Abstract
Introduction:
Budd-Chiari syndrome (BCS) is a rare condition affecting one in a million adults. BCS involves outflow obstruction in the hepatic venous system, which can occur anywhere between the small hepatic veins and the atrio-caval junction and cannot be due to heart, pericardial, or hepatic veno-occlusive disease.
Case Presentation:
We report an exceedingly rare form of BCS with less common initial clinical features in a young poor adult male patient which ignited a diagnostic uncertainty and a therapeutic challenge. The presence of the classical triad of BCS in the absence of major hepatic vein obstruction prompted the medical team to consider that the patient had a rare form of BCS. In this case, the financial condition of the patient and limited resources available restricted our ability to advance into the specific investigations. However, the patient was given symptomatic medical treatment and was followed up monthly. We also provided the patient with a statement that reaffirmed our inability to provide affordable surgical management options and called for an optimized national clinical guideline that could help the physicians deal with the challenges.
Conclusion:
An uncommon form of BCS in this patient provided a diagnostic challenge and therapeutic uncertainty in the low-resource settings. Primary care physicians should commence evidenced medical management based on clinical suspicion acknowledging the fact that obstruction of small hepatic veins is often not detected on an ultrasound.
Keywords: Budd-Chiari syndrome, small hepatic vein, low-resource setting
Introduction
Budd-Chiari syndrome (BCS) involves outflow obstruction in the hepatic venous system, which can occur anywhere between the small hepatic veins and the atrio-caval junction and cannot be due to heart, pericardial, or hepatic veno-occlusive disease.[1,2] BCS can occur as fulminant, acute, sub-acute, and chronic BCS (most common) based on the extension and rapidity of the occlusion and development of collateral circulation. BCS will mostly present with the classical triad of abdominal pain, hepatomegaly, and ascites.[1,3] BCS was first described in 1845 by The English physician, George Budd had described the first case of BCS in 1845 and then in 1989, Hans Chiari first described how it affects the body. Etiologically, BCS can be classified into primary BCS (due to a primary venous process e.g., thrombosis or phlebitis) and secondary BCS (due to compression or invasion external to the veins e.g., malignancy).[2] The most common causative factors are myeloproliferative neoplasms, Janus Kinase 2 gene[4] mutation, antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria (PNH), protein C and S deficiency, and oral contraceptives consumption.[5,6]
There is geographic variation in prevalence, etiology, and site of thrombosis of this rare syndrome. In Asia and Africa, 40% of the cases are due to membranous obstruction of the inferior vena cava, whereas hepatic vein thrombosis occurs commonly in western countries. The mean-age standardized incidence (1 per million per year) and prevalence rates (11 per million inhabitants) were reported in a recent systematic review.[7] However, the prevalence of BCS remains largely unknown in Bangladesh but estimates range between one in fifty thousand to one hundred thousand, which is higher than the global estimation. A past population-based epidemiologic study in an Asian country revealed a higher prevalence of BCS compared to western countries.[8]
BCS often gives a diagnostic and therapeutic challenge in low-resource settings such as Bangladesh, where the health system is stressed by an overwhelming number of patients and an overworked healthcare workforce. Here, we report such a case of BCS in a young poor adult male patient who presented to a low-resource setting in Bangladesh that imposed a diagnostic uncertainty and therapeutic challenge.
Case Presentation
A 29-year-old poor adult male from Rajbari district of Bangladesh, had presented to our tertiary care hospital in Faridpur district with severe abdominal pain and progressive abdominal distension associated with scanty micturition during the summer of 2016. The abdominal pain was located on the right hypochondriac region and was dull in nature with no relation with food intake and had no radiation. He had a history of admission to a primary care hospital three months earlier with jaundice and abdominal distension. He had no relevant family history of similar complaints.
