Abstract
Background:
The primary objective of this study was to intervene with vitamin D supplementation in rural-based women with pre-diabetes (impaired fasting glucose or impaired glucose tolerance) to prevent development of type 2 diabetes (T2DM).
Methods:
This was an open-label randomized placebo-controlled trial conducted in rural women with pre-diabetes and vitamin D deficiency (Clinicaltrials.gov NCT02513888). Women aged 20-60 years with pre-diabetes were selected from rural Haryana (north India) and followed up for two years. A semi-structured questionnaire was used to collect information on socio-demographic and behavioral details, like sun exposure, dietary habits, etc., The intervention group received vitamin D supplementation while control group received lactose granules as placebo. Equal doses of calcium carbonate were given to both the groups.
Results:
A total of 132 participants were recruited in the study (58 each in the intervention and control groups). It was observed that there was no statistical significance in the incidence of diabetes in the control group as compared to the intervention group at the end of 2 years (P = 0.701).
Conclusion:
Though during the first year there was some delay in development of DM in the intervention group but at the end of two years there was no significant effect of vitamin D supplementation in delaying the incidence of diabetes in these women after two years.
Trial registration:
(Clinicaltrials.gov NCT02513888).
Keywords: Community based RCT, diabetes, India, prediabetes, rural women, vitamin D.
Introduction
Vitamin D deficiency (VDD) is increasingly becoming a global health problem and is regarded as one of the most underdiagnosed and undertreated nutritional deficiencies in the world.[1] The VDD affects over 1 billion people worldwide.[2] Vitamin D, also known as the sunshine vitamin, is necessary for a lot of physiological functions and not just its role in the musculoskeletal system as previously believed.[3] The Indian subcontinent has adequate sunshine and ultraviolet rays for the production of vitamin D, so Asian Indians are presumed to have adequate levels of vitamin D. But nowadays, majority of the urban population (and some of the rural population) spend time indoors which results in minimum sun exposure.[4] According to the studies conducted in India, the prevalence of vitamin D deficiency ranges from 70% to 100%.[5] A baseline cross-sectional study conducted in Ballabgarh among the participants recruited for this trial showed the prevalence of vitamin D deficiency to be 91%.[6]
Vitamin D deficiency affects the body in many ways including some effects on diabetes as well.[7] Insulin resistance is the inability of the body to utilize insulin which can result in a higher risk of developing type 2 diabetes mellitus (T2DM). Various clinical and experimental studies have shown a relationship between serum 25 (OH) D and insulin sensitivity, hence, proving the role of vitamin D in preventing diabetes.[8,9,10] Vitamin D is essential for insulin synthesis and secretion from pancreatic B-cells.[11] A meta-analysis of the longitudinal observational studies showed 38% risk reduction in the incidence of diabetes in a population with a higher 25 (OH) D concentration.[12] The information from the longitudinal studies among a group with prediabetes is inconclusive.
This community-based rural clinical trial was conducted with the primary objective to intervene with vitamin D supplementation and certain lifestyle modification in women with pre-diabetes (impaired fasting glucose or impaired glucose tolerance) to prevent the development of type 2 diabetes (T2DM).
Materials and Methods
Trial design and participants
This study was an open-labelled randomized placebo-controlled trial conducted in selected women with pre-diabetes with vitamin D deficiency residing in Ballabgarh HDSS area. The study was registered with the Clinicaltrials.gov with trial number NCT02513888 (US, National Institute of Health, USA). The study was conducted in rural Haryana, field practice area of Comprehensive Rural Health Services Project (CRHSP-AIIMS) of All India Institute of Medical Sciences, New Delhi, India[13] from February 2013 to December 2017. Villages under the Health and Demographic Surveillance System (HDSS) were selected randomly for the intervention and control groups. The study participants were women aged 20 to 60 years who resided in the area for more than 6 months. A total of 2033 eligible women were screened for blood glucose levels as per the inclusion and exclusion criteria from the selected villages through health camps and out-patient department. Those who had pre-diabetes were screened for vitamin D deficiency, women with pre-diabetes and were vitamin D deficient were enrolled in the study and were followed for 2 years.
Ethical approval was taken from the Ethics committee of AIIMS, New Delhi before the start of the study. Subjects were eligible to participate if they met the following inclusion and exclusion criteria.
