Abstract
Introduction:
The purpose of this study is to identify issues faced by Federally Qualified Health Centers (FQHCs) in implementing lung cancer screening in low-resource settings.
Methods:
Medical directors of 258 FQHCs serving communities with tobacco use prevalence above the median of all 1,202 FQHCs nationally were sampled to participate in a web-based survey. Data were collected between August and October 2016. Data analysis was completed in June 2017.
Results:
There were 112 (43%) FQHC medical directors or surrogates who responded to the 2016 survey. Overall, 41% of respondents were aware of a lung cancer screening program within 30 miles of their system’s largest clinic. Although 43% reported that some providers in their system offer screening, it was typically at a very low volume (less than ten/month). Although FQHCs are required to collect tobacco use data, only 13% indicated these data can identify patients eligible for screening. Many FQHCs reported important patient financial barriers for screening, including lack of insurance (72%), preauthorization requirements (58%), and out-of-pocket cost burdens for follow-up procedures (73%). Only 51% indicated having adequate access to specialty providers to manage abnormal findings, and few reported that leadership had either committed resources to lung cancer screening (12%) or prioritized lung cancer screening (12%).
Conclusions:
FQHCs and other safety-net clinics, which predominantly serve low-socioeconomic populations with high proportions of smokers eligible for lung cancer screening, face significant economic and resource challenges to implementing lung cancer screening. Although these vulnerable patients are at increased risk for lung cancer, reducing patient financial burdens and appropriately managing abnormal findings are critical to ensure that offering screening does not inadvertently lead to harm and increase disparities
INTRODUCTION
Numerous professional organizations now recommend low-dose computed tomography (LDCT) lung cancer screening for high-risk smokers,1–6 based on the National Lung Screening Trial that showed that screening with LDCT reduced lung cancer mortality by 20% compared with chest radiography.7 In February 2015, Centers for Medicare and Medicaid Services began covering LDCT screening with a written prescription from a physician and documentation of shared decision making.8 Healthcare systems and providers are beginning to offer lung cancer screening to eligible patients but uptake has been slow. A survey of Society of Thoracic Radiology members showed an increase in the number of screening programs from 50 to 62 between 2013 and 2014, but less than half of the institutions reported screening more than 50 patients in the preceding year.9 Recent data from the 2015 National Health Interview Survey highlighted that only 5.8% of the target population has been screened for lung cancer.10
Evidence of barriers to implementation is also emerging.11–13 An early survey of pulmonologists identified that insufficient infrastructure and personnel are potential barriers to implementation.14 Additionally, a 2014 survey found that 11 states had no identified LDCT screening centers and many states with high rates of lung cancer incidence and mortality had limited screening capacity, particularly in rural areas.15
The high societal, health system, and individual patient costs of lung cancer screening have been recognized.16 Medicare costs could approach $7 billion over a 5-year time horizon.17 The Veterans Health Administration has estimated initial costs of $500 to $900 million to screen eligible veterans.18 Although current insurance regulations require that services recommended by U.S. Preventive Services Task Force are fully covered with no patient copay, insured patients can be responsible for substantial portions of costs associated with follow-up procedures. A recent review of 13 economic evaluations of lung cancer screening concluded that there is currently too much uncertainty about critical parameters of delivering lung cancer screening to quantify the true cost of screening and determine whether LDCT screening is cost effective.19 These parameters include the ability of screening programs to identify appropriate populations at risk for lung cancer and the frequency and costs of managing both suspicious lung cancer findings and incidental findings for non-cancer abnormalities.
