Abstract
Background
Exercise intolerance is common in people with heart failure and preserved ejection fraction (HFpEF). Right ventricular (RV) dysfunction has been shown at rest in HFpEF but little data are available regarding dynamic RV-pulmonary artery (PA) coupling during exercise.
Methods and results
Subjects with HFpEF (n = 50) and controls (n = 24) prospectively underwent invasive cardiopulmonary exercise testing using high-fidelity micromanometer catheters along with simultaneous assessment of RV and left ventricular (LV) mechanics by echocardiography. Compared with controls at rest, subjects with HFpEF displayed preserved RV systolic and diastolic mechanics (RV s′ and e′), impaired LV s′ and e′, higher biventricular filling pressures, and higher pulmonary artery pressures. On exercise, subjects with HFpEF displayed less increase in stroke volume, heart rate, and cardiac output (CO), with blunted increase in CO relative to O2 consumption (VO2). Enhancement in RV systolic and diastolic function on exercise was impaired in HFpEF compared with controls. Exercise-induced PA vasodilation was reduced in HFpEF in correlation with greater venous hypoxia. Elevations in biventricular filling pressures and limitations in CO reserve were strongly correlated with abnormal enhancement in ventricular mechanics in the RV and LV during stress.
Conclusions
In addition to limited LV reserve, patients with HFpEF display impaired RV reserve during exercise that is associated with high filling pressures and inadequate CO responses. These findings highlight the importance of biventricular dysfunction in HFpEF and suggest that novel therapies targeting myocardial reserve in both the left and right heart may be effective to improve clinical status.
Keywords: Heart failure, Heart failure with preserved ejection fraction, Diastolic function, Haemodynamics, Exercise, Pulmonary hypertension, Right ventricular function
Introduction
Roughly half of patients with heart failure (HF) have a preserved ejection fraction (HFpEF).1 Haemodynamically, HFpEF is characterized by pulmonary venous (and arterial) hypertension that is precipitated by or exacerbated by exercise.2–4 Heart failure with preserved ejection fraction was historically considered to be a primary problem of the left ventricle and systemic circulation, and there are clear limitations in left ventricular (LV) systolic and diastolic reserve that develop during exercise in patients with HFpEF.2–9
More recent studies have shown that pulmonary arterial (PA) and right ventricular (RV) function are also impaired at rest in some patients with HFpEF.10–13 However, it is unclear if right heart abnormalities are simply related to long-standing left heart disease, or if abnormalities in RV–PA coupling may be present even in the earlier stages of HFpEF. Demonstration of RV reserve impairment in addition to LV limitations would provide important evidence supporting the notion that perturbations in chamber and myocardial function are more global in HFpEF. If true, this would have implications for the design of novel therapies targeting the cardiac myocyte in both sides of the heart.
Accordingly, we performed this prospective study to thoroughly characterize rest and exercise ventricular-vascular function in both the right- and the left-sided circulations using invasive and non-invasive techniques with simultaneous expired gas analysis comparing subjects with HFpEF to control subjects without HF. The goals were to (1) determine whether myocardial dysfunction during exercise is present in the RV as well as the LV, (2) evaluate how myocardial limitations relate to the haemodynamic abnormalities that develop during stress in HFpEF, and (3) examine dynamic RV–PA coupling responses to exercise along with their physiologic correlates.
Methods
Subjects referred to the Mayo Clinic catheterization laboratory for invasive haemodynamic exercise stress testing for exertional dyspnoea were enrolled in this prospective study between August 2011 and July 2013. Written informed consent was provided by all patients prior to participation in study-related procedures. The study was approved by the Mayo Clinic Institutional Review Board and the study was registered (NCT01418248). Haemodynamic data from some of these subjects have been published elsewhere,14,15 but not as it relates biventricular function to exercise haemodynamics or exercise capacity as determined by expired gas analysis.
Study population
Heart failure with preserved ejection fraction was defined by clinical symptoms of chronic HF (dyspnoea, fatigue), normal EF (≥50%), and elevated left heart filling pressures (pulmonary capillary wedge pressure, PCWP) at rest (>15 mmHg) and/or with exercise (≥25 mmHg).3,14,15 Subjects with significant valvular heart disease (>mild stenosis, >moderate regurgitation), cor pulmonale, significant pulmonary disease, congenital heart disease, left-to-right shunt, unstable coronary artery disease, myocardial infarction within 60 days, hypertrophic or infiltrative cardiomyopathy, primary renal or hepatic disease, high output HF, and constrictive pericarditis were excluded. Control subjects were referred for invasive exercise assessment because of symptoms of exercise intolerance, but displayed no demonstrable cardiac aetiology for symptoms, with normal rest and exercise PA pressures (rest <25 mmHg, exercise <40 mmHg) and normal rest-exercise PCWP (<15 mmHg rest and <25 mmHg exercise).