Inclusion criteria
-
1) Women aged 20-60 years with pre-diabetes
- a) Fasting blood glucose ≥100 mg/dl and <126 mg/dl, or
- b) 2-h plasma glucose ≥140 mg/dl and <200 mg/dl (after ingestion of 75 g anhydrous oral glucose), and baseline level of 25 hydroxy vitamin D <30 ng/dl.
Exclusion criteria:
Received vitamin D and/or calcium supplementation in the previous six months.
On any medication within last one month which could potentially influence insulin secretion, insulin sensitivity, vitamin D or calcium metabolism (e.g. Metformin, thiazolidinediones, steroids, etc) and on medications like steroids, Calcitonin, etc.)
Pregnancy and lactation at the time of study.
Severe end-organ damage or chronic diseases
Known case of HIV infection.
Primary or tertiary hyperparathyroidism, granulomatous disorders (e.g. sarcoidosis), and any lymphomas.
Known case of diabetes mellitus.
First visit
A semi-structured and pre-tested interview schedule was administered to all enrolled participants. The interview schedule consisted of questions on socio-demographic factors, behavioral pattern (sun exposure, tobacco use, alcohol use, personal and family history for chronic diseases), anthropometric measurements (included height, weight, mid-arm circumference, neck circumference, and waist circumference), skinfold measurements (included biceps, triceps, subscapular, supra iliac), blood pressure, clinical examination, biochemical test results and physical activity. The information on sun exposure was collected through a semi-structured questionnaire with a 24-hour sun exposure recall. The duration of sun exposure was measured in minutes exposed per day, <5 minutes, 5-15 minutes, 16-30 minutes, and >30 minutes. Nutritional intake was measured using a food frequency questionnaire.
Standard procedures were followed for all the measurements. SECA 201 ergonomic, non-stretchable measuring tape (Medical Measuring Systems and Scales. SECA. GMBH and co. kg. Hammer Steindamm 9-25. 22089 Hamburg. Germany) was used to measure the circumferences. Height was measured, without shoes by SECA 213 portable stadiometer (Medical Measuring Systems and Scales. SECA. GMBH and co. kg. Hammer Steindamm 9-25. 22089 Hamburg. Germany). Standing height was assessed through maximum vertical stature for persons who can stand unassisted. Weight was recorded nearest to the 100 g using SECA 803 digital flat scale. Skinfolds were measured by using a Lange skinfold caliper (to nearest of 1 mm) from the right side of the participant. All the measurements were repeated three times in the same position and same conditions. The average of the three readings taken by the same observer was used for the final analysis.
Blood pressure was measured by digital BP instrument: OMRON HEM 8711. Body composition analysis was done using non-segmental TANITA BC 420. Nutritional assessment was done by two days 24-hour dietary recall method along with a validated semi-quantitative food frequency questionnaire (FFQ). The amount of food consumed was recorded using household measures and then converted to standard measures. The nutrients were calculated according to the standard values.
Biochemical tests at baseline included glycaemic profile by Oral Glucose Tolerance Test (OGTT), HbA1c, vitamin D levels by serum 25 (OH) D, serum parathyroid hormone, fasting serum insulin, calcium, phosphorus, lipid profile, and complete blood count (CBC). As per our previous procedures (reference) baseline fasting venous blood sample was collected according to the test schedule from all selected participants under aseptic conditions through health camps. A standard 2-hour OGTT was performed after a 12-hour of fasting. Participants were instructed to take measured (75 gms) carbohydrates in the form of glucose. Before ingestion of glucose, blood was drawn at 0 hr and then 2 hr after ingestion of 75 gms of glucose.
Samples were analyzed at designated labs. The biochemical analysis was done using an ELISA kit. Serum 25 (OH) D levels were measured by chemiluminescence method by DiaSorin LIAISON® 25 (OH) D. Lab reports were given to the participants after sample collection and adequate management was done at respective PHC and CRHSP Hospital. All the parameters were repeated after one year to see the effect of the intervention. 25 (OH) D and parathyroid hormone levels were done after every six months during the two-year intervention.
Interventions
Supplementation with vitamin D and oral calcium; Doses of cholecalciferol (commercial name, Calcirol, manufactured by CADILA pharmaceuticals) 60,000 IU (sachets, dissolved in half a glass of milk) once a week for eight weeks was given to the intervention group and placebo (Lactose Granules) was given to the control group. Equal doses of calcium carbonate (1 gm per day, commercial name Calcal) were given to both groups. After every 24 weeks, serum vitamin D levels were assessed.
