazurek MH, Yuen MM, Cahn BA, Shah JT, Ward A, et al. Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients. JAMA Neurol. 2020;e203263. [DOI] [PMC free article] [PubMed]
- 62.Freeman CW, Masur J, Hassankhani A, Wolf RL, Levine JM, Mohan S. COVID-19-related disseminated leukoencephalopathy (CRDL): a retrospective study of findings on brain MRI. Am J Roentgenol. 2020;216:1046–7. doi: 10.2214/AJR.20.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? N Microbes N Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delorme C, Paccoud O, Kas A, Hesters A, Bombois S, Shambrook P, et al. COVID-19-related encephalopathy: a case series with brain FDG-positron-emission tomography/computed tomography findings. Eur J Neurol. 2020;27:2651–7. doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, et al. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kremer S, Lersy F, de Sèze J, Ferré JC, Maamar A, Carsin-Nicol B, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297:E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coolen T, Lolli V, Sadeghi N, Rovai A, Trotta N, Taccone FS, et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95:e2016–e2027. doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- 69.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological features of COVID-19. N. Engl J Med. 2020;383:989–92. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen MP, Le Quesne J, Officer-Jones L, Teodòsio A, Thaventhiran J, Ficken C, et al. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol. 2021;47:17–25. doi: 10.1111/nan.12662. [DOI] [PubMed] [Google Scholar]
- 71.Roberto KT, Espiritu AI, Fernandez MLL, Gutierrez JC. Electroencephalographic findings in COVID-19 patients: a systematic review. Seizure. 2020;82:17–22. doi: 10.1016/j.seizure.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vespignani H, Colas D, Lavin BS, Soufflet C, Maillard L, Pourcher V, et al. Report on electroencephalographic findings in critically ill patients with COVID-19. Ann Neurol. 2020;88:626–30. doi: 10.1002/ana.25814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canham LJW, Staniaszek LE, Mortimer AM, Nouri LF, Kane NM. Electroencephalographic (EEG) features of encephalopathy in the setting of Covid-19: a case series. Clin Neurophysiol Pract. 2020;5:199–205. doi: 10.1016/j.cnp.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen W, Toprani S, Werbaneth K, Falco-Walter J. Status epilepticus and other EEG findings in patients with COVID-19: a case series. Seizure. 2020;81:198–200. doi: 10.1016/j.seizure.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louis S, Dhawan A, Newey C, Nair D, Jehi L, Hantus S, et al. Continuous electroencephalography characteristics and acute symptomatic seizures in COVID-19 patients. Clin Neurophysiol. 2020;131:2651–6. doi: 10.1016/j.clinph.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Stefano P, Nencha U, De Stefano L, Megevand P, Seeck M, Focal EEG. changes indicating critical illness associated cerebral microbleeds in a COVID-19 patient. Clin Neurophysiol Pract. 2020;5:125–9. doi: 10.1016/j.cnp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanberg N, Ashton NJ, Andersson LM, Yilmaz A, Lindh M, Nilsson S, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95:e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 78.Ameres M, Brandstetter S, Toncheva AA, Kabesch M, Leppert D, Kuhle J, et al. Association of neuronal injury blood marker neurofilament light chain with mild-to-moderate COVID-19. J Neurol. 2020;267:3476–8. doi: 10.1007/s00415-020-10050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prudencio M, Erben Y, Marquez CP, Jansen-West KR, Franco-Mesa C, Heckman MG, et al. Serum neurofilament light protein correlates with unfavorable clinical outcomes in hospitalized patients with COVID-19. Sci Transl Med. 2021;13:eabi7643. [DOI] [PMC free article] [PubMed]
- 80.Kanberg N, Simrén J, Edén A, Andersson LM, Nilsson S, Ashton NJ, et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine. 2021;70:103512. doi: 10.1016/j.ebiom.2021.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oussalah A, Gleye S, Urmes IC, Laugel E, Barbé F, Orlowski S, et al. The spectrum of biochemical alterations associated with organ dysfunction and inflammatory status and their association with disease outcomes in severe COVID-19: a longitudinal cohort and time-series design study. EClinicalMedicine. 2020;100554. [DOI] [PMC free article] [PubMed]
- 82.Edén A, Kanberg N, Gostner J, Fuchs D, Hagberg L, Andersson LM, et al. CSF biomarkers in patients with COVID-19 and neurological symptoms: a case series. Neurology. 2021;96:e294–e300. doi: 10.1212/WNL.0000000000010977. [DOI] [PubMed] [Google Scholar]
