Abstract
Purpose of the Study:
Assisted living (AL) residents with dementia require assistance with activities of daily living, encounter limited opportunities to engage in physical activity, and often exhibit challenging behavioral symptoms. The Function Focused Care Intervention for the Cognitively Impaired (FFC-CI) teaches and motivates direct care workers (DCWs) to engage residents with dementia in activities that optimize function and activity while minimizing behavioral symptoms. The purpose of this study was to test the impact of FFC-CI on function, physical activity, behavior, and falls.
Design and Methods:
A cluster-randomized trial included 96 residents with dementia and 76 DCWs from 4 ALs. Generalized estimating equations were used to evaluate outcomes at 3 and 6 months.
Results:
There were no treatment by time differences with regard to resident behavior, mood, counts of physical activity based on actigraphy, falls, and function. There were significant increases in physical activity based on kilocalories burned (p = .001), time spent in physical activity based on survey results (p = .001), and time spent in repetitive behaviors, such as wandering (p = .01) among the control group over time. There were no treatment by time differences with regard to DCW beliefs, knowledge, or performance of FFC, except for less decline in job satisfaction among the treatment group (p = .002). Treatment fidelity with regard to delivery and receipt were poor due to high staff attrition in the treatment group (46% vs. 16%) and limited site support.
Implications:
The findings from this study can be used to adapt future FFC intervention studies to improve treatment fidelity and optimize intervention efficacy.
Keywords: Dementia, Assisted living, Function, Physical activity, Workforce issues
It is estimated that 42% of older adults living in assisted living (AL) in the United States have moderate to severe cognitive impairment (CI) (Zimmerman, Sloane, & Reed, 2014), and some studies have found that the prevalence of dementia in AL is as high as 70% (Maust et al., 2006; Park-Lee, Sengupta, & Harris-Kojetin, 2013; Zimmerman et al., 2014). In addition to significant CI, the majority of AL residents require assistance with activities of daily living (ADLs) and instrumental activities of daily living (IADLs) (Resnick, Galik, Gruber-Baldini, & Zimmerman, 2010; Sloane et al., 2005). Many are sedentary and have limited opportunities to engage in physical activity (Resnick et al., 2010; Zimmerman et al., 2007). Approximately, one third of residents in AL exhibit agitated behaviors at least once a week with more than half of the population prescribed psychotropic medication (Gruber-Baldini, Boustani, Sloane, & Zimmerman, 2004; Zimmerman et al., 2014). Increasingly these individuals are aging-in-place, and the majority will continue to remain in the AL setting after 1 year (Kenny et al., 2008; Lyketsos et al., 2007). Given the combined cognitive and functional impairments and care needs of these individuals, innovative approaches are needed to help them gain or regain function during their residency in AL settings.
The care of older adults with moderate to severe CI in AL settings has traditionally followed a task based, “just get it done” approach (Galik, Resnick, & Pretzer-Aboff, 2009). These individuals commonly exhibit behavioral symptoms during care interactions such as resisting the care being provided, agitation, restlessness, and anxiety (Corbett & Ballard, 2012). This results in care that is custodial in nature and does not take into consideration the underlying functional ability of the resident. The focus of traditional care interactions is on task completion and minimization of behavioral problems (Bowers, Esmond, & Jacobson, 2000; Cohen-Mansfield et al., 2006). Residents are not encouraged to perform activities such as combing their own hair, brushing their teeth, or ambulating. Providing custodial care can facilitate a decline in physical capability and function, cause medical complications, exacerbate depression, increase the likelihood of sustaining a fall, and ultimately may result in a need to be transferred to higher levels of care such as acute care or skilled nursing care (Kenny et al., 2008; Lyketsos et al., 2007; Tighe et al., 2008).
Behavioral symptoms may be managed with established behavioral strategies such as person-centered care approaches, sensory stimulation, and exposure of residents to music or aromatherapy (Kolanowski, Fick, Frazer, & Penrod, 2010; Penrod et al., 2007); cognitive-emotional approaches such as the use of reminiscence therapy; and social contact approaches such as animal therapy (Ayalon, Gum, Feliciano, & Areán, 2006; Cohen-Mansfield, Thein, Marx, Dakheel-Ali, & Freedman, 2012; Kong et al., 2009; O’Connor, Ames, Gardner, & King, 2009; O’Neil et al., 2011). Although these approaches have been successful in minimizing agitated behaviors commonly encountered during routine bathing and dressing, they do not consider how to optimize and maintain physical function (Davison et al., 2007; Teri, Huda, Gibbons, Young, & van Leynseele, 2005). To provide care without exacerbating behavioral symptoms and contributing to functional decline, we propose the use of a function Focused Care (FFC) approach for those with moderate to severe CI (FFC-CI). Function Focused Care for the Cognitively Impaired (FFC-CI) is an intervention designed to change the philosophy of nursing care such that the focus is on optimizing physical and functional activities, whereas minimizing behavioral symptoms in residents rather than simply completing nursing care tasks (e.g., dressing, bathing, or feeding the individual) (Galik et al., 2008; Galik, Resnick, Hammersla, & Brightwater, 2014). FFC-CI is guided by the Social Ecological Model (SEM, Sallis et al., 2006) and Social Cognitive Theory (SCT, Bandura, 1997) and incorporates motivational techniques and individualized goals in order to optimally engage residents in functional tasks and physical activities.
Successful integration of new philosophies of care in AL settings are influenced by factors within the setting such as caregivers’ beliefs about the utility and feasibility of the new approach, their knowledge and training, administration’s recognition and support of the new care approach, sufficient amounts of staffing to get basic care provided, consistency of the staff, fit between the intervention and culture or philosophy of care within the community, and environmental resources (Banaszak-Holl, Castle, Lin, Shrivastwa, & Spreitzer, 2013; Grabowski, Elliot, Leitzell, Cohen, & Zimmerman, 2014; Liu, Liu, & Wang, 2011; Resnick et al., 2008). There are also challenges and barriers at the level of the resident. These include such things as acute medical problems that may cause delirium and exacerbation of behavioral symptoms, age-related changes, sociodemographic characteristics, medical comorbidities that affect function, apathy, depressed mood, lack of motivation, pain, fear of falling, body mass index, and polypharmacotherapy (Brown, Williams, Woodby, Davis, & Allman, 2007; Galik et al., 2008; Ouslander et al., 2005; Resnick et al., 2008).
