Abstract
Purpose:
Our goal is to develop a low-cost tool that can be used to create consistent, partial thickness defects in rabbit and other large animals with minimal surgical training and that can facilitate pre-clinical testing of lamellar and in situ-forming biosynthetic matrix materials for corneal repair.
Materials & Methods:
In this study, three modified trephines were designed to create deep corneal wound defects with consistent depth in large animals. The modified trephines incorporated either 3D printed parts made from photopolymerizable resins, or custom-cut commercially available Teflon sheets. Wound defects were imaged with optical coherence tomography (OCT), and the depth was analyzed based on the OCT images.
Results:
The results revealed that an inner-stopper guard trephine had the best performance in creating consistent and precise wound defect depth compared to modified vacuum trephine and custom guard vacuum trephine. A 75% ± 10% cut of the cornea was achieved with inner-stopper guard trephine. The wound defect depth by inner-stopper guard trephine was independent on the corneal thickness or size of the globes. Although the cut depth by inner-stopper guard trephine relied on the experiences of users, the consistency (standard deviation) of the depth was independent on the experience of users.
Conclusions:
Our studies provided three cost-efficient animal trephines that could create consistent corneal wound depth by lab researchers without extensive training in keratectomy.
Keywords: anterior lamellar keratectomy, anterior lamellar keratoplasty (ALK), rabbit corneal defect wound model, animal vacuum trephine, guarded trephine
Introduction
A biosynthetic matrix to fill stromal defects is an emerging and promising approach to treating corneal diseases where partial of the corneal tissue is damaged and needs to be replaced but the corneal endothelium is unaffected. A number of partial-thickness corneal substitute technologies are under development1–5, some involving pre-formed hydrogel buttons and scaffold materials (lamellar implants),6–8 and others involving curable materials that form on the wound bed (referred to here as in situ-forming extracellular matrix therapy).9–12 Several lamellar implants have entered clinical trials and shown great promise in the treatment of keratoconus, corneal scarring, or fungal corneal ulcers.13, 14 Further understanding of the role of biomaterials in corneal regeneration can accelerate the translation of these therapeutic concepts. The depth and area of the corneal defect affect repair mechanisms because these parameters affect the numbers of available corneal stromal stem cells and the overall pattern of epithelial cell migration and healing.5, 15 Therefore, creation of consistent corneal defects in animal models is critical to compare the wound healing effects of candidate biomaterials. However, the usual tools used for keratoplasty procedures in human patients are not always accessible to researchers who conduct pre-clinical animal studies. These tools include vacuum trephines, microkeratomes, intraoperative Optical Coherence Tomography (OCT), and lasers.16 While arguably the most cost-effective of these, vacuum trephines are not readily available in sizes that fit rabbit or smaller animal corneas. Excimer lasers and femtosecond lasers have been used to create precise and consistent corneal wounds in rabbits,17, 18 but these lasers are not widely used in animals due to their high cost. The most popular and least costly method to create corneal wound is using biopsy punch or trephine followed by manual keratectomy with a surgical knife or spatula.
Manual keratectomies can be performed with surgical training without the aid of OCT, however, getting repeatable and consistent depth with this approach is very difficult. This technique faces especially significant difficulties when deep defect is required. The corneal perforation ratio will be significantly increased with desired defect depth due to the small thickness of corneas—only approximately 540 μm in human.19 Therefore, to increase the successful ratio of Deep Anterior Lamellar Keratoplasty (DALK), Anwar’s “big-bubble” technique and auxiliary “small-bubble” techniques have been developed to remove the entirety of pre-Descemet’s stroma in human patients.20, 21 OCT guidance can also be used in conjunction with these approaches to improve successful ratio. Given these assistant techniques are available now, the successful ratio will still highly depend on the experiences of the surgeon. For pre-clinical animal studies, the animal cornea is usually thinner than human, for example the average rabbit cornea is only 356 μm.22 And the surgeons are usually roughly trained students or technicians in research labs. So, it is even difficult to create deep keratectomy on animals. Additionally, in cases where a subtotal, Anterior Lamellar Keratoplasty (ALK)-type of stromal wound that does not extend to Descemet’s membrane is desired, achieving consistent depth- especially in the absence of the aforementioned technologies, through simple, repeatable procedure while minimizing the risk of perforation remains elusive. Therefore, there is an unmet need in pre-clinical research to develop an inexpensive tool that can create consistent corneal wound defect, which is the goal of this work.
Herein, we will introduce three types of modified trephines that create a deep, circular cut on the cornea of rabbits and pigs. The three designs are a modified vacuum trephine, a custom guarded vacuum trephine, and an inner-stopper guarded trephine. 3D printing was used to make the components to assemble the three trephine designs. The detailed manufacture and assembling procedures will be described in the Materials & Methods. The corneal wound depth on ex vivo rabbit eyes were evaluated with OCT. The wound depth consistency of these trephines was compared when used by the same surgeon. Correlation between the cut depth, globe size, and corneal thickness were analysed. The inner-stopper guarded trephine created the most consistent corneal wound defect. The depth of corneal wound defect created with the inner-stopper guarded trephine varied with the experience of surgeons. However, the standard deviations were independent of the surgeons.
