Abstract
Phytosphingosine (PHS) is a naturally occurring bioactive sphingolipid molecule. Intermediates such as sphingolipid long‐chain bases (LCBs) in sphingolipid biosynthesis have been shown to have important roles as signaling molecules. PHS treatment caused rapid cell damage and upregulated the generation of reactive oxygen species (ROS) and ethylene in tobacco plants. These events were followed by the induction of sphingosine kinase (SphK) in a biphasic manner, which metabolized PHS to phytosphingosine‐1‐phosphate (PHS‐1‐P). On the other hand, a PHS treatment with a virulent pathogen, Phytophthora parasitica var. nicotianae (Ppn), alleviated the pathogen‐induced cell damage and reduced the growth of Ppn. A Ppn infection increased the PHS and PHS‐1‐P levels significantly in the upper part of the leaves at the infection site at the later stage. In addition, Ppn increased the transcription levels of serine palmitoyltransferase (LCB1 and LCB2) for sphingolipid biosynthesis at the later stage, which was enhanced further by PHS. Moreover, the PHS treatment increased the transcription and activity of SphK, which was accompanied by prominent increases in the transcription levels of ROS‐detoxifying enzymes and PR proteins in the later phase of the pathogen infection. Overall, the PHS‐induced resistant effects were prominent during the necrotic stage of this hemibiotrophic infection, indicating that it is more beneficial for inhibiting the pathogenicity on necrotic cell death. Phosphorylated LCBs reduced the pathogen‐induced cell damage significantly in this stage. These results suggest that the selective channeling of sphingolipids into phosphorylated forms has a pro‐survival effect on plant immunity.
Keywords: pathogenesis‐related proteins, phosphorylated phytosphingosine, phytosphingosine, reactive oxygen species, ROS‐detoxifying enzyme, sphingosine kinase
1. INTRODUCTION
Sphingolipids are essential structural components of the cellular membrane system, which includes the plasma membrane, tonoplast, and other endomembranes of plant cells that provide mechanical stability (Markham et al., 2006). It is estimated that sphingolipids constitute up to 30% of the tonoplast and plasma membrane lipids in plants (Lynch & Dunn, 2004). Further, sphingolipids are reported to participate as bioactive molecules in the regulation of intracellular processes including cell proliferation, differentiation, development, apoptosis, angiogenesis, and immunity in all eukaryotic and some prokaryotic cells (Aguilera‐Romero et al., 2013). Moreover, sphingolipid metabolites have drawn attention as second messengers for stomatal closure, programmed cell death (PCD), and defense against pathogen attack in plants (Glenz et al., 2019; Magnin‐Robert et al., 2015). Although the physiological roles of sphingolipid have been extensively studied in animals (Mashima et al., 2020), it remains unclear what mechanism is responsible for their physiological action in plants.
Long‐chain bases (LCBs) are unique building blocks in all sphingolipids including ceramide, sphingomyelin, dihydrosphingosine, and phytosphingosine (PHS). Sphingolipid biosynthesis is initiated in the endoplasmic reticulum (ER) by condensation of serine with palmitoyl‐coenzyme A catalyzed by serine palmitoyltransferase (SPT), resulting in the production of the LCB 3‐ketodihydrosphinganine, which is then reduced to dihydrosphingosine (d18:0) (Chao et al., 2011). Dihydrosphingosine is further modified to PHS (t18:0) by the introduction of a double bond into the LCB in plants and fungi (Berkey et al., 2012), as well as to sphingosine, a free form of long‐chain sphingoid base, in mammals (Mashima et al., 2020). The highest proportion of PHS, a trihydroxylated LCB, has been reported in the leaves of tobacco (Nicotiana tabacum and Nicotiana benthamiana) with large proportions of d18:2 and t18:1 (Cacas et al., 2012). Therefore, the composition of sphingolipids in plants is notably different from those in animals and fungi, in which the major LCBs are t18:1 and d18:2.
One very important sphingolipid metabolite is sphingosine‐1‐phosphate (S1P), which is formed from sphingosine by sphingosine kinase (SphK). The activity of S1P has been described in a wide spectrum of organisms, ranging from Arabidopsis thaliana through Saccharomyces cerevisiae to Homo sapiens (Bourquin et al., 2010; Michaelson et al., 2009). S1P has received attention due to its regulation of many biological processes such as cell growth and survival, proliferation, differentiation, and immune function in eukaryotes (Proia & Hla, 2015; Spiegel & Milstien, 2003). S1P functions as an intracellular second messenger and a ligand for G‐protein‐coupled cell‐surface receptors belonging to the lysophospholipid receptor family in mammals, which functions that have been implicated in cell growth and inhibition of apoptosis (Spiegel & Milstien, 2003). It was recently reported that S1P signaling via sphingosine 1‐phosphate receptor‐1 (S1PR1), a cell‐surface receptor, can enhance tumorigenesis and stimulate growth, expansion, angiogenesis, metastasis, and survival of cancer cells (Cartier et al., 2020). Therefore, potential uses of S1P signaling modulators as pharmaceutical and therapeutic targets in cancer therapy have been suggested.
The evidence for S1P as a signaling molecule in mammals has been extended to plants. S1P is involved in abscisic acid (ABA)‐mediated guard cell signal transduction in response to chilling (Dutilleul et al., 2012) in Arabidopsis. Phytosphingosine‐1‐phosphate (PHS1P) is active in stress signaling, which is mediated by the G‐protein α‐subunit in Arabidopsis (Coursol et al., 2005). More recent studies have observed that they participate as signaling molecules in the defense pathways of the hypersensitive response (HR) (Glenz et al., 2019).
The final step in the sphingolipid degradative pathway is mediated by S1P lyase (DPL1), which irreversibly converts S1P to hexadecenal and phosphoethanoamine (Magnin‐Robert et al., 2015; Nishikawa et al., 2008). It has been suggested that DPL1 silences the alarms from the immune system by removing the available S1P signaling pools. However, there is the reversible dephosphorylation of S1P back to sphingosine, which is mediated by S1P phosphatase (SPP) (Zhang et al., 2012). Recent studies showing that both DPL1 and SPP regulate LCB/LCBP homeostasis have suggested new opportunities for the use of S1P‐metabolizing enzymes as antiviral drugs by targeting the host enzymes (Wolf et al., 2019). Similar findings that modification of sphingolipid metabolite content can affect plant defense responses have been reported in Arabidopsis (Magnin‐Robert et al., 2015).
PHS is a major LCB in some plants and is involved in cell signaling. Sphingolipidomic profiling revealed that PHS accumulates as early as 1 h after pathogen inoculation with virulent and avirulent strains of Pseudomonas syringae pv. tomato in Arabidopsis leaves (Peer et al., 2010). We previously reported that PHS levels rapidly increased in susceptible tobacco (N. tabacum L. cv Wisconsin 38) plants at 1 and 48 h after shoot inoculation with the hemibiotrophic pathogen P. parasitica var. nicotianae (Ppn), which was determined by performing ultraperformance liquid chromatography‐quadrupole‐time of flight/mass spectrometry (Cho et al., 2012). In this study, we tried to establish a pathophysiological link between pathogen infection and sphingolipid metabolites such as PHS and PHS1P in tobacco plants. Further, we analyzed their contribution to plant defense.
2. RESULTS
2.1. PHS‐induced rapid responses
Although the physiological roles of sphingolipids are not fully described, several studies have indicated that they have crucial roles in the induction of apoptotic‐like cell death in plants (Berkey et al., 2012). Initially, we determined the rate of cell damage in PHS‐treated tobacco plant stems with five to six leaves, followed by photography after staining with lactophenol trypan blue. The leaves were treated with PHS for 12 h at concentrations of 1–10 μM. The results showed that the PHS treatment induced rapid cell death from 1‐μM PHS (Figure 1a). An investigation of cell damage after the PHS treatment revealed severe damage to the leaves, even at 1‐μM concentration, indicating that PHS initiates PCD through the HR response.
FIGURE 1.
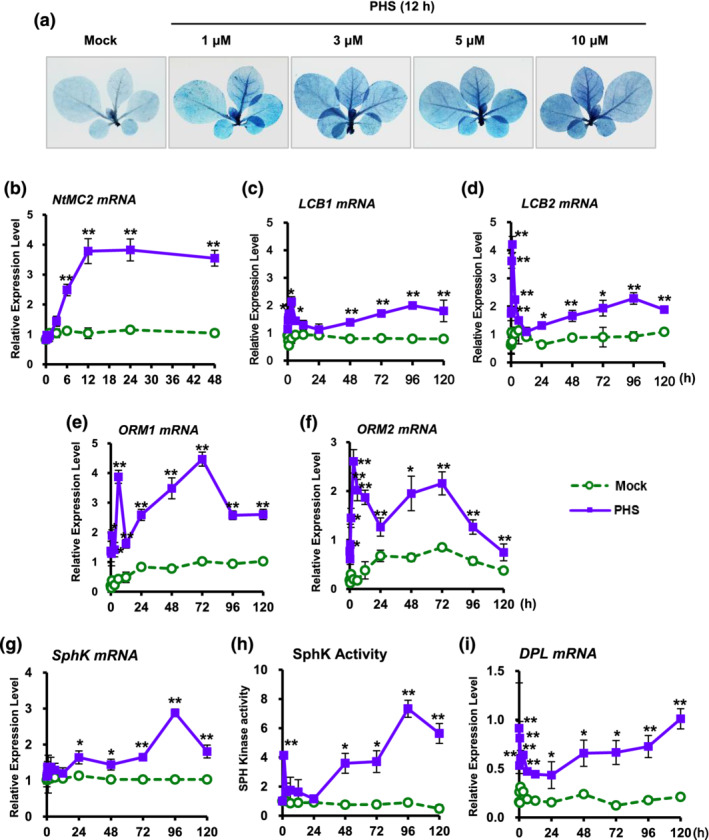
Effect of phytosphingosine (PHS) treatment on cell damage and transcription of sphingolipid biosynthesis‐related genes in tobacco leaves. (a) Mature tobacco leaves were treated with different concentrations of PHS for 12 h, and after the indicated time, necrotic areas were stained with trypan blue and then imaged with a digital camera. (b) transcription levels of tobacco NtMC2 gene in tobacco plants after PHS treatment for 48 h. results of real‐time qRT‐PCR analysis of NtMC2 transcription after 1‐μM PHS treatment using total RNAs from tobacco leaves. (c–f) Transcription levels of LCB1 (c) and LCB2 (d) of serine palmitoyltransferase and ORM1 (e) and ORM2 (f) of orosomucoid in tobacco leaves after 1‐μM PHS treatment. (g and h) Transcription level (g) and activity (h) of sphingosine kinase in tobacco leaves after 1‐μM PHS treatment. (i) Transcription levels of DPL1 of sphingosine‐1‐phoshpate lyase in tobacco leaves after 1 μM PHS treatment. Transcription levels are expressed relative to the reference gene β‐actin after real‐time qRT‐PCR. Relative mRNA expression levels are expressed as means ± SD. One asterisk (P < .05) or two asterisks (P < .01) indicate a significant difference between mock‐ and PHS‐treated cases at the same indicated time
Therefore, we investigated the expression of genes related to cell damage after treatment with 1‐μM PHS. We first determined the effects of PHS treatment on the expression of the metacaspase type II gene (MC2), which mediates biotic and abiotic stress‐induced PCD (Watanabe & Lam, 2011). Following PHS treatment, the transcription level of NtMC2 gradually increased to a maximum level at 12 h (Figure 1b), after which it decreased somewhat until 48 h. Therefore, the absence of a further increase in NtMC2 transcription after 12 h indicates that cell damage did not progress into a further severe state, which is in accordance with the PHS‐induced cell death pattern (Figure 1a). These results indicate that a rapid upregulation of NtMC2 expression is responsible for the PHS‐mediated HRs at an early stage.