In 2016, the Society of Behavioral Medicine highlighted that disparities endemic to lung cancer will remain and may be exacerbated by gaps in implementation of high-quality screening among high-risk populations.5 This is because a disproportionate burden of lung cancer incidence and mortality largely tracks disparities associated with higher tobacco use among individuals with fewer socioeconomic resources; some racial/ethnic minority groups; individuals residing in rural areas; the lesbian, gay, bisexual, transgender, and questioning community; and individuals with psychiatric comorbidity. They emphasized the importance of targeting efforts to reach underserved populations and provided a specific recommendation to expand the resource capacity for lung cancer screening within Federally Qualified Health Centers (FQHCs). FQHCs are safety-net clinics, often located in rural areas, whose underserved populations have a high burden of tobacco use. FQHCs comprise about 90% of the Community Health Centers program, which cared for more than 24 million low-income patients in the U.S. in 2015.20 Although FQHCs are mandated to provide preventive services, they may face substantial challenges to implementing lung cancer screening given their unique patient population of underinsured and uninsured individuals. Recognizing the need for guidance about implementing screening in high-risk, underserved populations, this study surveyed a national sample of FQHC medical directors to explore potential barriers that impact screening access, uptake, and adherence in FQHC populations.
METHODS
Using data from the 2013 Uniform Data System (UDS), 258 of the 1,202 FQHCs serving a catchment area with a patient population above the median of tobacco use (>26% of adult patients) were selected for the survey. Because this study’s budget was limited, this approach was used to select clinics with the patient populations most likely to be eligible for lung cancer screening rather than a random national sample of clinics. The medical director for each FQHC was individually invited by email to complete the web-based survey. Up to five follow-up emails were sent. If the email for a medical director could not be identified, the survey was redirected to be completed by the chief operating officer, quality officer, or another individual knowledgeable about the site’s tobacco assessment and assistance practices. All survey recipients were advised to pass the survey on to someone more knowledgeable of current practices, if applicable. Only one survey per FQHC was accepted. Upon completion, participants received a $100 gift card as an incentive. Data collection began in August 2016 and concluded in October 2016. Data analysis was completed in June 2017.
The survey assessed FQHCs’ current tobacco assessment and assistance practices, the degree to which they utilize the electronic health record (EHR) for documentation and tracking, and their connection to resources to conduct lung cancer screening using LDCT for high-risk patients. Early versions of the survey were pilot tested with clinicians practicing at FQHCs to provide feedback on the item content, wording, and the overall length and flow of the survey. The study protocol was reviewed and approved by the Case Western Reserve University IRB.
Descriptive statistics of responses are reported. Bivariate associations were used to examine characteristics of two groups: FQHCs that reported they were aware of providers offering lung cancer screening versus FQHCs that reported not offering lung cancer screening. Respondents who reported being unsure whether lung cancer screening was offered at their FQHC were combined with the not offering screening group. Associations were evaluated using p<0.05 and were tested using chi-square and ANOVA. Multivariable analyses were conducted to determine which variables were independently associated with report of FQHC engagement in lung cancer screening. Descriptive characteristics of the population served by each FQHC and number of full-time equivalent employee clinicians and clinical sites were drawn from the UDS indicators reported by each FQHC in 2013.
RESULTS
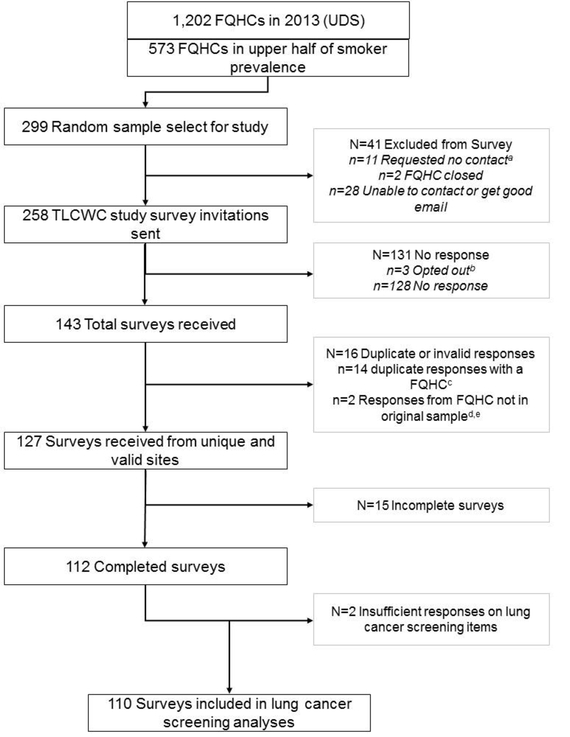
Of the 299 FQHCs selected for the random sample, 258 were invited for the survey after excluding 41 determined to be closed or involved in data collection for a related study of colorectal cancer screening. Representatives from 110 FQHCs completed the survey for a 43% response rate. Based on clinic characteristics in the UDS data, there were no statistically significant differences between responders and nonresponders with regard to the number of sites, the proportion of adults aged 55–74 years, the proportion of adults using tobacco, and the proportion located in an urban setting.