Study protocol
After providing consent, subjects underwent history and physical examination and comprehensive resting echocardiogram. Cardiac catheterization was then performed with simultaneous echocardiography and expired gas analysis at rest and during supine exercise. The first stage of exercise (20 Watts, W) was performed for 5 min to allow for adequate echocardiographic data acquisition (below) and was followed by graded 10 W increments in workload (3 min stages) to subject-reported exhaustion (peak workload).
Catheterization protocol
Subjects were studied on chronic medications in the fasted state after minimal sedation in the supine position as previously described.3,14,15 Right heart catheterization was performed through a 9 Fr sheath via the right internal jugular vein. Transducers were zeroed at mid-axilla, measured by laser calipers. Pressures in the right atrium, PA, and PCWP were measured at end expiration (mean of ≥3 beats) using 2 Fr high-fidelity, micromanometer-tipped catheters (Millar Instruments, Houston, TX, USA) advanced through the lumen of a 7 Fr fluid-filled catheter (Balloon wedge, Arrow). Pulmonary capillary wedge pressure position was verified by fluoroscopy, typical waveforms, and direct oximetry (PCWP blood saturation ≥94%). Right atrial pressure and PCWP were measured at mid a wave.
Pressure tracings from the entire study were recorded, digitized (240 Hz), and stored for offline analysis by one investigator (B.A.B.). Arterial blood pressure (BP) was measured continuously through a 4–6 Fr radial arterial cannula. Oxygen consumption (VO2) was measured directly and continuously throughout the study (below). Systemic and PA O2 contents were determined by oximetry (saturation × haemoglobin × 1.34 × 10) to calculate direct arterial-venous O2 content difference (AVO2diff = systemic – PA O2 content).
Cardiac output (CO) was determined by the direct Fick method (CO = VO2/AVO2diff) at baseline, 20 W, and peak exercise. Stroke volume (SV) was determined from the quotient of CO and heart rate (HR). Pulmonary vascular resistance (PVR = [mean PA − PCWP]/CO) and PA compliance (PAC = SV/[PA pulse pressure]) were calculated.
Echocardiography
Two-dimensional, M-mode, Doppler, and tissue Doppler echocardiography was performed according to the contemporary guidelines by experienced sonographers.16–18 Echocardiographic data were obtained simultaneously with invasive assessment at rest and during all stages of exercise. All studies were interpreted offline and in blinded fashion by a single investigator with experience in resting and exercise echocardiographic assessment (G.C.K.). Left ventricular systolic and diastolic function was assessed using mitral annular tissue velocities using the average of values measured at the lateral and septal annulus.16,17 Right ventricular systolic and diastolic function was assessed using lateral tricuspid annular tissue velocities.18 Right ventricular length and systolic/diastolic diameters were measured at the base and mid-ventricle perpendicularly to septum.
Expired gas analysis
Oxygen consumed (VO2), carbon dioxide produced (VCO2), and minute ventilation (VE) were measured by mouth piece and pneumotachograph (MedGraphics, St. Paul, MN, USA) throughout exercise as previously described.14,15 Respiratory exchange ratio was calculated as VCO2/VO2. Manual calibration was performed immediately prior to each test. Data were averaged over the last 30 s of each stage. Peak VO2 was defined as the mean of the last 30 s of the exercise test. Gas exchange data were interpreted offline in a blinded fashion by a single investigator with experience in cardiopulmonary exercise test interpretation (T.P.O.).14,15
Statistical analysis
Results are reported as mean (SD), median (IQR), or number (%). Within-group differences are assessed by paired t-test. Between-group differences were compared by ANOVA, χ 2, or Fisher's exact test (single time point) or two-way repeated measures ANOVA incorporating baseline, submaximal (20 W), and peak exercise responses. Multivariable linear regression analysis was used to adjust for relevant baseline group differences. For non-normally distributed variables entered into regression models, the assumption of normally distributed residuals was verified by Quantile plots, and no violations were observed. A mixed effects model was used for regression analyses where repeated measures were assessed. Analyses were performed using JMP 10.0.0 SAS Institute, Cary, NC, USA.
Results
Of 106 subjects enrolled consecutively, 50 and 24 subjects met our prespecified criteria defining HFpEF and control, respectively. In the HFpEF cohort, 32 subjects (64%) had elevated PCWP both at rest and exercise, while 18 (36%) displayed elevated PCWP only during exercise. Similar to previous studies, subjects with HFpEF were older, heavier, and more likely to have a history of systemic hypertension, kidney disease, and anaemia when compared with controls (Table 1 ). Sex, medication use, and prevalence of coronary disease and diabetes were similar. As expected, HFpEF subjects displayed more congestion when compared with controls, evidenced by higher NT-proBNP levels, greater jugular distention, and more peripheral oedema.
Table 1.
