Abstract
Background and Aim:
Inadequate nutrition during fetal development resulting from poor dietary habits leads to reprogramming within fetal tissues and poses as a risk factor for non-communicable diseases in later life. This study was conducted to determine the dietary habits, diversity, and predictors among pregnant women in Lagos, Nigeria.
Methods:
A descriptive cross-sectional study was conducted using a structured interviewer-administered questionnaire to obtain data from pregnant women attending primary health care centers in Lagos, Nigeria. A multistage sampling method was used to select 350 pregnant women. A food frequency questionnaire was used to assess the dietary habits while dietary diversity was measured using non-quantifiable 24-hour recall. Data were analyzed using Epi-Info version 7.2 computer software. Chi-square and t-test were used to test for associations and P value < 0.05 was considered to be statistically significant.
Results:
Only 16.7% of respondents consumed five servings of fruits and vegetables daily while the rice was the most frequent meal taken (45.4%). Meat was the commonest animal protein (20.3%) and only 30.8% had a high dietary diversity score (DDS). High DDS was significantly associated with parity of 1–3, living in a duplex or detached house, completion of at least secondary school education, and highly skilled professionals.
Conclusion:
Healthy dietary habits and high DDS were low and associated with low parity and higher socio-economic status. Nutrition intervention that encourages higher dietary diversity is needed especially among women of higher parity and lower socioeconomic status in Lagos.
Keywords: Antenatal care, dietary diversity, dietary habits, predictors, pregnant women, primary health care centers
Introduction
Inadequate nutrition during crucial periods of fetal development resulting from poor dietary habits may lead to reprogramming within fetal tissues and pose as a risk factor for non-communicable diseases in later life.[1] Fetal malnutrition can result from either a surplus or deficiency in the mother. Surpluses result in overweight and complicate pregnancy and labor, leading to serious birth defects.[1] Deficiency of nutrients on the other hand increases the risk for intrauterine growth retardation (IUGR) which usually predicts early life growth curves and survival, besides age and sex.[2]
Maternal undernutrition ranges from 10% to 19% in most countries in sub-Saharan Africa, south-central, and south-eastern Asia. The situation is critical in Ethiopia with a prevalence rate of 38%.[3] The prevalence of overweight and obesity has been rising in all regions, together reaching more than 70% in the Americas and the Caribbean and more than 40% in Africa with economic development, nutritional shifts, and cultural factors often cited as drivers.[4]
According to Nigeria Demographic and Health Survey (NDHS), the prevalence of undernutrition and overnutrition among women aged 15 to 49 years in Nigeria are 11% and 25%, respectively.[5] Although the World Health Organization (WHO) and Food and Agricultural organization of the United Nations (FAO) recommend consumption of at least two servings of fruits and three servings of vegetables per day, approximately 16.0 million (1.0%) disability-adjusted life years and 2.8% of deaths worldwide (1.7 million) have been attributed to low fruits and vegetable consumption.[6] Most pregnant women in Nigeria have been shown to consume vegetables but almost half of them still avoid eggs, fish, meat, and chocolate beverage which are high in protein needed by the fetus because of taboos.[7,8] Maternal and child undernutrition is highly prevalent in low and middle-income countries, resulting in substantial increases in mortality and overall disease burden. In Nigeria, 32% of children younger than five years are stunted, 3.5% are severely wasted, 20.2% are underweight.[3] More than a third of child deaths and 11% of the total disease burden worldwide are due to maternal and child undernutrition.[3] Maternal undernutrition increases the probability of poor pregnancy outcomes and contributes to at least a fifth of maternal death globally.[9] According to World Food Program, approximately half of all pregnant women in developing countries are anemic which causes about 110,000 deaths during childbirth each year. Moreover, underweight babies from malnourished mothers are 20% more likely to die before the age of five.[10] Other causes of maternal death are all strongly associated with maternal malnutrition.[11]
Widespread global micronutrient deficiencies (MNDs) and their consequences exist with pregnant women and under 5-year-old children at the highest risk.[3,12,13,14] Consequences of maternal and child undernutrition were responsible for 2·2 million deaths and 21% of disability-adjusted life-years (DALYs) for children younger than 5 years.[3] Studies in Nigeria have shown spina bifida to constitute about 72.7% of neural tube defects diagnosed in pregnant women, perinatal mortality of 81.8%, and low birth weight (mostly due to maternal malnutrition) accounting for almost 30% of all births. Malnutrition determines survival, poverty, and development.[15,16,17] Malnutrition due to dietary habits is higher during pregnancy than at any other stage of the life cycle.[16] A pregnant woman's nutritional intake plays a major role in determining fetal health, predisposition to some diseases (more than genetic factors), and influences the fetus for the rest of its life. Hence, high dietary diversity is important as good sources of nutrients to support proper growth and development.[18]
Studies on the dietary habits and diversity of pregnant women in different regions in Nigeria showed that many pregnant women eat three meals and few eat fruits and vegetables daily, while many of them avoid egg, milk, fish, snail, and beans during pregnancy.[8,15,19] The past studies did not assess the dietary habits using the food frequency table, did not obtain any information on craving for non-nutritious items, snacking, consumption of alcohol during pregnancy, and the factors affecting the variety of foods being eaten. In Lagos, 57.7% of the women had a high dietary diversity and it was associated with employment status but the predictors of the dietary diversity were not determined.[20] This study, therefore, determined the dietary habits, diversity, and their predictors among pregnant women attending primary health care centers for antenatal care in Lagos, Nigeria. Information from the study would be useful to primary care physicians in counseling pregnant women to improve their dietary habits and diversity.