On examination, he was anemic, mildly icteric, had ankle oedema, along with ascites. His vital signs were normal. However, he had visible engorged veins over the chest and abdomen with the flow of direction being away from the umbilicus [Figure 1]. On abdominal examination, the fluid thrill test was found to be positive with a palpable liver and spleen. During the examination, his testes were found to be atrophied and there was a loss of male secondary sexual characteristics. The routine laboratory investigations revealed a hemoglobin level of 61 g/L with a normal total leukocyte count (9000 cells/mm3) and platelet count (2.6 × 105/mm3). His liver function tests revealed a serum bilirubin level of 32 g/L, total serum protein of 52 g/L, and a serum albumin level of 30 g/L. His viral markers were found negative. The ascitic fluid examination showed 4800 cells/mm3 and biochemical examination showed the protein and sugar levels to be 38 g/L and 0.22 g/L, respectively. An USG of the abdomen revealed that the liver was enlarged but had normal texture [Figure 2]. However, the portal vein was normal in diameter (9 mm) without any notable thrombus. Furthermore, a doppler study of hepatic and portal system revealed that the portal vein, hepatic artery, and hepatic veins were intact; showing well-filling of blood and normal flow velocity spectrum, apparently there was no evidence of portal hypertension [Figure 3 and 4]. Also, multiple air-fluid levels were noted in the X-ray abdomen, suggestive of obstruction [Figure 5]. The radiological diagnosis of BCS could not be established; however, in this case, there was evidence of the presence of a collateral circulation despite a normal diameter and blood flow through the major hepatic veins.
Figure 1.
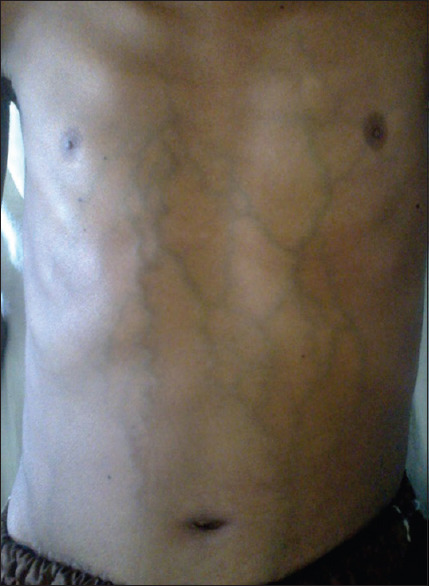
Patients' abdomen during clinical examination
Figure 2.

Ultrasonogram Report of the Whole Abdomen with Special Attention to the Hepatobiliary System before treatment
Figure 3.
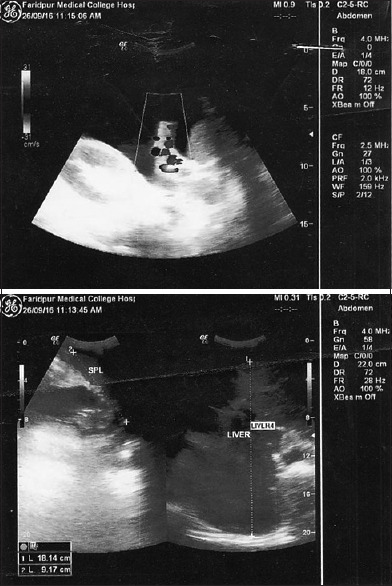
Doppler ultrasound of Porto-Hepatic Venous System
Figure 4.

Doppler Ultrasound Report of Portal and Hepatic Venous System
Figure 5.
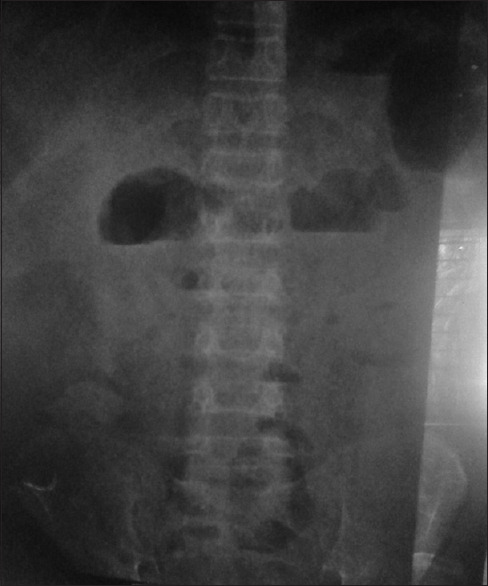
X-ray of the patients Abdomen in Anterior Posterior view before starting treatment
The patient was diagnosed as BCS based on the classical triad of abdominal pain, liver enlargement, and ascites. A symptomatic and supportive treatment plan was established, and the patient was counseled about possible surgical treatment options in BCS such as liver transplantation or portosystemic shunts. Stenting could not be a treatment option as the site of obstruction could not be identified without further costly investigations and the patient was not able to afford liver transplantation. However, his ascites was reduced after conservative treatment with propranolol, lactulose, and paracentesis for the ascetic fluid. The patient got discharged after a 15-day hospitalization and advised for a monthly follow-up at the outpatient department.