We next determined whether alteration of sphingolipid biosynthesis‐related gene expression occurs after PHS treatment. LCB molecules, which are unique components of sphingolipids, are formed by condensation of serine and palmitoyl‐coenzyme A (Dietrich et al., 2008). This reaction is the first distinctive step in sphingolipid biosynthesis, which is catalyzed by the pyridoxal phosphate‐dependent enzyme SPT (EC 2.3.1.50). SPT, an ER‐associated heterodimeric protein consisting of LCB1 and LCB2 subunits, is thought to be a rate‐limiting enzyme in the sphingolipid biosynthetic pathway (Dietrich et al., 2008). It was recently reported that PHS is sufficient to induce ER stress surveillance phenotypes in budding yeast, S. cerevisiae, which in turn elevates the PHS level, suggesting the presence of feedback activation in pathogen‐induced sphingolipid biosynthesis.
Gene expression levels of LCB1 and LCB2 subunits were determined in order to elucidate the effects of PHS in sphingolipid biosynthetic pathways (Figure 1c,d). Although transcriptions of LCB1 and LCB2 were unaltered in mock‐treated control plants, transcription levels of both genes were rapidly upregulated from 15 min after PHS treatment, with higher expression of LCB2 than LCB1. PHS‐induced activation had the strongest effects on LCB1 at 3 h and on LCB2 at 1 h. The amounts of both transcripts and their abundance patterns from 24 to 120 h were similar, with second peaks occurring at 96 h after PHS treatment. The similar biphasic pattern was also detected in the transcript changes of ORM1 and ORM2 of tobacco orosomucoid (Figure 1e,f), which mediate SPT oligomerization and its subcellular localization, but may directly or indirectly inhibit its activity (Han et al., 2019). In particular, although the ORM proteins negatively regulate SPT, the regulatory mechanisms of these proteins in the sphingolipid biosynthetic pathway are unclear (Alsiyabi et al., 2021).
We next investigated the effects of PHS treatment on the transcription and activity of SphK, which catalyzes PHS to PHS1P, which is abundant in fungi and plants (Dutilleul et al., 2012). PSH1P has a fundamental role as an intracellular signaling molecule in development and stress responses in eukaryotic cells (Piña et al., 2018). Although the transcription level of SphK was immediately increased after PHS treatment, it returned to the basal level after 12 h (Figure 1g). The amount of SphK transcripts began to increase again after 24 h of PHS treatment, reaching a maximum at 96 h and then decreasing. However, no change was observed for the entire period following mock treatment.
The level of SphK activity responded to PHS treatment in a biphasic manner (Figure 1h). SphK activity began to increase rapidly and peaked at 1 h, after which it returned to the basal level. However, it increased again after 24 h and reached a peak at 96 h, similar to the maximum pattern level of the SphK transcript (Figure 1h). These results suggest that the PHS‐induced activation of SphK occurs at the transcriptional level. It is suggested that exogenously added PHS can be metabolized to PHS1P at a later stage, which is known to be involved in intracellular signaling (Coursol et al., 2005).
The final step in sphingolipid metabolism is mediated by DPL1, which degrades long‐chain base‐1‐phosphates (LCBPs) such as PHS1P and is the only reported route for the destruction of sphingolipids (Magnin‐Robert et al., 2015). The amount of DPL1 mRNA induced after PHS treatment was biphasic (Figure 1i). The amount of DPL1 mRNA rapidly increased to a peak at 0.5 h after PHS treatment; after which, it lowered to basal levels before gradually increasing up to 120 h.
2.2. NADPH oxidase‐dependent transient ROS accumulation at an early stage after PHS treatment
It has been recognized that reactive oxygen species (ROS) generation and signaling can activate HR‐related cell death in plants (Zurbriggen et al., 2010). PHS is considered a powerful contributor to oxidative damage in eukaryotic cells. To investigate ROS generation in tobacco leaves following treatment with 1‐μM PHS, we histochemically monitored the levels of two important ROS, superoxide anion and hydrogen peroxide, by using 3,3′‐diaminobenzidine (DAB) and nitrotetrazolium blue chloride (NBT) as chromogenic substrates, respectively, in tobacco leaves for up to 48 h. NBT reacts with O2˙− to form a dark blue insoluble formazan compound, and DAB is oxidized by hydrogen to produce a reddish‐brown precipitate (Kumar et al., 2014 ). Both ROS were significantly detected from 15 min after PHS treatment and abundance peaked at 1 h, suggesting the ROS were rapidly and transiently generated (Figure 2a,b). Surprisingly, only very low levels of both ROS were produced after 3 h (Figure S1). These results imply that PHS‐induced ROS generation is only HR‐relevant at an early stage.
FIGURE 2.
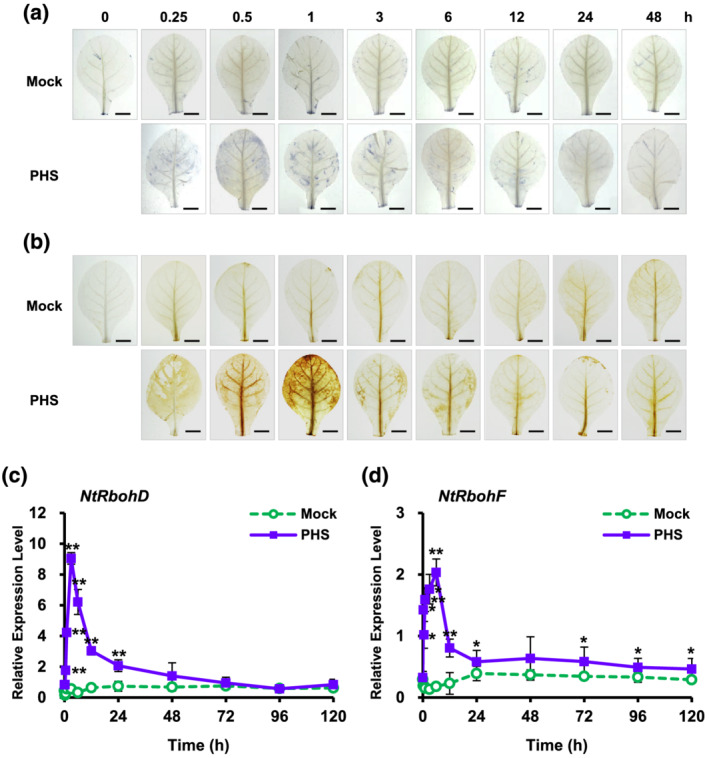
Effects of phytosphingosine (PHS) treatment on reactive oxygen species (ROS) accumulation and NADPH oxidase gene (NtRbohD and NtRbohF) transcription in tobacco leaves. (a and b) Histochemical analysis of intracellular ROS accumulation in PHS‐treated tobacco leaves. After mature tobacco leaves were treated with 1‐μM PHS for 48 h, superoxide anion level was determined by nitrotetrazolium blue chloride (NBT) staining (a), and H2O2 level was detected by 3,3′‐diaminobenzidine (DAB) staining (b). Staining images of leaves were photographed by a digital camera. (c and d) Relative mRNA levels of NtRbohD and NtRbohF genes in mature tobacco leaves treated with 1‐μM PHS. Transcription levels of NtRbohD (c) or NtRbohF (d) are expressed as means ± SD. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. An asterisk indicates a significant difference between mock‐ and PHS‐treated cases (one asterisk [P < .05] or two asterisks at the same time point [P < .01])
In aerobic organisms, ROS are produced during normal cellular metabolism as by‐products of metabolic pathways and electron flow in both mitochondria and chloroplasts. The neutrophil nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, RboH, is a transmembrane protein that generates superoxide radicals in plant cells. Its isoforms, NtRbohD and NtRbohF, are expressed throughout tobacco plants and produce superoxide anions, which are unable to permeate cell membranes under ambient pH conditions due to the presence of a negative charge.
To provide further insight into ROS accumulation in PHS‐treated tobacco leaves, we examined gene expression profiles of tobacco plants in the context of ROS production following PHS treatment. To determine the expression of NtRboh genes in PHS‐induced ROS generation, we measured the transcription levels of two NtRboh genes in tobacco leaves using real‐time quantitative reverse transcription polymerase chain reaction (qRT‐PCR) analysis. Transcription of NtRbohD and NtRbohF occurred in a time‐dependent manner; more specifically, NtRbohD and NtRbohF transcription showed monophasic kinetics upon PHS treatment, displaying early transient peaks at 3 and 6 h, respectively (Figure 2c,d). The transcription level of NtRbohD increased by about 7.4‐fold in the PHS‐treated tobacco plants compared with that in mock‐treated plants at 1 h after PHS treatment. Soon thereafter, NtRbohD transcription gradually decreased, returning to almost basal level after 48 h. The relative transcription level of NtRbohD at 1 h normalized to the expression level of β‐actin was about four times higher than that of NtRbohF at 1 h, suggesting that NtRbohD is more responsive than NtRbohF during PHS‐induced ROS generation.
2.3. PHS‐induced ethylene production is dependent on HR‐related NtACS2 and NtACS4 expression
Ethylene is implicated as a virulence factor of pathogens as well as a signaling molecule in disease resistance (Wi et al., 2012). Pathogen infection was shown to induce typical responses, including biphasic production of ROS and ethylene, in which synergism between ROS and ethylene constitutes an important regulatory mechanism in tobacco leaves (Wi et al., 2012). Therefore, we propose that ethylene production could be a central component of a self‐amplifying loop wherein transient biphasic ROS bursts have a critical regulatory role.
To further determine whether or not ethylene functions as a physiological amplifier in PHS‐induced cell death, we measured ethylene production after PHS treatment. Monophasic ethylene production was observed in tobacco leaves after PHS treatment (Figure 3a), which was in accordance with the observation of monophasic ROS production (Figure 2a,b). Ethylene production rapidly increased at 30 min, reached a transient peak at about 1 h, and declined thereafter. After 30 h of PHS treatment, ethylene production had completely returned to the basal level. Taken together, the results show that PHS‐induced ROS generation is followed by PHS‐induced ethylene production, suggesting that ROS generation functions upstream of ethylene generation in response to PHS treatment.
FIGURE 3.