- 83.Paterson RW, Benjamin LA, Mehta PR, Brown RL, Athauda D, Ashton NJ, et al. Serum and cerebrospinal fluid biomarker profiles in acute SARS-CoV-2-associated neurological syndromes. Brain Commun. 2021;3:fcab099. doi: 10.1093/braincomms/fcab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benjamin LA, Paterson RW, Moll R, Pericleous C, Brown R, Mehta PR, et al. Antiphospholipid antibodies and neurological manifestations in acute COVID-19: a single-centre cross-sectional study. EClinicalMedicine. 2021;101070. [DOI] [PMC free article] [PubMed]
- 85.Alexopoulos H, Magira E, Bitzogli K, Kafasi N, Vlachoyiannopoulos P, Tzioufas A, et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm. 2020;7:e893. doi: 10.1212/NXI.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Domingues RB, Mendes-Correa MC, de Moura Leite F, Sabino EC, Salarini DZ, Claro I, et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. 2020;267:3154–6. doi: 10.1007/s00415-020-09996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–6. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Panciani PP, Saraceno G, Zanin L, Renisi G, Signorini L, Battaglia L, et al. SARS-CoV-2: “Three-steps” infection model and CSF diagnostic implication. Brain Behav Immun. 2020;87:128–9. doi: 10.1016/j.bbi.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Espíndola OM, Siqueira M, Soares CN, Lima M, Leite A, Araujo A, et al. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int J Infect Dis. 2020;96:567–9. doi: 10.1016/j.ijid.2020.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harschnitz O, Studer L. Human stem cell models to study host-virus interactions in the central nervous system. Nat Rev Immunol. 2021;21:441–53. doi: 10.1038/s41577-020-00474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramani A, Pranty AI, Gopalakrishnan J. Neurotropic effects of SARS-CoV-2 modeled by the human brain organoids. Stem Cell Rep. 2021;16:373–84. doi: 10.1016/j.stemcr.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen KG, Park K, Spence JR. Studying SARS-CoV-2 infectivity and therapeutic responses with complex organoids. Nat Cell Biol. 2021;23:822–33. doi: 10.1038/s41556-021-00721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mao XY, Jin WL. iPSCs-derived platform: a feasible tool for probing the neurotropism of SARS-CoV-2. ACS Chem Neurosci. 2020;11:2489–91. doi: 10.1021/acschemneuro.0c00512. [DOI] [PubMed] [Google Scholar]
- 96.Zhang BZ, Chu H, Han S, Shuai H, Deng J, Hu YF, et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928–31. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang C, Zhang M, Garcia G JR, Tian E, Cui Q, Chen X, et al. ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell. 2021;28:331–42. doi: 10.1016/j.stem.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ng JH, Sun A, Je HS, Tan EK. Unravelling pathophysiology of neurological and psychiatric complications of COVID-19 using brain organoids. Neuroscientist. 2021;10738584211015136. [DOI] [PMC free article] [PubMed]
- 99.Jacob F, Pather SR, Huang WK, Zhang F, Wong S, Zhou H, et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell. 2020;27:937–50. doi: 10.1016/j.stem.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McMahon CL, Staples H, Gazi M, Carrion R, Hsieh J. SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Rep. 2021;16:1156–64. doi: 10.1016/j.stemcr.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, et al. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27:951–61. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang L, Sievert D, Clark AE, Lee S, Federman H, Gastfriend B, et al. A human three-dimensional neural-perivascular ‘assembloid’ promotes astrocytic development and enables modeling of SARS-CoV-2 neuropathology. Nat Med. 2021;27:1600–06. doi: 10.1038/s41591-021-01443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bauer L, Lendemeijer B, Leijten L, Embregts C, Rockx B, Kushner SA, et al. Replication kinetics, cell tropism, and associated immune responses in SARS-CoV-2- and H5N1 virus-infected human induced pluripotent stem cell-derived neural models. mSphere. 2021;6:e0027021. doi: 10.1128/mSphere.00270-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pedrosa C, Goto-Silva L, Temerozo JR, Souza L, Vitória G, Ornelas IM, et al. Non-permissive SARS-CoV-2 infection in human neurospheres. Stem Cell Res. 2021;54:102436. doi: 10.1016/j.scr.2021.102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bullen CK, Hogberg HT, Bahadirli-Talbott A, Bishai WR, Hartung T, Keuthan C, et al. Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2. ALTEX. 2020;37:665–71. doi: 10.14573/altex.2006111. [DOI] [PubMed] [Google Scholar]