Baseline characteristics
| Control (n = 24) | HFpEF (n = 50) | P | |
|---|---|---|---|
| Age (years) | 61 ± 12 | 70 ± 11 | 0.002 |
| Female, n (%) | 11 (46) | 27 (54) | 0.5 |
| Body mass index (kg/m2) | 27.2 ± 4.4 | 34.4 ± 6.9 | <0.001 |
| Comorbidities | |||
| Coronary disease, n (%) | 6 (25) | 18 (36) | 0.3 |
| Diabetes mellitus, n (%) | 5 (21) | 18 (36) | 0.3 |
| Hypertension, n (%) | 15 (63) | 47 (94) | 0.001 |
| Medications | |||
| ACE or ARB, n (%) | 10 (42) | 33 (66) | 0.048 |
| β-blocker, n (%) | 11 (46) | 33 (66) | 0.10 |
| Loop diuretic, n (%) | 5 (21) | 20 (40) | 0.12 |
| Laboratories and exam | |||
| Haemoglobin (g/dL) | 13.9 ± 1.2 | 12.6 ± 1.3 | <0.001 |
| Creatinine (g/dL) | 1.0 (0.8, 1.1) | 1.2 (0.9,1,6) | 0.011 |
| NT-proBNP (pg/mL) | 76 (38, 228) | 549 (161, 2126) | <0.001 |
| JVP (<8/8-12/12-16/>16 cm) | 24/0/0/0 | 26/15/2/7 | <0.001 |
| Oedema (none/mild/mod-sev) | 23/1/0 | 30/13/7 | 0.004 |
| LV structure | |||
| LV diastolic dimension (mm) | 48 ± 6 | 48 ± 6 | 0.7 |
| LV end diastolic volume (mL) | 110 ± 40 | 110 ± 53 | 0.9 |
| LV mass index (gm/m2) | 88 ± 20 | 86 ± 23 | 0.7 |
| LV ejection fraction (%) | 60 ± 9 | 62 ± 8 | 0.4 |
| RV structure | |||
| RV diastolic diameter, mid (mm) | 29 ± 7 | 31 ± 6 | 0.4 |
| RV diastolic diameter, base (mm) | 34 ± 8 | 38 ± 6 | 0.043 |
Resting ventricular structure and regional mechanics
Left ventricular dimensions, mass, volumes, and EF were similar in HFpEF and control subjects (Table 1 ). In contrast, LV diastolic (e′) and systolic (s′) tissue velocities were lower in HFpEF subjects while E/e′ ratios were higher compared with controls (Table 2 ). These differences persisted after adjusting for age and BMI (all P < 0.05). Right ventricular basal diameter was somewhat greater in HFpEF but all other measures of RV systolic and diastolic function were similar in HFpEF and controls when measured at rest (Tables 1 and 2).
Table 2.
Baseline and exercise haemodynamics, metabolism, and ventricular function
| Baseline |
20 W exercise |
Peak exercise |
P-valueª | ||||
|---|---|---|---|---|---|---|---|
| Control | HFpEF | Control | HFpEF | Control | HFpEF | ||
| HR (bpm) | 68 ± 13 | 67 ± 10 | 91 ± 14 | 88 ± 14 | 121 ± 18 | 97 ± 15 | <0.001 |
| SBP (mmHg) | 139 ± 24 | 149 ± 21 | 167 ± 29 | 175 ± 26 | 184 ± 25 | 185 ± 30 | 0.2 |
| MBP (mmHg) | 91 ± 13 | 96 ± 13 | 106 ± 15 | 108 ± 15 | 111 ± 12 | 111 ± 15 | 0.3 |
| Central haemodynamics | |||||||
| RAP (mmHg) | 4 ± 2 | 10 ± 4* | 8 ± 3 | 21 ± 5* | 8 ± 4 | 22 ± 6* | <0.001 |
| PASP (mmHg) | 27 ± 6 | 41 ± 12* | 37 ± 10 | 66 ± 12* | 41 ± 9 | 70 ± 13* | <0.001 |
| mPAP (mmHg) | 16 ± 4 | 27 ± 8* | 25 ± 7 | 47 ± 10* | 27 ± 6 | 48 ± 8* | <0.001 |
| PCWP (mmHg) | 7 ± 3 | 17 ± 6* | 13 ± 5 | 31 ± 5* | 14 ± 5 | 34 ± 6* | <0.001 |
| PVR (WU) | 1.9 ± 0.8 | 2.0 ± 1.2 | 1.5 ± 0.7 | 2.3 ± 1.2† | 1.3 ± 0.6 | 2.1 ± 1.2 | 0.016 |
| PAC (mL/mmHg) | 4.4 ± 1.5 | 3.6 ± 1.3‡ | 4.8 ± 2.2 | 2.6 ± 1.2* | 4.3 ± 1.6 | 2.6 ± 1.4* | <0.001 |
| Integrated function | |||||||
| VO2 (mL/min) | 211 ± 61 | 242 ± 54‡ | 640 ± 99 | 629 ± 130 | 1162 ± 389 | 809 ± 220* | <0.001 |