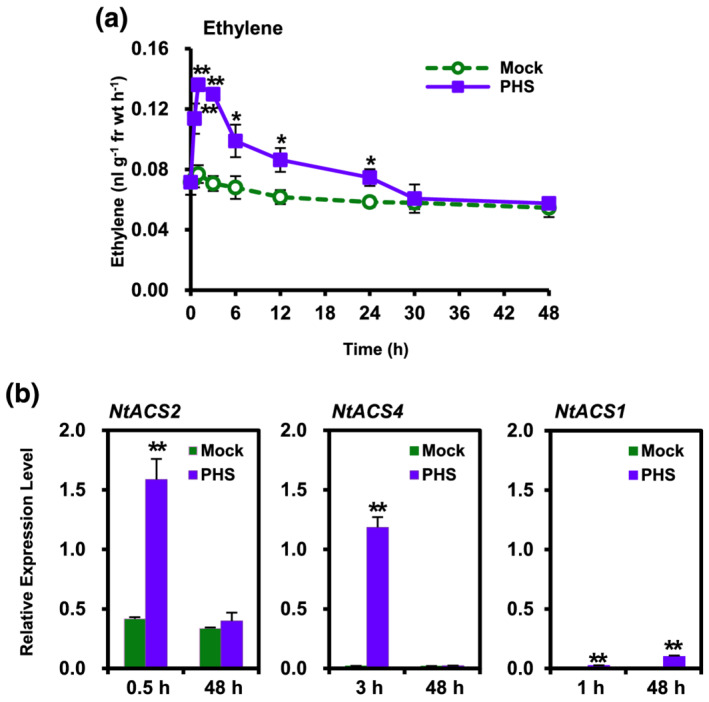
Kinetics of ethylene production and expression profiles of ACS isoforms in response to phytosphingosine (PHS) treatment. (a) Ethylene production in tobacco leaves after treatment with 1‐μM PHS. Whole leaves of tobacco plants were treated with 1‐μM PHS for 48 h. (b) Transcription levels of NtACS gene family members NtACS2 (left panel), NtACS4 (middle panel) and NtACS1 (right panel) at the indicated times after treatment with 1‐μM PHS in tobacco leaves. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. Ethylene levels and relative mRNA expression levels are expressed as means ± SD. An asterisk indicates a significant difference between mock‐ and PHS‐treated cases (one asterisk [P < .05] or two asterisks [P < .01] at the same indicated time)
Abiotic stresses are known to induce ethylene production through gene‐specific expression of 1‐aminocyclopropane‐1‐carboxylic acid (ACC) synthase (ACS) members in a time‐dependent manner (Wi et al., 2010). HR‐related NtACS2 and NtACS4 expressions have been shown to induce early ethylene production, as early as after 1 h, in response to either abiotic/oxidative or biotic stress induced by the compatible pathogen Ppn, whereas necrosis‐ or senescence‐related NtACS1 expression was shown to be responsible for late‐phase ethylene production at 24 or 72 h for induction of oxidative or biotic stress, respectively (Wi et al., 2010, 2012).
In this study, we measured the transcription levels of NtACS1, NtACS2, and NtACS4 in response to PHS treatment by performing qRT‐PCR. Although NtACS2 transcription was detected at a relatively low level in mock‐treated plants at 0.5 and 48 h, it was upregulated by about 3.8‐fold at 0.5 h in PHS‐treated tobacco plants compared with that in mock‐treated plants (Figure 3b). However, it returned to the basal level at 48 h, at which time there was no significant difference in NtACS2 expression between PHS‐ and mock‐treated plants. On the other hand, NtACS4 expression was remarkably upregulated, by approximately 59‐fold, at 3 h compared with mock‐treated tobacco plants. However, HR‐related NtACS4 transcription was almost absent at 48 h in both mock‐ and PHS‐treated plants. These results are consistent with our previous finding that apoptotic‐like cell death, which was dependent on NtACS2 and NtACS4, was not further induced at 48 h after PHS treatment.
We previously reported that expression of NtACS1, a senescence‐ or necrosis‐related ACS gene member, increased starting at 30 h and peaked at 72 h after compatible pathogen inoculation, indicating NtACS1 is active in plant death during the later phase of pathogen infection and has a role different from those of NtACS2 and NtACS4 (Wi et al., 2012). PHS‐induced NtACS1 transcription was hardly increased at 1 and 48 h (Figure 3b). Therefore, based on the low levels of NtACS1 transcription and ethylene production at 48 h after PHS treatment, PHS‐induced cell death was not accompanied by senescence‐related plant death. This observation corresponds well with the monophasic production patterns of ROS and ethylene at an early stage following PHS treatment, indicating that additional necrotic cell death did not occur at a later stage (Figures 1a, 2a,b, and 3a). These results further suggest that cell death induced by PHS is related to the early massive increases in ROS production and signaling for resistance induction.
2.4. Effect of PHS on pathogen growth and cell damage under the infection of a hemibiotrophic pathogen
In order to investigate why cell damage was not serious following PHS treatment, we examined the effects of PHS on pathogenicity after infection with the hemibiotrophic pathogen P. parasitica, which induces biotrophic activity during early infection and necrotrophic activity in the later stage of colonization (Shibata et al., 2010).
PHS‐treated tobacco plants did not develop additional cell damage even until 120 h in bottles containing culture media (Figure 4a). Therefore, we first investigated the effect of cell damage in the presence of PHS at the necrotic stage up to 120 h after pathogen infection. Hemibiotrophic pathogen Ppn gradually increased cell damage in tobacco plants, whereas the PHS treatment did not promote cell damage following pathogen invasion until 120 h, compared with that in plants treated only with the pathogens (Figure 4b). Trypan blue staining results revealed that PHS treatment did not further aggravate cell damage between 3 and 120 h after pathogen infection, implying the presence of a resistance mechanism working against the pathogen infection.
FIGURE 4.
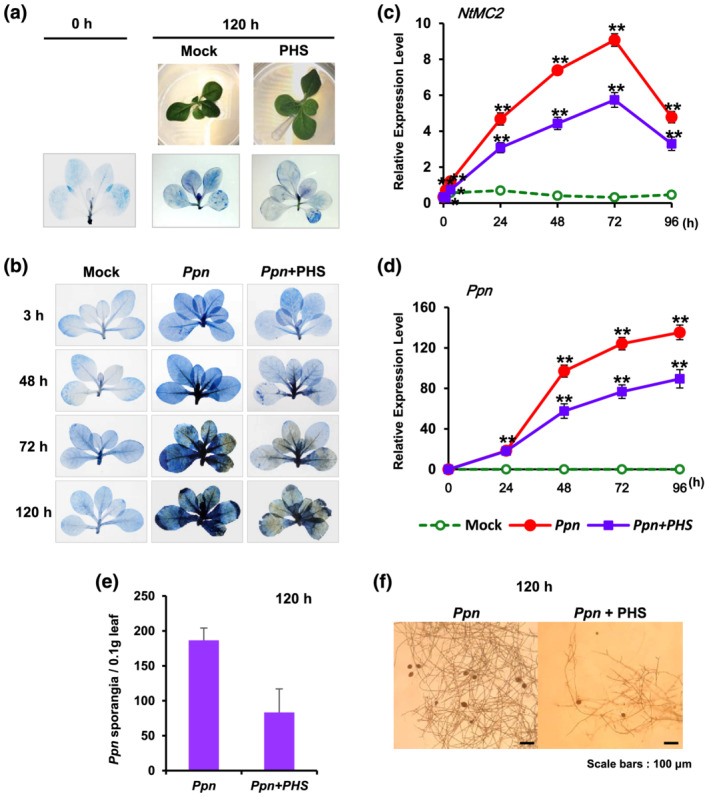
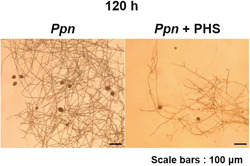
Effect of phytosphingosine (PHS) treatment on cell damage and pathogen growth in Ppn‐inoculated tobacco leaves. (a) Necrotic areas in tobacco plants were determined after 1‐μM PHS treatment using trypan blue staining and then imaged with a digital camera. Cell damage was detected after treatment with 100 μl of 1‐μM PHS was slowly dropped on the shoot area using a micropipette tip. First line: mock treatment; second line: photograph of plants treated with PHS for 120 h in culture bottles; third line: cell damages in tobacco plants after treatment with PHS after trypan blue staining. (b) Necrotic areas in Ppn‐inoculated tobacco plants with or without 1‐μM PHS treatment were determined using trypan blue staining and then imaged with a digital camera. Cell damage was detected at the indicated time after shoot inoculation with Ppn until 120 h, during which 100 μl of 1‐μM PHS. (c) Transcription levels of the tobacco NtMC2 gene in Ppn‐inoculated tobacco plants after PHS treatment for 96 h. Real‐time qRT‐PCR analysis of NtMC2 transcription was performed using total RNAs from pathogen‐infected tobacco leaves with PHS treatment. (d) Detection of Ppn in wild‐type plants after inoculation by real‐time qRT‐PCR of the 5.8S rRNA of Ppn using total RNAs from pathogen‐infected tobacco leaves with PHS treatment. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. Relative mRNA expression levels are expressed as means ± SD. Asterisks indicate a significant difference in PHS‐treated or co‐treated cases with PHS and Ppn infection from mock‐treated cases at the same time point (one asterisk [P < .05] or two asterisks [P < .01]). (e and f) Determination of the number of sporangia in Ppn‐infected tobacco leaves with or without PHS treatment. (e) The number of sporangia was counted from .1 g of tobacco leaves after 120 h of Ppn inoculation. (f) The hypha and sporangia were photographed in Ppn‐infected tobacco leaves with (right) or without (left) PHS treatment
The transcription level of metacaspase 2 in N. tabacum (NtMC2), which is a fundamental part of the cell death machinery in response to abiotic and biotic stresses (Watanabe & Lam, 2011), was significantly induced, reaching a peak at 72 h after Ppn inoculation. PHS significantly inhibited Ppn‐induced transcription of NtMC2, suggesting that PHS contributes to reducing the pro‐cell death role of metacaspase (Figure 4c).
Real‐time qRT‐PCR is a reliable technique for the detection and quantification of plant pathogens, and it is increasingly being used in plant pathology investigations. We previously reported that qRT‐PCR was a highly sensitive method for the quantification of Ppn in plants (Wi et al., 2012). We designed primers specific to 5.8S rRNA (GenBank AY769953) as an internal control for monitoring Ppn growth. Using qRT‐PCR, expression of the 5.8S rRNA gene was compared with that of tobacco β‐actin, which was used as the internal plant reference gene. Pathogen growth was continuous until 96 h, revealing an S‐shaped growth curve after Ppn inoculation, which was significantly inhibited by PHS treatment; moreover, the inhibitory effect was more prominent during the necrotrophic stage (Figure 4d).
Next, we determined the numbers of sporangia in tobacco leaves after Ppn infection with or without PHS. After 120 h of Ppn infection, PHS significantly reduced the numbers of Ppn‐produced sporangia (Figure 4e). In addition, PHS significantly inhibited the growth of mycelium with sporangia from Ppn inoculates in tobacco leaves (Figure 4f). Taken together, the results are in agreement with the observed abrogation of cell damage and pathogen growth upon co‐treatment with pathogen and PHS, suggesting the presence of PHS‐related pathogen resistance.
2.5. Induced resistance by the treatment with PHS and PHS1P under virulent pathogen infection
Although PHS caused rapid cell death, PHS exhibited pathogen resistance at the necrotic stage, which was the later stage of a Ppn infection, but the reason for this is unclear. This study examined the significant increases in the transcript level and enzyme activity of SphK at 96 h after a PHS treatment (Figure 1g,h). Therefore, under a Ppn infection, the sphingolipids, particularly PHS and PHS1P, were analyzed quantitatively by ultraperformance liquid chromatography‐tandem mass spectrometry (UPLC‐MS/MS). As expected, the levels of PHS and PHS1P were significantly higher in the necrotic stage after 96 h with Ppn infection than in the early stage (0 and 1 h) (Figure 5).
FIGURE 5.