Materials and Methods
Study design
A descriptive cross-sectional study was conducted among pregnant women attending primary health care centers for antenatal care in Lagos State, Nigeria. The minimum sample size was calculated using Cochran's formula and prevalence of pregnant women with high nutritional knowledge towards good dietary practices as 65%[15] was 350.
Selection and description of participants
Multistage sampling technique was used to determine the sample. One local government area was selected using simple random sampling (by balloting), which was Surulere, and four PHCs were selected from seven by simple random sampling. In each PHC, pregnant women were recruited consecutively until the sample size of 88 for each PHC was complete to obtain 352 pregnant women.
Data collection
A semi-structured questionnaire was administered by two trained research assistants to obtain data. Pretesting of the questionnaire was done in Mushin Local government area and necessary adjustments were made to the questionnaires using the result of the pre-test.
Data processing and analysis
The data obtained were analyzed using the Epi-info 7 statistical software (version 2.2.6). The analyzed data were presented as frequency tables. Chi-square and t-tests were used to test for associations between categorical and continuous variables, respectively. Dietary diversity was scored on a 9-point scale. Those who scored between 0–3 had low dietary diversity, 4–5 had medium dietary diversity, and 6–9 had high dietary diversity.[20,21]
Ethical considerations
Research was conducted according to standard ethical standards as declared by Helsinki. Ethics approval was obtained from the Health Research and Ethics Committee of Lagos University Teaching Hospital (HREC NO: ADM/DCST/HREC/APP/274). Permission was obtained from the Chairman and the Medical Officer of Health of Surulere Local Government Area. Informed written consent was obtained from each participant and confidentiality was maintained throughout the study.
Results
The mean age of the respondents was 28.6 ± 4.9 years. The majority of them were married (88.9%) and had a tertiary level of education (58.0%). The predominant estimated monthly income was between N20,000 and N50,000.
More than half (52.0%) of the respondents ate 3 times per day, 43.1% skipped meals, and lunch (37.4%) was the most skipped meals. The majority (71.7%) ate outside their homes, only 16.7% ate five servings of fruits and vegetables daily, 5.6% drank alcohol while 38% avoided some foods or engaged in a special diet in the current pregnancy [Table 1].
Table 1.
Dietary habits of respondents
| Habits | Frequency | Percentage |
|---|---|---|
| Frequency of food consumption daily | ||
| 1-2 times | 40 | 11.4 |
| 3 times | 182 | 52.0 |
| 4 or more times | 59 | 16.9 |
| Irregular | 69 | 19.7 |
| No of days meals are skipped in a week | ||
| None | 199 | 56.9 |
| 1-2 | 78 | 22.3 |
| 3-4 | 56 | 16.0 |
| 5-6 | 16 | 4.6 |
| 7 | 1 | 0.3 |
| Skip breakfast | 111 | 31.7 |
| Skip lunch | 131 | 37.4 |
| Skip dinner | 104 | 29.7 |
| Eat in restaurants outside the home | 251 | 71.7 |
| Consume adequate servings of fruits and vegetables daily (5 and above) | 58 | 16.7 |
| Drink adequate amount of water daily | 44 | 12.7 |
| Drink alcohol daily | 20 | 5.7 |
| Eat pastry snacks | 200 | 57.1 |
| Avoiding any food or diet in the current pregnancy | 133 | 38.0 |
| Reasons for avoiding food | ||
| Religion | 4 | 1.1 |
| Taboo/culture | 6 | 1.7 |
| Dislike/discomfort | 95 | 27.1 |
| To avoid big baby | 25 | 7.1 |
| Labor difficulty | 10 | 2.9 |
| Others | 9 | 2.6 |
| Cravings for the following items | ||
| Calabar chalk | 17 | 4.9 |
| Dry ground | 22 | 6.3 |
| Cold drinks | 150 | 42.9 |
| Ice blocks | 48 | 13.7 |
| Bitter kola | 43 | 12.3 |
| Raw sweet potato | 46 | 13.1 |
| Attitude to nutrition in pregnancy | ||
| Nutrition does not matter | 72 | 20.6 |
| Pregnant women should eat less to avoid big babies | 168 | 48.0 |
| Pregnant women should eat more to have healthy babies | 251 | 71.7 |
| Pregnant women should avoid animal-based food | 124 | 35.4 |
Rice (45.6%) was the commonest cereals consumed while plantain (27.8%) and cassava (19.1%) were the main roots and tubers consumed. Animal protein intake was mostly from meat (20.3%) [Figure 1].