- 106.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218:e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yi SA, Nam KH, Yun J, Gim D, Joe D, Kim YH, et al. Infection of brain organoids and 2D cortical neurons with SARS-CoV-2 pseudovirus. Viruses. 2020;12:1004. doi: 10.3390/v12091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-Islam P, Müller-Schiffmann A, et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;e106230. [DOI] [PMC free article] [PubMed]
- 109.Tiwari SK, Wang S, Smith D, Carlin AF, Rana TM. Revealing tissue-specific SARS-CoV-2 infection and host responses using human stem cell-derived lung and cerebral organoids. Stem Cell Rep. 2021;16:437–45. doi: 10.1016/j.stemcr.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–36. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andrews MG, Mukhtar T, Eze UC, Simoneau CR, Perez Y, Mostajo-Radji MA, et al. Tropism of SARS-CoV-2 for developing human cortical astrocytes. bioRxiv. 2021;2021.01.17.427024. 10.1101/2021.01.17.427024. Preprint
- 112.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–00120. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–92. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–2. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482–94. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lechien JR, Radulesco T, Calvo-Henriquez C, Chiesa-Estomba CM, Hans S, Barillari MR, et al. ACE2 & TMPRSS2 expressions in head & neck tissues: a systematic review. Head Neck Pathol. 2021;15:225–35. doi: 10.1007/s12105-020-01212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–35. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muus C, Luecken MD, Eraslan G, Sikkema L, Waghray A, Heimberg G, et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat Med. 2021;27:546–59. doi: 10.1038/s41591-020-01227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–8. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 122.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–75. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toljan K. Letter to the Editor regarding the viewpoint “Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanism”. ACS Chem Neurosci. 2020;11:1192–4. doi: 10.1021/acschemneuro.0c00174. [DOI] [PubMed] [Google Scholar]
- 124.Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41:1089–96. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ortiz ME, Thurman A, Pezzulo AA, Leidinger MR, Klesney-Tait JA, Karp PH, et al. Heterogeneous expression of the SARS-coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine. 2020;60:102976. doi: 10.1016/j.ebiom.2020.102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deffner F, Scharr M, Klingenstein S, Klingenstein M, Milazzo A, Scherer S, et al. Histological evidence for the enteric nervous system and the choroid plexus as alternative routes of neuroinvasion by SARS-CoV2. Front Neuroanat. 2020;14:596439. doi: 10.3389/fnana.2020.596439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen R, Wang K, Yu J, Howard D, French L, Chen Z, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 2020;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6:eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matsuda K, Park CH, Sunden Y, Kimura T, Ochiai K, Kida H, et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet Pathol. 2004;41:101–7. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- 131.Fagre A, Lewis J, Eckley M, Zhan S, Rocha SM, Sexton NR, et al. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLoS Pathog. 2021;17:e1009585. doi: 10.1371/journal.ppat.1009585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiao L, Yang Y, Yu W, Zhao Y, Long H, Gao J, et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal Transduct Target Ther. 2021;6:169. doi: 10.1038/s41392-021-00591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–75. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 134.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–29. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Sarnelli G. Can the enteric nervous system be an alternative entrance door in SARS-CoV2 neuroinvasion? Brain Behav Immun. 2020;87:93–94. doi: 10.1016/j.bbi.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang AC, Kern F, Losada PM, Agam MR, Maat CA, Schmartz GP, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–71. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alquisiras-Burgos I, Peralta-Arrieta I, Alonso-Palomares LA, Zacapala-Gómez AE, Salmerón-Bárcenas EG, Aguilera P. Neurological complications associated with the blood-brain barrier damage induced by the inflammatory response during SARS-CoV-2 infection. Mol Neurobiol. 2021;58:520–35. doi: 10.1007/s12035-020-02134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9:e016219. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z, Liu T, Yang N, Han D, Mi X, Li Y, et al. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. 2020;14:533–41. doi: 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Radnis C, Qiu S, Jhaveri M, Da Silva I, Szewka A, Koffman L. Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J Neurol Sci. 2020;418:117119. doi: 10.1016/j.jns.2020.117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Battaglini D, Brunetti I, Anania P, Fiaschi P, Zona G, Ball L, et al. Neurological manifestations of severe SARS-CoV-2 infection: Potential mechanisms and implications of individualized mechanical ventilation settings. Front Neurol. 2020;11:845. doi: 10.3389/fneur.2020.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mohammadi S, Moosaie F, Aarabi MH. Understanding the immunologic characteristics of neurologic manifestations of SARS-CoV-2 and potential immunological mechanisms. Mol Neurobiol. 2020;57:5263–75. doi: 10.1007/s12035-020-02094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ramlall V, Thangaraj PM, Meydan C, Foox J, Butler D, Kim J, et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat Med. 2020;26:1609–15. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Murta V, Villarreal A, Ramos AJ. Severe acute respiratory syndrome coronavirus 2 impact on the central nervous system: Are astrocytes and microglia main players or merely bystanders? ASN Neuro. 2020;12:1759091420954960. doi: 10.1177/1759091420954960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sariol A, Mackin S, Allred MG, Ma C, Zhou Y, Zhang Q, et al. Microglia depletion exacerbates demyelination and impairs remyelination in a neurotropic coronavirus infection. Proc Natl Acad Sci USA. 2020;117:24464–74. doi: 10.1073/pnas.2007814117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perrin P, Collongues N, Baloglu S, Bedo D, Bassand X, Lavaux T, et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur J Neurol. 2021;28:248–58. doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Steardo L, Jr., Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10:261. doi: 10.1038/s41398-020-00949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–43. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623–35. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 152.Farhadian S, Glick LR, Vogels C, Thomas J, Chiarella J, Casanovas-Massana A, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20:248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Heming M, Li X, Räuber S, Mausberg AK, Börsch AL, Hartlehnert M, et al. Neurological manifestations of COVID-19 feature T cell exhaustion and dedifferentiated monocytes in cerebrospinal fluid. Immunity. 2021;54:164–75. doi: 10.1016/j.immuni.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwabenland M, Salié H, Tanevski J, Killmer S, Lago MS, Schlaak AE, et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54:1594–610. doi: 10.1016/j.immuni.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Webb BJ, Peltan ID, Jensen P, Hoda D, Hunter B, Silver A, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92:1733–4. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–68. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kreye J, Reincke SM, Pruss H. Do cross-reactive antibodies cause neuropathology in COVID-19? Nat Rev Immunol. 2020;20:645–6. doi: 10.1038/s41577-020-00458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Park MD. Sex differences in immune responses in COVID-19. Nat Rev Immunol. 2020;20:461. doi: 10.1038/s41577-020-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Song E, Bartley CM, Chow RD, Ngo TT, Jiang R, Zamecnik CR, et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep. Med. 2021;2:100288. doi: 10.1016/j.xcrm.2021.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khodamoradi Z, Hosseini SA, Gholampoor Saadi MH, Mehrabi Z, Sasani MR, Yaghoubi S. COVID-19 meningitis without pulmonary involvement with positive cerebrospinal fluid PCR. Eur J Neurol. 2020;27:2668–9. doi: 10.1111/ene.14536. [DOI] [PubMed] [Google Scholar]
- 164.Wang M, Li T, Qiao F, Wang L, Li C, Gong Y. Coronavirus disease 2019 associated with aggressive neurological and mental abnormalities confirmed based on cerebrospinal fluid antibodies: a case report. Medicine. 2020;99:e21428. doi: 10.1097/MD.0000000000021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Steinman JB, Lum FM, Ho PP, Kaminski N, Steinman L. Reduced development of COVID-19 in children reveals molecular checkpoints gating pathogenesis illuminating potential therapeutics. Proc Natl Acad Sci USA. 2020;117:24620–6. doi: 10.1073/pnas.2012358117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Martinez OM, Bridges ND, Goldmuntz E, Pascual V. The immune roadmap for understanding multi-system inflammatory syndrome in children: opportunities and challenges. Nat Med. 2020;26:1819–24. doi: 10.1038/s41591-020-1140-9. [DOI] [PubMed] [Google Scholar]