Characteristics of the participating sites are reported in Table 1. Among the respondents, 47 (43%) FQHCs reported providers are offering some lung cancer screening, 43 (39%) reported providers are not currently offering screening and 22 (20%) reported they did not know if providers were offering screening. All clinics were asked about resources and infrastructure to support lung cancer screening. Overall, 45 (41%) reported they were aware of a LDCT screening center ≤30 miles of their sites main clinic. Although current smoking status is ascertained by all clinics, only 59 (54%) indicated that pack-year history, which is required to determine eligibility for LDCT screening, is routinely documented in their EHR for all eligible patients. Of sites with pack-year history information available, only 29% indicated the information was reliably very accurate (e.g., would use for patient care decisions), and only 13% indicated the EHR data could be queried to identify eligible patients. With the exception of greater access to a LDCT screening center, the resources to support lung cancer screening were similar across groups. Notably, of the 47 FQHCs indicating providers offer screening, only three clinics reported screening more than ten patients per month. Of the sites that are offering screening, eight reported using a reminder in their EHR to alert providers about a patient’s eligibility and only three reported using a patient reminder system to encourage adherence to repeat LDCT visits. Of those clinics offering screening, ten (21%) reported actively tracking abnormal findings, nine (19%) reported being aware that the referring screening site tracked abnormal findings, and 28 (60%) reported that abnormal findings were not systematically tracked.
Table 1.
Characteristics of Responding FQHCs, and Reported Resources to Support Lung Cancer Screening Stratified by Current Implementation of Screening (N=110)
| Characteristics | Total | Providers offer screening (n=47) | Providers do not offer screening (n=42) | Don’t know if screening is offered (n=21) | p-valuea |
|---|---|---|---|---|---|
|
| |||||
| Site characteristicsb | |||||
| # of sites, Median | 4.0 | 6.0 | 3.0 | 4.0 | 0.06 |
| % of adults aged 55–74 years, M (SD) | 17.5 (5.1) | 17.6 (6.0) | 17.3 (4.4) | 17.4 (4.7) | 0.96 |
| % of adults using tobacco, M (SD) | 39.6 (9.9) | 39.3 (9.1) | 38.9 (10.3) | 41.5 (11.2) | 0.60 |
| Urban, n (% yes) | 54 (49.5) | 26 (55.3) | 16 (38.1) | 12 (57.1) | 0.19 |
| Respondent characteristics | |||||
| Role, n (% yes) | <0.001 | ||||
| Chief medical officer or clinical director | 72 (65.5) | 41 (87.2) | 23 (54.8) | 8 (38.1) | |
| CEO or COO | 19 (17.3) | 1 (2.1) | 11 (26.2) | 7 (33.3) | |
| Quality officer | 5 (4.5) | 2 (4.3) | 1 (2.4) | 2 (9.5) | |
| Other | 14 (12.7) | 3 (6.4) | 7 (16.7) | 4 (19.0) | |
| Time in position, n (% yes) | 0.90 | ||||
| Less than 1 year | 21 (19.1) | 9 (19.1) | 7 (16.7) | 5 (23.8) | |
| 1–3 years | 46 (41.8) | 21 (44.7) | 18 (42.9) | 7 (33.3) | |
| More than 3 years | 43 (39.1) | 17 (36.2) | 17 (40.5) | 9 (42.9) | |
| Resources to support screening, n (% yes) | |||||
| LDCT screening center within 30 miles | 45 (40.9) | 28 (59.6) | 12 (28.6) | 5 (23.8) | <0.001 |
| EHR lung cancer screening best practice alert | 6 (5.5) | 3 (6.4) | 3 (7.1) | 0 (0.0) | 0.47 |
| Routinely document pack-year smoking history | 59 (53.6) | 25 (53.2) | 21 (50.0) | 13 (61.9) | 0.67 |
| Pack-year smoking history accuracyc | 0.67 | ||||
| Very accurate | 17 (28.8) | 7 (28.0) | 5 (23.8) | 5 (38.5) | |
| Somewhat | 30 (50.8) | 12 (48.0) | 13 (61.9) | 5 (38.5) | |
| Not at all accurate | 4 (6.8) | 3 (12.0) | 0 (0.0) | 1 (7.7) | |
| Don’t know | 8 (13.6) | 3 (12.0) | 3 (14.3) | 2 (15.4) | |
| >1 smoking cessation resource that meets patient needs (e.g., referral to Quitline) | 81 (73.6) | 33 (70.2) | 33 (78.6) | 15 (71.4) | 0.65 |
Notes: Boldface indicates statistical significance (p<0.05). Percentages may not add to 100 due to rounding.