Materials & Methods
Materials
Photopolymerizing resin was purchased from FormLabs™ (Clear Resin version FLGPCLO2). Iso-propyl alcohol was obtained from FisherScientific. Teflon polytetrafluoroethylene (PTFE) sheets with thickness at 2.4 mm and 254, 127, 76, and 51 μm were obtained from McMaster-Carr, trephine blades with 3.5 mm diameter (Item # 33–0350) were purchased from Ambler surgical. Rabbit eyes were obtained from VisionTech (Sunnyvale, Texas) and Pel-Freeze Biologicals (Rogers, Arizona). Pig eyes were obtained from VisionTech (Sunnyvale, Texas).
Trephine parts Designing and Prototyping
SolidWorks®, a 3-Dimensional computer aided design (3D-CAD) software, was used to design parts for corneal trephines. These designs were created as a triangle mesh file and then output as a stereolithography file. A Form2™ stereolithography 3D printer was used to prototype the designs. In order to program the stereolithography file for this printer, a slicing program PreForm™ (FormLabs™) was used to orientate, generate supports, and create G-Code for the part. A photopolymerizing resin was used as the raw material to build the part layer by layer. The printing parameters were set to 96 mW, 405 nm wavelength, and 140 μm laser spot size. After the printing was finished, the excess resin was washed off with isopropyl alcohol. The parts were then separated with the supports, cured with ultraviolet light to finish polymerizing, and at last polished with sandpapers.
Trephine Installation
Modified vacuum trephine
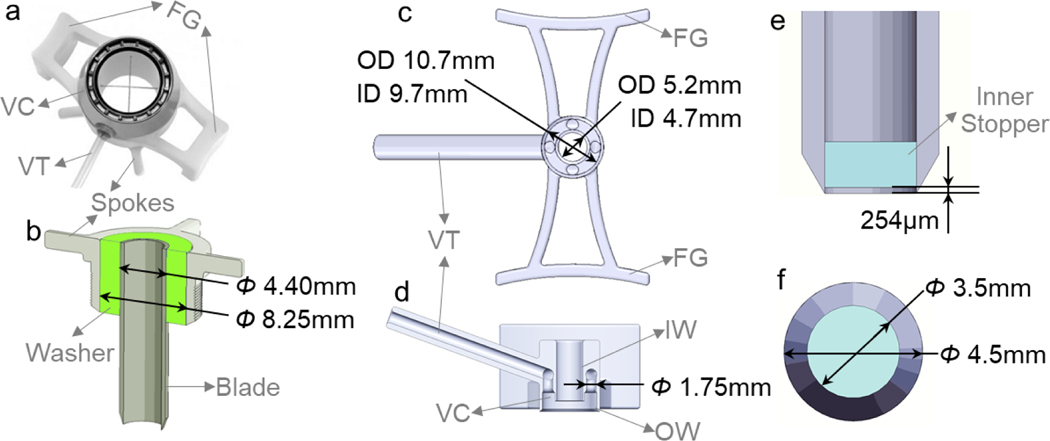
The modified vacuum trephine was remodelled based on an 8-mm-diameter Hessburg-Barron Vacuum Corneal trephine for human (Figure 1 a) by replacing the 8 mm blade with a 3.5 mm blade (Ambler Surgical). The original 8-mm-diamter trephine blade was replaced with a 3.5-mm-diamter trephine blade. The new trephine blade was installed with the assistance of a 3D printed washer (Figure 1 b). This washer is designed to ensure that the trephine blade is parallel to the vacuum body and cuts perpendicularly into the cornea so that the incision on the cornea is concentric with the inner wall of the vacuum chamber.
Figure 1.
Design of trephines. (a) Structure of 8-mm-diameter Hessburg-Barron Vacuum Corneal trephine for human. (b) Installation of a 3.5-mm trephine blade for modified vacuum trephine with a 3D-printed washer (green) that fit the trephine blade concentric into the part with spokes. (c) Top view and (d) sagittal view of the 3D printed guard of the custom guarded vacuum trephine. (e) Frontal view and (f) top view of inner-stopper guarded trephine. The inner stopper (cyan) was created by cutting a 2.4 mm Teflon PTFE plate. FG: finger grip; VC: vacuum chamber; VT: vacuum tube; IW: inner wall; OW: outer wall.
Custom guarded vacuum trephine
This trephine used a blade guard to control the distance that the trephine blade can cut into the cornea. The guard design adopted the vacuum feature of the Hessburg-Barron Vacuum Corneal trephine to immobilize the globes during surgery. The design and dimensions of the guard is shown in Figure 1 c & d. The height between inner and outside walls of the vacuum chamber was matched to corneal curvature of mature rabbits.
Inner-stopper guarded trephine
The inner stopper was created by cutting the 2.4 mm Teflon PTFE plate with a 3.5-mm trephine blade (Figure 1 e & f). A distance of 254 μm between the edge of the blade and the inner stopper was calibrated by stamping the blade on and then cutting through a 254 μm Teflon PTFE calibration sheet. The inner stopper was then pushed towards the calibration sheet so that there was no vacancy between the inner stopper and the calibration sheet. Finally, the inner stopper was fixed with super glue and the calibration sheet in the trephine was removed, leaving a 254 μm gap between the edge of the blade and the inner stopper.
Corneal wound defect creation
Ex vivo keratectomy was performed on dissected rabbit or pig globes to compare the corneal wound defect created by these trephines. The trephine and other surgical tools were sterilized with povidone iodine and 70% pure ethanol in advance. In vivo keratectomies were performed on New Zealand White rabbits. Animal experiments were designed to conform with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and were reviewed and approved by the Stanford University Institutional Animal Care and Use Committee. All anesthesia techniques were performed by the veterinary service center (VSC) at Stanford University.