If the subjects were still deficient in vitamin D, supplementation of cholecalciferol 60,000 IU per week for eight weeks was repeated. If the 25 (OH) D level was normal, cholecalciferol supplementation of 200 IU per day was given as a maintenance dose.
Lifestyle modification counselling for benefits of sun exposure, vitamin D rich and balanced diet was given to both the groups through various modes like lectures, workshops, group discussions, leaflets/booklets distribution. Women were told about the benefits of a balanced diet, dietary intake of calcium, vitamin D, benefits of sun exposure, and adverse health effects of vitamin D deficiency. At the end of the study, the incidence of T2DM in both groups was compared.
Randomization
Villages under the Health and Demographic Surveillance System (HDSS) were selected randomly for intervention and control groups by random number sequence generator. Eligible women were recruited in both groups from the respective villages. For this study women with prediabetes aged 20-60 years with vitamin D deficiency were recruited from the selected villages. After randomization, they were given lifestyle modification counselling with either vitamin D or placebo. This was a single-blind study and women were not aware of being in either intervention or control group.
Follow up visits and compliance
All the participants recruited in the study were followed up for two years. During the follow-up, participants were visited a minimum of 5 times (at 0 month-baseline, 6 months, 12 months, 18 months, and 24 months). Information was collected every 6 months for food frequency, nutritional intake, anthropometric measurements, skinfold measurements, sun exposure, physical examination, and body composition analysis. All the blood parameters were repeated after one year to see the effects of the intervention. Compliance for the intervention was ascertained by telephone calls to participants, a bi-monthly home visit by health workers and a six-monthly personal session for motivation. The level of compliance expected was at least 85%. To ensure 100% compliance, all investigational medication was taken under supervision.
Sample size calculation
To determine the change in outcome with an alpha error of 5%, 90% power, and 20% dropouts, the effective sample size was calculated to be 150 subjects, 75 each in intervention and control groups. We aimed to recruit 150 participants but we were able to find 132 eligible participants and recruited 116 participants, 58 each in the intervention and control group. Required data and information were available for these 116 participants only.
Outcome variables
The primary outcome variable was the incidence of T2DM. Secondary outcomes included changes in lipid profile, calcium, phosphorus and parathyroid hormone.
Statistical analysis
Data were entered in an excel sheet for a different time interval. Distribution of demographic details, clinical anthropometric findings, behavioral characteristics, and other parameters were presented as mean and SD and number with percentages as relevant. Bivariate analysis was done using the Chi-square test. All statistical analyses were performed using Stata-12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP). For all the variables, a P value < 0.05 was considered statistically significant. The incidence of diabetes in both groups was calculated by both including and excluding the loss to follow up in total N.
Results
A total of 2033 participants were assessed for eligibility and 132 were found eligible and included in the study and followed for 24 months. [Figure 1].
Figure 1.
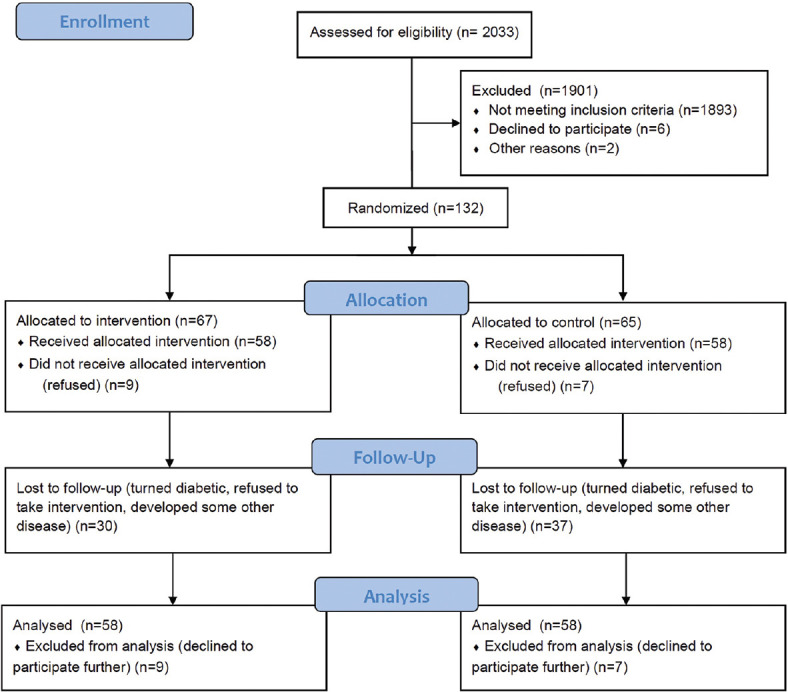
Flowchart showing recruitment and follow up of participants
General characteristics
Fifty-eight participants were recruited in both the intervention and control groups. The baseline characteristics and clinical/anthropological findings of both groups are mentioned in Tables 1 and 2 respectively.