| VO2 (mL/min/kg) | 2.60 ± 0.49 | 2.49 ± 0.54 | 8.13 ± 1.62 | 6.54 ± 1.60† | 14.4 ± 4.1 | 8.3 ± 2.1* | <0.001 |
| Sa O2 (mL/dL) | 16.8 ± 1.7 | 15.0 ± 1.9† | 17.2 ± 1.7 | 15.5 ± 2.0† | 17.8 ± 1.7 | 15.9 ± 2.0† | 0.9 |
| PA O2 (mL/dL) | 12.6 ± 1.7 | 10.2 ± 1.6* | 9.4 ± 2.2 | 6.1 ± 1.8* | 7.9 ± 2.4 | 5.6 ± 1.9* | <0.001 |
| AVO2diff (mL/dL) | 4.1 ± 0.9 | 4.8 ± 0.9† | 7.9 ± 1.4 | 9.3 ± 1.9† | 9.9 ± 1.7 | 10.4 ± 2.3 | 0.002 |
| CO (L/min) | 5.2 ± 1.8 | 5.1 ± 1.2 | 8.2 ± 1.8 | 6.8 ± 2.0† | 11.8 ± 3.9 | 8.1 ± 2.8* | <0.001 |
| SV (mL) | 77 ± 23 | 77 ± 19 | 93 ± 24 | 78 ± 21† | 98 ± 28 | 83 ± 27‡ | 0.007 |
| Ventricular mechanics | |||||||
| RV e′ (cm/s) | 11 ± 5 | 12 ± 9 | 13 ± 5 | 11 ± 5 | 19 ± 9 | 12 ± 6 | 0.010 |
| RV s′ (cm/s) | 12 ± 3 | 11 ± 3 | 13 ± 4 | 10 ± 3 | 14 ± 4 | 11 ± 3 | 0.044 |
| LV e′ (cm/s) | 8.5 ± 2.1 | 6.9 ± 2.0† | 10 ± 3 | 7 ± 2 | 13 ± 5 | 8 ± 3* | <0.001 |
| LV s′ (cm/s) | 7.5 ± 1.9 | 6.4 ± 1.5† | 8 ± 2 | 7 ± 2‡ | 11 ± 3 | 8 ± 2† | 0.025 |
ªTwo-way RMANOVA for comparing the exercise-dependent change in each variable between groups.
*P<0.001 vs. control.
†P<0.01 vs. control.
‡P<0.05 vs. control for single time point comparisons.
Resting haemodynamics
Baseline HR and systemic BP were similar in HFpEF and control subjects (Table 2 ). Right and left heart filling pressures and PA pressures were normal in control subjects and mildly elevated at rest in HFpEF subjects (all P < 0.001 vs. controls in univariate analyses and after adjusting for age and BMI). Pulmonary artery compliance was lower in HFpEF subjects at rest while PVR was similar (Table 2 ).
Oxygen consumption was mildly elevated at rest in HFpEF but not after adjusting for body mass (Table 2 ). Baseline systemic and PA O2 contents were lower in HFpEF subjects. Resting SV and CO were similar in HFpEF and control subjects, though AVO2diff was higher in HFpEF (Table 2 ) even after adjusting for haemoglobin (P < 0.001), suggesting relative tissue hypoperfusion despite similar CO in HFpEF compared with control subjects at rest.
Exercise performance and integrated function
Exercise capacity was impaired in HFpEF compared with controls (Table 2 ). Borg symptoms scores were more severe in HFpEF than controls for dyspnoea (5 ± 2 vs. 3 ± 2, P = 0.015) and fatigue (13 ± 3 vs. 11 ± 2, P = 0.006) during submaximal exercise (20W), but there were no differences in perceived ratings of dyspnoea (7 ± 2 vs. 7 ± 2, P = 0.9) or fatigue (17 ± 2 vs. 17 ± 3, P = 0.9) at peak exercise. Heart failure and preserved ejection fraction subjects tended to display lower peak respiratory exchange ratios with exercise (1.05 [IQR 1.01–1.13] vs. 1.03 [IQR 0.95–1.10], P = 0.12) and higher VE/VCO2 slopes (31.5 [IQR 28.5–36.4] vs. 34.5 [IQR 30.6–40.8], P = 0.11). Heart failure and preserved ejection fraction patients reached lower peak workload compared with controls (36 ± 15 vs. 70 ± 29 W, P < 0.001). Total VO2 (mL/min) at 20 W exercise was similar in cases and controls but VO2/kg at 20 W was lower in HFpEF subjects. Total and weight-scaled VO2 at peak exercise were 30–40% lower in HFpEF compared with controls (P < 0.001, Table 2 ).