Using modified vacuum trephine
The trephine blade was retracted eight quarter turns from the edge of the inner wall of the vacuum chamber. The modified vacuum trephine was first placed on a globe with the blade aimed at its center. This could be verified under a dissection microscope because center of the trephine blade could be seen through. Once the trephine was placed on the cornea, vacuum was applied by completely compressing and then releasing a 5-mL suction syringe. The vacuum was confirmed by a plunger reading smaller than 5 mL. The blade was slowly advanced 8 quarter turns and then retracted 8 quarter turns. This step was repeated once before releasing the vacuum and removing the trephine. The trephination resulted in a circular cut of consistent depth circumferentially. The edge of the incised cornea was then lifted with a pair of fine tip forceps. A blunt spatula was then used to carefully dissect and excise the anterior stroma along the plane created by the posterior-most margin of the trephine incision. Finally, the resulting partial thickness corneal button was then removed. The above procedures are shown in Video S1.
Using custom guarded vacuum trephine
The custom guarded vacuum trephine was a simplified vacuum trephine. The guard confined the depth of the trephine blade that could go into the cornea. The vacuum chamber was first placed on a globe with its center aligned with the central cornea under dissection microscope. Vacuum was applied by completely compressing and then releasing a 5-mL suction syringe. The vacuum was confirmed by a plunger reading within 3–5 mL. Next, the trephine blade was inserted to the guard and pushed all the way down with rotation that assisted the cutting. The trephine blade and then the vacuum chamber was removed by releasing the vacuum. The trephination would leave a circular cut and a corneal flap on the central cornea. Finally, this corneal flap was removed with a blunt spatula.
Using inner-stopper guarded trephine
Unlike the vacuum trephines, inner-stopper guarded trephine could not be seen through from the center. However, its outside was clear and therefore its cut position could be observed from the outside. For the trephination, the inner-stopper guarded trephine was placed directly onto central cornea. The trephine blade was pressed into cornea and then rotated so that it cut into the stromal tissue. The maximal cut depth was constrained by the distance between the edge of the blade and the inner stopper. The rotation was stopped when the trephine could not go further. The trephine was retracted, leaving a circular cut and a corneal flap on the central cornea. The flap was then removed with a blunt spatula. The surgical procedures are shown in Video S2. For the in vivo anterior lamellar keratectomies on rabbits, the experimental eye was fixed with a pair of ring forceps (item no 11106–09, Fine Science Tools) during trephination.
Determination of the globe size
Moisture on the globes was removed with paper towel after they were removed from storage saline. Then the size and weight of each globe were measured. The side-to-side diameter perpendicular to axial of the globe was measured with a caliber. Each globe was weighed with a digital balance.
Measurement of the corneal wound defect depth
After the keratectomy, the corneal wound defect was imaged with optical coherence tomography (OCT). The images were then analyzed with ImageJ. The thickness of the residual cornea at the keratectomy area and the intact cornea adjacent to keratectomy area were measured. The percentage of excised cornea was calculated using the formula: 100*(thickness of intact cornea–thickness of residual stromal bed)/thickness of intact cornea %. The rabbit cornea was found to have uniform thickness,23 therefore the thickness of the intact corneas was normalized to central corneal thickness of mature rabbits which is 356 μm.22 The absolute depth of cut was then calculated based on the formula: excised percentage*intact cornea*(356/average thickness of intact cornea).
Data analysis
Data means, standard deviations, and p values were calculated in Microsoft Excel 2016. A two-tailed Student’s t-test was used for significance and p values < 0.05 were considered as significant. Person’s coefficient was calculated with the PEARSON function in Microsoft Excel 2016.
Results
Consistency of corneal defect depth with modified vacuum trephine
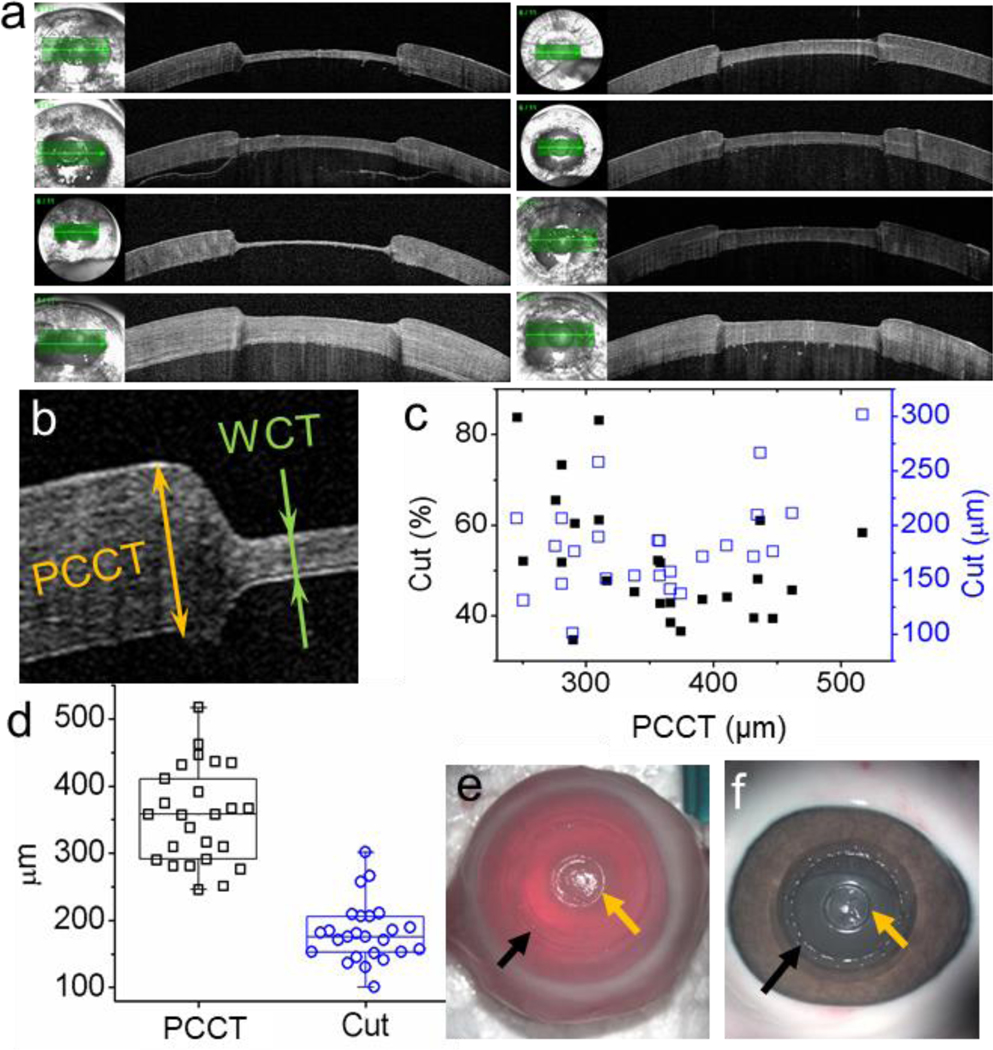