Table 1.
Distribution of All Study Participants by Socio-Demographic Characteristics
| Socio-demographic characteristics | Total N | Mean (SD)/% | Intervention Group Total N | Mean (SD)/% | Control Group Total N | Mean (SD)/% | P | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 116 | 47.1 (7.5) | 58 | 48.1 (6.7) | 58 | 46.1 (8.1) | 0.1501 | |
| Age Groups | 20-30 years | 2 | 1.7 | 0 | 0.0 | 2 | 3.5 | 0.662 |
| 31-40 years | 19 | 16.3 | 9 | 15.5 | 10 | 17.2 | ||
| 41-50 years | 58 | 50.0 | 29 | 50.0 | 29 | 50.0 | ||
| 51-60 years | 37 | 31.9 | 20 | 34.5 | 17 | 29.3 | ||
| Marital status | Married | 96 | 82.8 | 48 | 82.8 | 48 | 82.8 | 0.840 |
| Divorced/Separated | 3 | 2.6 | 1 | 1.7 | 2 | 3.5 | ||
| Widow | 16 | 13.8 | 9 | 15.5 | 7 | 12.0 | ||
| Unmarried | 1 | 0.8 | - | - | 1 | 1.7 | ||
| Education Category | Illiterate | 66 | 56.9 | 34 | 58.6 | 32 | 55.1 | 0.956 |
| Primary | 42 | 36.2 | 21 | 36.2 | 21 | 36.2 | ||
| Secondary | 7 | 6.0 | 3 | 5.2 | 4 | 7.0 | ||
| Graduate | 1 | 0.9 | 0 | 0.0 | 1 | 1.7 | ||
| Occupational status | Student | 1 | 0.9 | 0 | 0.0 | 1 | 1.7 | 0.166 |
| House Work | 99 | 85.3 | 52 | 89.6 | 47 | 81.0 | ||
| Service/Business | 2 | 1.7 | 1 | 1.7 | 1 | 1.7 | ||
| On job | 6 | 5.2 | 4 | 7.0 | 2 | 3.5 | ||
| Labor | 7 | 6.0 | 1 | 1.7 | 6 | 10.3 | ||
| Don’t do any work | 1 | 0.9 | 0 | 0.0 | 1 | 1.7 | ||
| Average duration of Sun exposure in a day | Minutes/day | 116 | 112 (109) | 58 | 116 (100) | 58 | 108 (118) | |
| Tobacco intake | Never | 95 | 82 | 50 | 86.2 | 45 | 77.6 | 0.449 |
| Occasional | 7 | 6 | 3 | 5.2 | 4 | 6.9 | ||
| Regular | 14 | 12 | 5 | 8.6 | 9 | 15.5 | ||
| Dietary intake | Calories intake (Kcal) | 115 | 1672 (465) | 57 | 1676 (481) | 58 | 1668 (452) | 0.9310 |
| Protein intake (gm) | 114 | 50 (15.4) | 56 | 49 (14.6) | 58 | 50.4 (16.2) | 0.6751 | |
| Physical activity | Minutes/day | 116 | 221 (435) | 58 | 194 (300) | 58 | 247 (538) | |
| Total | 116 | 100 | 58 | 100 | 58 | 100 | ||
Table 2.