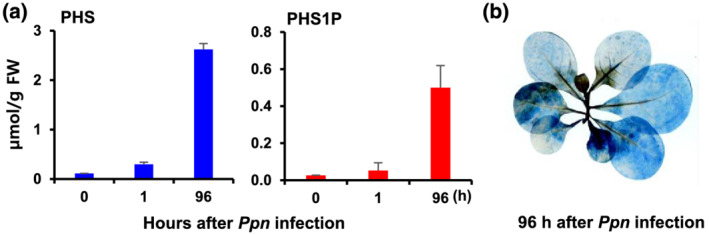
Contents of phytosphingosine (PHS) and phytosphingosine‐1‐phosphate (PHS1P) and necrosis in tobacco leaves in Ppn‐inoculated tobacco leaves. (a) Amount of PHS and PHS1P produced by the upper third and fourth leaves of the tobacco plants at 1 and 96 h after inoculating the stem with Ppn. (b) Ppn‐inoculated tobacco plants determined using trypan blue staining and imaging with a digital camera
The LCBPs treatment was performed to examine the effects on Ppn‐induced cell damage. LCBPs were treated with 1 μM of S1P or PHS1P and Ppn pathogens on tobacco plant stems with approximately five leaves. Both the S1P and PHS1P treatment inhibited cell damage effectively in plants at 24 and 72 h after a pathogen infection (Figure 6). In addition, although the PHS treatment was at a relatively weak level, it still effectively reduced Ppn‐induced cell damage. Despite the administration of PHS1P and S1P at the base of the stem, even the leaves at the top of the stem showed remarkably weak cell damage. These results suggest that PHS1P and S1P can induce SAR, increasing the resistance of the whole plant. Overall, these observations suggest that sphingolipids might be involved in preventing necrosis at the necrotic stage.
FIGURE 6.
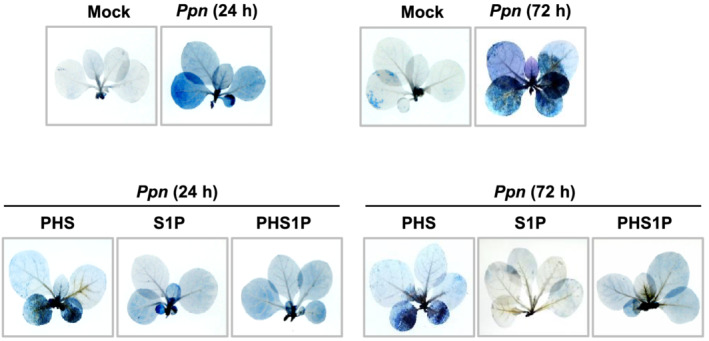
Effect of sphingosine‐1‐phosphate (S1P) and phytosphingosine‐1‐phosphate (PHS1P) treatment on cell damage in Ppn‐inoculated tobacco leaves. Necrotic areas in Ppn‐inoculated tobacco plants were determined after 1 μM of S1P or PHS1P treatment by using trypan blue staining and imaging with a digital camera. Cell damage was detected after shoot inoculation with Ppn for 24 and 72 h, during which 100 μl of 1‐μM PHS was slowly dropped on the shoot area using a micropipette tip
SphK also phosphorylates sphingosine and other sphingoid LCBs. We next determined the transcription level of SphK after PHS treatment and under pathogen infection by performing qRT‐PCR. The mRNA level of SphK was significantly elevated upon PHS treatment in Ppn‐inoculated tobacco plants compared with that in mock‐treated controls (Figure 7a). After PHS treatment, transcription of the SphK gene continuously increased until 96 h, and PHS was more effective from 48 to 96 h during the necrotic stage of hemibiotrophic pathogen infection.
FIGURE 7.
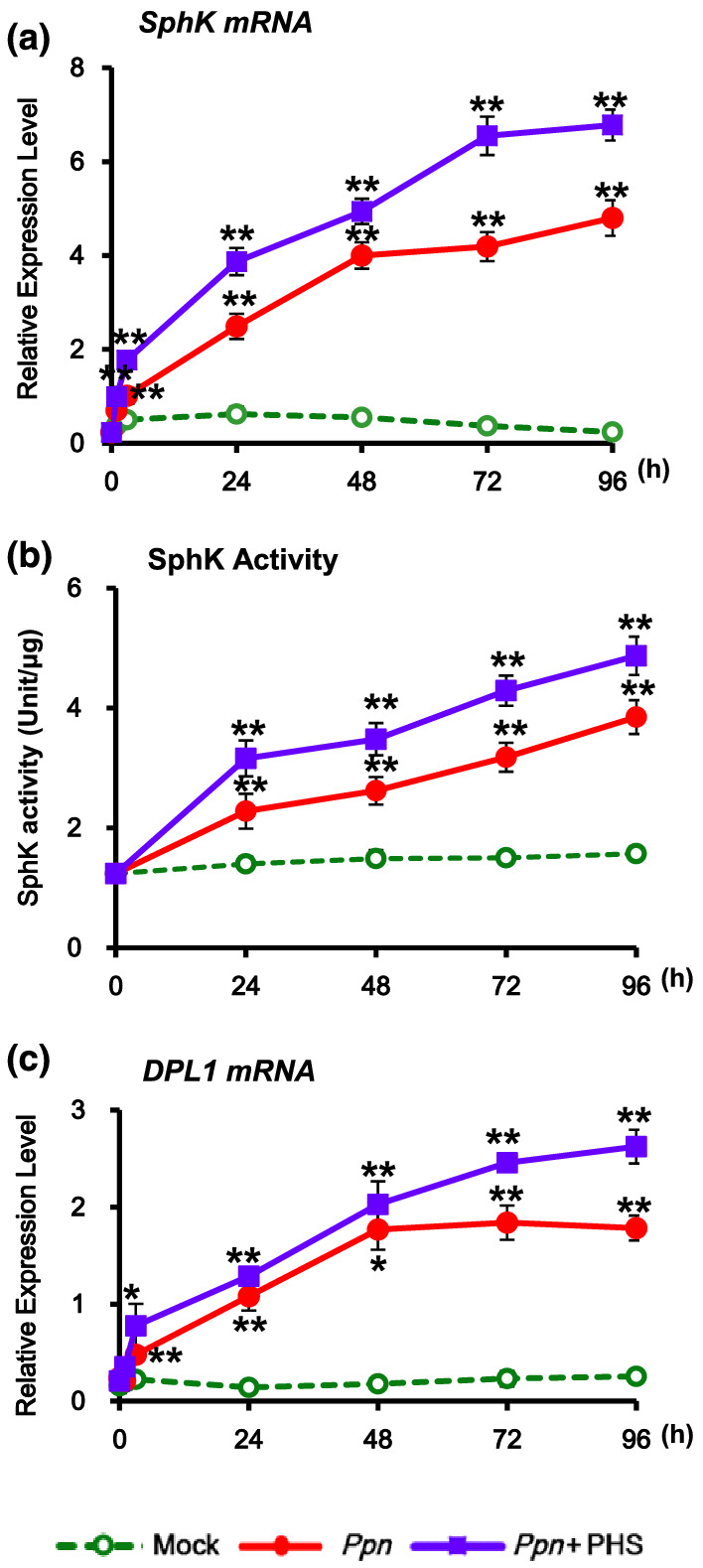
Effect of phytosphingosine (PHS) treatment on transcription of SphK and DPL1 genes and activity of sphingosine kinase (SphK). Relative transcription levels of SphK, serine palmitoyltransferase (SPT), and ceramidase in response to PHS treatment. Transcription levels of the SphK and DPL1 were determined in Ppn‐inoculated tobacco leaves after treatment with 1‐μM PHS for 96 h. (a and c) Real‐time qRT‐PCR analysis of SphK (a) and DPL1 (c) transcription was performed in total RNAs from pathogen‐infected tobacco leaves with PHS treatment. (b) The activity of SphK was determined in Ppn‐infected WT tobacco leaves with PHS treatment for 96 h. Asterisks indicate a significant difference in PHS‐treated or co‐treated cases with PHS and Ppn infection from mock‐treated cases at the same indicated time (one asterisk [P < .05] or two asterisks [P < .01])
PHS treatment further elevated the pathogen‐induced increase of SphK activity in tobacco plants during the entire treatment period, compared with that in mock‐treated leaves (Figure 7b). The increases in the patterns of SphK gene expression and enzyme activity induced by PHS treatment were similar, suggesting that they are regulated at the transcriptional stage. The observation that upregulation of SphK gene expression and SphK activity might be related to the attenuation of necrotic cell death by PHS at the both stages of Ppn treatment (24 and 72 h) (Figure 6) suggests that PHS can act as a signaling molecule against pathogen attack through the activation of SphK transcription in plants.
In accordance with the SphK activity change, DPL1 transcript of the enzyme responsible for degradation to PHS1P or S1P was more induced in PHS‐treated tobacco plants after 48 h of Ppn infection, which is during the necrotic stage of the hemibiotrophic pathogen (Figure 7c).
We next determined whether alteration of gene expressions for sphingolipid metabolism occurs after PHS treatment in Ppn‐infected tobacco plants. Although transcription of LCB1 and LCB2 was unaltered in mock‐treated control plants, transcription levels of both genes were significantly upregulated from 24 h after Ppn inoculation, with higher expression of LCB2 than LCB1. PHS treatment resulted in further significant elevations of LCB1 and LCB2 transcription during the entire period of pathogen infection; PHS‐induced activation had the strongest effect on LCB1 at 72 h and on LCB2 at 48 h (Figure 8a,b). However, ORM1 and ORM2 transcription rapidly increased after PHS treatment (Figure 8c,d), suggesting transcriptional changes may also occur in sphingolipid homeostatic regulation.
FIGURE 8.
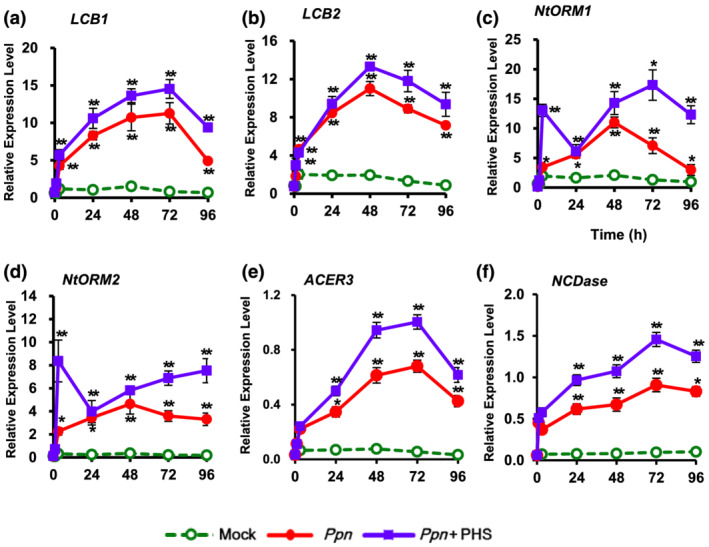
Effect of PHS treatment on transcription of sphingolipid biosynthesis‐related genes. (a and b) Transcription levels of two subunits, LCB1 (a) and LCB2 (B), of serine palmitoyltransferase (SPT) were determined for 96 h after phytosphingosine (PHS) treatment. (c and d) Transcription of two genes, ORM1 (c) and ORM2 (d), of orosomucoid were determined after PHS treatment. Real‐time qRT‐PCR analysis of each gene was performed using total RNAs from pathogen‐infected tobacco leaves with PHS treatment. (e and f) Transcription levels of ceramidases ACER3 (e) and NCDase (f) were determined for 96 h after PHS treatment. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. Relative mRNA expression levels are expressed as means ± SD. Asterisks indicate a significant difference in PHS‐treated or co‐treated cases with PHS and Ppn infection from mock‐treated cases at the same time point (one asterisk [P < .05] or two asterisks [P < .01])
We further investigated whether PHS affects the transcription of genes next to SPT in sphingolipid biosynthesis. Transcription of alkaline ceramidase (ACER3), which hydrates unsaturated ceramides, dihydroceramides, and phytoceramides to form LCBs such as PHS or sphingosine (Mashima et al., 2020), was effectively elevated, by approximately 63%, after 48 h of PHS treatment compared to that after Ppn inoculation (Figure 8e). Moreover, transcription of neutral ceramidase (NCDase), which may protect against apoptotic cell death by preventing the accumulation of ceramides in cells (Osawa et al., 2005), was elevated by about 53% at 48 h after co‐treatment with Ppn and PHS (Figure 8f). Collectively, these results suggest that PHS‐induced elevation of LCB1, LCB2, ACER3, and NCDase transcription in accordance with increased SphK expression and SphK activity can contribute to attenuation of pathogen‐induced necrotic cell death at a later stage.