Ex vivo anterior lamellar keratectomies were performed on 25 rabbit eyes by one researcher. The average diameter of these globes was 18.81 ± 1.02 mm, and their average weight was 3.56 ± 0.52 g. The modified vacuum trephine and spatula method created obvious and smooth corneal defect as shown in Figure 2 a, which exhibited eight randomly selected keratectomy. To determine the consistency of corneal defect depth, the paracentral corneal thickness (PCCT), excised percentage of the cornea, and the wound depth of each globe were measured based on the OCT images. As shown in Figure 2 b, the paracentral corneal thickness was the thickness of intact cornea adjacent to the corneal defect. It was normalized so that the average paracentral corneal thickness was equal to reported 356 μm.22, 23 Standard deviation of paracentral corneal thickness of these globes was 72 μm (Figure 2 d). The percentage and thickness of cut were independent on paracentral corneal thickness (Figure 2 c). With the method described for modified vacuum trephine, the thickness of cut was 182 ± 45 μm (RSD = 25%) (Figure 2 d). The minimum and maximum wound depth with the modified vacuum trephine were 101 and 301 μm. The anterior lamellar keratectomies with the modified vacuum trephine removed 52% ± 13% of the central cornea. Noticeably, there were 5 incidents of perforation (20% perforation) during trephination. Figure 2 e showed the rabbit cornea after keratectomy, a dent was observed from the trephination. The dent was clearer on a pig eye (Figure 2 f), which matches the pattern of the vacuum chamber shown in Figure 1 a.
Figure 2.
OCT images and photos of corneal wound defect created with 3.5-mm modified vacuum trephine. (a) OCT images of randomly selected corneas showed that the modified vacuum trephine and spatula method created obvious and smooth corneal defect. (b) Paracentral corneal thickness (PCCT) and residual stromal bed (bed) were measured to determine the corneal defect depth. (c) Paracentral corneal thickness dependence of percentage of cut (black solid squares) and thickness of cut (blue open square). (d) Distributions of PCCT (black) and thickness of cut (blue). The whisks present minimums and maximums. Photos of (e) rabbit and (f) pig cornea after anterior lamellar keratectomy with the 3.5-mm modified vacuum trephine. The orange arrows pointed at the keratectomy and the black arrows pointed at the undesired dent marker by vacuum body.
Consistency of corneal defect depth with custom guarded vacuum trephine
Twenty keratectomies were performed ex vivo with the custom guarded vacuum trephine by the same surgeon from the modified vacuum trephine. Two of the corneas were perforated (10% perforation). The defect shape by custom guarded vacuum trephine was slightly different with that by the modified vacuum trephine. The edges of corneal defects were less steep compared to the defects created with modified vacuum trephine (Figure 3 a). The percentage and thickness of cut by the custom guarded vacuum trephine were independent on paracentral corneal thickness (Figure 3 b). The paracentral corneal thickness was also normalized so that the average paracentral corneal thickness was equal to reported 356 μm.22, 23 Standard deviation of paracentral corneal thickness of these globes was 33 μm (Figure 3 c). With the method described for custom guarded vacuum trephine, the thickness of cut was 182 ± 55 μm (RSD = 34%) (Figure 3 c). The minimum and maximum wound depth with the modified vacuum trephine were 93 and 271 μm. The anterior lamellar keratectomies with the custom guarded vacuum trephine removed 52% ± 16% of the central cornea. There was also dented marker left on the cornea after keratectomy with the custom guarded vacuum trephine (Figure 3 d). This dented marker was caused by the outer wall of the vacuum channel (Figure 1 d).
Figure 3.
OCT images and photos of corneal wound defect created with the guarded trephine. (a) OCT images of randomly selected corneas showed that the custom guarded vacuum trephine and spatula method created obvious and smooth corneal defect. (b) Paracentral corneal thickness (PCCT) dependence of percentage of cut (black solid squares) and thickness of cut (blue open square). (c) Distributions of PCCT (black) and thickness of cut (blue). The whisks present minimums and maximums. (d) Photo of rabbit cornea after anterior lamellar keratectomy with the guarded trephine. The orange arrows pointed at the keratectomy and the black arrows pointed at the undesired dent marker by vacuum channel.