Distribution of All Study Participants by Clinical and Anthropometric Findings
| Characteristics | Total Mean (SD)/% | Intervention Group Mean (SD)/% | Control Group Mean (SD)/% | |
|---|---|---|---|---|
| Clinical examination (mm/Hg) | Pulse | 81 (9) | 82 (10) | 80 (9) |
| Systolic BP | 124 (16) | 128 (18) | 121 (13) | |
| Diastolic BP | 79 (9) | 80 (10) | 77 (7.9) | |
| Physical Measurements | Waist circumference (cm) | 90.4 (20.2) | 91.5 (20.1) | 83.4 (22.7) |
| Hip circumference (cm) | 98.4 (21.8) | 95.9 (20.3) | 92.2 (23.2) | |
| Waist Hip ratio | 0.9 (0.2) | 0.9 (0.2) | 0.8 (0.2) | |
| Skin fold measurements (mm) | Biceps | 18 (10) | 19 (10) | 15 (9) |
| Triceps | 30 (10) | 29 (9) | 28 (11) | |
| Sub scapular | 26 (10) | 25 (9) | 26 (10) | |
| Supra iliac | 23 (10) | 22.2 (8.1) | 23 (11) | |
| Body composition analysis | Fat mass | 22.8 (9) | 24.1 (8.1) | 19.2 (8.8) |
| Fat free mass | 29.1 (10) | 35.9 (6.3) | 35 (12.8) | |
| Fat % | 37 (10) | 37.7 (7) | 32.5 (11.5) | |
| Total Body water | 26.1 (7) | 25.4 (5.8) | 23.6 (8.1) |
Primary outcome
It was observed that the incidence of diabetes was higher in the control group as compared to the intervention group at 1 year duration but the difference was not statistically significant (p -0.43). At the end of 2 years as well, the difference in both the groups was statistically non-significant (p-0.7. [Table 3].
Table 3.
Incidence of Diabetes Among Participants in Intervention and Control Groups at 12 and 24 Months
| Incidence of diabetes including loss to follow up | Incidence of diabetes excluding loss to follow up | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Experimental (%) (N=58) | Control (%) (N=58) | Total (%) (N=112) | Experimental (%) (N=49) | Control (%) (N=45) | Total (%) (N=104) | |
| 12 months | 8 (13.79) | 12 (20.69) | 20 (17.24) | 8 (16%) | 12 (27%) | 20 (19.2%) |
|
| ||||||
| Experimental (%) (N=58) | Control (%) (N=58) | Total (%) (N=112) | Experimental (%) (N=47) | Control (%) (N=42) | Total (%) (N=89) | |
|
| ||||||
| 24 months | 16 (27.59) | 16 (27.59) | 32 (27.59) | 16 (34%) | 16 (38%) | 32 (35.9%) |
At the end of twenty-four months, 23 women in the intervention group and 20 women in the control group became euglycemic, but the difference was not statistically significant (p-0.56). Mean HbA1c levels were also calculated and are given in Table 4.
Table 4.
Effects of Vitamin D Supplementation on HbA1c and Lipids (Cholesterol) in Intervention and Control Groups at 0, 12 and 24 Months
| Serum HbA1c and Cholesterol | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Time period | HbA1c (%) | Cholesterol (mg/dl) | ||||
|
| ||||||
| 0 month | Total Mean (SD) | Intervention Mean (SD) | Control Mean (SD) | Total Mean (SD) | Intervention Mean (SD) | Control Mean (SD) |
| 12 months | N=116 6.71 (1.91) | N=58 6.81 (2.01) | N=58 6.61 (1.81) | N=116 202.2 (43.3) | N=58 209.4 (39.3) | N=58 195 (46) |
| 24 months | N=92 5.55 (0.78) | N=50 5.58 (0.70) | N=42 5.51 (0.88) | N=92 170.6 (45.3) | N=50 176 (42.5) | N=42 164 (48) |
| Total | N=65 5.92 (0.41) | N=37 5.98 (0.37) | N=28 5.84 (0.45) | N=65 170 (53.6) | N=37 176.7 (47) | N=28 162 (61) |
Secondary outcomes
Lipid and HbA1C levels
The mean serum concentration of cholesterol in both the intervention and control group was higher during the recruitment and came down in subsequent visits. There was no statistically significant association of vitamin D supplementation on serum cholesterol level. [Table 4].
Parathyroid hormone, calcium, and phosphorus
At the end of two years mean serum vitamin D, PTH, calcium, and phosphorus were higher in the intervention group when compared with the control group, but the difference was not statistically significant. [Table 5].
Table 5.