Figure 1.
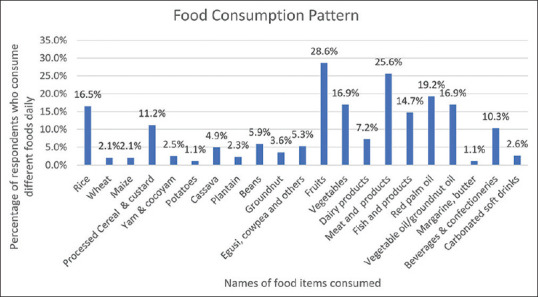
Food consumption pattern of respondents
Starchy staple (94.6%), meat, and fish (73.1%) were the most common foods consumed based on 24 hours diet recalls while the least was organ meat (26.3%). About 30.8% of the respondents had a high dietary diversity score and the means score ± SD was 5.0 ± 1.7 [Table 2, Figure 2].
Table 2.
Respondents’ food consumption on 24 h diet recall
| Food Group | Frequency | Percentage |
|---|---|---|
| Grains/Roots and Tubers | 331 | 94.6 |
| Dark green leafy vegetables | 168 | 48.0 |
| Vitamin A rich fruits and vegetables | 151 | 43.1 |
| Other fruits and vegetables | 216 | 61.7 |
| Organ meat | 92 | 26.3 |
| Meat and fish | 256 | 73.1 |
| Eggs | 161 | 46.0 |
| Legumes, nuts, and seeds | 172 | 49.1 |
| Milk and milk products | 190 | 54.3 |
Figure 2.
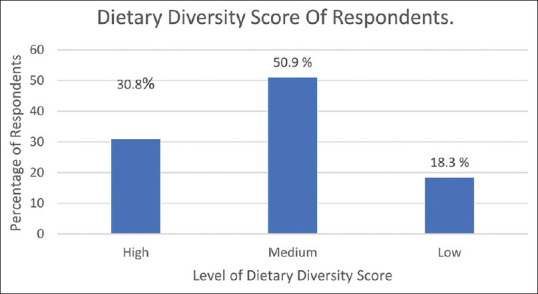
Dietary diversity score of respondents
Singles were more likely to have a higher dietary diversity score than married women. The Hausas were more likely to have a high dietary diversity score than the Yorubas or Igbos. Civil servants were more likely to have a high dietary diversity score than highly skilled professionals. Those who earn above N250,000 were more likely to have a higher score than the rest [Table 3].
Table 3.
Association between respondents’ sociodemographic characteristics and dietary diversity
| Socio-demographic characteristics | Diversity score | χ2 | df | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| High (%) | Medium (%) | Low (%) | ||||
| Marital status | ||||||
| Single | 17 (48.6) | 10 (28.6) | 8 (22.9) | 20.24 | 6 | 0.001* |
| Married | 86 (27.7) | 168 (54.2) | 56 (18.1) | |||
| Divorced | 4 (100.0) | 0 (0.0) | 0 (0.0) | |||
| Widowed | 1 (100.0) | 0 (0.0) | 0 (0.0) | |||
| Ethnicity | ||||||
| Igbo | 20 (20.8) | 53 (55.2) | 23 (24.0) | 27.11 | 6 | 0.000* |
| Yoruba | 69 (33.0) | 37 (18.7) | 39 (18.7) | |||
| Hausa | 14 (70.0) | 5 (25.0) | 1 (5.0) | |||
| Others | 5 (20.0) | 19 (76.0) | 1 (4.0) | |||
| Religion | ||||||
| Christian | 67 (27.6) | 124 (51.0) | 52 (21.4) | 16.45 | 4 | 0.004* |
| Islam | 36 (35.3) | 54 (52.9) | 12 (11.8) | |||
| Traditional | 5 (100.0) | 0 (0.0) | 0 (0.0) | |||
| Highest level of education | ||||||
| None | 8 (66.7) | 3 (25.0) | 1 (8.3) | 14.98 | 6 | 0.013* |
| Primary | 3 (30.0) | 5 (50.0) | 2 (20.0) | |||
| Secondary | 47 (37.9) | 57 (46.0) | 20 (16.1) | |||
| Tertiary | 48 (23.7) | 113 (55.9) | 41 (20.3) | |||
| Level of education (husband) | ||||||
| None | 11 (55.0) | 9 (45.0) | 0 (0.0) | 36.19 | 8 | 0.000* |
| Primary | 2 (22.0) | 2 (22.2) | 5 (55.6) | |||
| Secondary | 30 (34.9) | 41 (47.7) | 15 (17.4) | |||
| Tertiary | 44 (22.2) | 116 (58.6) | 38 (19.2) | |||
| Others | 21 (56.8) | 10 (27.0) | 0 (0.0) | |||
| Number of children | ||||||
| 0 | 29 (24.8) | 59 (50.4) | 29 (24.8) | 6.83 | 4 | 0.035* |
| 1-3 | 77 (34.2) | 115 (51.1) | 33 (14.7) | |||
| 4 and above | 2 (25.0) | 4 (50.0) | 2 (25.0) | |||
| Occupation | ||||||
| Highly skilled professional | 37 (37.0) | 47 (47.0) | 16 (16.0) | 28.67 | 8 | 0.000 |
| Civil servant | 30 (44.1) | 35 (51.5) | 3 (4.4) | |||
| Artisan | 8 (14.6) | 31 (56.4) | 16 (29.1) | |||
| Trader | 26 (28.0) | 50 (53.8) | 17 (18.3) | |||
| Unemployed | 7 (20.6) | 12 (35.3) | 12 (35.3) | |||
| Occupation of husband | ||||||
| Highly skilled professional | 32 (27.6) | 65 (56.0) | 19 (16.4) | 18.69 | 8 | 0.01* |
| Civil servant | 37 (37.4) | 48 (48.5) | 14 (14.1) | |||
| Artisan | 11 (22.9) | 29 (60.4) | 8 (16.7) | |||
| Trader | 7 (14.9) | 23 (48.9) | 17 (36.2) | |||