Consistency of corneal defect depth with inner-stopper guarded trephine
Twenty keratectomies were performed ex vivo with the inner-stopper guarded trephine by the same surgeon from the vacuum trephines. Only one of the corneas were perforated during trephination (5% perforation). The edges of corneal defects created by inner-stopper guarded trephine were shown in Figure 4 a. These defects had steeper edge than defects by custom guarded vacuum trephine. The percentage and thickness of cut by the inner-stopper guarded trephine were independent on paracentral corneal thickness (Figure 4 b). The paracentral corneal thickness was also normalized so that its average was equal to reported 356 μm.22 Standard deviation of paracentral corneal thickness of these globes was 33 μm (Figure 4 c). With the method described for inner-stopper guarded trephine, the thickness of cut by the same researcher was 265 ± 37 μm (RSD = 14%) (Figure 3 c). The minimum and maximum wound depth with the modified vacuum trephine were 207 and 347 μm. The anterior lamellar keratectomies with the inner-stopper guarded trephine removed 75% ± 10% of the central cornea. There was only a clear circular cut but no other damage to the cornea by the inner-stopper guarded trephine blade after trephination (Figure 4 d & e).
Figure 4.
OCT images and photos of corneal wound defect created with the inner-stopper guarded trephine. (a) OCT images of randomly selected corneas showed that the inner-stopper guarded trephine and spatula method created obvious and smooth corneal defect. (b) Paracentral corneal thickness (PCCT) dependence of percentage of cut (black solid squares) and thickness of cut (blue open square). (c) Distributions of PCCT (black) and thickness of cut (blue). The whisks present minimums and maximums. Photos of (d) rabbit and (e) pig cornea after trephination with the inner-stopper guarded trephine. Only trephination cut was seen on the cornea (pointed by orange arrows).
In vivo anterior lamellar keratectomies were performed by the same researcher on 38 rabbit eyes, among which 3 corneas were perforated, led to a perforation ratio of 7.9%. The average cut of the unperforated corneas was 62% ± 8% (RSD=13%), which equals to an average cut depth of approximately 225 ± 29 μm. The minimum and maximum cut were 47% and 85%, corresponding to 173 and 310 μm. The average cut in vivo (both in percentage and micrometer) was significantly different with that ex vivo (p < 0.0005, 2-tail heteroscedastic student t-test).
Correlations between corneal defect depth with inner-stopper guarded trephine and globe size
Three parameters were used to assess globe size: side-to-side diameter of the globes perpendicular to axial, the weight of globes, and central corneal thickness. The linear correlation between the corneal defect depth by inner-stopper guarded trephine and these parameters were shown in Figure 5. The defect depth showed a mild positive dependence on globe diameter (Pearson’s r = 0.24, Figure 5a) and paracentral corneal thickness (Pearson’s r = 0.34, Figure 5c). The linear correlation between the corneal defect depth and the globe weight was very weak (Pearson’s r = −0.08, Figure 5b). The percentage of removed cornea was independent on the paracentral corneal thickness (Pearson’s r = −0.16, Figure 5d).
Figure 5.
Correlations between the corneal defect created by inner-stopper guarded trephine with globe diameter (a), globe weight (b), and paracentral corneal thickness (c & d). The linear dependence was considered to be mild (a, c) and weak (b, d) when the absolute value of Pearson’s r was smaller than 0.4 and 0.2.
Inter personal consistency of corneal defect depth with inner-stopper guarded trephine
The related results now read as: “The dependence of the corneal defect depth through the inner-stopper guarded trephine on the experience of the users was also studied. Figure 6 showed the corneal defect depth created by four users from the same research lab. User 1 is a postdoctoral scholar with a background in Engineering, user 2 is a post-baccalaureate research associate with a background in Biology, user 3 is a medical student, and user 4 is a resident with one year of general medicine that has not yet started ophthalmologic surgical training. User 1 has been intensively trained and has practiced corneal keratectomy surgery while the other users have been trained but have had very limited experience with corneal keratectomy: user 2 had 1-month of experience, and Users 3 and 4 had no experience. With the ISGT, user1 to user4 removed 75% ± 10% (RSD = 14%), 54% ± 8% (RSD = 15%), 53% ± 11% (RSD = 21%), and 53% ± 14% (RSD = 26%) of the ex vivo rabbit cornea respectively, which corresponded to cut depths of approximately 265, 192, 190, and 188 μm.
Figure 6.
Comparison of the thickness of cut by users with different experiences in keratectomy by inner-stopper guarded trephine (ISGT). User 1 and user 2 had 6-month and 1-month experiences respectively in keratectomy. The whisks present standard deviations.
Discussion
The ability to achieve consistent stromal wound depth in a rabbit animal corneal stromal defect model through manual, trephine-assisted keratectomy would help researchers evaluate regenerative therapies, for the eventual benefit of patients at risk of or suffering from corneal blindness. With the goal of developing such an animal model, we have designed three types of modified trephines that can control the trephination depth, including a modified vacuum trephine, a custom guarded vacuum trephine, and an inner-stopper guarded trephine with diameters of 3.5-mm. We used 3D printing to make the trephine parts for the vacuum trephines.