Theoretical Framework
To address the many challenges and barriers to implementation of new care approaches, FFC-CI was developed using a SEM, which incorporates intrapersonal, interpersonal, environment, and institutional/policy factors (Sallis et al., 2006). Further, the interpersonal interactions in FFC-CI are guided by SCT (Bandura, 1997). SCT suggests that the stronger an individual’s self-efficacy and outcome expectations, the more likely it is that he or she will initiate and persist with a given activity. Self-efficacy expectations are the individuals’ beliefs in their capabilities to perform a course of action to attain a desired outcome; and outcome expectations are the beliefs that a certain consequence will be produced by personal action. Efficacy expectations are dynamic and enhanced by four mechanisms: (a) successful performance of the activity; (b) verbal persuasion; (c) seeing like individuals perform an activity; and (d) pleasant and unpleasant physiological and affective states (e.g., pain) associated with an activity. In addition to making appropriate changes in the environment and policies that serve as barriers to engaging residents in function and physical activity, these four mechanisms were used in FFC-CI to motivate residents to participate in functional activities and be physically active.
Intervention
FFC-CI consists of four components, all of which are described in Table 1. The components of the intervention were implemented by a Function Focused Care Nurse (FFCN), a study supported registered nurse with experience working with residents with CI. The FFCN worked within each treatment AL for 10hr a week for 6 months. Component 1, referred to as Evaluation of Person-Environment Fit, comprehensively assessed the environment and made changes to increase opportunities for functional and physical activities within the setting (e.g., making pleasant walkways). Component 2 focused on educating DCWs, other members of the health care team, families, and residents (as appropriate) about FFC and ways in which to increase time the residents spent in functional and physical activities. Component 3 involved the FFCN working with a FFC Champion identified by each participating setting to establish individualized FFC goals for the participants. Lastly, Component 4 focused on mentoring and motivating the DCWs, and other staff in the settings, to engage residents in appropriate FFC activities as per their goals. Small incentives (i.e., pens, drinks, and snacks) were provided in formal class instruction. No monetary incentives were used. Further details and content of the intervention have been previously published (Resnick, 2011).
Table 1.
The FFC for Cognitively Impaired Intervention
| Component | Description of activities |
|---|---|
| Component 1: Evaluation of Person-Environment Fit | A baseline assessment of the Person-Environment Fit was completed and the FFCN used this to alter the environment within the setting to optimize function and physical activity for residents (e.g., availability of walking space areas, rest areas for walks, appropriate bed and chair heights to facilitate transfers). |
| Component 2: Education | Education of DCWs, other members of the health care team, families, and residents (as appropriate) was provided. Education of the DCWs was provided by an advanced practice nurse and given at times convenient for the staff and in a way that best met their schedules. Content included: (a) information about the philosophy of FFC; (b) optimal ways in which to motivate cognitively impaired residents to engage in FFC during ADLs and exercise; (c) optimal ways in which to use the environment and integrate FFC into the resident’s daily life, and (d) documentation of FFC activities on a monthly flow sheet. Education of the interdisciplinary team, families/informal caregivers, and residents (when appropriate) was offered (separately one for staff, one for families, one for residents) during a single one time class and available via a handout. |
| Component 3: Establishing FFC Goals for Residents | The FFCN and setting identified FFC Champion (FFCC) worked together to establish appropriate FFC goals for participants. The State required AL Resident Assessment Form, recent therapy notes, input from DCWs working with the resident, families, and direct assessment of basic range of motion and ability to following single step commands were all used to establish goals. Goals were placed in a location that was easily accessible to all staff working with the resident and were given to the family/LAR. Goals were re-evaluated monthly by the FFCN/FFCC and revised as indicated. |
| Component 4: Mentoring/Monitoring and Sustainability of the Intervention | The FFCN worked with the FFCC and staff 10hr a week for 6 months and provided ongoing encouragement related to engaging residents in FFC activities (e.g., performing ADLs, walking to the dining room, going to exercise classes). Motivational strategies included (a) positive reinforcement, (b) addressing positive experiences or unpleasant feelings and experiences associated with implementing FFC (e.g., frustration, discouragement, fear or pain among residents), (c)providing information to strengthen the beliefs of the DCWs and others about the benefits of functional and physical activities with older adults with dementia, (d) helping all participants to integrate function focused care activities into routine care. |
Note: ADL = activities of daily living; DCW = direct care workers; FFC = Function Focused Care; FFCC = Function Focused Care Champion; FFCN = Function Focused Care Nurse; LAR = legally authorized representatives.
The sites randomized to FFC-ED were exposed to the same educational intervention as described for FFC-CI (Component 2 of FFC-CI); however, they were not exposed to environmental and policy recommendations or ongoing mentoring and motivating of AL staff by the FFCN and FFC Champion. The educational program in FFC-ED was given by an advanced practice nurse with experience implementing FFC interventions. Following the education, however, there was no additional interaction between the nurse and the facility.
The specific aims of this study were to positively impact the function, physical activity, mood, and behavior of AL residents with moderate to severe CI. We hypothesized that (a) residents exposed to FFC-CI would maintain or improve functional performance/physical activity and demonstrate fewer depressive symptoms, decreased anxiety, and decreased apathy when compared with residents exposed to FFC-Education only (FFC-ED), and (b) direct care workers (DCWs) exposed to FFC-CI would have stronger efficacy beliefs, greater knowledge of FFC, and spend more time providing FFC types of interactions with residents when compared with DCWs exposed to FFC-ED.
Methods
This was a 6-month cluster-randomized controlled trial. Four dementia-specific ALs were randomly assigned to treatment (FFC-CI) or attention control (FFC-ED). All four of the ALs were owned and operated by the same for-profit company, provided dementia special care units, were similar in size (50–70 residents), were located in the Baltimore Washington metropolitan area, and utilized the same organizational policies and procedures regarding staffing and care practices. A randomized cluster design was utilized with randomization occurring at the facility level rather than the AL unit/floor or individual level in order to control for carryover of learned FFC skills by staff to units randomized to the educational control.
Sample
The study was approved by a university-based institutional review board. The research team obtained a list of all residents who were cognitively eligible to participate (as per the facility staff) and their legally authorized representatives (LARs) from each facility. The AL facility administrator sent a letter to all LARs prior to recruitment, which gave LARs an opportunity to decline contact by the research staff. Residents were deemed eligible to participate if they were 55 years of age or older, lived in the AL at the time of recruitment, were known to have a Mini-Mental State Exam (MMSE) score of 15 or less (Folstein, Folstein, & McHugh, 1975), and had an anticipated length of stay of 6 months or greater. To consent residents, the research evaluator first contacted the LAR to obtain his or her consent in a face-to-face encounter. Following consent from the LAR, potential resident participants were then approached for a face-to-face encounter in order to complete the Evaluation to Sign Consent (Resnick, Rogers, Galik, & Gruber-Baldini, 2007). Residents who were unable to pass the Evaluation to Sign Consent were asked to sign an assent form and were then enrolled in the study. If residents passed the Evaluation to Sign Consent, they were also asked to sign a consent form in addition to their LAR.