| Unemployed | 0 (0.0) | 2 (100.0) | 0 (0.0) | |||
| Type of residence | ||||||
| Bungalow | 12 (46.1) | 10 (38.5) | 4 (15.4) | 17.77 | 6 | 0.01 |
| Duplex detached | 13 (61.9) | 6 (28.6) | 2 (9.5) | |||
| Flat | 59 (31.4) | 95 (50.5) | 34 (18.1) | |||
| Room apartment | 24 (20.9) | 67 (58.3) | 24 (20.9) | |||
| Estimated monthly income | ||||||
| <N10,000 | 17 (30.9) | 23 (41.8) | 15 (27.3) | 19.68 | 10 | 0.008 |
| N10,000-N20,000 | 21 (30.4) | 34 (49.3) | 14 (20.3) | |||
| N20,001-N50,000 | 32 (29.4) | 59 (54.1) | 18 (16.5) | |||
| N50,001-N100,000 | 17 (28.3) | 33 (55.0) | 10 (16.7) | |||
| N100,001-N250,000 | 9 (25.0) | 25 (69.4) | 2 (5.6) | |||
| Above N250,000 | 12 (57.1) | 4 (19.1) | 5 (23.8) | |||
*Fischer’s exact
Marital status, ethnicity, and the number of children were predictors of the dietary diversity of the respondents [Table 4].
Table 4.
Predictors of dietary diversity in respondents
| P | Odd ratio | 95% C.I. for Odd ratio | ||
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Marital Status | ||||
| Single* | 0 | 0 | ||
| Married | 0.004 | 0.045 | 0.005 | 0.380 |
| Ethnicity | ||||
| Yoruba * | 0.040 | |||
| Igbo | 0.023 | 0.162 | 0.034 | 0.781 |
| Hausa | 0.011 | 0.121 | 0.024 | 0.618 |
| Religion | ||||
| Christian * | 0 | 0 | ||
| Islam | 0.099 | 0.498 | 0.217 | 1.139 |
| Level of education | ||||
| No formal | 0.146 | |||
| Primary/secondary | 0.820 | 0.733 | 0.050 | 10.662 |
| Tertiary | 0.054 | 2.231 | 0.987 | 5.040 |
| Level of education of husband | ||||
| No formal * | 0.136 | 0 | ||
| Primary/secondary | 0.073 | 9.233 | 0.810 | 105.185 |
| Tertiary | 0.405 | 0.689 | 0.287 | 1.656 |
| No. of children | ||||
| None * | 0 | |||
| 1-3 | 0.057 | 0.484 | 0.230 | 1.021 |
| Occupation | ||||
| Highly skilled * | 0.378 | |||
| Civil servant/artisan/trader | 0.825 | 1.141 | 0.353 | 3.688 |
| Unemployed | 0.280 | 1.782 | 0.625 | 5.079 |
| Occupation of husband | ||||
| Highly skilled* | 0.474 | |||
| Civil servant/artisan/trader | 0.473 | 0.433 | 0.044 | 4.276 |
| Unemployed | 0.315 | 0.309 | 0.031 | 3.055 |
| Type of residence | ||||
| Room apartment * | 0.770 | |||
| Flat | 0.791 | 0.862 | 0.288 | 2.580 |
| Bungalow and duplex | 0.835 | 1.117 | 0.394 | 3.164 |
| Estimated monthly income | ||||
| 0-20,000* | 0.176 | |||
| 21,000-100,000 | 0.092 | 0.396 | 0.135 | 1.165 |
| >100,000 | 0.482 | 0.701 | 0.260 | 1.888 |
*Reference value
Discussion
More than half of the respondents (52.0%) ate three times daily. Prevalence of at least 3 meals daily was higher than that obtained in a similar study in Odeda, Ogun state where 38% of women ate three times daily; however, other reports from Port Harcourt (60%), Imo (71%), and Kano (80%) showed higher prevalence.[8,19,22,23]
In this current study, most women avoided food because of dislike/discomfort (27.1%) and only a few did so because of religion (1.1%), cultural taboos (1.7%), and “avoiding big babies” (7.1%). This contrasts with a report from Ethiopia where the majority of the participants (74.4%) avoided food because of religion and only 13.3% did so because of dislike/discomfort.[24] Women in this study are more enlightened about the benefits of food in pregnancy and did not allow religious or cultural taboos to debar them. Foods prone to cultural taboos such as meat from wild animals chicken, eggs, and snails are rich in proteins. Avoidance of certain food(s) and the inadequate knowledge about their benefits can deprive women of adequate nutrition especially during crucial periods of pregnancy when it is highly beneficial to both mother and fetus. In a similar study in Ile-Ife where respondents reported dietary restrictions during pregnancy, most of the foods that were avoided were related to physical discomfort during gestation.[25]