- 167.De Paulis M, Oliveira D, Vieira RP, Pinto IC, Machado R, Cavalcanti MP, et al. Multisystem inflammatory syndrome associated with COVID-19 with neurologic manifestations in a child: A brief report. Pediatr Infect Dis J. 2020;39:e321–e324. doi: 10.1097/INF.0000000000002834. [DOI] [PubMed] [Google Scholar]
- 168.Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5:323–31. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–8. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vasistha NA, Pardo-Navarro M, Gasthaus J, Weijers D, Müller MK, García-González D, et al. Maternal inflammation has a profound effect on cortical interneuron development in a stage and subtype-specific manner. Mol Psychiatry. 2020;25:2313–29. doi: 10.1038/s41380-019-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ploubidis GB, Batty GD, Patalay P, Bann D, Goodman A. Association of early-life mental health with biomarkers in midlife and premature mortality: evidence from the 1958 British Birth Cohort. JAMA Psychiatry. 2021;78:38–46. doi: 10.1001/jamapsychiatry.2020.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hofmann H, Geier M, Marzi A, Krumbiegel M, Peipp M, Fey GH, et al. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem Biophys Res Commun. 2004;319:1216–21. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–60. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–81. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Matsuzawa Y, Ogawa H, Kimura K, Konishi M, Kirigaya J, Fukui K, et al. Renin-angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020;43:1257–66. doi: 10.1038/s41440-020-00535-8. [DOI] [PubMed] [Google Scholar]
- 176.De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21:909–14. doi: 10.1016/j.jamda.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–36. doi: 10.1001/jama.2019.20153. [DOI] [PubMed] [Google Scholar]
- 178.Onweni CL, Zhang YS, Caulfield T, Hopkins CE, Fairweather L, Freeman WD. ACEI/ARB therapy in COVID-19: the double-edged sword of ACE2 and SARS-CoV-2 viral docking. Crit Care. 2020;24:475. doi: 10.1186/s13054-020-03195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luzzi S, Giotta Lucifero A, Marasco S, Del Maestro M, Bellantoni G, Gragnaniello C. Targeting of renin-angiotensin system in COVID-19 patients affected by stroke: emerging concerns about detrimental vs. benefit effect. Interdiscip Neurosurg. 2020;22:100822. doi: 10.1016/j.inat.2020.100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thevarajan I, Nguyen T, Koutsakos M, Druce J, Caly L, van de Sandt CE, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–5. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–67. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 183.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–5. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu J, Huang J, Zhu G, Liu Y, Xiao H, Zhou Q, et al. Systemic corticosteroids and mortality in severe and critical COVID-19 patients in Wuhan, China. J Clin Endocrinol Metab. 2020;105:dgaa627. doi: 10.1210/clinem/dgz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA. 2020;324:1298–306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, Merchant HA, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14:1149–63. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–41. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–20. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dastan F, Saffaei A, Haseli S, Marjani M, Moniri A, Abtahian Z, et al. Promising effects of tocilizumab in COVID-19: a non-controlled, prospective clinical trial. Int Immunopharmacol. 2020;88:106869. doi: 10.1016/j.intimp.2020.106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Masiá M, Fernández-González M, Padilla S, Ortega P, García JA, Agulló V, et al. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: a prospective cohort study. EBioMedicine. 2020;60:102999. doi: 10.1016/j.ebiom.2020.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Malgie J, Schoones JW, Pijls BG. Decreased mortality in COVID-19 patients treated with Tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin Infect Dis. 2021;72:e742–e749. doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Muccioli L, Pensato U, Cani I, Guerra L, Provini F, Bordin G, et al. COVID-19-related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J Neuroimmunol. 