Increases in CO with submaximal (20 W) and peak exercise were depressed in HFpEF subjects compared with controls secondary to limitations in SV reserve and chronotropic incompetence, resulting in blunted increase in CO relative to metabolic demand (lower ΔCO/ΔVO2) (Figure 1A–D and Table 2 ). Each of these differences persisted after adjusting for age and BMI, except for peak exercise SV (P = 0.09). Systemic and PA O2 contents were lower in HFpEF compared with controls at all stages, while AVO2diff was greater in HFpEF at 20 W but similar at peak (Table 2 and Figure 1E and F).
Figure 1.
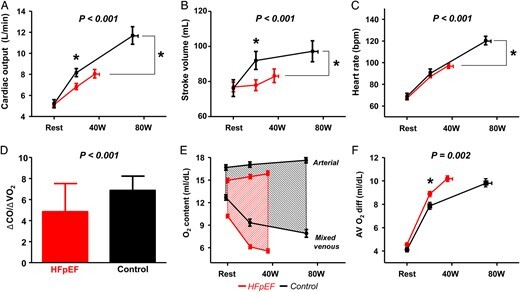
Baseline, low-level exercise (20 W), and peak exercise haemodynamics shown in heart failure with preserved ejection fraction subjects (red) and controls (black) for cardiac output (A), stroke volume (B), heart rate (C), cardiac output reserve relative to oxygen consumption (ΔCO/ΔVO2) (D), and arterial-venous oxygen contents and difference (AVO2diff) (E and F). P-value refers to group–exercise interaction comparison (RMANOVA) characterizing exercise reserve. *P < 0.01 between groups for single time point comparisons.
Exercise haemodynamic responses
At submaximal and peak exercise, HFpEF subjects displayed two- to three-fold higher right and left heart filling pressures (Table 2 ). Pulmonary artery pressures rose approximately two-fold more in HFpEF compared with controls in association with impaired pulmonary vasodilation, manifest by lesser decreases in PVR and greater reduction in PA compliance (Figure 2A and B). These differences remained significant after adjusting for age and BMI. Accordingly, subjects with HFpEF displayed a three-fold steeper PA pressure-flow relationship compared with controls (7.0 [IQR 5.3–12.7] vs. 2.0 [IQR 0.9–2.8] mmHg/L/min, P < 0.001; Figure 2C). Pulmonary vascular resistances at rest and during exercise varied inversely with PA O2 saturation in HFpEF subjects, but not in controls (Figure 2D).
Figure 2.
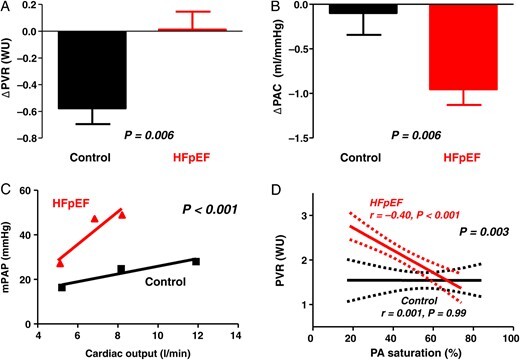
When compared with controls (black), subjects with heart failure with preserved ejection fraction (red) displayed impaired pulmonary vasodilation, with less reduction in pulmonary vascular resistance (A), greater reduction in pulmonary artery compliance (B), and steeper slope of pulmonary artery pressure-flow relationship (C). In heart failure with preserved ejection fraction subjects, pulmonary vascular resistance increased with decreasing mixed venous oxygen saturation (pulmonary artery), whereas no such relationship was observed in controls (D).
There were no differences between HFpEF and control subjects in systemic arterial BP responses to exercise. Peak VO2 was correlated with peak exercise SV (r = 0.74, P < 0.001), PA compliance (r = 0.73, P < 0.001), PVR (r = −0.56, P < 0.001), HR (r = 0.49, P < 0.001), RAP (r = −0.48, P < 0.001), mean PA pressure (r = −0.45, P < 0.001), and PCWP (r = −0.44, P < 0.001).
Ventricular mechanics and exercise haemodynamics
Compared with controls, subjects with HFpEF displayed impaired right and left ventricular reserve with exercise, manifest by less increase in RV and LV systolic (s′) and diastolic (e′) tissue velocities (Table 2 and Figures 3 and 4). Each of these differences persisted after adjusting for age and BMI. Inability to enhance peak exercise systolic mechanics was correlated with impaired diastolic reserve in both the RV and LV (Figure 3 ). The inability to enhance diastolic mechanics in the RV and LV with exercise was directly correlated with greater increases in right and left heart filling pressures (Figure 4 ). Diastolic ventricular reserve responses in the left and right ventricles were also strongly correlated with exercise CO reserve (r = 0.8, P < 0.001 for LV e′; r = 0.6, P < 0.001 for RV e′).