For continuous variables, mean values were compared across the 3 LDCT groups (Yes, No, Don’t Know) using one-way ANOVAs (or Welch’s ANOVAs, which adjust for unequal variances). For categorical variables, chi-square tests were used to compare the proportions in the different categories across the 3 LDCT groups.
From FQHC Uniform Data Source, 2013 reporting.
Only reported for the sites that routinely document pack-year smoking history.
LDCT, Low-Dose Computed Tomography; FQHC, Federally Qualified Health Center; CEO, chief executive officer; COO, chief operating officer; EHR, electronic health record.
Survey respondents were asked about barriers to offering lung cancer screening at their sites and about their personal perceptions about screening (Table 2). The majority of barriers were financial, including patients’ lack of insurance (72%), challenges obtaining prior authorization (58%), and coverage denials (30%). More than half of sites also reported that transportation to LDCT facilities was a major challenge for some patients. Only seven (6%) sites reported not having any barriers to offering screening. Of note, there were no significant differences in perceptions of barriers for those sites that offer screening and those that do not offer screening.
Table 2.
Perceptions Lung Cancer Screening and Barriers to Implementing Screening in FQHCs Stratified by Current Implementation of Screening (n=110)
| Barriers and perceptions | Total | Providers offer screening (n=47) | Providers do not offer screening or don’t know if screening is offered (n=63) | p-valuea |
|---|---|---|---|---|
|
| ||||
| Barriers to offering lung cancer screening, n, (% yes) | ||||
| Lack of insurance coverage | 79 (71.8) | 33 (70.2) | 46 (73.0) | 0.75 |
| Prior authorization by health insurance is required | 64 (58.2) | 27 (57.4) | 37 (58.7) | 0.89 |
| Transportation challenges for patients | 60 (54.5) | 28 (59.6) | 32 (50.8) | 0.36 |
| Difficult to refer certain patient populations | 43 (39.1) | 17 (36.2) | 26 (41.3) | 0.59 |
| Coverage denials received | 33 (30.0) | 18 (38.3) | 15 (23.8) | 0.10 |
| Services for non-English speaking patients are limited or unavailable | 32 (29.1) | 11 (23.4) | 21 (33.3) | 0.26 |
| Other | 21 (19.1) | 6 (12.8) | 15 (23.8) | 0.15 |
| We do not have any barriers to offering LDCT | 7 (6.4) | 3 (6.4) | 4 (6.3) | 0.99 |
| Lung cancer screening perceptionsb, n, (% agree or strongly agree) | ||||
| Evidence from randomized trials show that lung cancer screening with LDCT scans prevents lung cancer deaths | 73 (67.0) | 40 (85.1) | 33 (53.2) | <0.001 |
| Available clinical evidence about lung cancer screening will be applicable to our patient population | 89 (81.7) | 40 (85.1) | 49 (79.0) | 0.42 |
| Lung cancer is an important clinical concern for our patient population | 92 (84.4) | 42 (89.4) | 50 (80.6) | 0.21 |
| Clinicians believe that other clinical priorities are more important than lung cancer screening for our patients | 37 (33.9) | 15 (31.9) | 22 (35.5) | 0.70 |
| Senior leadership at our clinical site has made lung cancer screening a priority | 13 (11.9) | 7 (14.9) | 6 (9.7) | 0.40 |
| Senior leadership at our clinical site has committed resources to support lung cancer screening | 13 (11.9) | 8 (17.0) | 5 (8.1) | 0.15 |
| Our clinical site has adequate access to specialty providers to appropriately manage abnormal findings on lung cancer screening tests | 56 (51.4) | 29 (61.7) | 27 (43.5) | 0.06 |
| Patients frequently ask for lung cancer screening | 2 (1.8) | 0 (0.0) | 2 (3.2) | 0.50 |
| The benefits of lung cancer screening with LDCT outweigh the potential harms | 59 (54.1) | 36 (76.6) | 23 (37.1) | <0.001 |
| Under-insured patients are less likely to be referred for lung cancer screening with LDCT | 66 (60.6) | 33 (70.2) | 33 (53.2) | 0.07 |
| Out-of-pocket costs for follow-up procedures of suspicious screening findings will be a significant financial burden for our patients | 79 (72.5) | 35 (74.5) | 44 (71.0) | 0.68 |