Most of the respondents (56.9%) did not skip meals and the most commonly skipped meal was lunch (37.4%). In a similar study conducted in Imo State, Nigeria, most of the participants (74%) did not skip meals and lunch was also the most skipped meal.[8] Lunch is probably skipped because of the difficulty of getting food during working hours, perceived stress involved in cooking, misconceptions about weight gain, or a dietary habit acquired long before pregnancy. In contrast, reports from Northwestern Ethiopia showed that only 38.3% did not skip meals and the most commonly skipped meal was breakfast (70%).[24] The difference in the type of meal skipped could have been due to socio-economic characteristics or cultural differences.
Only 16.7% of the pregnant women consumed five or more servings of fruits and vegetables (F and V). This is similar to the report of another study in Ghana where only 3.8% take F and V daily but contrasts with report from similar studies in Ireland (76%) and India (47%).[26] Many Nigerian women might not have information about the recommended number of servings of F and V or be unable to afford the adequate amount. Unfortunately, several micronutrients, which are important for mothers' health as well as baby's growth and development are largely obtained from fruits and vegetables.
Pastry snacks usually contain high levels of trans fats, sugar, and salt which can be hazardous to the mother and developing fetus. More than half of the respondents (57.1%) ate snacks between meals. This is slightly higher than reports from Ogun state (30%) and Guto Gida Woreda, Ethiopia (40.1%) [22,27] but lower than obtained from North-western, Ethiopia where as much as 80.2% consume snacks.[24] This discrepancy is due to socio-economic differences. Studies have shown that an interaction between maternal high fat diet and the genotype during early life development can enhance the susceptibility to cardio-renal diseases later in life, hence potentiating the need to discourage or limit unhealthy snacking among pregnant women.[28]
Pica is an eating disorder characterized by a deliberate desire for substances that are largely non-nutritive such as paper, clay, metal, chalk, soil, or glass at an age when this behavior is developmentally inappropriate. Only 4.9% of the pregnant women in this study ate dry clay unlike in Cameroon where 85% of pregnant women consumed dry clay (”Calabar chalk”).[29] However, almost half (42.9%) of them craved for and consumed cold drinks similar to reports from Cameroon, Pakistan, Ethiopia, and Northern Ghana where 31%, 78%, and 47% craved unusual food items.[29,30,31] Pica and consumption of empty nutrient foods have been occasionally associated with micronutrient deficiencies. Since most women in this study did not achieve the recommended daily serving of fruits and vegetables, the chances of being anemic and having low hemoglobin relative to the general population are high.[32]
Only 5.7% consumed alcohol either as beer or wine similar to a Polish study that reported consumption of alcohol to be rare among its respondents.[1] Other Nigerian studies conducted in Imo state (19%) and Port Harcourt (20%) reported higher rates of consumption of alcohol among pregnant women.[8,19] Alcohol consumption should be discouraged especially during pregnancy because it can cause fetal alcohol syndrome in the fetus leading to irreversible mental and physical retardation.