2020;349:577400. doi: 10.1016/j.jneuroim.2020.577400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Talluri K, Lall N, Moreno MA, Nichols L, Bande D. Posterior reversible encephalopathy syndrome in a patient with SARS-CoV-2 infection treated with tocilizumab. Cureus. 2021;13:e13475. doi: 10.7759/cureus.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muccioli L, Pensato U, Bernabè G, Ferri L, Tappatà M, Volpi L, et al. Intravenous immunoglobulin therapy in COVID-19-related encephalopathy. J Neurol. 2021;268:2671–5. doi: 10.1007/s00415-020-10248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Manganotti P, Furlanis G, Ajčević M, Moras C, Bonzi L, Pesavento V, et al. Intravenous immunoglobulin response in new-onset refractory status epilepticus (NORSE) COVID-19 adult patients. J Neurol. 2021;268:3569–73. doi: 10.1007/s00415-021-10468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Khaja M, Gomez G, Santana Y, Hernandez N, Haider A, Lara J, et al. A 44-year-old hispanic man with loss of taste and bilateral facial weakness diagnosed with Guillain-Barre syndrome and Bell’s Palsy associated with SARS-CoV-2 infection treated with intravenous immunoglobulin. Am J Case Rep. 2020;21:e927956. doi: 10.12659/AJCR.927956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hoang TN, Pino M, Boddapati AK, Viox EG, Starke CE, Upadhyay AA, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184:460–75. doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vatsalya V, Li F, Frimodig J, Gala KS, Srivastava S, Kong M, et al. Repurposing treatment of Wernicke-Korsakoff syndrome for Th-17 cell immune storm syndrome and neurological symptoms in COVID-19: Thiamine efficacy and safety, in-vitro evidence and pharmacokinetic profile. Front Pharm. 2020;11:598128. doi: 10.3389/fphar.2020.598128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shields GS, Spahr CM, Slavich GM. Psychosocial interventions and immune system function: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2020;77:1031–43. doi: 10.1001/jamapsychiatry.2020.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yue JL, Yan W, Sun YK, Yuan K, Su SZ, Han Y, et al. Mental health services for infectious disease outbreaks including COVID-19: a rapid systematic review. Psychol Med. 2020;50:2498–513. doi: 10.1017/S0033291720003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Riva L, Yuan S, Yin X, Martin-Sancho L, Matsunaga N, Pache L, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–9. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ong WY, Go ML, Wang DY, Cheah IK, Halliwell B. Effects of antimalarial drugs on neuroinflammation-potential use for treatment of COVID-19-related neurologic complications. Mol Neurobiol. 2021;58:106–17. doi: 10.1007/s12035-020-02093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Abella BS, Jolkovsky EL, Biney BT, Uspal JE, Hyman MC, Frank I, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 2021;181:195–202. doi: 10.1001/jamainternmed.2020.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Axfors C, Schmitt AM, Janiaud P, Van't Hooft J, Abd-Elsalam S, Abdo EF, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021;12:2349. doi: 10.1038/s41467-021-22446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hamm BS, Rosenthal LJ. Psychiatric aspects of chloroquine and hydroxychloroquine treatment in the wake of coronavirus disease-2019: psychopharmacological interactions and neuropsychiatric sequelae. Psychosomatics. 2020;61:597–606. doi: 10.1016/j.psym.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ambar Akkaoui M, Lejoyeux M, Geoffroy PA. Chloroquine-induced first-episode psychosis in a patient self-medicated for COVID-19. Biol Psychiatry. 2021;89:e9. doi: 10.1016/j.biopsych.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Garcia P, Revet A, Yrondi A, Rousseau V, Degboe Y, Montastruc F. Psychiatric disorders and hydroxychloroquine for coronavirus disease 2019 (COVID-19): a VigiBase study. Drug Saf. 2020;43:1315–22. doi: 10.1007/s40264-020-01013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature. 2020;586:594–9. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 210.Azabou E, Bao G, Heming N, Bounab R, Moine P, Chevallier S, et al. Randomized controlled study evaluating efficiency of low intensity transcranial direct current stimulation (tDCS) for dyspnea relief in mechanically ventilated COVID-19 patients in ICU: The tDCS-DYSP-COVID protocol. Front Med. 2020;7:372. doi: 10.3389/fmed.2020.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Siddiqui R, Khan NA. Proposed intranasal route for drug administration in the management of central nervous system manifestations of COVID-19. ACS Chem Neurosci. 2020;11:1523–4. doi: 10.1021/acschemneuro.0c00288. [DOI] [PubMed] [Google Scholar]