| Lung cancer screening may undermine smoking cessation efforts with our patient population | 10 (9.2) | 2 (4.3) | 8 (12.9) | 0.18 |
| We need to provide lung cancer screening to be a leader in cancer prevention | 60 (55.0) | 31 (66.0) | 29 (46.8) | 0.05 |
| Engaging patients in shared decision making for lung cancer screening is challenging | 55 (50.5) | 23 (48.9) | 32 (51.6) | 0.78 |
Notes: Boldface indicates statistical significance (p<0.05). Percentages may not add to 100 due to rounding.
Chi-square tests (or Fisher’s Exact Test if >20% of cells had expected count <5) were used to compare the proportion(s) across the LDCT groups (Yes and No/Don’t Know).
Due to missing data on perceptions for one site, Total N=109 and No LDCT or Don’t Know n=62.
FQHC, Federally Qualified Health Center; LDCT, Low-Dose Computed Tomography.
Financial obstacles were also common among responders’ perceptions of lung cancer screening with 73% indicating they felt out-of-pocket costs for follow-up procedures will be a significant burden to patients. Only 12% of sites indicated senior leadership had made lung cancer screening a priority, and 13% reported leadership had committed resources to screening. Notably, just half of responders felt their site has adequate access to specialty providers to adequately manage abnormal findings.
Responders from sites offering screening were more enthusiastic about the evidence base supporting screening, with 87% agreeing that the evidence from RCTs demonstrates a mortality benefit for lung cancer screening, and 75% agreeing that the benefits of screening outweigh harms, compared with 54% (p<0.001) and 39% (p<0.001), respectively, among sites not offering or not aware of providers offering screening. Responders were asked to select the two most important barriers to implementing screening. The ranking exercise highlighted out-of-pocket cost burdens for follow-up procedures (54%), underinsurance (38%), having other more important clinical priorities (27%), challenges engaging patients in shared decision making (19%), concerns about screening potentially undermining smoking cessation efforts (13%), and having limited access to specialty providers for managing abnormal findings (12%; data not shown).
DISCUSSION
The survey found very low reported use of lung cancer screening by the end of 2016 among FQHC clinic sites with a high proportion of smokers. Although uptake of lung cancer screening has generally been slow nationally following the publication of National Lung Screening Trial results and guideline recommendations,10,12 the low adoption of screening by safety-net clinics who care for low-SES individuals that include high numbers of smokers, suggests that disparities in access to screening are likely to emerge. These findings demonstrate several pathways for disparities. First, the patient population served by safety-net clinics currently has limited access to dedicated lung cancer screening programs. Second, many FQHCs lack infrastructure and capacity to document and query variables in the EHR to identify eligible populations, to monitor abnormal findings, and to remind patients of follow-up procedures or annual repeat screening. FQHCs have limited access to specialty providers to manage necessary follow-up care, and potential gaps in smoking cessation resources to fully address patient needs. These elements are considered essential by Centers for Medicare and Medicaid Services and professional society guidelines for successfully offering lung cancer screening, as there are concerns that the benefit of screening observed in the clinical trial setting may be diminished in community practice if high-quality screening and appropriate management of suspicious findings is not available. Thus, disparities may emerge by either limited access to providers offering screening, by offering screening with poor quality shared decision making and smoking cessation counseling processes, and inadequate management of abnormal pulmonary findings and incidental non-pulmonary findings.