A Consort diagram for resident recruitment and retention is presented in Figure 1. There were 207 residents screened to participate in the study, and no residents were able to provide their own consent. Twenty-five residents who were approached were not eligible to participate because they did not meet study inclusion criteria (hospice, non-communicative, and so on). Of the 182 residents who were eligible at the time of recruitment, both consent and assent to participate in the study was obtained from 106 LARs and residents, respectively. Twenty-five LARs were not available to provide face-to-face consent, 46 LARs refused consent, and 12 residents refused assent. Following the completion of consent procedures, 10 consented residents were ineligible due to cognition (screening MMSE >15). Ninety-six residents were enrolled in the study with 48 residents in the treatment group and 48 residents in the educational control group.
Figure 1.
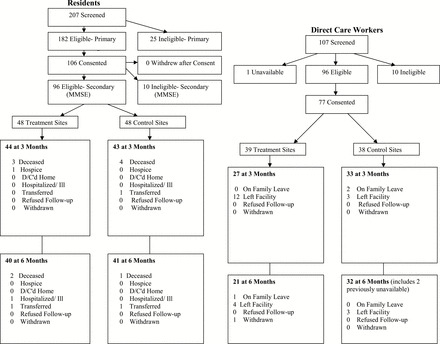
Consort Diagram for Residents and Direct Care Workers.
DCWs were invited to participate in the study during “meet and greet” sessions held during shift changes on several different days. DCWs were nursing assistants and medication technicians and did not include LPNs and RNs whose presence was limited in these sites. During the “meet and greet” sessions, they were provided with information about the study. DCWs were eligible to participate if they were able to read and write English and worked 16hr a week or more at the AL at the time of recruitment.
A consort diagram for DCW recruitment and retention is presented in Figure 1. There were 109 DCWs screened to participate in the study. Ninety-six DCWs were eligible to participate. Seventy-seven (71%) of the approached DCWs consented to participate with 39 DCWs in the treatment group and 38 DCWs in the educational control group.
Measures
Descriptive information was obtained for residents and included age, gender, race, marital status, years of education, cognitive status, and number of medical comorbidities. For DCWs, descriptive information included age, race, gender, education, years of experience, and years of employment within the AL. All of the measures that were utilized have established evidence of reliability and validity as described in Table 2. All outcome measures, including actigraphy, were collected by trained research evaluators who were not associated with research intervention staff. Survey instruments that required proxy verbal report on the resident’s activity and behavior were obtained from the DCW assigned to the resident’s care on the day of measurement.
Table 2.
Description and Psychometric Properties of Resident and Direct Care Worker Measures
| Measures | Description | Validity and reliability |
|---|---|---|
| Residents | ||
| Barthel Index (Mahoney & Barthel, 1965) | A 14-item measure of physical function that assesses ability for self-care. Verbal report of function was obtained from the NA who was assigned to the resident’s care on the day of testing. Score ranges from 0 (dependence) to 100 (independence). | Alpha coefficients range from .62–.80 and test–retest reliability (r = .82). There is evidence of validity based on a correlation between direct observation of the Barthel Index and the Functional Inventory Measure (r = .97, p < .05) among older adults (Mahoney & Barthel, 1965; Resnick & Daly, 1998). There is evidence of reliability and validity when caregiver report data have been utilized to report the functional abilities of dementia patients (Ranhoff, 1997). |
| ActiGraph (Actigraph, 2004) | An accelerometer that objectively measures physical activity. | Actigraphy data with older adults provided evidence of test–retest reliability (r = .98), and validity based on a significant relationship with oxygen uptake (r = .73), heart rate (r = .71), physical activities (r = .46), and sedentary activities (r = .35) (Actigraph, 2004). |
| Physical Activity for Long-Term Care (Resnick & Galik, 2007) | An observational survey related to resident time spent in physical activity according to six subscales, which include locomotion, personal care activities, structured exercise, recreational activities, caretaking activities, and repetitive behaviors. | There was evidence of criterion-referenced validity based on bivariate correlations between the physical activity survey for long-term care and the ActiGraph (r = .55–.60, p < .05). Interrater reliability was based on bivariate correlations between DCWs (r = .82–.94, p < .05) (Resnick & Galik, 2007). |
| Cohen-Mansfield Agitation Inventory, Short Form (Cohen-Mansfield et al., 1989) | Based on the factor structure of the original CMAI inventory (long form) (Cohen- Mansfield et al., 1989). Uses a five-point Likert scale to rate the frequency of agitated behaviors in patients with cognitive impairment. | Interrater reliability testing showed a correlation of .82 for exact agreement and .93 for a one point discrepancy. Factor analysis provided evidence of validity (Cohen- Mansfield, 1991). |
| Cornell Scale for Depression in Dementia (Alexopoulos et al., 1988) | A 19-item survey designed to assess depressive symptoms in individuals with dementia. | Commonly used and has evidence of interrater reliability, internal consistency (.84) and validity (Alexopoulos et al., 1988). |
| Apathy Evaluation Scale (Marin et al., 1991) | A survey of global apathy. | Has evidence of adequate internal consistency, item reliability, interrater reliability, and concurrent validity (Robert et al., 2002). |
| DCWs | ||
| Restorative Care Behavior Checklist (Resnick et al., 2007) | An observation measure of nursing assistant performance of function focused care. The care interactions of nursing assistants and residents were observed across a variety of activities (e.g., bathing, dressing, eating) were evaluated. | There is evidence of interrater reliability (.93–.94.). Validity was based on evidence of the internal structure of the measure using Rasch analysis and convergent validity with a good fit of the items supporting the uni-dimensional nature of the measure (Resnick et al., 2007). |
| Theoretical Knowledge of Restorative Care Activities Test (Resnick et al., 2008; Resnick & Simpson, 2003) | An 11-item paper and pencil test that measures nursing assistants knowledge of function focused care. | There was evidence for tes–retest reliability at 2-week intervals with a Pearson correlation of .85, p < .05. Validity was based on contrasted groups (Resnick & Simpson, 2003). |
| Nursing Assistants’ Self-Efficacy for Restorative Care Activities (Resnick et al., 2008; Resnick & Simpson, 2003) | A 10-item survey that measures the nursing assistants’ confidence in performing specific function focused care activities in the face of obstacles. | There is evidence of internal consistency with alpha coefficients that ranged from .80 to .91 and validity was based on contrasted groups (Resnick & Simpson, 2003) and Rasch analysis (Resnick et al., 2008). |
| Nursing Assistant Outcome Expectations for Restorative Care Activities (Resnick et al., 2008; Resnick & Simpson, 2003) | A 9-item survey that focuses on nursing assistant beliefs in outcomes associated with performing function focused care activities with residents. | Validity was based on confirmatory factor analysis and item fit using Rasch analysis, and the fact that the items all fit the respective measurement model (Resnick et al., 2008). |
| The Job Attitude Scale (Helmer et al., 1995) | A 17-item survey that measures five factors believed to influence work satisfaction: pay, organizational factors, task requirements, job status, and autonomy. | Using test theory and Rasch analysis, there was evidence of reliability, validity, and generalizability when the Job Attitude Scale was used with nursing assistants in long-term care settings (Flannery et al., 2012). |
Note: CMAI = Cohen-Mansfield Agitation Inventory, Short Form; DCW = direct care workers.