Levels of Vitamin D, PTH, Calcium, Phosphorus in Intervention and Control Groups at 0, 12 and 24 Months
| Serum Vitamin D, PTH, Calcium and Phosphorus | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Time Period | Vitamin D (ng/ml) | PTH (pg/ml) | ||||
|
|
|
|||||
| Total Mean (SD) | Intervention Mean (SD) | Control Mean (SD) | Total Mean (SD) | Intervention Mean (SD) | Control Mean (SD) | |
| 0 month | N=116 | N=58 | N=58 | N=116 | N=58 | N=58 |
| 22.8 | 27.6 | 27.7 | 49.5 | 48 | 50.9 | |
| (9) | (12.9) | (14.6) | (27) | (23.6) | (30.3) | |
| 12 months | N=92 | N=50 | N=42 | N=92 | N=50 | N=42 |
| 45.62 | 51.63 | 24.4 | 57.9 | 63.9 | 51.39 | |
| (23.6) | 22.9 | (12.3) | (27.47) | (21.3) | (29.4) | |
| 24 months | N=65 | N=37 | N=28 | N=65 | N=37 | N=28 |
| 58.3 | 69.9 | 42.8 | 46.2 | 40.8 | 53.3 | |
| (34.8) | (34.1) | (29.6) | (23.2) | (19.4) | 26.1 | |
|
| ||||||
| Time Period | Calcium (mg/dl) | Phosphorus (mg/dl) | ||||
|
|
|
|||||
| Total Mean (SD) | Intervention Mean (SD) | Control Mean (SD) | Total Mean (SD) | Intervention Mean (SD) | Control Mean (SD) | |
|
| ||||||
| 0 month | N=116 | N=58 | N=58 | N=116 | N=58 | N=58 |
| 9.26 | 9.6 | 8.7 | 3.57 | 3.6 | 3.4 | |
| (2) | (2.5) | (1.1) | (0.8) | (1) | (0.6) | |
| 12 months | N=92 | N=50 | N=42 | N=92 | N=50 | N=42 |
| 10.2 | 10.8 | 9.4 | 3.8 | 4 | 3.62 | |
| (8) | (10.7) | (1.02) | (0.8) | (0.8) | (0.8) | |
| 24 months | N=62 | N=34 | N=28 | N=65 | N=37 | N=28 |
| 9.3 | 9.07 | 9.6 | 3.78 | 3.8 | 3.71 | |
| (1.1) | (2.8) | (0.9) | (0.9) | (1) | (0.6) | |
Seasonal variation
The sun exposure was similar in both the groups; 116 mins/day and 108 mins/day in the intervention and control group respectively. The serum vitamin D level was recorded during every visit. We calculated the difference in mean levels of vitamin D in all the readings during different seasons. The mean serum vitamin D level in summers and winter was 42.57 (20.22) and 43.2 (28) respectively and the difference was not statistically significant.
Discussion
We found that the incidence of Type 2 DM among women with prediabetes who received vitamin D supplementation was lower than those who did not receive the vitamin D, at the end of one year of follow up. But this difference was non-existent at the end of two years. There was no significant difference in studied variables in both groups. Various studies conducted all over the world have shown varied results regarding the effect of vitamin D in the progression of prediabetes to diabetes. The intervention group received vitamin D 60000 IU per week followed by 200 IU per day after vitamin D level became normal. The control group received calcium tablets 500 mg per day (which contained 250 IU of vitamin D). A large trial conducted in Norway with 511 adults with pre-diabetes to study the effect of vitamin D supplementation for the prevention of type 2 diabetes with 20,000 IU/wk (~2900 IU/d) of vitamin D3 or placebo found that the rate of incident diabetes was lower than the control group throughout the study. But the difference was not statistically significant (hazard ratio, 0.90; 95% CI, 0.69 to 1.18).[14] These findings were consistent with findings from other RCTs conducted worldwide.[15,16,17,18] A study conducted in the urban population of Delhi, India has shown that supplementation with vitamin D decreased the fasting blood glucose and HbA1c in women.[19]
The cases of diabetes in India are gradually increasing. Diabetes is more dangerous in India as it affects the younger population resulting in an increased chance of complications and decreased quality of life.[20] Larger studies with more precision are required to capture the effect of vitamin D supplementation on the incidence of diabetes. The primary care physicians can be of great use in managing this huge problem. The counselling for dietary modification and physical activities should be done at primary care level so that timely management can be done.