Third, safety-net providers are reporting substantial financial burden to patients, including lack of insurance coverage for some patients, and significant obstacles even to those with insurance including challenges with pre-authorization, denial of claims, underinsurance, and out-of-pocket costs for follow-up procedures. The Affordable Care Act currently provides first-dollar payment for A- and B-grade recommended screening tests from the U.S. Preventive Services Task Force, but not for subsequent diagnostic tests or treatments. Again, these barriers may limit both access to screening, as well as lead to poor adherence with repeat screening, recommended follow-up care, and treatment.
There are some examples of inferior cancer screening quality among low-SES patients and low-resource settings in the breast and colon cancer screening context, including reduced detection rates and inferior bowel prep.21–26 These studies highlight that ensuring access to high-quality screening services is a parallel goal to achieving high levels of participation in screening. Many of the quality concerns for breast and colon cancer screening reflect technical performance of the mammogram or colonoscopy. Concerns about variation in mammography quality led to Congress passing the Mammography Quality Standards Act,27 and there have been calls for national quality monitoring for colonoscopy.28 However, the quality concerns for lung cancer screening go beyond the technical performance of the CT imaging. Notably, mammographers and gastroenterologists typically take primary responsibility for managing follow-up of abnormal screening findings, which is not necessarily true for radiologists performing chest CT, even for some dedicated lung cancer screening programs.
The findings from this survey of FQHCs are similar to previously described concerns about implementing lung cancer screening.16 The quality of smoking history information in electronic records of many health systems is inadequate to identify eligible patients,29 which is a target for improvement that can be addressed by FQHCs. Harris et al.30 highlighted the potential harm of financial consequences associated with screening and the cascade of care associated with suspicious findings, especially for low-SES individuals who are underinsured and uninsured. A qualitative study of primary care providers focusing on implementation of screening among high-risk patients identified cost, the potential for false positive test results, and the complexity of follow-up for abnormal results among the barriers to screening.31 Kinsinger and colleagues18 described the experience of 2,106 patients from eight medical centers who participated in the Lung Cancer Screening Demonstration Project in the Veterans Health Administration. This project found that a large number of screening participants will be identified with both suspicious pulmonary findings and incidental findings that require careful coordination of care with specialty services including oncology, pulmonary, and cardiology to determine if the findings require additional diagnostic evaluation. The Veterans Health Administration experience reinforces the concern expressed by respondents from FQHCs in this survey that a lack of access to specialty care providers to appropriately manage findings from lung cancer screening tests will likely be a challenge for their patient population.
Other countries have acknowledged the challenge of offering screening to underserved and hard to reach populations, and have not yet adopted national lung cancer screening programs.32,33 Concerns exist about being able to safely and broadly offer screening have been voiced by the American Academy of Family Physicians, which is one professional society that has not endorsed widespread screening.34 The Medicare Evidence Development & Coverage Advisory Committee, who advised Centers for Medicare and Medicaid Services, was concerned that the benefits of screening observed in the National Lung Screening Trial may not be realized in the Medicare population, in part because of variation in quality, and called for more research on the matter.35
Limitations
The response rate was less than 50% and participants from screening sites may been more likely to respond to the email invitation and may be overrepresented in the study sample. This would imply that an even smaller proportion of FQHC sites have adequate access and capacity to offer screening. Only one FHQC site representative was surveyed, generally the medical director, and this person’s views might not represent the perceptions broadly held by clinicians at the FQHC site. Data from the 2013 UDS was used to sample clinics based on tobacco use and compare respondent and nonrespondents. This data was the most recent dataset available, however, it may not reflect clinic characteristics in 2016 if characteristics of the clinics had changed substantially. Clinics reporting offering screening were more often completed by clinic directors (Table 1), which may indicate potential biases, such as clinic directors being more familiar with services in the clinic than if a chief executive officer completed the survey, or that a clinic director may be unwilling to indicate a clinic service is not being offered. Although the goal of the survey was to identify the person most familiar with tobacco and lung cancer screening activities, the study findings may have been influenced by the individual respondent and may not reflect the true services being offered.