Resident Outcomes
Physical function of the residents was measured using the Barthel Index, a 14-item measure of physical function that assesses an individual’s ability for self-care (Mahoney & Barthel, 1965). To complete the Barthel Index, verbal report of the resident’s functional abilities was obtained from the DCW assigned to the resident’s care on the day of measurement. Physical activity was objectively measured for 24hr using the ActiGraph, an accelerometer that records activity in set epochs of time (Actigraph, 2004). Anxiety/agitation was measured using the Cohen-Mansfield Agitation Inventory, Short Form, which is a survey of behavioral symptoms commonly found in long-term care residents with dementia (Cohen-Mansfield, Marx, & Rosenthal, 1989). Depression was measured using the Cornell Scale for Depression in Dementia, a 19-item survey that identifies if the resident shows evidence of depressive symptoms (Alexopoulos, Abrams, Young, & Shamoian, 1988). Apathy was measured using the Apathy Inventory, a rating scale that also is based on input from the DCW. The items on the Apathy Inventory consider if the resident demonstrates emotional blunting, lack of initiative, and lack of interest (Marin, Biedrzycki, & Firinciogullari, 1991).
Adverse events were measured using a cumulative count of participant falls, falls with injuries, emergency room transfers, and death obtained from the AL manager or delegating nurse in each setting. Resident outcome measures were completed at baseline, and at 3 and 6 months after initiation of the intervention.
DCW Outcomes
Survey data were obtained from DCWs at baseline, and at 3- and 6-months postintervention. Data included knowledge about FFC (this was only obtained at baseline and 6 months), self-efficacy and outcome expectations associated with performing FFC, job satisfaction, and observed performance of FFC. Knowledge of FFC was evaluated using an 11-item multiple choice test (Resnick, Galik, Pretzer-Aboff, Rogers, & Gruber-Baldini, 2008; Resnick & Simpson, 2003). Self-efficacy and outcome expectations associated with providing FFC to cognitively impaired residents were evaluated using the Self-efficacy for Restorative Care Activities and Outcome Expectations for Restorative Care Activities Scales (Resnick et al., 2008; Resnick & Simpson, 2003). Job satisfaction was examined using the 17-item Job Attitude Scale (Helmer, Olson, & Heim, 1995). Lastly, the Restorative Care Behavior Checklist (RCBC) was used to observe consented DCWs during care interactions with consented residents to determine if DCWs were or were not providing FFC (Resnick et al., 2007). As per the RCBC instrument, a variety of care-related interactions (e.g., chair mobility, range of motion, bathing, dressing, grooming, ambulating) were observed at each measurement time point. Typically, 7–9 different care interactions were observed out of a possible 19 and observations of each DCW lasted approximately 45min.
Data Analysis
Descriptive analysis of the data by experimental group at each follow-up time point was done with regard to demographics and baseline variables to assess potential bias created by differential attrition. Generalized estimating equations were used to perform repeated measures analyses with outcome measures as the dependent variable. An intention-to-treat paradigm was followed.
For each outcome, exploratory analyses (scatterplots, frequencies, and boxplots) were performed to assess model assumptions. There were no differences in demographic, outcome, or descriptive variables between the residents based on group status with the exception of the treatment group having more residents who sustained at least one fall at baseline (29 [60%] in the treatment group and 17 [35%] in the control group). Among the DCWs, there was a significant difference between the groups with regard to age (p = .04) and performance of FFC (p = .03) and these variables were controlled for in all analyses. Chi-square analysis and analysis of variance were done to determine if there were treatment differences during the study period with regard to whether a resident sustained a fall or injury, visited an emergency department for a fall, was transferred to the hospital for reasons other than a fall, or died. All tests were two-sided with a 5% significance level, and all were adjusted for clustering within settings.
Results
Direct Care Workers
The majority of the DCWs were women (n = 74; 96%), non-White (n = 71, 92%), and their mean age was 37.20 (standard deviation [SD] = 11.15, with a range of 21–65). The majority (n = 75, 98%) of the DCWs had a high school education or higher. Overall, the DCW participants reported they had worked within the facilities for 2.71 (SD = 2.26) years and had 5.96 (SD = 5.58) years of experience as DCWs. Table 3 provides a comparison of demographic data of DCWs in the control and treatment groups. At baseline, there was a statistically significant difference between the DCWs such that the DCWs in the treatment sites were younger than those in control sites (39.9 [SD = 11.20] vs. 34.6 [SD = 10.59] years of age, p = .04).
Table 3.
Description of Direct Care Workers (N = 77) and Residents (N = 96) According to Treatment Group
| Variable | Control | FFC-CI intervention | p Value (from t test or chi-square) |
|---|---|---|---|
| DCWs, N = 38 | DCWs, N = 39 | ||
| Age | 39.9±11.2 | 34.6±10.6 | .04 |
| Female gender | 35 (92%) | 39 (100%) | .12 |
| African American and mixed race | 35 (92%) | 36 (92%) | .99 |
| Years of education | 13.6±1.8 | 13.2±1.1 | .21 |
| Years of experience as DCW | 6.1±5.2 | 5.8±5.9 | .79 |
| Residents, N = 48 | Residents, N = 48 | ||
| Age | 84.3±7.0 | 83.0±7.3 | .37 |
| Female gender | 35 (73%) | 33 (69%) | .82 |
| Caucasian | 43 (90%) | 45 (94%) | .58 |
| Not married (widowed, divorced, separated, never married) | 37 (77%) | 36 (75%) | .53 |
| Mini-mental state exam | 5.6±4.6 | 6.1±5.2 | .65 |
Note: FFC-CI = Function Focused Care Intervention for the Cognitively Impaired.
DCW Beliefs and Performance of FFC
As shown in Table 4, at baseline, DCWs had fairly strong beliefs in the benefit of FFC and some confidence in their ability to provide FFC to residents. However, they were not very knowledgeable about FFC based on a paper and pencil test. Overall, they had fair job satisfaction. Those in the treatment site performed more FFC at baseline than those in the control site (69% [SD = 20%] vs. 80% [SD = 23%], p = .03). There was no treatment by time differences in the two groups with regard to self-efficacy or outcome expectations associated with FFC or knowledge of FFC. There was significant decline in job satisfaction over time among both groups with those in the treatment group showing less decline (χ2 = 18.63, p = .002). After controlling for baseline differences among the groups, there was no difference over time in percentage of FFC performed. Both groups increased the amount of FFC they provided at the 3-month testing time point, but this was not maintained over time.