Figure 3.
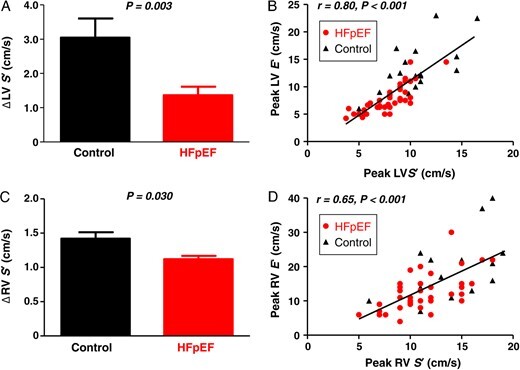
(A–D) Compared with controls (black), subjects with heart failure with preserved ejection fraction (red) showed less increase in left and right ventricular systolic velocities (LV S′, RV S′) during exercise that were correlated with reduced diastolic reserve in the respective chambers (LV/RV E′).
Figure 4.
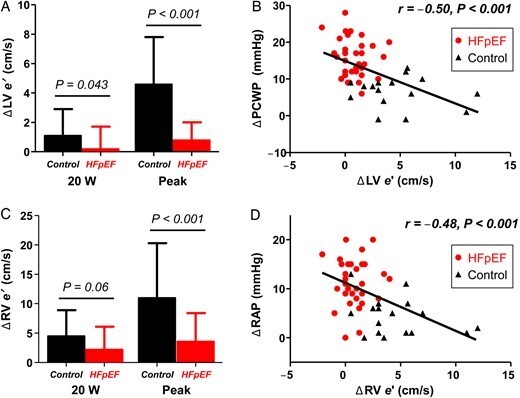
Compared with controls (black), subjects with heart failure with preserved ejection fraction displayed less increase in left ventricular (A) and right ventricular (C) diastolic mechanics (E′ velocities) at low level (20 W) and peak exercise. Impaired enhancement in diastolic mechanics was associated with greater increases in pulmonary capillary wedge pressure (B) and right atrial pressure (D).
Discussion
We show for the first time that similar to the LV, RV reserve during exercise is impaired in people with HFpEF, even in the early stages of the disease where right-sided structural remodelling is absent. Impaired RV reserve in HFpEF is coupled with exercise-induced pulmonary hypertension caused by passive LV congestion as well as inadequate pulmonary vasodilatation associated with venous hypoxia. We further demonstrate that the characteristic haemodynamic perturbations developing during exercise in HFpEF (high filling pressures, depressed CO reserve) are strongly correlated with inadequate enhancement in diastolic mechanics in the RV and LV, which in turn is strongly associated with impairments in systolic reserve in the respective chambers. These data provide compelling evidence that right heart disease is not restricted to patients with advanced LV failure. Rather, these data show that myocardial dysfunction in HFpEF is a biventricular process that affects both diastole and systole. As such, therapies that improve cardiac myocyte function throughout the cardiac cycle and in both the right and left heart would be expected to be most effective to improve the cardiac limitations that contribute to exercise intolerance in people with HFpEF.
Right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction
Similar to other abnormalities in early stage HFpEF,3,9 pulmonary vascular limitations were not apparent at rest in the current study but were unmasked during exercise, manifest by abnormal changes in pulmonary resistance and compliance (Figure 2 ). Coupled with greater elevation in pulmonary venous pressures, these changes culminate in steeper PA pressure-flow relationship in HFpEF subjects compared with controls, which we demonstrate for the first time in HFpEF. The latter index of PA vascular function is superior to single time point measures like PVR because it also incorporates pulmonary vascular distensibility and vascular recruitment that are not assessable at rest. The steeper PA pressure-flow slope in HFpEF increases RV afterload for any given CO19,20 and may serve as a novel endpoint for pharmacologic therapies targeting abnormal haemodynamics in HFpEF, as recently demonstrated.21
Venous hypoxia during exercise was more profound in HFpEF subjects, and intriguingly PVR varied inversely with PA O2 saturation in HFpEF subjects but not controls, suggesting a possible role of hypoxia-induced pulmonary vasoconstriction in HFpEF. This may partly explain why some HFpEF patients go on to develop a ‘mixed’ PH phenotype, with greater PVR, lower PA compliance, and worse outcomes despite similar PCWP.22
We show for the first time that exercise-induced PH in HFpEF was associated with impaired RV systolic and diastolic reserve (Figures 3 and 4). While this is consistent with depressed RV reserve in response to dobutamine stimulation in HFpEF as recently reported,15 there are important differences between these two stresses. In contrast to β-adrenergic stimulation which unloads the RV through reduction in PA pressures, exercise markedly elevates RV afterload through increases in downstream PCWP and PA pressures in HFpEF. Right ventricular preload is also challenged more by exercise via greater increases in CO and venous return compared with dobutamine. Because physical activity is the most common stress encountered by the cardiovascular system in daily life, cardiovascular responses to exercise are much more physiologically relevant than changes with isolated adrenergic stimulation. The current data suggest a potentially important role for right-sided afterload reduction in HFpEF at rest and during exercise, which could be accomplished using pulmonary vasodilators15 or simply reduction in exercise PCWP.23
In the current study, RV structure and function were similar to controls at rest, in contrast to recent studies in more advanced HFpEF patients.11,13 We speculate that this was related to the earlier stage of HFpEF severity in the current sample. Importantly, the clear abnormalities shown in RV–PA interaction during exercise suggest that there may be an opportunity to alter the course of HFpEF progression using novel therapies targeting coupling reserve at the level of the right ventricle or pulmonary artery.