CONCLUSIONS
The investment of resources to safely and effectively offer lung cancer screening is emerging as a challenge for safety-net clinics serving low-SES patients, which include a high proportion of smokers eligible for lung cancer screening. Many settings serving vulnerable patients lack the resources necessary for broadly adopting high-quality lung cancer screening, which may potentially lead to future disparities in health outcomes.
Figure 1.
Schema of sample and participation by FQHCs nationally
FQHC, Federally Qualified Health Center; UDS, Uniform Data System
ACKNOWLEDGMENTS
This research is the result of work conducted by six of the Cancer Prevention and Control Research Network sites funded by the Centers for Disease Control and Prevention and the National Cancer Institute. We wish to thank other members of the Tobacco and Lung Cancer Screening Workgroup and staff that supported the conduct of this research. We particularly thank Brittany Lavanty, MS and Rebecca Williams, PhD for assisting with programming the web-based survey. The research was supported by the following cooperative agreements from the Centers for Disease Control and Prevention, Prevention Research Program and the National Cancer Institute: U48DP005030, U48DP005013, U48DP005014, U48DP005017, U48DP005021, and U48DP005000. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Moyer V US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;160(5):330–338. 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 2.Wood D, Kazerooni E, Baum S, et al. Lung cancer screening, version 1.2015: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2015;13(1):23–34. 10.6004/jnccn.2015.0006. [DOI] [PubMed] [Google Scholar]
- 3.Wiener R, Gould M, Arenberg D, et al. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192(7):881–891. 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wender R, Fontham E, Barrera E, et al. American Cancer Society lung cancer screening guidelines. Ca Cancer J Clin. 2013;63(2):106–117. 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson K, Blok A, Buscemi J, et al. Society of Behavioral Medicine supports implementation of high quality lung cancer screening in high-risk populations. Transl Behav Med. 2016;6(4):669–671. 10.1007/s13142-016-0440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samet JM, Crowell R, Estepar RJ. Providing guidance on lung cancer screening to patients and physicians. American Lung Association; 2015.
- 7.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. New Eng J Med. 2011;365(5):395–409. 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT); 2015.
- 9.Eberth J, Sercy E. Implementation of lung cancer screening in the United States: changing trends based on a survey of Society of Thoracic Radiology members. J Thorac Imaging. 2015;30(6):W60–62. 10.1097/RTI.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 10.Huo J, Shen C, Volk R, Shih Y-C. Use of CT and chest radiography for lung cancer screening before and after publication of screening guidelines: intended and unintended uptake. JAMA Intern Med. 2017;177(3):439–441. 10.1001/jamainternmed.2016.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis J, Petty, Tooze J, et al. Low-dose CT lung cancer screening practices and attitudes among primary care providers at an academic medical center. Cancer Epidemiol Biomarkers Prev. 2015;24(4):664–670. 10.1158/1055-9965.EPI-14-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raz D, Wu G, Consunji M, et al. Perceptions and utilization of lung cancer screening among primary care physicians. J Thoracic Oncol. 2016;11(11):1856–1862. 10.1016/j.jtho.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ersek J, Eberth J, McDonnell K, et al. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324–2331. 10.1002/cncr.29944. [DOI] [PubMed] [Google Scholar]
- 14.Iaccarino J, Clark J, Bolton R, et al. A national survey of pulmonologists’ views on low-dose computed tomography screening for lung cancer. Ann Am Thorac Soc. 2015;12(11):1667–1675. 10.1513/AnnalsATS.201507-467OC. [DOI] [PubMed] [Google Scholar]
- 15.Eberth J, Qiu R, Adams S, et al. Lung cancer screening using low-dose CT: the current national landscape. Lung Cancer. 2014;85(3):379–384. 10.1016/j.lungcan.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Mulshine J D’Amico T. Issues with implementing a high-quality lung cancer screening program. CA Cancer J Clin. 2014;64(5):352–363. 10.3322/caac.21239. [DOI] [PubMed] [Google Scholar]
- 17.Roth J, Sullivan S, Goulart B, Ravelo A, Sanderson J, Ramsey S. Projected clinical, resource use, and fiscal impacts of implementing low-dose computed tomography lung cancer screening in Medicare. J Oncol Pract. 2015;11(4):267–272. 10.1200/JOP.2014.002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinsinger L, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399–406. 10.1001/jamainternmed.2016.9022. [DOI] [PubMed] [Google Scholar]
- 19.Raymakers A, Mayo J, Lam S, FitzGerald M, Whitehurst D, Lynd L. Cost-effectiveness analyses of lung cancer screening strategies using low-dose computed tomography: a systematic review. Appl Health Econ Health Policy. 2016;14(4):409–418. 10.1007/s40258-016-0226-5. [DOI] [PubMed] [Google Scholar]
- 20.Health Resources & Services Administration. Health Center Program Fact Sheet. www.bphc.hrsa.gov/about/healthcenterfactsheet.pdf.