2.6. PHS‐induced expression profiles of ROS‐detoxifying enzymes and PR genes under pathogen infection
Next, the effects of PHS on ROS generation were determined in tobacco plants during the necrotic stage of hemibiotrophic pathogen infection. We previously reported a second massive ROS peak indicating necrosis between 36 and 48 h in a compatible interaction with Ppn, in which pathogen growth is extensive (Wi et al., 2012). During the necrotic stage under a compatible Ppn pathogen infection, PHS treatment inhibited ROS accumulation by approximately 40% at 48 h and reduced the transcription of NtRbohD and NtRbohF in Ppn‐inoculated tobacco plants (Figures 9 and S2). This result is contrary to the PHS‐induced upregulation of NtRbohD transcription in the HR‐relevant stage at 1 h without Ppn infection (Figure 2c). In addition, PHS did not affect Ppn‐induced expression of NtACS2 and NtACS4 for ethylene production at the early stage but slightly reduce that of NtACS1 at the later stage (Figure S3).
FIGURE 9.
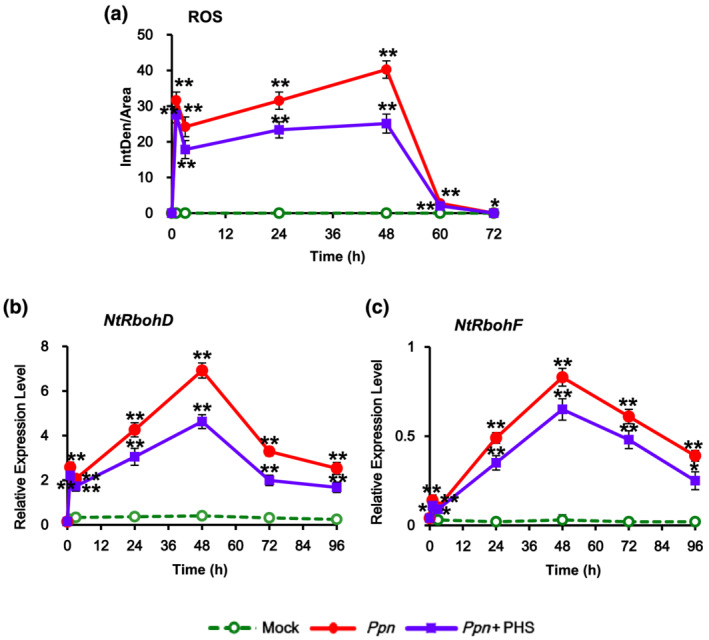
Effects of phytosphingosine (PHS) treatment on reactive oxygen species (ROS) production and NADPH oxidase gene (NtRbohD and NtRbohF) transcription in pathogen‐infected tobacco leaves. (a) After histochemical analysis of intracellular ROS accumulation in Ppn‐infected tobacco leaves after PHS treatment, the intensity of fluorescence was quantified by ImageJ software. After mature tobacco leaves were treated with 1‐μM PHS for the indicated time, ROS was determined by incubation with DCFH‐DA for 10 min. Staining images of leaves were obtained by confocal microscopy and then quantified by ImageJ software. (b and c) Relative mRNA levels of NtRbohD and NtRbohF genes in mature tobacco leaves infected with Ppn and then treated with PHS. Transcription levels of NtRbohD (b) or NtRbohF (c) are expressed as means ± SD. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. Relative mRNA expression levels are expressed as means ± SD. Asterisks indicate a significant difference in PHS‐treated or co‐treated cases with PHS and Ppn infection from mock‐treated cases at the same indicated time (one asterisk [P < .05] or two asterisks [P < .01])
ROS production at a later stage might be a byproduct or harmful substance of cell damage in the necrotic stage of compatible pathogen infection (Wi et al., 2012). Therefore, we investigated whether PHS contributes to the expression of ROS‐detoxifying enzymes during the later stage of Ppn infection. An enzymatic dismutation reaction converts superoxide into a more stable, membrane‐permeable hydrogen peroxide (H2O2) derivative, which is required for cell‐to‐cell signaling. ROS‐scavenging enzymes such as superoxide dismutase (SOD), ascorbate peroxide (APX), and catalase (CAT) provide cells with highly efficient mechanisms for detoxifying superoxide and H2O2 (Foyer & Noctor, 2005).
PHS treatment markedly upregulated the gene expression of enzymes involved in ROS‐detoxifying pathways, including mitochondrial manganese‐SOD (MnSODmi), cytosolic copper/zinc SOD (CuZnSODc), cytosolic APX (APXc), CAT (CAT1 and CAT2), and phi glutathione‐S‐transferase (GSTF) (Figure 10). Although CAT1 and CAT2 expressions were induced biphasically, expressions of other ROS‐detoxifying enzymes were significantly induced monophasically in the later stage of PHS treatment under Ppn infection. PHS treatment upregulated transcription of the SODs MnSOMmi and CuZnSODc, reaching maximum peaks at 72 and 48 h, respectively, and resulted in an elevation of APXc transcription, which peaked at 48 h for detoxification of H2O2, compared with that in only Ppn infection. Expression of GSTF, a plant‐specific phi class of stress‐induced GSTs, reached a peaked at 72 h under Ppn inoculation, which was elevated further by PHS treatment (Figure 10f). Therefore, it can be suggested that PHS activates the ROS detoxification pathway, resulting in the prevention of severe necrosis at a later stage of compatible pathogen infection. Therefore, it is suggested that PHS participates in plant survival, or rather, it prohibits further progress of cell damage in a time‐dependent manner suggests.
FIGURE 10.
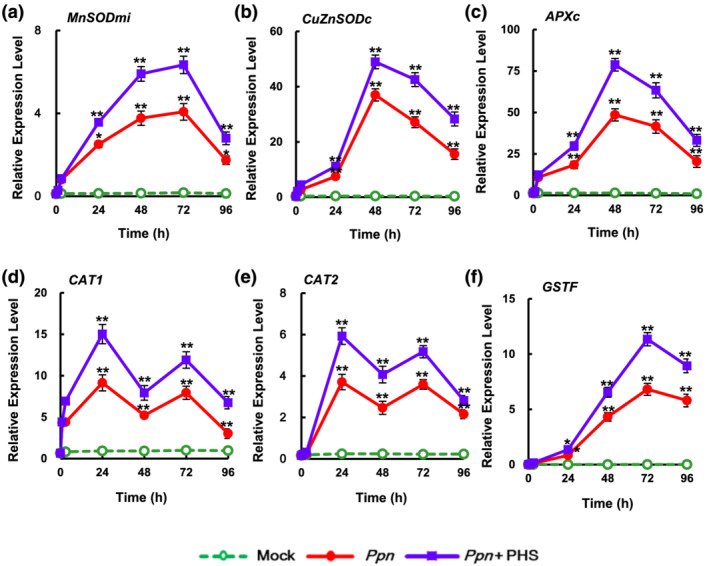
Relative transcription levels of endogenous reactive oxygen species (ROS) detoxification enzymes, CAT1, CAT2, MnSODmi, CuZnSODc, APXc, and GSTF, after 1‐μM phytosphingosine (PHS) treatment for 96 h in leaves of tobacco plants. PHS‐treated whole leaves were subjected to real‐time qRT‐PCR analysis. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. Relative mRNA expression levels are expressed as means ± SD. Asterisks indicate a significant difference in PHS‐treated or co‐treated cases with PHS and Ppn infection from mock‐treated cases at the same time point (one asterisk [P < .05] or two asterisks [P < .01])
We next investigated whether PHS can affect the expression of PR genes, which are responsible for the defense response against pathogen infection. As shown in Figure 11, PHS treatment significantly elevated mRNA levels of PR‐1a, PR‐3, PR‐4b, PR 5 (OSM), and SAR 8.2 at 48 h, as well as PR 5 and nonexpressor of PR1 (NPR1) at 72 h in pathogen‐infected tobacco plants compared with that in pathogen infection alone plants. In addition, transcription patterns of PR genes were characterized by a transient elevation followed by a gradual reduction to significantly lower levels after 96 h under the compatible pathogen infection. Transcriptions of osmotin‐like (OSM) and taumatin‐like (TLP) proteins, which belong to the PR5 family, were more markedly upregulated by PHS treatment after 48 and 72 h of Ppn inoculation, respectively, suggesting enhancement of the PHS effect on pathogen resistance. Transcription of SAR8.2, which has antifungal activity (Lee & Hwang, 2006), was most significantly elevated at 48 h after PHS treatment under pathogen infection, indicating that PHS possesses effective antifungal activity in response to fungal pathogens. These results indicate that upregulation of PR gene expression is responsible for PHS‐induced attenuation at the later stage of pathogenesis.
FIGURE 11.
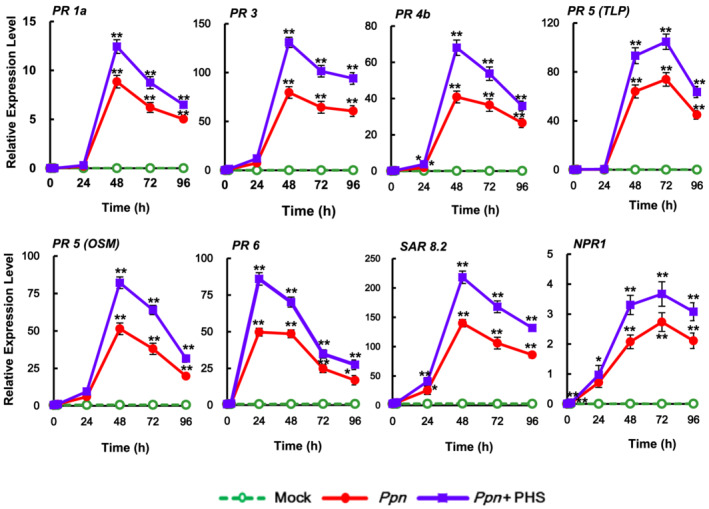
Effects of phytosphingosine (PHS) treatment on the transcription of PR genes. Relative transcription levels of PR proteins under pathogen infection in response to PHS treatment for 96 h. transcription levels of the PR genes PR‐1a, PR‐3, PR‐4b, taumatin‐like proteins (TLP), and osmotin (OSM) for PR5, PR‐6, SAR8.2, and NPR1 were determined in tobacco leaves after treatment with 1‐μM PHS. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. Relative mRNA expression levels are expressed as means ± SD. Asterisks indicate a significant difference in PHS‐treated or co‐treated cases with PHS and Ppn infection from mock‐treated cases at the same time point (one asterisk [P < .05] or two asterisks [P < .01])
3. DISCUSSION
Sphingolipids, a class of well‐known lipids in mammal cells, have been shown to have important functions in plants in recent years (Aguilera‐Romero et al., 2013). The mono‐unsaturated dihydroxy LCB (d18:1) and the saturated or mono‐unsaturated trihydroxy LCB (t18:0 and t18:1, respectively) are the main LCBs in plants (Cacas et al., 2012). Although the cis (E) and trans (Z) isomers of 4‐hydroxy‐8‐sphingenine (t18:1) comprise the major forms in Arabidopsis and tomato (Dietrich et al., 2008), PHS (t18:0) is a major LCB in tobacco plants, and there are quite similar LCB compositions in N. benthamiana and N. tabacum leaves (Cacas et al., 2012).