Table 4.
Direct Care Workers Outcomes by Treatment Site at 3 and 6 Months
| Outcome | Range | Control | FFC-CI intervention | χ2 (p) | ||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| Outcome expectations | 0–45 | 2.71 (.74) | ||||
| Baseline | 37.78 | 0.95 | 36.42 | 0.74 | .26 | |
| 3 months | 36.84 | 1.28 | 35.88 | 1.10 | .56 | |
| 6 months | 36.09 | 1.00 | 36.09 | 1.92 | .83 | |
| Self-efficacy | 0–100 | 4.98 (.42) | ||||
| Baseline | 79.05 | 2.87 | 80.26 | 2.42 | .75 | |
| 3 months | 74.59 | 3.95 | 82.64 | 3.95 | .08 | |
| 6 months | 72.38 | 5.33 | 76.66 | 4.90 | .55 | |
| Job satisfaction | 0–51 | 18.63 (.002) | ||||
| Baseline | 38.26 | 1.04 | 37.97 | 0.84 | .83 | |
| 3 months | 35.43 | 1.19 | 36.76 | 1.07 | .41 | |
| 6 months | 34.61 | 0.99 | 35.30 | 1.07 | .64 | |
| Knowledge | 0–11 | 7.11 (.06) | ||||
| Baseline | 5.78 | 0.28 | 6.63 | 0.33 | .05 | |
| 6 months | 5.97 | 0.33 | 7.15 | 0.47 | .04 | |
| Observed performance | 0%–100% | 1.1 (.75) | ||||
| Baseline | .69 | .03 | .80 | .04 | .03 | |
| 3 months | .82 | .07 | .85 | .07 | .74 | |
| 6 months | .75 | .09 | .74 | .09 | .91 | |
Note: FFC-CI = Function Focused Care Intervention for the Cognitively Impaired.
Resident Outcomes
The residents were mostly women (n = 68, [71%]) and White (n = 88, [92%]). Most were unmarried (n = 73 [76%]; unmarried included those who were widowed, never married, divorced, or separated) with a smaller number of individuals still married (n = 23, [24%]). The mean age of residents was 83.7 (SD = 7.1) and overall they had a moderate to severe level of CI as per study design with a mean MMSE of 5.8 (SD = 4.9). Residents had multiple comorbid conditions (mean of 6.9 [SD = 2.1] medical diagnoses) and had fairly good physical capability (mean 9.6 in the Physical Capability Scale, SD = 3.1 with a range of 0–11) but engaged in only 2.11 (SD = 3.2) min of moderate level of physical activity over a 24-hr period. Table 3 provides a comparison of demographic data of DCWs in the control and treatment group.
Resident Physical Activity (Survey)
Study-related outcomes of residents are shown within Table 5. Residents in the control group showed a significant increase in overall time spent in physical activity based on survey results over time (control group increased from 186.46 [standard error (SE) = 18.21] min at baseline to 225.33 [SE = 22.12] min, whereas those in the treatment group decreased from 225.58 [SE = 19.80] to 124.55 [SE = 15.89], p = .001). Repetitive behavior was the only subscale of the physical activity survey in which there were significant differences. Specifically, the control group increased from 50.04 (SE = 10.76) to 63.36 (SE = 13.66) min spent in repetitive activity, whereas time spent in repetitive activity decreased among those in the treatment group (66.63 [SE = 16.32] to 25.80 [SE = 13.49] min, p = .05) over time.
Table 5.
Primary Resident Outcomes by Treatment Site at 3 and 6 Months
| Outcome | Control | FFC-CI intervention | χ2 (p) | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Physical activity results | |||||
| Physical activity survey | 32.72 (.001) | ||||
| Baseline | 186.46 | 18.21 | 225.58 | 19.8 | .15 |
| 3 months | 208.12 | 19.37 | 223.55 | 28.02 | .65 |
| 6 months | 225.33 | 22.12 | 124.55 | 15.89 | .001 |
| Physical activity: repetitive behavior | 11.16 (.01) | ||||
| Baseline | 50.04 | 10.76 | 66.63 | 16.32 | .39 |
| 3 months | 65.88 | 15.44 | 57.75 | 13.19 | .69 |
| 6 months | 63.36 | 13.66 | 25.80 | 13.49 | .05 |
| Actigraph: counts | 9.03 (.11) | ||||
| Baseline | 37,667 | 4,390 | 34,998 | 3,961 | .65 |
| 3 months | 71,606 | 15,233 | 43,572 | 7,232 | .10 |
| 6 months | 35,951 | 4,707 | 22,976 | 9,398 | .22 |
| Actigraphy: kcals | 46.76 (.001) | ||||
| Baseline | 50.72 | 6.86 | 43.85 | 5.52 | .44 |
| 3 months | 56.37 | 17.93 | 30.04 | 3.74 | .07 |
| 6 months | 30.12 | 3.69 | 11.42 | 5.73 | .01 |
| Barthel index | 3.25 (.20) | ||||
| Baseline | 66.97 | 3.70 | 70.52 | 2.65 | .44 |
| 3 months | 66.60 | 0.75 | 72.97 | 3.21 | .20 |
| 6 months | 60.87 | 4.33 | 59.37 | 3.36 | .79 |
| Psychosocial outcomes | |||||
| Agitation | 7.94 (.16) | ||||
| Baseline | 21.00 | 1.06 | 21.39 | 1.03 | .79 |
| 3 months | 23.14 | 1.37 | 20.81 | 1.09 | .18 |
| 6 months | 20.15 | 1.05 | 19.12 | 1.04 | .49 |
| Depression | 0.43 (.51) | ||||
| Baseline | 3.65 | 0.47 | 4.75 | 0.49 | .67 |
| 3 months | 5.05 | 0.70 | 5.89 | 0.75 | .41 |
| 6 months | 3.26 | 0.59 | 2.90 | 0.53 | .67 |
| Apathy | 3.72 (.16) | ||||
| Baseline | 16.41 | 0.72 | 17.04 | 0.70 | .53 |
| 3 months | 15.48 | 0.74 | 15.04 | 0.77 | .94 |
| 6 months | 14.92 | 0.76 | 13.44 | 0.70 | .15 |
Note: FFC-CI = Function Focused Care Intervention for the Cognitively Impaired.