To design the parts, CAD software was used because it allowed us to custom-design parametric parts for modelling, simulations and rapid prototyping. With Solidworks software, we could create a triangle-based mesh of the designs, and output them as stereolithography (STL) files, which is the format for the FormLabs™ 2 stereolithography 3D printer. FormLabs 2 stereolithography 3D printer prints objects upside down by submerging a platform into a tank of liquid resin. As such, generating supports for the part is a vital aspect to the success of each print. The following three step are essential to the success of the 3D printing process. First, a 45o orientation of the part is recommended in order to minimize surface area in contact with the tank. Generating a G-code is the numerical control programming language that relays how to print the part to the 3D printer. It is required in order to orchestrate the movement of the servomotors which direct the laser beam to specific areas on the build platform. In addition, creating efficient support points for the part to be fully supported in order to overcome the shear force the part will exhibit due to the contact patch between it and the tank.
The three trephines had different depth control mechanisms. The defects created by these trephines showed different degrees of perpendicularity of the defect edge and intactness of the peripheral corneal epithelium, which could affect the migration behavior and viability of adjacent intact corneal epithelial cells during corneal wound healing process. The modified vacuum trephine controlled its trephination depth by screw-guided rotation of the trephine blade. The cut depth made by the modified vacuum trephine can be adjusted by changing the number of turning cycles—this mechanism of the Hessburg-Barron trephine was left unmodified; thus, each quarter-turn of the dial is designed to go approximately 60 microns. However, given that the diameter and curvature of the rabbit cornea as well as the wound diameter being created is different, the depth calibration of the modified device was also different: each quarter-turn of the dial resulted in approximately 23 microns of cut-depth. The modified vacuum trephine can hold the cornea by uninterrupted vacuum chamber during the trephining process and therefore allows for a perfect perpendicular cut into the cornea. A drawback of the modified vacuum trephine is that it presses on the cornea and produce undesired damage to the cornea epithelium. In the rabbit, this creates a peripheral indentation near the limbus, whereas the circular cut is in the central 3.5 mm zone.
The custom guarded vacuum trephine also caused undesired damage to the peripheral corneal epithelium due to its own vacuum suction mechanism. The distance between the trephine blade and the guard determined the cut depth. Therefore, the depth by custom guarded vacuum trephine could not be tuned once the trephine was installed, which offered less flexibility in cut depth compared to the modified vacuum trephine. The custom guarded vacuum trephine created a much less perpendicular cut edge for the corneal defect compared to modified vacuum trephine. This was likely due to the free rotation of the trephine blade featured in the custom guarded vacuum trephine, unlike the screw-guided rotation.
The inner-stopper guarded trephine did not use any 3D printed parts but utilized Teflon PTFE sheets to control the cut depth. Therefore, it is the simplest and most affordable among three types of trephines. Its cut depth was determined by the distance between the blade and the inner stopper, which could be adjusted by using Teflon PTFE sheets with different thickness. Of note, spacer materials can also be 3D printed to the desired thickness and used in the same way. The defect edges by the inner-stopper guarded trephine was perpendicular to the residual bed, and the perpendicularity was between those by modified vacuum trephine and custom guarded vacuum trephine. Moreover, the inner-stopper guarded trephine did not create any damage to the peripheral corneal because the stopper contacted only the to-be-removed corneal epithelium within the inner diameter of the trephine, and there is no vacuum mechanism that grasps the peripheral epithelium.
The inner-stopper guarded trephine showed the highest consistency in defect depth (Figure 7 a). The corneal defect depth consistency from low to high was provided by the custom guarded vacuum trephine (RSD: 34%, std. dev.: 51 μm), modified vacuum trephine (RSD: 25%, std. dev.: 45 μm), and inner-stopper guarded trephine (RSD: 14%, std. dev.: 37 μm). The defect depth by inner-stopper guarded trephine was the most precise as it had the lowest perforation ratio (Figure 7 b) at a much higher thickness of cut than the other two trephines.
Figure 7.
Comparison of the three trephine designs on their performance in creating consistent wound defect depth. (a) Wound defect depth and (b) perforation ratio by the three trephines. MVT: modified vacuum trephine; CGVT: custom guard vacuum trephine; ISGT: inner-stopper guarded trephine.
The in vivo and ex vivo anterior lamellar keratectomies by the same surgeon showed significant differences in cut depth, which could be due to that the ex vivo eye’s characteristics (such as intraocular pressure and tear film) and corneal mechanical properties differ from in vivo situations. Nevertheless, the unperforated in vivo keratectomies by the ISGT (average cut = 225 ± 29 μm, percentage of cut = 62% ± 8%, RSD: 13%) were slightly more precise than ex vivo keratectomies (average cut = 265 ± 37 μm, percentage of cut = 75% ± 10%, RSD: 14%). Of note, the corneal perforation was likely because that the ISGT was pressed too hard on the cornea. To avoid perforation, the user should gently press the ISGT against the cornea and then repeatedly rotate the ISGT on the cornea alternatively between clockwise and counter-clockwise without indenting the cornea until the blade stops cutting. The cut depth of the ISGT can be marked outside the trephine blade to help users monitor progress.
The highest consistency in defect depth by inner-stopper guarded trephine was likely because it did not use vacuum. The vacuum decided the degree of “lifting” of the cornea and thus changed the depth at the time of cutting. While vacuum strength differences could be decreased by using the same syringe and same volume, it was hard to control the contact between the cornea and vacuum chamber as well as the normal forces on the cornea before the vacuum formation. The inner-stopper guarded trephine did not use vacuum and therefore excluded the variance caused by vacuum. The inner-stopper guarded trephine would only cut how much was left by the inner-stopper and therefore, the defect depth was almost independent on the globe size including the diameter, weight, and central corneal thickness.