Myocardial reserve and haemodynamics in heart failure with preserved ejection fraction
Cardiac output limitation in the current study was related to both chronotropic incompetence and inadequate SV reserve, confirming and extending upon prior studies.2–5,8,9,24 Despite preserved LVEF, there are subtle deficits in LV systolic function in HFpEF that are associated with adverse outcome.7,25–28 These mild systolic limitations at rest may become dramatic during exercise, as observed in the current study and others—leading to marked systolic reserve limitation.5,7–9,27 During ejection, the mitral and tricuspid annuli shorten towards the apex of the heart. The velocity of this motion (s′) serves as a measure of contractile function. During early diastole, the mitral and tricuspid annuli recoil away from the apex. This motion enhances transfer of blood from atrium to ventricle during early diastole, minimizing the requirement for an increase in atrial pressure to drive ventricular filling.29
Systolic reserve is intimately related to diastolic reserve (Figure 3 ). With more vigorous contraction below the ‘unstressed’ right or left ventricular volume, there is greater elastic recoil in the subsequent diastole serving to augment diastolic motion. This enhancement in e′ plays an important role in generating early diastolic ‘suction gradient’ to aid ventricular filling.7,27,30 These invasive data obtained using gold standard techniques importantly extend upon what has been reported from non-invasive studies,7,27 showing for the first time that inadequate enhancement in ventricular mechanics during systole and particularly diastole contributes to greater increases in atrial pressure as well as impairments in CO reserve that importantly contribute to limitations in exercise capacity, though the strength of association with PCWP was relatively modest (r = 0.5).
The combined limitation in systolic and diastolic reserve, which was observed in both the right and left side of the heart points to the likelihood that systemic processes affecting the heart are central in HFpEF. Candidate mechanisms in this regard include abnormalities in nitric oxide metabolism, oxidative stress, altered myocardial energetic availability, or cardiovascular senescence.31–33 These findings support development and testing of novel small molecule drugs that affect cardiomyocyte function in both ventricles, for example, agents affecting the sarcomeres, calcium handling, or mitochondrial respiration. Further study is required to better understand the candidate targets in this regard.
Cardiac limitations and implications for therapy
According to the Fick principle, VO2 is equal to the product of CO and arterial-venous O2 content difference (AVO2diff).34 Thus, a reduction in VO2 could be caused by impairments in CO, AVO2diff, or both. Total VO2 (unscaled to body weight) was similar in HFpEF and controls during matched submaximal exercise (20 W) despite lower CO in HFpEF. To maintain a similar total VO2, HFpEF patients relied on greater increases in AVO2diff, possibly reflecting enhanced O2 extraction. This may be an adaptation to chronic hypoperfusion in patients with HFpEF, or simply a repercussion of greater time available in the gas diffusion out of the skeletal muscle capillaries when flow is depressed.4
Recent studies have reported that VO2 limitations in HFpEF may also be predominantly related to abnormalities in AVO2diff reserve.24,34–36 In contrast, we observed that the increase in CO relative to metabolic demand (ΔCO/ΔVO2) was substantially lower in HFpEF compared with controls, similar to previous studies from our group and as well as others.4,20 We speculate that the discrepant findings between studies may relate to pathophysiologic heterogeneity.34 Patients in the current study as well as others were clearly limited by the heart,4,20 whereas others appear to be limited more by the periphery.24,35,36 This heterogeneity may partly explain the absent response to standard HF therapies noted in previous trials in HFpEF,37 and it suggests a potential role for exercise testing to appropriately phenotype individual patients with HFpEF.34
New therapies hold promise to improve cardiac abnormalities present in the LV and RV in HFpEF. A recent placebo-controlled trial demonstrated that sodium nitrite, which is reduced to nitric oxide in vivo, selectively reduces LV and RV filling pressures in HFpEF during exercise while restoring CO reserve and improving pulmonary artery pressure-flow relationships.21 This study also showed that ventricular stroke work improved with nitrite during exercise, indicating a direct myocardial effect independent of changes in load. Longer term studies testing inorganic nitrite in HFpEF are currently in the planning stages. Chronotropic incompetence was also an important contributor to depressed CO reserve in HFpEF, as previously reported.4,5,9,24 An ongoing study is testing whether rate-adaptive atrial pacing can improve exercise capacity in HFpEF (NCT02145351).