Resident Physical Activity and Function
With regard to objective measurements of physical activity, there were no significant differences noted among the two groups in overall counts of physical activity. There was a significant overall difference between the groups in the amount of kilocalories burned (p = .001). Although both groups showed a decrease in the amount of kilocalories burned at 6 months based on actigraphy, the treatment group had a greater decline than those in the control group (control decreased from 50.72 [SE = 6.86] to 30.12 [SE = 3.69] kcals burned and the treatment group declined from 43.85 [SE = 5.52] to 11.42 [SE = 5.73] kcals burned, p = .01]. Similarly, at 3-months postimplementation of the intervention, the control group showed an increase in time spent in moderate level physical activity based on actigraphy, whereas the treatment group declined (control group started with 2.75 [SE = 0 .67] min of moderate level physical activity and increased to 3.48 [SE = 1.48] min of moderate level physical activity and the treatment group decreased from 1.62min [SE = 0.39] to 0.50min [SE = 0 .15], p = .04). There was no significant difference between the two groups with regard to function (i.e., performance of ADL) over the 6-month study period.
Resident Mood and Behavior
There was no significant difference between groups with regard to agitation or apathy. Both groups initially showed a non-significant increase in depressive symptoms, but then this decreased by 6 months. From a safety perspective, there was not a significant difference in total number of falls that occurred during the treatment period based on group status. Although, non-significant, there was a smaller percentage of residents falling in the control group than in the treatment group (27% in control vs. 46% in treatment, χ2 = 3.64, p = .06). There was no difference between the groups with regard to deaths, and there were no hospitalizations and only one injury that occurred during the treatment period.
Treatment Fidelity
To maximize fidelity, different interventionists were used for the different arms of the study. As per our intervention protocol, in treatment sites multiple teaching modalities were used (formal in-service class, printed copy of materials, one-on-one review of material on the units) and 100% of the recruited DCWs were exposed to the initial educational materials. The FFC Nurse spent 10hr a week in each treatment AL and completed on average 8–10 mentoring sessions per week. Conversely, in control communities, DCWs were offered a formal class given at multiple time periods to accommodate all shifts over a 3-week period for FFC-ED. Formal in-service classes were offered 10–12 different times for both treatment and control sites. Using this approach, we reached 67% of the recruited DCWs in control sites. All other aspects of the intervention were implemented as intended by the FFCN as delineated in Components 1–4 with the exception of consistent, active engagement of the facility-based champion due to staff turnover. Although the FFCN was able to work with the facility champion and staff to develop goals on all participants and to implement motivational interventions geared toward engaging the residents in FFC activities, DCW turnover was evident in the treatment sites. Specifically, 46% DCWs in the treatment group left their settings (primarily due to resignation), whereas only 16% of recruited DCWs left in the control group. A policy change occurred in the treatment sites that allowed residents more opportunities to engage in exercise without being required to remain in a seated position.
Discussion
The hypotheses in this study were not fully supported. Exposure to our FFC intervention did not result in positive findings with regard to psychosocial outcomes (depressive symptoms, anxiety, or apathy), nor did we demonstrate maintenance or improvement in overall function and physical activity among residents in the treatment group when compared with control. There was actually a slight decline in overall physical activity among residents in the treatment group when compared with control based on subjective reporting of time spent in physical activity as well as objective data via actigraphy. Further examination of the subsections within the Physical Activity Survey for Long-term Care provided evidence that repetitive behavior was the only type of activity that decreased in the treatment site. We anticipate that decrease in time in all activity and energy expended was due to the decrease in repetitive behavior. Repetitive behaviors included such things as wandering, dressing and undressing, folding and unfolding papers or clothing. In the treatment sites, the FFCN worked closely with nursing and activity staff to engage the residents in group activities that facilitated some function, and thus, we anticipate that the decrease in repetitive behaviors may have been due to this involvement in a group activity and less aimless wandering.
Wandering has been repeatedly noted to have advantages and disadvantages for older adults with CI (Algase et al., 2008; King-Kallimanis et al., 2010; Lee, Algase, & McConnell, 2014; Moore, Algase, Powell-Cope, Applegarth, & Beattie, 2009). Wandering has positive implications with regard to maintaining optimal physical function and increasing time spent in physical activity. In addition, wandering has been noted to be enjoyable and calming for some residents. Conversely, residents may engage in wandering as a way in which to deal with pain, shortness of breath, constipation, anxiety, or boredom. Wandering may result in physical harm to residents as it can cause excessive weight loss or fatigue and can increase the risk of elopement. Ongoing research is needed to explore the impact of FFC on wandering and assure that FFC only eliminates wandering when there are negative implications versus eliminating these behaviors when they may have positive benefits for the resident.
Overall the residents engaged in only a very small amount of physical activity, 2.11min (SD = 3.2) over a 24-hr period versus the recommended 30min daily of moderate level physical activity (American College of Sports Medicine and the American Heart Association, 2013). These findings are similar to those noted among other groups of residents in AL settings (Król-Zielińska, Kusy, Zieliński, & Osiński, 2011; Resnick et al., 2010). Many factors have been identified by residents and through observations that are anticipated to influence performance of physical activity. These include individual factors such as laziness, boredom, a decline in vision and hearing, pain and associated musculoskeletal problems, poor balance, fear of falling, and acute illness (Phillips & Flesner, 2013; Schutzer & Graves, 2004).
Environmental factors were also noted to have a significant impact on residents’ participation in physical activity (Lu, 2010; Phillips & Flesner, 2013; Wang & Lee, 2010). Even when physical activity programs are offered in these settings, only a small number are willing to participate (Johnson et al., 2013). Those that do participate, however, were noted to be able to achieve a moderate level of physical activity, although they needed time to build up to a full 30min of moderate level activity daily (Johnson et al., 2013). Thus, continued testing of motivational interventions are needed to eliminate the known barriers and engage residents, particularly those with moderate to severe CI to engage in physical and functional activities. The motivational techniques used should be individualized and innovative and utilize previous physical and functional activity experiences that are familiar to the resident such as house cleaning or delivering materials door-to-door (Galik et al., 2009).
With regard to DCW outcomes, we were likewise unable to fully support our hypotheses with the only difference between groups being less decline in job satisfaction among those in the treatment group. However, the finding that job dissatisfaction declined less in the intervention group could have implications for improving care delivery. This finding is consistent with other work, which has demonstrated that increased opportunities for training and supportive supervision models in long-term care settings are positively associated with job satisfaction among DCWs (Choi & Johantgen, 2012; Han et al., 2014) and may result in greater career commitment (Coogle, Parham, & Rachel, 2011). Additionally, delivery of high-quality care that also supports the development of relationships between DCWs and residents, such as FFC, is also associated with improvements in job satisfaction (Chung, 2012; Lerner, Resnick, Galik, & Flynn, 2011).