Sphingolipids, as bioactive molecules, have been shown to have physiological functions in the stress responses and PCD in plants (Berkey et al., 2012). Sphingolipid accumulation is an intrinsic early step in HR activation during the pathogen response. Sphingolipids derived from the fungal pathogen Magnaporthe grisea have been shown to induce phytoalexin accumulation, PCD, and resistance to infection in rice (Koga et al., 1998). It was reported that sphingolipids such as PHS and PHS1P have important roles in signal transduction pathways that regulate physiological functions and stress responses in plants, especially the immune response (Rivas‐San Vicente et al., 2013).
In N. benthamiana, sphingolipid biosynthesis is crucial during the early stages of defense responses to the non‐host pathogen Pseudomonas cichorii as well as the host pathogen P. syringae pv. tabaci (Takahashi et al., 2009). We previously reported an elevation of PHS in response to the virulent pathogen Ppn in tobacco leaves after 1 h and 48 h (Cho et al., 2012). A previous study showed that PHS is a beneficial regulator in the pathogenesis process (Berkey et al., 2012). Therefore, it is necessary to examine how PHS plays this role. It can be suggested that the rapid accumulation of sphingolipid bases such as sphingosine and PHS is an important step for the resistance response against pathogen attack. However, exactly how pathogens trigger PHS accumulation has not been described, and whether or not PHS accumulation has a role in the restriction of bacterial growth during the pathogen response also remains to be elucidated. Further, the physiological significance of PHS accumulation in pathogen‐infected plants requires explanation. On that basis, our study first focused on the pathophysiological significance of PHS.
Although PHS induced acute transient accumulation of ROS and ethylene (Figures 2 and 3), which are associated with HR‐related cell death (Figure 1a,b), the transcription and activation of SphK increased in a biphasic pattern under PHS treatment (Figure 1g,h). In the later stage of the Ppn infection, pathogen growth and pathogen‐induced cell damage were significantly suppressed by PHS treatment (Figure 4b–f). In addition, the levels of PHS and PHS1P were increased dramatically at 96 h after Ppn inoculation (Figure 5). These observations are consistent with the results showing that an exogenous treatment with PHS1P and S1P can suppress pathogen‐induced plant damage significantly (Figure 6).
A biphasic defense response against the hemibiotroph, such as Ppn, initially induces Ca2+ signaling and the ROS‐mediated pathway before 6 h, which is followed by ROS detoxification and PR gene‐related defenses from 18 to 24 h after pathogen infection (van den Berg et al., 2018; Wi et al., 2012). This suggests that these inductions of a later stage could play a key role in the resistance to hemibiotroph pathogen. Therefore, it could be suggested that these characteristics were related to the abrogation of the late phase of ROS accumulation and necrotic cell death. As PHS and its phosphorylated form have been reported to mediate pathogen resistance in several plants against necrotrophic pathogens (Magnin‐Robert et al., 2015), these results suggest that PHS might be involved in the development of protective machinery against pathogen‐induced cell death through conversion of PHS into other protective compounds such as PHS1P.
Interestingly, alteration of the balance between PHS and phosphorylated PHS is commonly known to affect sphingolipid‐mediated PCD or cell survival in plants and animals (Sánchez‐Rangel et al., 2015). These results indicate that the magnitude of SphK transcription is related to the progression of plant tolerance against pathogenicity. Moreover, PHS upregulated the expression of ROS‐detoxifying enzymes, which may be responsible for decreasing ROS levels, and the expression of PR genes at the later stage of Ppn infection (Figures 10 and 11). Elevation of PR gene expression resulted in disease resistance such as SAR in the necrotic stages of Ppn infection in PHS‐treated tobacco leaves.
A previous study reported that the PHS level increased biphasically (by 1.8‐fold at 1 h and 2.36‐fold at 48 h) (Cho et al., 2012), and the SphK activity increased continuously up to 96 h after virulent pathogen inoculation of tobacco plants (Figure 7b). In another study, the ceramide kinase mRNA level was upregulated by about fivefold at 24 h in plants infected with virulent P. syrangae compared to the level in an uninfected control (Liang et al., 2003). Collectively, these observations indicate that PHS can act as a signaling molecule by activating SphK transcription for the suppression of necrotic cell death when infected by the hemibiotrophic pathogen P. parasitica. Therefore, based on the profile of SphK transcription, increased induction of SphK is notably advantageous to plant immunity.
Interesting evidence has indicated that phosphorylated sphingosines such as S1P can act as potent bioactive lipids for the survival of cancer cells (Riccitelli et al., 2013). Other studies have shown that SphK inhibition results in apoptosis in animal xenografts (Kapitonov et al., 2009). Further, exogenous S1P supplementation can propel murine splenocytes toward a pro‐survival outcome in response to hypoxia‐induced injury (Chawla et al., 2014). Moreover, PHS1P is known to increase the cell viability of human dermal fibroblasts via the c‐Jun N‐terminal kinase/Akt pathway (Lee et al., 2012).
It has also been reported that SphK is activated by ABA in A. thaliana for the inhibition of stomatal opening and the promotion of stomatal closure, suggesting that S1P is a signaling molecule involved in ABA regulation of guard cell turgor (Coursol et al., 2005). In addition, overexpression of the rice S1P lyase gene OsDPL1 in transgenic tobacco was shown to result in reduced tolerance to salt and oxidative stresses compared to that in WT plants (Zhang et al., 2012). ROS generation induced by LCBs is specifically blocked by their phosphorylated forms, indicating that maintenance of homeostasis between a free sphingolipid base and a phosphorylated derivative is critical to determining cell fate (Shi et al., 2007).
In conclusion, these observations in this study suggest that elevated gene expressions of long‐chain sphingolipid bases for de novo synthesis of a sphingolipid is an important determinant for preventing necrotic cell death in response to pathogen attack. These observations also suggest that an elevated ratio of phosphorylated/unphosphorylated long‐chain sphingolipid bases induced by SphK may be a more significant determinant for promoting resistance during plant–pathogen interactions. Therefore, the rapid induction of PHS is beneficial for the activation of SphK and inhibition of pathogenicity in the necrotic stage of a hemibiotrophic infection, resulting in the development of SAR in plant immunity. Taken together, our results indicate that the selective channeling of sphingolipids into their phosphorylated forms in conjunction with detoxification of stress‐induced ROS has physiological pro‐survival effects related to resistance in plants exposed to biotic stresses.
4. MATERIALS AND METHODS
4.1. Plant materials, growth conditions, PHS treatment, and fungal inoculation
Surface‐sterilized seeds of tobacco (N. tabacum L. Wisconsin 38) plants were cultured on solid Murashige and Skoog (MS) medium (pH 5.8) under a light cycle (16‐h light/8‐h dark, 100 μmol photons m−2 s−1) at room temperature (25 ± 5°C). PHS was obtained from Santa Cruz Biotech (Dallas, Texas, USA). PHS1P and NBD sphingosine were purchased from Avanti Polar Lipids (Alabaster, Alabama, USA) and sphingosine‐1‐P (S1P) was purchased from Sigma‐Aldrich (St. Louis, Missouri, USA. Solutions of PHS, PHS1P, and S1P with 20‐mM MES buffer (pH 6.1) were applied to whole leaves through petioles or stems with five leaves. Tobacco shoots with five to six leaves were inoculated directly with a pathogen plug (1 cm diameter) in a culture bottle containing solid half‐strength MS medium. The mock treatment was the control group in the absence of PHS (Figures 1, 2, 3 and 4a). In addition, the mock treatment was a control tobacco shoot on the MS media plug without Ppn (Figures 4b–f and 5, 6, 7, 8, 9, 10, 11).
4.2. RNA isolation and real‐time qRT‐PCR
Total RNA isolation was performed as described previously (Seo et al., 2020). After 1 μg of total RNA from leaves was reverse transcribed using a High Fidelity PrimeScript™ RT‐PCR Kit (Takara, Japan), and real‐time qRT‐PCR was performed using a Thermal Cycler Dice® Real Time System III T950 (Takara, Japan) with gene‐specific PCR primers (Table S1). Relative expression levels in each cDNA sample were normalized to the reference gene β‐actin.
4.3. SphK activity assay
The activity of SphK was measured by performing the fluorescence‐based in vivo assay with NBD sphingosine (Pitman et al., 2017). To extract crude proteins from .3 g of tobacco leaves, frozen leaves were ground into powder and suspended in 300 μl of lysis buffer (50‐mM Tris–HCl, pH 7.4, 150‐mM NaCl, 10% glycerol [w/v], 1‐mM dithiothreitol, 2‐mM Na3VO4, 10‐mM NaF, 10‐mM β‐glycerophosphate, 1‐mM EDTA, and 0.5‐mM phenylmethylsulfonyl fluoride). Then, 200 μl of protein extracts were incubated for 1 h at 37°C with 10‐μM NBD sphingosine. The reaction was initiated by adding 10‐μM ATP in final concentration to the reaction buffer. At the end of the reaction, the reaction was stopped by adding 270 μl of chloroform/methanol/HCl (100:200:1, v/v/v). After adding 20 μl of 5‐M KCl, phase separation was made by adding 70 μl of chloroform. After spotting the lower chloroform phase onto the TLC plate, the TLC plate was developed with 1‐butanol/ethanol/glacial acetic acid/H2O (8:2:1:2) in a glass tank. The S1P spot, which has a relative migration (Rf) of approximately 0.72, was quantified after adding 600 μl of chloroform/methanol/water (5:5:1) using a fluorophotometer (excitation 460 nM, emission 534 nM). SphK activity was calculated based on the S1P spot intensity. All samples were prepared in triplicate, and the assay was repeated at least three times.
4.4. Measurement of ROS
Superoxide anion level was determined using NBT solution (.2%) in 50‐mM sodium phosphate buffer (pH 7.5), and H2O2 level was determined using DAB staining solution (1 mg/ml) in distilled water. For total ROS determination, leaf epidermal strips were peeled from tobacco leaves and were floated on a solution of 50‐μM DCFH‐DA (Sigma Chemicals, St Louis, MO, USA). The leaf stripe samples were also co‐treated with a solution consisting of 1‐μM PHS. ROS was observed by fluorescence microscopy (excitation: 450 ± 490 nm; barrier 520 ± 560 nm) equipped with a cooled CCD camera (OLYMPUS, FV300, Japan).
4.5. Ethylene measurement
Ethylene production by PHS‐treated plants was measured by gas chromatography (Hewlett Packard 5890 Series II, Wilmington, DE, USA) using an activated alumina column at 250°C and a flame ionization detector.