Daily use of palm oil (19.2%) during food preparation was the major source of fat and oil and close to a quarter (23.6%) take beverages regularly. This is in contrast to the study in urban slum Lagos where the use of vegetable oil (47%) was the major source of fat and oil; however, a similar proportion of respondents (28.8%) took beverages regularly.[20] Palm oil is a rich source of bioavailable vitamin A and could be used for improving vitamin A status in pregnancy.
Rice was the most frequently consumed cereal, similar to several other studies conducted in Port Harcourt, Ogun, and Ethiopia.[19,22,27] However, whole wheat bread was the most frequently consumed cereal in an earlier study in Lagos.[20] Rice and other cereals are the most common staple foods in many regions of the world; therefore, availability could be the major reason for consuming it more than other foods.
In this current study, meat (20.3%) was the most common animal protein consumed unlike the reports from earlier studies in Lagos (76%) and Ogun (49.3%) where animal protein was majorly from fish.[20,22] Fish is a good source of omega-3 fatty acid which has been shown to reduce the risk of preterm labor in high-risk populations, prevent perinatal depression, and important for fetal brain development but respondents might not be aware of these benefits.[33]
The most common food eaten is a starchy staple (94.6%) and the least common is organ meat (26.3%). Starchy staple was also the commonest food eaten in other studies from Lagos (99.1%), South-western Bangladesh (99), Ethiopia (100), and Kenya (99.2%) and organ meats were the least.[20,21,34,35] Organ meats are rich in B vitamins and form an excellent protein source. Starchy staples (cereals and roots) are the cheapest sources of calories while cereals are the cheapest sources of protein, hence constituting the largest proportion of food intake in most developing countries.
Only 30.8% of the pregnant women had a high dietary diversity score. Similarly, low rates were recorded in Central Ethiopia (25.4%) and Pakistan (5%).[34,36] However, other studies in urban slums Lagos (57.7%) and Kenya (60.6%) reported higher rates.[20,35] Respondents in regions where low rates were recorded might not be well informed about the importance of a diverse diet in achieving nutrient adequacy, optimal growth, and development in the mother and fetus. Going out to purchase diverse foods or preparing them at home might be challenging for pregnant women; therefore, limiting them to only what is readily available.
The mean DDS of these pregnant women was 5.0 ± 1.7 SD which is much lower than the result obtained from the Kenya and Pakistan studies[35,36] although higher than the result from Bangladesh and Central Ethiopia.[21,34] Sociodemographic, socioeconomic, and seasonal variation might be the reasons for the discrepancy. Women in low and middle-income countries tend to increase their DDS during harvest season.
There was a significant relationship between ethnicity, type of residence, occupation of women, monthly income, and the dietary diversity of the women. The Hausas and Muslims had the highest dietary diversity score. This may be due to the cultural practices of the Hausas eating a wide variety of foods. Civil servants had higher DDS than the highly skilled professionals who had higher scores than the artisans, traders, and unemployed. This agrees with a study done in Kenya where the employed had the highest odds of attaining the minimum dietary diversity than the unemployed.[37] Women of higher socioeconomic status had higher dietary diversity scores compared to others. A high monthly income is associated with increased purchasing power thereby increasing the possibility of consuming a diversified diet compared to women with a low monthly income. Similar results were also obtained from Bangladesh and Kenya.[21,37]
Higher educational level was associated with higher DSS. Higher education increases the possibility of acquiring relevant information on dietary choices and practices beneficial to maternal well-being. This correlated with findings in Central Ethiopia where those who had tertiary and secondary education are more likely to achieve adequate dietary diversity.[34]
Single pregnant women were more likely to have a high dietary diversity than married women. This might be because of freedom in making a decision or spending the available money or getting multiple supports from family and friends. This was in contrast with the study in Ethiopia and Tanzania where married women had higher DDS.[34,38] Difference might be because Ethiopia and Tanzania are more developed than Nigeria and married women had more freedom, receiving support from their husbands rather than giving out.
Women who had no child were also more likely to have a high DDS. This is probably because of less burden, more time, and money available to be focused on themselves. Moreover, being first-time mothers, they might want to take their diet and nutrition more seriously compared to others. However, other studies in Pakistan, East Gojjam Zone, and Northwest Ethiopia did not show any association between parity and dietary diversity score.[36,39]
Conclusion
Healthy dietary habits and high DDS were low and associated with low parity and higher socio-economic status. Nutrition intervention that encourages higher dietary diversity is needed especially among women of higher parity and lower socioeconomic status in Lagos.