As might be anticipated in these settings, there was significant attrition of staff, with this being particularly evident in the treatment sites. Specifically, 18 (46%) DCWs in the treatment group left their settings, whereas only 6 (16%) recruited DCWs left in the control group. Despite attempts to continually assure some level of education related to FFC in the treatment settings among newly hired DCWs, it was challenging for the FFCN and champions to assure that the information was received and that there was consistent delivery of the study intervention. Moreover, in the treatment sites, it was noted that DCW positions remained vacant for a period of time and remaining staff expressed feeling stressed due to workload. To our knowledge, there was no historical event, such as leadership change or poor state survey result that may have influenced the greater staff turnover in the treatment group.
As noted previously, the recruited residents had severe CI and moderate levels of behavioral symptoms that challenged DCWs during care interactions. Although exercise interventions are an appropriate and useful approach to management of behavioral symptoms in dementia (Ayalon et al., 2006; Cohen-Mansfield, 2001; Kong, Evans, & Guevara, 2009), we anticipate that the DCWs may not strongly believe this and/or fear that exercise and engagement in physical activity may actually cause an exacerbation of behavioral symptoms and/or put residents at risk for cardiovascular events and/or falls. Ongoing education and role modeling are needed to help DCWs believe that even residents with CI and behavioral symptoms and those who may also have end stage cardiovascular disease can benefit from exercise (Santos et al., 2010). Moreover, ongoing assurance is needed to help the DCWs also believe that there is no reason to anticipate an increased risk for falls (Galik et al., 2014; Gruber-Baldini, Resnick, Hebel, Galik, & Zimmerman, 2011.
Limitations
The findings from this study are limited by the inclusion of a small, select sample of residents and DCWs in a single regional area. The settings were not reflective of the many different types of AL settings across the country. The measures used in this study were mostly objective in nature; however, the proxy reports for resident survey measures were provided by DCWs and thus may have been biased. Although controlled for in statistical analyses, there was some attrition noted over the 6-month period among residents (similar between the groups) and staff (greater attrition in the treatment group). Staffing levels were not collected as part of this study as all facilities were owned by the same parent company; however, given the unequal attrition of staff, this would be an important factor to measure in future work. Finally, the cost of the intervention was not calculated as part of the study, but primarily consisted of the salary of the FFC Nurse for 10hr a week over a 6-month period. The training of a facility-based champion was designed to address sustainability of the intervention over time. Future work could consider whether the cost of the nurse interventionist is offset by resident outcomes.
Despite the limitations and lack of significance in this study, the information obtained will help guide future interventions and revision of FFC-CI and will facilitate further testing of this intervention. Specifically, we plan in future studies to focus on the impact of FFC on resistance to care and transitioning negative behaviors such as wandering to more appropriate behaviors such as participation in group activities. Likewise, we will need to put plans for attrition in place, such as integration of FFC-ED in new employee orientation, and identification of more than one facility-based champion. We will continue to work with DCWs to optimize function and physical activity as a way to decrease fall risk and prevent infections and need for re-hospitalization.
Funding
This study was supported by the Robert Wood Johnson Foundation Nurse Faculty Scholar Grant (66520).
References
- Actigraph. (2004). Actigraph analysis software. Retrieved from http://www.theactigraph.com.
- Alexopoulos G. S., Abrams R. C., Young R. C., Shamoian C. A. (1988). Cornell scale for depression in dementia. Biological Psychiatry, 23, 271–284. [DOI] [PubMed] [Google Scholar]
- Algase D. L., Antonakos C., Yao L., Beattie E. R., Hong G. R., Beel-Bates C. A. (2008). Are wandering and physically nonaggressive agitation equivalent? The American Journal of Geriatric Psychiatry, 16, 293–299. doi:10.1097/JGP.0b013e3181629943 [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine and the American Heart Association. (2013). Guidelines for physical activity. Retrieved from http://www.heart.org/HEARTORG/GettingHealthy/PhysicalActivity/StartWalking/American-Heart-Association-Recommendations-for-Physical-Activity-in-Adults_UCM_307976_Article.jsp. www.americanheart.org
- Ayalon L., Gum A. M., Feliciano L., Areán P. A. (2006). Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: A systematic review. Archives of Internal Medicine, 166, 2182–2188. doi:10.1001/archinte.166.20.2182 [DOI] [PubMed] [Google Scholar]
- Banaszak-Holl, J., Castle, N. G., Lin, M. K., Shrivastwa, N., & Spreitzer, G. (2013). The role of organizational culture in retaining nursing workforce. The Gerontologist. doi:10.1093/geront/gnt129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. (1997). Self-efficacy: The exercise of control. New York: WH Freeman Company. [Google Scholar]
- Bowers B. J., Esmond S., Jacobson N. (2000). The relationship between staffing and quality in long-term care facilities: Exploring the views of nurse aides. Journal of Nursing Care Quality, 14, 55–64. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Williams B. R., Woodby L. L., Davis L. L., Allman R. M. (2007). Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. Journal of Hospital Medicine, 2, 305–313. doi:10.1002/jhm.209 [DOI] [PubMed] [Google Scholar]
- Choi, J., & Johantgen, M. (2012). The importance of supervision in retention of CNAs. Research in Nursing & Health, 35, 187–199. doi:10.1002/nur.21461 [DOI] [PubMed] [Google Scholar]
- Chung, G. (2012). Understanding nursing home worker conceptualizations about good care. The Gerontologist. doi:10.1093/geront/gns117 [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J. (1991). Instruction manual for the Cohen-Mansfield agitation inventory. The Research Institute of the Hebrew Home of Greater Washington. Retrieved from http://www.dementia-assessment.com.au/symptoms/CMAI_Manual.pdf [Google Scholar]
- Cohen-Mansfield J. (2001). Nonpharmacologic interventions for inappropriate behaviors in dementia: A review, summary, and critique. American Journal of Geriatric Psychiatry, 9, 361–381. [PubMed] [Google Scholar]