The cut depth of the inner-stopper guarded trephine was user-dependent. The cut-depth was designed to be 254 μm between the blade and the inner stopper. The more experienced user created an average depth of 265 μm with a 5% perforation ratio; the less experienced operators created an average depth of approximately 190 μm with a 0% perforation ratio. The corneal wound depth by the more experienced user was significantly higher than that by the less experienced user. The difference was likely due to the precautions used while performing keratectomies: the more experienced user was more aggressive in terms of cut depth and had a higher perforation ratio than the less experienced users. Moreover, increased practice time decreased the RSD of the cut depth.
The guarded trephine concept has translational potential in a number of ways. First, it is a low-cost, simple, and reproducible way for pre-clinical researchers to perform partial thickness keratectomies on animal eyes. This is important because there are currently no vacuum trephines available designed specifically for animal corneas, which are smaller than human corneas. Moreover, OCTs and lasers dedicated to animal use are not always available to researchers due to their cost. To fill this need for ways to reproducibly create corneal stromal wounds in animal eyes, we have described here a “do-it-yourself” (DIY) stopper element that can be added to an existing trephine or biopsy punch at a specific distance between the trephine blade edge and a stopper can help create a cut with the desired depth. Future iterations of this can include an outer stopper as well which may further improve consistency. The device concept discussed here is analogous to guarded blades used for deep, sub-total corneal incisions such as those used for astigmatic keratotomies. With the emergence of extracellular matrix therapies and lamellar biosynthetic grafts as promising new ways of addressing corneal blindness, simple, low-cost ways of excising scarred corneal stroma could also be useful in under-resourced settings for human patients as well.
Conclusions
We have designed, manufactured, and compared three modified trephines that allow lab researchers to create consistent corneal stromal defects on large animals with ex vivo eyes. All trephines could be easily made in research labs at a low cost. The modified vacuum trephine showed the highest perpendicularity of the defect edge. The inner-stopper guarded trephine showed the highest defect depth consistency, followed by modified vacuum trephine and custom guarded vacuum trephine. Only the inner-stopper guarded trephine caused no damage to the peripheral corneal epithelium. The in vivo keratectomies done using the inner-stopper guarded trephine was slightly more precise than those done on corneas ex vivo. The inner-stopper guarded trephine is useful for creating corneal defects with consistent depth for corneal would healing studies and is particularly suitable for the researchers without substantial training and practice in performing keratectomies, which is the case for most research labs. Therefore, this tool can benefit the biomaterials research community in the development of the corneal replacement materials by making this surgical technique more accessible and reproducible at low incremental cost. Moreover, the simple design and depth consistency can also provide potential insights into low cost, guarded human trephine design. For example, using a pressure sensor in the inner stopper could help precisely control the trephination depth. This stopper design can be applied to commercially available trephines and biopsy punches, which can benefit the clinic settings to remove only the damaged part of cornea and its corresponding corneal surface. The low cost of stoppers also allows the guarded trephine concept to be used as a disposable product.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the support from the National Institutes of Health (National Eye Institute K08EY028176 and a Departmental P30EY026877 core grant), the Stanford SPARK Translational Research Grant and Maternal & Child Health Research Institute (MCHRI) (D.M.), the core grant and Career Development Award from Research to Prevent Blindness (RPB), the Matilda Ziegler Foundation, the VA Rehabilitation Research and Development Small Projects in Rehabilitation Effectiveness (SPiRE) program (I21 RX003179), and the Byers Eye Institute at Stanford. The authors also acknowledge Dr. Fernandes-Cunha, Gabriella M., Ignacio J. Blanco, Kristina Russano, Dr. Yang Hu, Dr. Liang Li, Dr. Fang Fang, and Haoliang Huang from Department of Ophthalmology at Stanford.
Footnotes
Data availability
All CAD drawings will be published and downloadable from the journal and our lab website.