The patient was reasonably doing well without any significant complaints until one-month after being discharged. He then presented back to our hospital subsequently with similar complaints and ascites. An USG of the abdomen confirmed his large ascites and 2.5 L of ascitic fluid was drawn out by paracentesis. He was then discharged from the hospital with the same medication and has remained reasonably well for 7 months from his last admission as seen during his monthly visits at the outpatient department.
He was followed up for a social visit 22 months after this initial admission and shared his subjective experience of being in good health. We had the privilege to request the patient to share his opinion regarding his condition. The patient kindly stated that “he hates the fact that his circumstance has no affordable permanent cure at the moment, but his general health and well-being are alright because he believes his family is supportive enough to ensure he takes his medications regularly”.
Discussion
In this young adult male with abdominal pain, ascites, and hepatomegaly coupled with the presence of collateral circulation, the diagnosis was most suggestive of BCS. The collaterals developed most likely due to an obstruction in the small hepatic veins shunting blood through the caudate lobe.[9] BCS is usually diagnosed clinically and radiologically demonstrating obstruction in the hepatic venous outflow tract. The obstruction is then localized usually through a doppler USG, CT scan, venography, or MRI.[9]
The angiographic study of major hepatic veins and IVC is the current gold standard; however, this facility is largely available only in super-specialized centers and was not a feasible solution for this poor patient. The sensitivity and specificity for the diagnosis of BCS with doppler USG is 85%, indicating that major hepatic venous patency may not rule it out.[10] This might have been a plausible reason in our patient who could have an obstruction in small intra-hepatic veins, which were undetectable on doppler USG. Exceedingly rare forms of BCS were previously reported where the obstruction is found to be limited to small intra-hepatic veins and with a normal diameter and normal large hepatic venous blood flow.[9] The diagnosis of such a small vessel outflow block requires a histological confirmation by liver biopsy. Again, in low-resource settings, this is often a therapeutic challenge complicated by a diagnostic uncertainty, where an extensive diagnostic workout will not position this poor patient to avail any of the other expensive surgical treatments such as a liver transplantation with life-long anticoagulation.
There can be a variable presentation of BCS according to the forms of BCS. For example, progressive severe upper abdominal pain, elevated liver enzymes, jaundice, vomiting, and ascites are common findings in fulminant cases with high mortality.[11] The patients with the acute syndrome typically present with a short period of resistant ascites, hepatic necrosis without the formation of venous collaterals, and usually causing with major hepatic vein thrombosis. The sub-acute form has a more insidious onset, with the patients having minimal necrosis and ascites due to the establishment of hepatic venous collateral circulation. This form of BCS is more common, which is suspected to have happened in the case of this patient.[12] Chronically, caudate lobe of the liver get enlarged, compressing the IVC while passing behind the liver. Chronic cases can also be presented as a complication of cirrhosis and ascites.[13] Consequent to portal hypertension, patients may develop hematemesis and/or melaena due to gastric varices, hemorrhoids, and can accompany splenomegaly, which was evident via the presentation of anemia in this patient, even though the liver texture was normal. This patient's cause for testicular atrophy and lack of secondary sexual characteristics could not be revealed, but further work-up could reveal any underlying etiological relation with the BCS pathogenesis. However, previous studies had reported the presence of testicular atrophy in patients with BCS.[14]
A treated patient with BCS has a good prognosis with a 90% overall 5-year prognosis rate. Restoration of hepatic venous flow is the aim of treatment in BCS. The initial medical treatment in these patients is an anticoagulant therapy, and in most cases long term continuation has been recommended due to better prognosis. Along with it, dietary salt restriction, potassium-sparing diuretics and a large volume paracentesis with intravenous albumin infusion may be required. Advanced management (e.g., porto-systemic shunts and liver transplantation) could be an option when anticoagulation therapy fails, or patients develop decompensated liver cirrhosis. In this patient, anticoagulant therapy could not be started in view of unclear indication in the current guidelines.
In developing countries including Bangladesh, sophisticated diagnostic workups and treatments are mostly concentrated in corporate healthcare centers and patients often need to make out-of-pocket health expenditures. This is rightly reflected in this patient's statement which calls for nationwide efforts to bring affordable and curable solutions, at the same time re-emphasizes the need for a national health insurance scheme.