Declaration of patient consent
The authors certify that they have obtained all appropriate participant consent forms. In the form, the participants have given their consent their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Książek P, Kozłowiec J, Kozłowiec M. The nutritional knowledge of pregnant women. Pol J Public Health. 2015;124:191–4. [Google Scholar]
- 2.Alisjahbana B, Rivami DS, Octavia L, Susilawati N, Pangaribuan M, Alisjahbana A, et al. Intrauterine growth retardation (IUGR) as determinant and environment as modulator of infant mortality and morbidity: The Tanjungsari Cohort Study in Indonesia. Asia Pac J Clin Nutr. 2019;28:S17–31. doi: 10.6133/apjcn.201901_28(S1).0002. [DOI] [PubMed] [Google Scholar]
- 3.Abera SF, Kantelhardt EJ, Bezabih AM, Tsadik M, Lauvai J, Ejeta G, et al. What factors are associated with maternal undernutrition in eastern zone of Tigray, Ethiopia.Evidence for nutritional well-being of lactating mothers? BMC Public Health. 2020;20:1214. doi: 10.1186/s12889-020-09313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 5.NDHS. Nigeria Demographic and Health Survey 2013. Niger Demogr Heal Surv 2013 Natl Popul Comm Fed Repub Niger. 2013:175–199. [Google Scholar]
- 6.WHO | Unhealthy diet. Available from: https://www.who.int/gho/ncd/risk_factors/unhealthy_diet_text/en/
- 7.Adinma J, Umeononihu OS, Umeh MN. Maternal nutrition in Nigeria. Trop J Obstet Gynaecol. 2017;34:79–84. [Google Scholar]
- 8.Fasola O, Abosede O, Fasola FA. Knowledge, attitude and practice of good nutrition among women of childbearing age in Somolu Local Government, Lagos State. J Public Health Afr. 2018;9:793. doi: 10.4081/jphia.2018.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhutta ZA. Maternal Malnutrition Globally: Epidemiology and Links to Childhood Malnutrition. 2013 [Google Scholar]
- 10.Malnutrition - Mother, Infant and Young Child Nutrition & Malnutrition - Feeding practices including micronutrient deficiencies prevention, control of wasting, stunting and underweight. Available from: http://motherchildnutrition.org/malnutrition/
- 11.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: A WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 12.Bailey RL, West KP, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66:22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 13.CDC. Micronutrient Facts | IMMPaCt | CDC. 2015. [[Last accessed on 2018 Jun 06]]. Available from: http://www.cdc.gov/immpact/micronutrients/
- 14.Horino M, Bahar L, Al-Jadba G, Habash R, Akihiro S, West KP., Jr Dietary inadequacy, micronutrient deficiencies, and approaches to preventing poor nutrition in the Gaza Strip. Food Nutr Bull. 2020;41:503–11. doi: 10.1177/0379572120967819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nnadi D, Singh S. The prevalence of neural tube defects in North-West Nigeria. Saudi J Health Sci. 2016;5:6–10. [Google Scholar]
- 16.Kever RT, Martins SD, Lola N, Dathini H, Habu H, Fatima AA, et al. Knowledge and attitude of pregnant Women towards dietary practices in Yerwa Clinic, Maiduguri Metropolitan Council Borno State. Journal of Research in Nursing and Midwifery. 2015;4:12–9. [Google Scholar]
- 17.Daba G, Beyene F, Fekadu H, Garoma W. Assessment of knowledge of pregnant mothers on maternal nutrition and associated factors in Guto Gida Woreda, East Wollega Zone, Ethiopia. J Nutr Food Sci. 2013;3:235. [Google Scholar]
- 18.Diop L, Becquey E, Turowska Z, Huybregts L, Ruel MT, Gelli A. Standard minimum dietary diversity indicators for women or infants and young children are good predictors of adequate micronutrient intakes in 24-59-month-old children and their nonpregnant non-breastfeeding mothers in rural Burkina Faso. J Nutr. 2021;151:412–22. doi: 10.1093/jn/nxaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wordu GO, Akusu MO. Dietary Practices and Food Preferences of Pregnant Women in Port Harcourt Metropolis, Nigeria”. Acta Scientific Nutritional Health 4.3. 2020:45–8. [Google Scholar]
- 20.Okunaiya GA, Fadupin GT, Oladeji D. Knowledge, attitude and practice of maternal and child food-based dietary guidelines among pregnant women in urban slum of Lagos State. Clin Mother Child Health. 2016;13:240. [Google Scholar]
- 21.Shamim AA, Mashreky SR, Ferdous T, Tegenfeldt K, Roy S, Rahman AK, et al. Pregnant women diet quality and its sociodemographic determinants in Southwestern Bangladesh. Food Nutr Bull. 2016;37:14–26. doi: 10.1177/0379572116632137. [DOI] [PubMed] [Google Scholar]