4.6. Trypan blue staining
To monitor plant cell death, tobacco leaves were stained as previously described (Seo et al., 2020). PHS‐treated tobacco whole leaves were immersed for 1 min in a boiling solution of 10 ml of lactic acid, 10 ml of glycerol, 10 g of phenol, and .4% (w/v) trypan blue. Stained plants were decolorized overnight and then photographed using a digital camera.
4.7. Quantitation of sporangia
Pathogen isolation from Ppn‐infected tobacco leaves was performed following a modified method from that in a previously reported protocol (Sharma & Ghosh, 2016). The pathogen‐infected tobacco leaves were sterilized outside using a 2% NaClO (v/v) solution. After sterilized leaves were flooded with phosphate‐buffered saline (PBS) at 4°C for 30 min and then incubated at 25°C for 16–20 h. After sonication, the number of sporangia was determined by using a hemocytometer.
4.8. Quantification of PHS and PHS1P in tobacco leaves
The leaves samples (100 mg) were homogenized in 300 μl of isopropyl alcohol using a Tissuelyser (Qiagen, Hilden, Germany) and then incubated at −20°C for 60 min. After centrifugation of the mixture at 15,000×g for 15 min, 5 μl of the supernatants were injected to UPLC‐MS/MS system. The chromatographic system consisted of a Waters Acquity UPLC system (Waters, Milford, USA) coupled with a quadrupole time‐of‐flight (Q‐TOF) mass spectrometer (Synapt G2‐Si, Waters, Milford, USA). The chromatographic separation was carried out on an Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm). The mobile phases (delivered at .4 ml/min) consisted of (A) 5‐mM ammonium acetate in water adjusted to pH 4 with formic acid and (B) 5‐mM ammonium acetate in water/acetonitrile (95:5, v/v). The MS analysis was performed in a positive scan mode from m/z 50 to 8,000, and the mass spectrometry parameters were set as follow: capillary voltage = 2,000 V; cone voltage = 30 V; source temperature = 110°C; desolvation temperature = 550°C; and desolvation gas flow = 900 L/h. To ensure the accuracy of the measured mass, leucine‐encephalin (m/z 556.2771 in positive mode) was used as a reference lock‐mass compound. The quantitative analysis of metabolites was performed in multiple reaction monitoring (MRM) mode. The data were processed by using MassLynx™ 4.1 software.
CONFLICT OF INTEREST
The authors declare no conflict of interest associated with the work described in this manuscript.
FUNDING INFORMATION
This work was supported by grants to K.Y.P. from the Korea Research Institute of Bioscience & Biotechnology (Project No. 2018‐0208) and the National Research Foundation of Korea (Project No. 2018‐0075).
RESPONSIBILITY OF THE AUTHOR
All authors agree on the author responsible for contact.
AUTHOR CONTRIBUTION
K.Y.P. designed and supervised the experiments; S.Y.S. and Y.J.K. performed most of the experiments; S.Y.S. performed ROS fluorescence and qRT‐PCR experiments; J.K. and M.H.N. performed the measurement of PHS and PHS1P; K.Y.P. wrote all of the article. M.H.N. participated in topic‐related discussions and agreed to serve as the author responsible for contact and communication.
Supporting information
Table S1. Primers used in this study for real‐time qRT‐PCR.
Figure S1. Effect of PHS on ROS accumulation in guard cells of tobacco leaves. A, ROS accumulation in guard cells was determined using confocal laser scanning microscopy (CLSM) after staining with 50 μM DCFH‐DA. The CLSM images of DCF fluorescence (green) were merged with the bright‐field images in the third column. Scale bars = 20 μm. B, Quantitation of DCF fluorescence intensity was calculated using ImageJ software.
Figure S2. Effect of PHS on ROS accumulation in guard cells of Ppn‐inoculated tobacco leaves. The CLSM images of DCF fluorescence (green; DCFDA staining) and chlorophyll (Chl) autofluorescence (red) were determined at the indicated time after PHS treatment under Ppn inoculation. Both CLSM images were merged in the third column while the images in the bright field were merged in the fourth column. White boxes denote nuclei. Images are representative of three independent experiments with more than ten CLSM images at each indicated time. Scale Bars = 20 μm.
Figure S3. Effect of PHS on expression profiles of ACS isoforms in Ppn‐inoculated tobacco leaves. Transcription levels of NtACS gene family members NtACS1 (left panel), NtACS2 (middle panel), and NtACS4 (right panel) at the indicated times after treatment with 1 μM PHS in Ppn‐infected tobacco leaves. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. Ethylene levels and relative mRNA expression levels are expressed as means ± SD. An asterisk indicates a significant difference in PHS‐treated cases or PHS and Ppn inoculation co‐treated cases from mock‐treated cases (one asterisk (P < .05) or two asterisks at the same time point (P < .01)).
ACKNOWLEDGMENTS
This work was supported by grants from the National Research Foundation of Korea (Project No. NRF‐2020R1AC1012652) and Korean Research Institute of Biology and Biotechnology (Project No. 2021‐0166) to K.Y.P. This Research is supported by the MSIT, Korea, under the Grand Information Technology Research Center Support Program (IITP‐2021‐0‐01489) supervised by the IITP (Institute for Information & Communication Technology Planning & Evaluation).
Seo, S. Y., Kim, Y. J., Kim, J., Nam, M. H., & Park, K. Y. (2021). Phytosphingosine induces systemic acquired resistance through activation of sphingosine kinase. Plant Direct, 5(10), e351. 10.1002/pld3.351
REFERENCES
- Aguilera‐Romero, A., Gehin, C., & Riezman, H. (2013). Sphingolipid homeostasis in the web of metabolic routes. Biochimica et Biophysica Acta, 1841, 647–656. 10.1016/j.bbalip.2013.10.014 [DOI] [PubMed] [Google Scholar]
- Alsiyabi, A., Solis, A. G., Cahoon, E. B., & Saha, R. (2021). Dissecting the regulatory roles of ORM proteins in the sphingolipid pathway of plants. PLoS Computational Biology, 17(1), e1008284. 10.1371/journal.pcbi.1008284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey, R., Bendigeri, D., & Xiao, S. (2012). Sphingolipids and plant defense/disease: The “death” connection and beyond. Frontiers in Plant Science, 3, 68. 10.3389/fpls.2012.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin, F., Riezman, H., Capitani, G., & Grütter, M. G. (2010). Structure and function of sphingosine‐1‐phosphate lyase, a key enzyme of sphingolipid metabolism. Structure, 18, 1054–1065. 10.1016/j.str.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Cacas, J. L., Melser, S., Domergue, F., Joubès, J., Bourdenx, B., Schmitter, J. M., & Mongrand, S. (2012). Rapid nanoscale quantitative analysis of plant sphingolipid long‐chain bases by GC–MS. Analytical and Bioanalytical Chemistry, 403, 2745–2755. 10.1007/s00216-012-6060-1 [DOI] [PubMed] [Google Scholar]
- Cartier, A., Leigh, T., Liu, C. H., & Hla, T. (2020). Endothelial sphingosine 1‐phosphate receptors promote vascular normalization and antitumor therapy. Proceedings of the National Academy of Sciences of the United States of America, 117, 3157–3166. 10.1073/pnas.1906246117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, D. Y., Gable, K., Chen, M., Baxter, I., Dietrich, C. R., Cahoon, E. B., Guerinot, M. L., Lahner, B., Lü, S., Markham, J. E., Morrissey, J., Han, G., Gupta, S. D., Harmon, J. M., Jaworski, J. G., Dunn, T. M., & Salt, D. E. (2011). Sphingolipids in the root play an important role in regulating the leaf ionome in Arabidopsis thaliana . Plant Cell, 23, 1061–1081. 10.1105/tpc.110.079095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla, S., Sahni, C., Tulsawani, R., Singh, M., Saraswat, D., Bansal, A., & Saxena, S. (2014). Exogenous sphingosine 1‐phosphate protects murine splenocytes against hypoxia‐induced injury. Lipids, 49, 191–202. 10.1007/s11745-013-3860-9 [DOI] [PubMed] [Google Scholar]
- Cho, K., Kim, Y., Wi, S. J., Seo, J. B., Kwon, J., Chung, J. H., Park, K. Y., & Nam, M. H. (2012). Nontargeted metabolite profiling in compatible pathogen‐inoculated tobacco (Nicotiana tabacum L. cv. Wisconsin 38) using UPLC‐Q‐TOF/MS. Journal of Agricultural and Food Chemistry, 60, 11015–11028. 10.1021/jf303702j [DOI] [PubMed] [Google Scholar]
- Coursol, S., Le Stunff, H., Lynch, D. V., Gilroy, S., Assmann, S. M., & Spiegel, S. (2005). Arabidopsis sphingosine kinase and the effects of phytosphingosine‐1‐phosphate on stomatal aperture. Plant Physiology, 137, 724–737. 10.1104/pp.104.055806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, C. R., Han, G., Chen, M., Berg, R. H., Dunn, T. M., & Cahoon, E. B. (2008). Loss‐of‐function mutations and inducible RNAi suppression of Arabidopsis LCB2 genes reveal the critical role of sphingolipids in gametophytic and sporophytic cell viability. The Plant Journal, 54, 284–298. 10.1111/j.1365-313X.2008.03420.x [DOI] [PubMed] [Google Scholar]
- Dutilleul, C., Benhassaine‐Kesri, G., Demandre, C., Rézé, N., Launay, A., Pelletier, S., Renou, J. P., Zachowski, A., Baudouin, E., & Guillas, I. (2012). Phytosphingosine‐phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling. The New Phytologist, 194, 181–191. 10.1111/j.1469-8137.2011.04017.x [DOI] [PubMed] [Google Scholar]
- Foyer, C. H., & Noctor, G. (2005). Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell, 17, 1866–1875. 10.1105/tpc.105.033589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenz, R., Schmalhaus, D., Krischke, M., Mueller, M. J., & Waller, F. (2019). Elevated levels of phosphorylated sphingobases do not antagonize sphingobase‐ or fumonisin b1‐induced plant cell death. Plant & Cell Physiology, 60, 1109–1119. 10.1093/pcp/pcz033 [DOI] [PubMed] [Google Scholar]
- Han, G., Gupta, S. D., Gable, K., Bacikova, D., Sengupta, N., Somashekarappa, N., Proia, R. L., Harmon, J. M., & Dunn, T. M. (2019). The ORMs interact with transmembrane domain 1 of Lcb1 and regulate serine palmitoyltransferase oligomerization, activity and localization. Biochimica et Biophysica Acta ‐ Molecular and Cell Biology of Lipids, 1864, 245–259. 10.1016/j.bbalip.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitonov, D., Allegood, J. C., Mitchell, C., Hait, N. C., Almenara, J. A., Adams, J. K., Zipkin, R. E., Dent, P., Kordula, T., Milstien, S., & Spiegel, S. (2009). Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Research, 69, 6915–6923. 10.1158/0008-5472.CAN-09-0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga, J., Yamauchi, T., Shimura, M., Ogawa, N., Oshima, K., Umemura, K., Kikuchi, M., & Ogasawara, N. (1998). Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. The Journal of Biological Chemistry, 273, 31985–31991. 10.1074/jbc.273.48.31985 [DOI] [PubMed] [Google Scholar]