- 21.Rauscher G, Murphy A, Orsi J, Dupuy D, Grabler P, Weldon C. Beyond the Mammography Quality Standards Act: measuring the quality of breast cancer screening programs. Am J Roentgenol. 2014;202(1):145–151. 10.2214/AJR.13.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadel M, Royalty J, Shapiro J, et al. Assessing screening quality in the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer. 2013;119(suppl 15):2834–2841. 10.1002/cncr.28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher R, Nadel M, Allen J, et al. The quality of colonoscopy services—responsibilities of referring clinicians. J Gen Intern Med. 2010;25(11):1230–1234. 10.1007/s11606-010-1446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauscher G, Allgood K, Whitman S, Conant E. Disparities in screening mammography services by race/ethnicity and health insurance. J Womens Health (Larchmt). 2012;21(2):154–160. 10.1089/jwh.2010.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebwohl B, Wang T, Neugut A. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014–2020. 10.1007/s10620-009–1079-7. [DOI] [PubMed] [Google Scholar]
- 26.Rex D, Imperiale T, Latinovich D, Bratcher. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97(7):1696–1700. 10.1111/j.1572-0241.2002.05827.x. [DOI] [PubMed] [Google Scholar]
- 27.Houn F, Elliott M, McCrohan J. The Mammography Quality Standards Act of 1992. History and philosophy. Radiol Clin North Am. 1995;33(6):1059–1065. [PubMed] [Google Scholar]
- 28.Lieberman D, Nadel M, Smith R, et al. Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc. 2007;65(6):757–766. 10.1016/j.gie.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 29.Modin H, Fathi J, Gilbert C, et al. Pack-year cigarette smoking history for determination of lung cancer screening eligibility: comparison of the electronic medical record versus a shared decision making conversation. Ann Am Thorac Soc. 2017;14(8):1320–1325. 10.1513/AnnalsATS.201612-984OC. [DOI] [PubMed] [Google Scholar]
- 30.Harris R, Sheridan S, Lewis C, et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA Intern Med. 2014;174(2):281–286. 10.1001/jamainternmed.2013.12745. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman RM, Sussman AL, Getrich CM, et al. Attitudes and beliefs of primary care providers in New Mexico about lung cancer screening using low-dose computed tomography. Prev Chron Dis. 2015;12:E108. 10.5888/pcd12.150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field Devaraj, Duffy Baldwin. CT screening for lung cancer: Is the evidence strong enough? Lung Cancer. 2016;91:29–35. 10.1016/j.lungcan.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 33.On Care C, Lewin G, Morissette K, et al. Recommendations on screening for lung cancer. CMAJ. 2016;188(6):425–432. 10.1503/cmaj.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gates T. Screening for cancer: concepts and controversies. Am Fam Physician. 2014;90(9):625–631. [PubMed] [Google Scholar]
- 35.Centers for Medicare & Medicaid Services. MEDCAC Meeting 4/30/2014 - Lung Cancer Screening with Low Dose Computed Tomography. 2017.