Declaration of Interest
The authors report no conflicts of interest
References
- 1.Moffatt SL, Cartwright VA, Stumpf TH. Centennial Review of Corneal Transplantation. Clin Exp Ophthalmol 2005; 33(6): 642–657. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Lu C, Wang L, Chen M, White J, Hao X, McLean KM, Chen H, Hughes TC. Gelatin-Based Photocurable Hydrogels for Corneal Wound Repair. ACS Appl Mater Interfaces 2018; 10(16): 13283–13292. [DOI] [PubMed] [Google Scholar]
- 3.Xu HL, Tong MQ, Wang LF, Chen R, Li XZ, Sohawon Y, Yao Q, Xiao J, Zhao YZ. Thiolated Gamma-Polyglutamic Acid as a Bioadhesive Hydrogel-Forming Material: Evaluation of Gelation, Bioadhesive Properties and Sustained Release of Kgf in the Repair of Injured Corneas. Biomater Sci 2019; 7(6): 2582–2599. [DOI] [PubMed] [Google Scholar]
- 4.Shirzaei Sani E, Kheirkhah A, Rana D, Sun Z, Foulsham W, Sheikhi A, Khademhosseini A, Dana R, Annabi N. Sutureless Repair of Corneal Injuries Using Naturally Derived Bioadhesive Hydrogels. Sci. Adv 2019; 5(3): eaav1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes-Cunha GM, Na KS, Putra I, Lee HJ, Hull S, Cheng YC, Blanco IJ, Eslani M, Djalilian AR, Myung D. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered within a Viscoelastic Gel Carrier. Stem Cells Transl Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myung D, Farooqui N, Waters D, Schaber S, Koh W, Carrasco M, Noolandi J, Frank CW, Ta CN. Glucose-Permeable Interpenetrating Polymer Network Hydrogels for Corneal Implant Applications: A Pilot Study. Curr Eye Res 2008; 33(1): 29–43. [DOI] [PubMed] [Google Scholar]
- 7.Guarnieri D, De Capua A, Ventre M, Borzacchiello A, Pedone C, Marasco D, Ruvo M, Netti PA. Covalently Immobilized Rgd Gradient on Peg Hydrogel Scaffold Influences Cell Migration Parameters. Acta Biomater. 2010; 6(7): 2532–2539. [DOI] [PubMed] [Google Scholar]
- 8.Islam MM, Buznyk O, Reddy JC, Pasyechnikova N, Alarcon EI, Hayes S, Lewis P, Fagerholm P, He C, Iakymenko S, Liu W, Meek KM, Sangwan VS, Griffith M. Biomaterials-Enabled Cornea Regeneration in Patients at High Risk for Rejection of Donor Tissue Transplantation. NPJ Regen Med 2018; 3(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HJ, Fernandes-Cunha GM, Na K-S, Hull SM, Myung D. Bio-Orthogonally Crosslinked, in Situ Forming Corneal Stromal Tissue Substitute. Adv. Healthc. Mater 2018; 7(19): 1800560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HJ, Fernandes-Cunha GM, Myung D. In Situ-Forming Hyaluronic Acid Hydrogel through Visible Light-Induced Thiol-Ene Reaction. React. Funct. Polym 2018; 131(29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F, Le P, Fernandes-Cunha GM, Heilshorn SC, Myung D. Bio-Orthogonally Crosslinked Hyaluronate-Collagen Hydrogel for Suture-Free Corneal Defect Repair. Biomaterials 2020; 255(120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, Le P, Lai K, Fernandes-Cunha GM, Myung D. Simultaneous Interpenetrating Polymer Network of Collagen and Hyaluronic Acid as an in Situ-Forming Corneal Defect Filler. Chem. Mater 2020; 32(12): 5208–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang MC, Liu X, Jin Y, Jiang DL, Wei XS, Xie HT. Lamellar Keratoplasty Treatment of Fungal Corneal Ulcers with Acellular Porcine Corneal Stroma. Am J Transplant 2015; 15(4): 1068–1075. [DOI] [PubMed] [Google Scholar]
- 14.Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Söderqvist M, Griffith M. A Biosynthetic Alternative to Human Donor Tissue for Inducing Corneal Regeneration: 24-Month Follow-up of a Phase 1 Clinical Study. Sci Transl Med 2010; 2(46): 46ra61–46ra61. [DOI] [PubMed] [Google Scholar]
- 15.Yazdani M, Shahdadfar A, Jackson CJ, Utheim TP. Hyaluronan-Based Hydrogel Scaffolds for Limbal Stem Cell Transplantation: A Review. Cells 2019; 8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borderie VM, Werthel AL, Touzeau O, Allouch C, Boutboul S, Laroche L. Comparison of Techniques Used for Removing the Recipient Stroma in Anterior Lamellar Keratoplasty. Arch Ophthalmol 2008; 126(1): 31–37. [DOI] [PubMed] [Google Scholar]
- 17.Raghunathan VK, Thomasy SM, Strøm P, Yañez-Soto B, Garland SP, Sermeno J, Reilly CM, Murphy CJ. Tissue and Cellular Biomechanics During Corneal Wound Injury and Repair. Acta Biomater. 2017; 58(291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koulikovska M, Rafat M, Petrovski G, Veréb Z, Akhtar S, Fagerholm P, Lagali N. Enhanced Regeneration of Corneal Tissue Via a Bioengineered Collagen Construct Implanted by a Nondisruptive Surgical Technique. Tissue Eng Part A 2015; 21(5–6): 1116–1130. [DOI] [PubMed] [Google Scholar]
- 19.Doughty MJ, Zaman ML. Human Corneal Thickness and Its Impact on Intraocular Pressure Measures: A Review and Meta-Analysis Approach. Surv. Ophthalmol 2000; 44(5): 367–408. [DOI] [PubMed] [Google Scholar]
- 20.Anwar M, Teichmann KD. Big-Bubble Technique to Bare Descemet’s Membrane in Anterior Lamellar Keratoplasty. J Cataract Refract Surg 2002; 28(3): 398–403. [DOI] [PubMed] [Google Scholar]
- 21.Parthasarathy A, Por YM, Tan DTH. Use of a “Small-Bubble Technique” to Increase the Success of Anwar’s “Big-Bubble Technique” for Deep Lamellar Keratoplasty with Complete Baring of Descemet’s Membrane. Br. J. Ophthalmol 2007; 91(10): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz D, Iliev ME, Frueh BE, Goldblum D. In Vivo Pachymetry in Normal Eyes of Rats, Mice and Rabbits with the Optical Low Coherence Reflectometer. Vision Res. 2003; 43(6): 723–728. [DOI] [PubMed] [Google Scholar]
- 23.Chan T, Payor S, Holden BA. Corneal Thickness Profiles in Rabbits Using an Ultrasonic Pachometer. Invest Ophthalmol Vis Sci 1983; 24(10): 1408–1410. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.