- 22.Ademuyiwa MO, Sanni SA. Consumption pattern and dietary practices of pregnant women in Odeda local government area of Ogun State. World Acad Sci Eng Technol Int J Food Sci Eng. 2013;7:489–93. [Google Scholar]
- 23.Ugwa EA. Nutritional practices and taboos among pregnant women attending antenatal care at general hospital in Kano, Northwest Nigeria. Ann Med Health Sci Res. 2016;6:109–14. doi: 10.4103/2141-9248.181846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nana A, Zema T. Dietary practices and associated factors during pregnancy in northwestern Ethiopia. BMC Pregnancy Childbirth. 2018;18:183. doi: 10.1186/s12884-018-1822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oluleke MO, Ogunwale AO, Arulogun OS, Adelekan AL. Dietary intake knowledge and reasons for food restriction during pregnancy among pregnant women attending primary health care centers in Ile-Ife, Nigeria. Int J Popul Stud. 2016;2:103–16. [Google Scholar]
- 26.Appiah PK, Naa Korklu AR, Bonchel DA, Fenu GA, Wadga-Mieza Yankey F. Nutritional knowledge and dietary intake habits among pregnant adolescents attending antenatal care clinics in urban community in Ghana. J Nutr Metab. 2021;2021:8835704. doi: 10.1155/2021/8835704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daba G, Beyene F, Garoma W, Fekadu H. Assessment of nutritional practices of pregnant mothers on maternal nutrition and associated factors in Guto Gida Woreda, East Wollega Zone, Ethiopia. Sci Technol Arts Res J. 2013;2:105–13. [Google Scholar]
- 28.Kruse M, Fiallo A, Tao J, Susztak K, Amann K, Katz EB, et al. A high fat diet during pregnancy and lactation induces cardiac and renal abnormalities in GLUT4+/- male mice. Kidney Blood Press Res. 2017;42:468–82. doi: 10.1159/000479383. [DOI] [PubMed] [Google Scholar]
- 29.Nchang Mugyia AS, Kien Tanya AN, Njotang PN, Ndombo PK. Knowledge and attitudes of pregnant mothers towards maternal dietary practices during pregnancy at the Etoug-Ebe Baptist Hospital Yaounde. Health Sci Dis. 2016;17:24–9. [Google Scholar]
- 30.Qureshi Z, Khan R. Diet intake trends among pregnant women in rural area of Rawalpindi, Pakistan. J Ayub Med Coll Abbottabad. 2015;27:684–8. [PubMed] [Google Scholar]
- 31.Abubakari A, Jahn A. Maternal dietary patterns and practices and birth weight in Northern Ghana. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0162285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao D, Young SL, Golden CD. A meta-analysis of pica and micronutrient status. Am J Hum Biol. 2015;27:84–93. doi: 10.1002/ajhb.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Seymour JV, Simmonds LA, Gould J, Makrides M, Middleton P. Omega-3 fatty acids to prevent preterm birth: Australian pregnant women's preterm birth awareness and intentions to increase omega-3 fatty acid intake. Nutr J. 2019;18:74. doi: 10.1186/s12937-019-0499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melaku Desta, Mohammed Akibu, Mesfin Tadese, Meskerem Tesfaye. Dietary diversity and associated factors among pregnant women attending antenatal clinic in Shashemane, Oromia, Central Ethiopia: A cross-sectional study. J Nutr Metab. 2019;2019:3916864. doi: 10.1155/2019/3916864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willy K, Judith K, Peter C. Dietary diversity, nutrient intake and nutritional status among pregnant women in Laikipia County, Kenya. Int J Health Sci Res. 2016;6:378–85. [Google Scholar]
- 36.Ali F, Thaver I, Ali Khan S. Assessment of dietary diversity and nutritional status of pregnant women in Islamabad, Pakistan. J Ayub Med Coll Abbottabad. 2014;26:506–9. [PubMed] [Google Scholar]
- 37.Kiboi W, Kimiywe J, Chege P. Determinants of dietary diversity among pregnant women in Laikipia County, Kenya: A cross-sectional study. BMC Nutr. 2017;3:12. [Google Scholar]
- 38.Ochieng J, Victor AS, Lukumay PJ, Dubois T. Determinants of dietary diversity and the potential role of men in improving household nutrition in Tanzania. PLoS One. 2017;12:e0189022. doi: 10.1371/journal.pone.0189022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeneabat T, Adugna H, Asmamaw T, Wubetu M, Admas M, Hailu G, et al. Maternal dietary diversity and micronutrient adequacy during pregnancy and related factors in East Gojjam Zone, Northwest Ethiopia, 2016. BMC Pregnancy Childbirth. 2019;19:173. doi: 10.1186/s12884-019-2299-2. https://doi.org/10.1186/s12884-019-2299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