- Kumar, D., Yusuf, M. A., Singh, P., Sardar, M., & Sarin, N. B. (2014). Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio‐protocol, 4(8), e1108. 10.21769/BioProtoc.1108 [DOI] [Google Scholar]
- Lee, J. P., Cha, H. J., Lee, K. S., Lee, K. K., Son, J. H., Kim, K. N., Lee, D. K., & An, S. (2012). Phytosphingosine‐1‐phosphate represses the hydrogen peroxide‐induced activation of c‐Jun N‐terminal kinase in human dermal fibroblasts through the phosphatidylinositol 3‐kinase/Akt pathway. Archives of Dermatological Research, 304, 673–678. 10.1007/s00403-012-1241-5 [DOI] [PubMed] [Google Scholar]
- Lee, S. C., & Hwang, B. K. (2006). CASAR82A, a pathogen‐induced pepper SAR8.2, exhibits an antifungal activity and its overexpression enhances disease resistance and stress tolerance. Plant Molecular Biology, 61, 95–109. 10.1007/s11103-005-6102-6 [DOI] [PubMed] [Google Scholar]
- Liang, H., Yao, N., Song, J. T., Luo, S., Lu, H., & Greenberg, J. T. (2003). Ceramides modulate programmed cell death in plants. Genes & Development, 17, 2636–2641. 10.1101/gad.1140503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, D. V., & Dunn, T. M. (2004). An introduction of plant sphingolipids and a review of recent advances in understanding their metabolism and function. The New Phytologist, 161, 677–702. 10.1111/j.1469-8137.2004.00992.x [DOI] [PubMed] [Google Scholar]
- Magnin‐Robert, M., Le Bourse, D., Markham, J., Dorey, S., Clément, C., Baillieul, F., & Dhondt‐Cordelier, S. (2015). Modifications of sphingolipid content affect tolerance to hemibiotrophic and necrotrophic pathogens by modulating plant defense responses in Arabidopsis. Plant Physiology, 169, 2255–2274. 10.1104/pp.15.01126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham, J. E., Li, J., Cahoon, E. B., & Jaworski, J. G. (2006). Separation and identification of major plant sphingolipid classes from leaves. The Journal of Biological Chemistry, 281, 22684–22694. 10.1074/jbc.M604050200 [DOI] [PubMed] [Google Scholar]
- Mashima, R., Okuyama, T., & Ohira, M. (2020). Biosynthesis of long chain base in sphingolipids in animals, plants and fungi. Future Science OA, 6, FSO434. 10.2144/fsoa-2019-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson, L. V., Zäuner, S., Markham, J. E., Haslam, R. P., Desikan, R., Mugford, S., Albrecht, S., Warnecke, D., Sperling, P., Heinz, E., & Napier, J. A. (2009). Functional characterization of a higher plant Sphingolipid Δ4‐Desaturase: Defining the role of sphingosine and sphingosine‐1‐phosphate in Arabidopsis . Plant Physiology, 149, 487–498. 10.1104/pp.108.129411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, M., Hosokawa, K., Ishiguro, M., Minamioka, H., Tamura, K., Hara‐Nishimura, I., Takahashi, Y., Shimazaki, K., & Imai, H. (2008). Degradation of sphingoid long‐chain base 1‐phosphates (LCB‐1Ps): Functional characterization and expression of AtDPL1 encoding LCB‐1P lyase involved in the dehydration stress response in Arabidopsis . Plant & Cell Physiology, 49, 1758–1763. 10.1093/pcp/pcn149 [DOI] [PubMed] [Google Scholar]
- Osawa, Y., Uchinami, H., Bielawski, J., Schwabe, R. F., Hannun, Y. A., & Brenner, D. A. (2005). Roles for C16‐ceramide and sphingosine 1‐phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor‐alpha. The Journal of Biological Chemistry, 280, 27879–27887. 10.1074/jbc.M503002200 [DOI] [PubMed] [Google Scholar]
- Peer, M., Stegmann, M., Mueller, M. J., & Waller, F. (2010). Pseudomonas syringae infection triggers de novo synthesis of phytosphingosine from sphinganine in Arabidopsis thaliana . FEBS Letters, 584, 4053–4056. 10.1016/j.febslet.2010.08.027 [DOI] [PubMed] [Google Scholar]
- Piña, F., Yagisawa, F., Obara, K., Gregerson, J. D., Kihara, A., & Niwa, M. (2018). Sphingolipids activate the endoplasmic reticulum stress surveillance pathway. The Journal of Cell Biology, 217, 495–505. 10.1083/jcb.201708068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman, M. R., Davies, L. T., & Pitson, S. M. (2017). An improved isoform‐selective assay for Sphingosine kinase 1 activity. Methods in Molecular Biology, 1697, 9–20. 10.1007/7651_2017_41 [DOI] [PubMed] [Google Scholar]
- Proia, R. L., & Hla, T. (2015). Emerging biology of sphingosine‐1‐phosphate: Its role in pathogenesis and therapy. The Journal of Clinical Investigation, 125, 1379–1387. 10.1172/JCI76369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccitelli, E., Giussani, P., Di Vito, C., Condomitti, G., Tringali, C., Caroli, M., Galli, R., Viani, P., & Riboni, L. (2013). Extracellular sphingosine‐1‐phosphate: A novel actor in human glioblastoma stem cell survival. PLoS ONE, 8, e68229. 10.1371/journal.pone.0068229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas‐San Vicente, M., Larios‐Zarate, G., & Plasencia, J. (2013). Disruption of sphingolipid biosynthesis in Nicotiana benthamiana activates salicylic acid‐dependent responses and compromises resistance to Alternaria alternata f. sp. lycopersici. Planta, 237, 121–136. 10.1007/s00425-012-1758-z [DOI] [PubMed] [Google Scholar]
- Sánchez‐Rangel, D., Rivas‐San Vicente, M., de la Torre‐Hernández, M. E., Nájera‐Martínez, M., & Plasencia, J. (2015). Deciphering the link between salicylic acid signaling and sphingolipid metabolism. Frontiers in Plant Science, 6, 125. 10.3389/fpls.2015.00125 eCollection 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, S. Y., Wi, S. J., & Park, K. Y. (2020). Functional switching of NPR1 between chloroplast and nucleus for adaptive response to salt stress. Scientific Reports, 10, 4339. 10.1038/s41598-020-61379-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M., & Ghosh, R. (2016). A reliable method for Phytophthora cajani isolation, sporangia, zoospore production and in planta infection of Pigeonpea. Bio‐protocol, 6(2), e1706. 10.21769/BioProtoc.1706 [DOI] [Google Scholar]
- Shi, L., Bielawski, J., Mu, J., Dong, H., Teng, C., Zhang, J., Yang, X., Tomishige, N., Hanada, K., Hannun, Y. A., & Zuo, J. (2007). Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Research, 17, 1030–1040. 10.1038/cr.2007.100 [DOI] [PubMed] [Google Scholar]
- Shibata, Y., Kawakita, K., & Takemoto, D. (2010). Age‐related resistance of Nicotiana benthamiana against hemibiotrophic pathogen Phytophthora infestans requires both ethylene‐ and salicylic acid‐mediated signaling pathways. Molecular Plant‐Microbe Interactions, 23, 1130–1142. 10.1094/MPMI-23-9-1130 [DOI] [PubMed] [Google Scholar]
- Spiegel, S., & Milstien, S. (2003). Exogenous and intracellularly generated sphingosine 1‐phosphate can regulate cellular processes by divergent pathways. Biochemical Society Transactions, 31, 1216–1219. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y., Berberich, T., Kanzaki, H., Matsumura, H., Saitoh, H., Kusano, T., & Terauchi, R. (2009). Unraveling the roles of sphingolipids in plant innate immunity. Plant Signaling & Behavior, 4, 536–538. 10.4161/psb.4.6.8583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg, N., Mahomed, W., Olivier, N. A., Swart, V., & Crampton, B. G. (2018). Transcriptome analysis of an incompatible Persea americana‐Phytophthora cinnamomi interaction reveals the involvement of SA‐ and JA‐pathways in a successful defense response. PLoS ONE, 13(10), e0205705. 10.1371/journal.pone.0205705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., & Lam, E. (2011). Arabidopsis metacaspase 2d is a positive mediator of cell death induced during biotic and abiotic stresses. The Plant Journal, 66, 969–982. 10.1111/j.1365-313X.2011.04554.x [DOI] [PubMed] [Google Scholar]
- Wi, S. J., Jang, S. J., & Park, K. Y. (2010). Inhibition of biphasic ethylene production enhances tolerance to abiotic stress by reducing the accumulation of reactive oxygen species in Nicotiana tabacum. Molecules and Cells, 30, 37–49. 10.1007/s10059-010-0086-z [DOI] [PubMed] [Google Scholar]
- Wi, S. J., Ji, N. R., & Park, K. Y. (2012). Synergistic biosynthesis of biphasic ethylene and reactive oxygen species in response to hemibiotrophic Phytophthora parasitica in tobacco plants. Plant Physiology, 159, 251–265. 10.1104/pp.112.194654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, J. J., Studstill, C. J., & Hahm, B. (2019). Emerging connections of S1P‐metabolizing enzymes with host defense and immunity during virus infections. Viruses, 11(12), E1097. 10.3390/v11121097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Zhai, J., Mo, J., Li, D., & Song, F. (2012). Overexpression of rice sphingosine‐1‐phoshpate lyase gene OsSPL1 in transgenic tobacco reduces salt and oxidative stress tolerance. Journal of Integrative Plant Biology, 54, 652–662. 10.1111/j.1744-7909.2012.01150.x [DOI] [PubMed] [Google Scholar]
- Zurbriggen, M. D., Carrillo, N., & Hajirezaei, M. R. (2010). ROS signaling in the hypersensitive response: When, where and what for? Plant Signaling & Behavior, 5, 393–396. 10.4161/psb.5.4.10793 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used in this study for real‐time qRT‐PCR.
Figure S1. Effect of PHS on ROS accumulation in guard cells of tobacco leaves. A, ROS accumulation in guard cells was determined using confocal laser scanning microscopy (CLSM) after staining with 50 μM DCFH‐DA. The CLSM images of DCF fluorescence (green) were merged with the bright‐field images in the third column. Scale bars = 20 μm. B, Quantitation of DCF fluorescence intensity was calculated using ImageJ software.
Figure S2. Effect of PHS on ROS accumulation in guard cells of Ppn‐inoculated tobacco leaves. The CLSM images of DCF fluorescence (green; DCFDA staining) and chlorophyll (Chl) autofluorescence (red) were determined at the indicated time after PHS treatment under Ppn inoculation. Both CLSM images were merged in the third column while the images in the bright field were merged in the fourth column. White boxes denote nuclei. Images are representative of three independent experiments with more than ten CLSM images at each indicated time. Scale Bars = 20 μm.
Figure S3. Effect of PHS on expression profiles of ACS isoforms in Ppn‐inoculated tobacco leaves. Transcription levels of NtACS gene family members NtACS1 (left panel), NtACS2 (middle panel), and NtACS4 (right panel) at the indicated times after treatment with 1 μM PHS in Ppn‐infected tobacco leaves. Transcription levels are expressed relative to the reference gene β‐actin after qRT‐PCR. Ethylene levels and relative mRNA expression levels are expressed as means ± SD. An asterisk indicates a significant difference in PHS‐treated cases or PHS and Ppn inoculation co‐treated cases from mock‐treated cases (one asterisk (P < .05) or two asterisks at the same time point (P < .01)).