Conclusion
In this poor adult male, an exceedingly rare form of Budd-Chiari syndrome provided a diagnostic challenge and therapeutic uncertainty for our clinical team in the low-resource setting. Primary care physicians who clinically suspect patients to have BCS on basis of the classical triad should prompt the consideration of the small hepatic vein obstruction, despite normal major hepatic veins on an USG. As the absence of extensive diagnostic work-up facilities in a low-resource setting often poses diagnostic and therapeutic challenges for poor patients, management should be routinely commenced with anticoagulation, beta blockers, laxatives, potassium sparing diuretics, and dietary sodium restriction, with paracentesis if required. Clinical guidelines should tailor their recommendations considering the diagnostic and therapeutic challenges in low-resource settings. In the long run, national health insurance policies may contribute to providing affordable solutions to relieve small hepatic vein obstruction in such settings, which however, may be challenging due to the complexity of the surgical management options.
List of Abbreviations
BCS - Budd-Chiari Syndrome; IVC - Inferior Venacava; USG- Ultrasonogram
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors wish to thank the patient who kindly gave consent for the case to be presented in this manuscript. The corresponding author ES was an intern physician at the study site (Faridpur Medical College Hospital, Faridpur, Bangladesh) during the study.
References
- 1.Boyer TD, Wright TL, Manns MP. Zakim and Boyer's Hepatology E-Book: A Textbook of Liver Disease. Elsevier Health Sciences, Philadelphia. 2011 [Google Scholar]
- 2.Juan Carlos Garcia-Pagàn, Elizabetta Buscarini, Harry L.A. Janssen, Frank W.G. Leebeck, Aurelie Plessier. European Association for the Study of the Liver, EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Hoekstra J, Janssen HLA. Vascular liver disorders (I): Diagnosis, treatment and prognosis of Budd-Chiari syndrome. Neth J Med. 2008;66:334–9. [PubMed] [Google Scholar]
- 4.Patel RK, Lea NC, Heneghan MA, Westwood NB, Milojkovic D, Thanigaikumar M, et al. Prevalence of the activating JAK2 tyrosine kinase mutation V617F in the Budd–Chiari syndrome. Gastroenterology. 2006;130:2031–8. doi: 10.1053/j.gastro.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Brandt LJ, Feldman M, Friedman LS, Sleisenger MH. Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. Saunders, Philadelphia. 2006 [Google Scholar]
- 6.Rajani R, Melin T, Björnsson E, Broomé U, Sangfelt P, Danielsson Å, et al. Budd-Chiari syndrome in Sweden: Epidemiology, clinical characteristics and survival–an 18-year experience. Liver Int. 2009;29:253–9. doi: 10.1111/j.1478-3231.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, De Stefano V, Li H, Zheng K, Bai Z, Guo X, et al. Epidemiology of Budd-Chiari syndrome: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2019;43:468–74. doi: 10.1016/j.clinre.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Ki M, Choi HY, Kim K-A, Kim BH, Jang ES, Jeong S-H. Incidence, prevalence and complications of Budd-Chiari syndrome in South Korea: A nationwide, population-based study. Liver Int. 2016;36:1067–73. doi: 10.1111/liv.13008. [DOI] [PubMed] [Google Scholar]
- 9.Riggio O, Marzano C, Papa A, Pasquale C, Gasperini ML, Gigante A, et al. Small hepatic veins Budd-Chiari syndrome. J Thromb Thrombolysis. 2014;37:536–9. doi: 10.1007/s11239-013-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal V, Gupta P, Sinha S, Dhaka N, Kalra N, Vijayvergiya R, et al. Budd-Chiari syndrome: Imaging review. Br J Radiol. 2018;91:20180441. doi: 10.1259/bjr.20180441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horton JD, San Miguel FL, Ortiz JA. Budd–Chiari syndrome: Illustrated review of current management. Liver Int. 2008;28:455–66. doi: 10.1111/j.1478-3231.2008.01684.x. [DOI] [PubMed] [Google Scholar]
- 12.Menon KN, Shah V, Kamath PS. The Budd–Chiari syndrome. N Engl J Med. 2004;350:578–85. doi: 10.1056/NEJMra020282. [DOI] [PubMed] [Google Scholar]
- 13.De DH, Pezzella AT, Nguyenduy T. Membranous obstruction of hepatic venous flow. Tex Heart Inst J. 1995;22:320–3. [PMC free article] [PubMed] [Google Scholar]
- 14.Sutradhar SR, Sarker CB, Rahman S, Debnath CR, Siddiqui NI, Huq HM, et al. Budd-Chiari syndrome. Mymensingh Med J. 2005;14:84–7. [PubMed] [Google Scholar]