In this study, the prevalence of insulin resistance increased in both the intervention and control group at 12 months, but the prevalence decreased at 24 months. In a trial designed to assess bone-related outcomes with daily supplementation with 700 units of vitamin D3 and 500 mg calcium carbonate, post hoc analysis was done. It was seen that insulin resistance improved among those with pre-diabetes (impaired fasting glucose) at baseline.[21] In another study conducted by Harris et al.,[22] it was observed that Insulin sensitivity decreased by 4% in the intervention (Vit D group) while it increased by 12% in the control group (P = 0.034). Whereas in other studies[23,24,25,26] there was no significant effect of vitamin D on insulin sensitivity.
In this study, calcium levels were higher in both the intervention and control group when compared to baseline as both the groups received calcium supplementation. There was no significant difference in the levels of PTH in the intervention and control groups. The increase in vitamin D levels helps in increased calcium absorption from diet and resorption from bones, hence resulting in PTH suppression.[25]
It was seen that there was no significant difference in vitamin D levels in the summer and winter months. This finding is consistent with the findings recorded in previous studies.[26] Some studies have recorded significantly higher levels of vitamin D in the summer months.[27] This is because the ultraviolet B does not have an adequate intensity to synthesize vitamin D in winters.[28] Since we get good sun in both seasons might be the reason for no seasonal variability in this area.
There was no significant difference between the cholesterol level of participants in both the groups at baseline or at the end of the intervention. This finding was consistent with the meta-analysis of RCTs[29] which showed that there is no statistically significant effect of vitamin D supplementation on various lipids including cholesterol.
Strength and limitation
The strength of the study includes that cluster randomization was done according to the geographical distribution which decreases the crossing over from one group to another to decrease the chances of contamination. Also, to the best of our knowledge, it is the first community-based RCT study with a sample size of over 100 participants in India to assess the contribution of vitamin D supplementation on the progression of prediabetes to diabetes. Lifestyle modification counselling was done by the participants as a part of the intervention plan. Also, an extensive questionnaire was used which captured much important information about the participant.
The limitation of the study includes that the compliance of the supplementation given cannot be objectively confirmed. The results show that in the first year there is some effect of vitamin D in the prevention of development of T2DM, but the effect diminishes at end of two years, it might be due to leniency in listening to our advice and taking Vit D over an extended period. There was a 21% loss to follow up at 12 months and a 40% loss to follow up at end of 24 months. Also, most of the information was collected as reported by the participants. Hence, recall bias and social desirability bias cannot be excluded.
Conclusion
In this community based RCT, we could not find any significant effect of vitamin D supplementation in delaying the incidence of diabetes among women with prediabetes (20-60 years) of the HDSS Ballabgarh during two years follow up. There was no significant effect of Vitamin D supplementation on serum cholesterol or insulin sensitivity.
Summary of the article
Many studies conducted in India show that the prevalence of vitamin D deficiency ranges from 70% to 100. Vitamin D is required for insulin synthesis. Evidence is inconclusive whether vitamin D supplementation decreases the incidence of diabetes. In this community-based trial, the vitamin D supplementation among women with pre-diabetes did not show any statistically significant effect on the progression to diabetes when compared to placebo. There was no effect on the insulin resistance, cholesterol, calcium, and phosphorus as well. Larger studies with more precision are required to capture the effect of vitamin D supplementation on incidence of diabetes.