Limitations
The subjects enrolled in this prospective study were referred for cardiac catheterization, presenting referral bias. The control group was not completely ‘normal’ in that participants had prevalent cardiovascular diseases including hypertension, diabetes, and coronary disease. Control subjects displayed no objective cardiac limitations that could account for symptoms of exercise intolerance, proving that they did not have cardiac failure, but their peak VO2 values were lower than one would expect based upon population norms. Part of this difference relates to the lower VO2 achieved with supine ergometry as opposed to upright treadmill exercise, but there may also be non-cardiac pathophysiologic factors underpinning exercise limitation in the control population that remain unexplained. Importantly, the fact that the control group was not completely healthy only biases our results toward the null. The finding of impaired CO reserve was based upon direct Fick assessment of flow rather than indicator-dilution methods which may show greater variability during exercise. There were baseline differences which might confound our results, in particular older age and increased BMI in HFpEF, but all key observations remained significant after adjusting for these baseline differences.
Conclusion
The current results show that pathologic elevations in filling pressures and inadequate generation of forward output during exercise in HFpEF are related to limitations in myocardial reserve that is present not only in the left ventricle but also the right heart. This indicates that HFpEF is a biventricular disorder that affects both diastole and systole. It follows from this observation that common insults affecting cardiomyocytes in both sides of the heart drive the haemodynamic determinants of symptoms and exercise intolerance. Novel therapies targeting biventricular and pulmonary vascular reserve hold promise to improve exercise capacity and quality of life in people with HFpEF.
Authors’ contributions
B.B. performed statistical analysis, handled funding and supervision, and drafted the manuscript. All authors acquired the data, conceived and designed the research, and made critical revision of the manuscript for key intellectual content.
Funding
This research was supported by a competitive prospective grants award from the Mayo Clinic and Foundation.
Conflict of interest: none declared.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 2.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 2010;56:855–863. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013;15:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 2006;114:2138–2147. [DOI] [PubMed] [Google Scholar]
- 6.Ennezat PV, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, Bauchart JJ, Mounier-Vehier C, Jude B, Neviere R, Bauters C, Asseman P, de Groote P, Lejemtel TH. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail 2008;14:475–480. [DOI] [PubMed] [Google Scholar]
- 7.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 2009;54:36–46. [DOI] [PubMed] [Google Scholar]
- 8.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 2009;54:402–409. [DOI] [PubMed] [Google Scholar]
- 9.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009;53:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2014;7:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014;130:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail 2015;8:41–48. [DOI] [PubMed] [Google Scholar]
- 15.Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K, Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail 2015;8:542–550. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 18.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 19.Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013;128:1470–1479. [DOI] [PubMed] [Google Scholar]
- 20.Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB, Systrom DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: implications for heart failure with preserved ejection fraction. Circ Heart Fail 2015;8:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 2015;66:1672–1682. [DOI] [PubMed] [Google Scholar]
- 22.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012;126:975–990. [DOI] [PubMed] [Google Scholar]
- 23.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012;125:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 2011;58:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 2009;54:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohara T, Niebel CL, Stewart KC, Charonko JJ, Pu M, Vlachos PP, Little WC. Loss of adrenergic augmentation of diastolic intra-LV pressure difference in patients with diastolic dysfunction: evaluation by color M-mode echocardiography. JACC Cardiovascular Imaging 2012;5:861–870. [DOI] [PubMed] [Google Scholar]
- 28.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015;132:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little WC, Oh JK. Echocardiographic evaluation of diastolic function can be used to guide clinical care. Circulation 2009;120:802–809. [DOI] [PubMed] [Google Scholar]
- 30.Opdahl A, Remme EW, Helle-Valle T, Lyseggen E, Vartdal T, Pettersen E, Edvardsen T, Smiseth OA. Determinants of left ventricular early-diastolic lengthening velocity: independent contributions from left ventricular relaxation, restoring forces, and lengthening load. Circulation 2009;119:2578–2586. [DOI] [PubMed] [Google Scholar]
- 31.Rider OJ, Francis JM, Ali MK, Holloway C, Pegg T, Robson MD, Tyler D, Byrne J, Clarke K, Neubauer S. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation 2012;125:1511–1519. [DOI] [PubMed] [Google Scholar]
- 32.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 33.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res 2014;115:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little WC, Borlaug BA. Exercise intolerance in heart failure with preserved ejection fraction: what does the heart have to do with it? Circ Heart Fail 2015;8:233–235. [DOI] [PubMed] [Google Scholar]
- 35.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 2011;13:1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 2015;8:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011;32:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]