Financial support and sponsorship
This work was supported by Department of Science and Technology, Ministry of Science and Technology, India (SSD/WS/056/2011).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–20. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 3.Stöcklin E, Eggersdorfer M. Vitamin D, an essential nutrient with versatile functions in nearly all organs. Int J Vitam Nutr Res. 2013;83:92–100. doi: 10.1024/0300-9831/a000151. [DOI] [PubMed] [Google Scholar]
- 4.Harinarayan CV, Holick MF, Prasad UV, Vani PS, Himabindu G. Vitamin D status and sun exposure in India. Dermatoendocrinol. 2013;5:130–41. doi: 10.4161/derm.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.G R, Gupta A. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients. 2014;6:729–75. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra P, Srivastava R, Misra A, Kant S, Kardam P, Vikram NK. Vitamin D status of adult females residing in Ballabgarh health and demographic surveillance system: A community-based study. Indian J Public Health. 2017;61:194–8. doi: 10.4103/ijph.IJPH_176_16. [DOI] [PubMed] [Google Scholar]
- 7.Angellotti E, Pittas AG. The role of vitamin D in the prevention of type 2 diabetes: To D or not to D? Endocrinology. 2017;158:2013–21. doi: 10.1210/en.2017-00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi Rad E, Djalali M, Koohdahi F, Saboor-Yaraghi AA, Eshraghian MR, Javanbakht MH, et al. The effects of vitamin D supplementation on glucose control and insulin resistance in patients with diabetes Type 2: A randomized clinical trial study. Iran J Public Health. 2014;43:1651–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Nazarian S, St Peter JV, Boston RC, Jones SA, Mariash CN. Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose? Transl Res. 2011;158:276–81. doi: 10.1016/j.trsl.2011.05.002. doi: 10.1016/j.trsl.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Zhai Y, Shen S. Association between vitamin D and prediabetes: A PRISMA-compliant meta-analysis? Medicine (Baltimore) 2020;99:e19034. doi: 10.1097/MD.0000000000019034. doi: 10.1097/MD.0000000000019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson RR. Handbook of Vitamin D in Human Health. Netherlands: Wageningen Academic Publisher. 2013:503–604. [Google Scholar]
- 12.Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–8. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kant S, Misra P, Gupta S, Goswami K, Krishnan A, Nongkynrih B, et al. The Ballabgarh Health and Demographic Surveillance System (CRHSP-AIIMS) Int J Epidemiol. 2013;42:758–68. doi: 10.1093/ije/dyt055. [DOI] [PubMed] [Google Scholar]
- 14.Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, et al. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab. 2016;101:1647–55. doi: 10.1210/jc.2015-4013. [DOI] [PubMed] [Google Scholar]
- 15.Ganji V, Tangpricha V, Zhang X. Serum vitamin D concentration≥75 nmol/L is related to decreased cardiometabolic and inflammatory biomarkers, metabolic syndrome, and diabetes; and increased cardiorespiratory fitness in us adults. Nutrients. 2020;12:730. doi: 10.3390/nu12030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuomainen TP, Virtanen JK, Voutilainen S, Nurmi T, Mursu J, de Mello VD, et al. Glucose metabolism effects of vitamin D in prediabetes: The VitDmet randomized placebo-controlled supplementation study. J Diabetes Res. 2015;2015:672653. doi: 10.1155/2015/672653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchay MS, Laway BA, Bashir MI, Wani AI, Misgar RA, Shah ZA. Effect of Vitamin D supplementation on glycemic parameters and progression of prediabetes to diabetes: A 1-year, open-label randomized study. Indian J Endocrinol Metab. 2015;19:387–92. doi: 10.4103/2230-8210.152783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for diabetes mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94:486–94. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt SP, Misra A, Pandey RM, Upadhyay AD, Gulati S, Singh N. Vitamin D supplementation in overweight/obese Asian Indian women with prediabetes reduces glycemic measures and truncal subcutaneous fat: A 78 weeks randomized placebo-controlled trial (PREVENT-WIN Trial) Sci Rep. 2020;10:220. doi: 10.1038/s41598-019-56904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014;7:45–8. doi: 10.4066/AMJ.2013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 22.Harris SS, Pittas AG, Palermo NJ. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab. 2012;14:789–94. doi: 10.1111/j.1463-1326.2012.01605.x. [DOI] [PubMed] [Google Scholar]
- 23.Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36:260–6. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Thani M, Sadoun E, Sofroniou A, Jayyousi A, Baagar KA, Al Hammaq A, et al. The effect of vitamin D supplementation on the glycemic control of pre-diabetic Qatari patients in a randomized control trial. BMC Nutr. 2019;5:46. doi: 10.1186/s40795-019-0311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US); 2011. PMID: 21796828. [PubMed] [Google Scholar]
- 26.Cong E, Walker MD, Kepley A, Zhang C, McMahon DJ, Silverberg SJ. Seasonal variability in vitamin D levels no longer detectable in primary hyperparathyroidism. J Clin Endocrinol Metab. 2015;100:3452–9. doi: 10.1210/JC.2015-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidari B, Haji Mirghassemi MB. Seasonal variations in serum vitamin D according to age and sex. Casp J Intern Med. 2012;3:535–40. [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes AM, Lucas RM, Ponsonby AL, Chapman C, Coulthard A, Dear K, et al. The role of latitude, ultraviolet radiation exposure and vitamin D in childhood asthma and hayfever: An Australian multicenter study. Pediatr Allergy Immunol. 2011;22:327–33. doi: 10.1111/j.1399-3038.2010.01099.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: A meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11:42. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]