- Cohen-Mansfield J., Creedon M. A., Malone T., Parpura-Gill A., Dakheel-Ali M., Heasly C. (2006). Dressing of cognitively impaired nursing home residents: Description and analysis. The Gerontologist, 46, 89–96. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J., Marx M. S., Rosenthal A. S. (1989). A description of agitation in a nursing home. Journal of Gerontology, 44, M77–M84. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J., Thein K., Marx M. S., Dakheel-Ali M., Freedman L. (2012). Efficacy of nonpharmacologic interventions for agitation in advanced dementia: A randomized, placebo-controlled trial. The Journal of Clinical Psychiatry, 73, 1255–1261. doi:10.4088/JCP.12m07918 [DOI] [PubMed] [Google Scholar]
- Coogle, C. L., Parham, I. A., & Rachel, C. A. (2011). Job satisfaction and career commitment among Alzheimer’s care providers: Addressing turnover and improving staff empowerment. American Journal of Alzheimer’s Disease and Other Dementias, 26, 521–527. doi:10.1177/1533317511429322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett A., Ballard C. (2012). Antipsychotics and mortality in dementia. The American Journal of Psychiatry, 169, 7–9. doi:10.1176/appi.ajp.2011.11101488 [DOI] [PubMed] [Google Scholar]
- Davison T. E., McCabe M. P., Visser S., Hudgson C., Buchanan G., George K. (2007). Controlled trial of dementia training with a peer support group for aged care staff. International Journal of Geriatric Psychiatry, 22, 868–873. doi:10.1002/gps.1754 [DOI] [PubMed] [Google Scholar]
- Flannery K., Resnick B., Galik E., Lipscomb J., McPhaul K. (2012). Reliability and validity assessment of the job attitude scale. Geriatric Nursing (New York), 33, 465–472. doi:10.1016/j.gerinurse.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Galik E. M., Resnick B., Gruber-Baldini A., Nahm E. S., Pearson K., Pretzer-Aboff I. (2008). Pilot testing of the restorative care intervention for the cognitively impaired. Journal of the American Medical Directors Association, 9, 516–522 doi: 10.1016/j.jamda.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Galik E., Resnick B., Pretzer-Aboff I. (2009). ‘Knowing what makes them tick’: Motivating cognitively impaired older adults to participate in restorative care. International Journal of Nursing Practice, 15, 48–55 doi: 10.1111/j.1440-172X.2008.01721.x. [DOI] [PubMed] [Google Scholar]
- Galik E., Resnick B., Hammersla M., Brightwater J. (2014). Optimizing function and physical activity among nursing home residents with dementia: Testing the impact of function-focused care. The Gerontologist, 54, 930–943. doi:10.1093/geront/gnt1 [DOI] [PubMed] [Google Scholar]
- Grabowski, D. C., Elliot, A., Leitzell, B., Cohen, L. W., & Zimmerman, S. (2014). Who are the innovators? Nursing homes implementing culture change. The Gerontologist, 54, S65–S75. doi:10.1093/geront/gnt144 [DOI] [PubMed] [Google Scholar]
- Gruber-Baldini A. L., Boustani M., Sloane P. D., Zimmerman S. (2004). Behavioral symptoms in residential care/assisted living facilities: Prevalence, risk factors, and medication management. Journal of the American Geriatrics Society, 52, 1610–1617. doi:10.1111/j.1532-5415.2004.52451.x [DOI] [PubMed] [Google Scholar]
- Gruber-Baldini A. L., Resnick B., Hebel J. R., Galik E., Zimmerman S. (2011). Adverse events associated with the Res-Care Intervention. Journal of the American Medical Directors Association, 12, 584–589. doi:10.1016/j.jamda.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Han, K., Trinkoff, A. M., Storr, C. L., Lerner, N., Johantgen, M., & Gartrell, K. (2014). Associations between state regulations, training length, perceived quality and job satisfaction among certified nursing assistants: Cross-sectional secondary data analysis. International Journal of Nursing Studies, 51, 1135–1141. doi:10.1016/j.ijnurstu.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Helmer T.Olson S., & Heim R. (1995). Strategies for nurse aide job satisfaction. The Journal of Long-Term Care Administration, 1, 10–14. [PubMed] [Google Scholar]
- Johnson J. A., McIlroy W. E., Roy E., Papaioannou A., Thabane L., Giangregorio L. (2013). Feasibility study of walking for exercise in individuals living in assisted living settings. Journal of Geriatric Physical Therapy, 36, 175–181 doi:10.1519/JPT.0b013e318282d2d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. M., Bellantonio S., Fortinsky R. H., Dauser D., Kleppinger A., Robison J., … Walsh S. J. (2008). Factors associated with skilled nursing facility transfers in dementia-specific assisted living. Alzheimer Disease and Associated Disorders, 22, 255–260. doi:10.1097/WAD.0b013e31816c92d5 [DOI] [PubMed] [Google Scholar]
- King-Kallimanis B., Schonfeld L., Molinari V. A., Algase D., Brown L. M., Kearns W. D., … Nelson A. L. (2010). Longitudinal investigation of wandering behavior in Department of Veterans Affairs nursing home care units. International Journal of Geriatric Psychiatry, 25, 166–174. doi:10.1002/gps.2316 [DOI] [PubMed] [Google Scholar]
- Kolanowski A., Fick D., Frazer C., Penrod J. (2010). It’s about time: Use of nonpharmacological interventions in the nursing home. Journal of Nursing Scholarship, 42, 214–222. doi:10.1111/j.1547-5069.2010.01338.x [DOI] [PubMed] [Google Scholar]
- Kong E. H., Evans L. K., Guevara J. P. (2009). Nonpharmacological intervention for agitation in dementia: A systematic review and meta-analysis. Aging & Mental Health, 13, 512–520. doi:10.1080/13607860902774394 [DOI] [PubMed] [Google Scholar]
- Król-Zielińska M., Kusy K., Zieliński J., Osiński W. (2011). Physical activity and functional fitness in institutionalized vs. independently living elderly: A comparison of 70-80-year-old city-dwellers. Archives of Gerontology and Geriatrics, 53, e10–e16. doi:10.1016/j.archger.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Lerner, N., Resnick, B., Galik, E., & Flynn, L. (2011). Job satisfaction of nursing assistants. The Journal of Nursing Administration, 41, 473 -478. doi:10.1097/NNA.0b013e3182346e7a [DOI] [PubMed] [Google Scholar]
- Liu, L.-F., Liu, W.-P., & Wang, J.-Y. (2011). Work autonomy of certified nursing assistants in long-term care facilities: Discrepant perceptions between nursing supervisors and certified nursing assistants. Journal of the American Medical Directors Association, 12, 524–534. doi:10.1016/j.jamda.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Lee K. H., Algase D. L., McConnell E. S. (2014). Relationship between observable emotional expression and wandering behavior of people with dementia. International Journal of Geriatric Psychiatry, 29, 85–92. doi:10.1002/gps.3977 [DOI] [PubMed] [Google Scholar]
- Lu Z. (2010). Investigating walking environments in and around assisted living facilities: A facility visit study. HERD, 3, 58–74. [DOI] [PubMed] [Google Scholar]
- Lyketsos C. G., Samus Q. M., Baker A., McNabney M., Onyike C. U., Mayer L. S., … Rosenblatt A. (2007). Effect of dementia and treatment of dementia on time to discharge from assisted living facilities: The Maryland assisted living study. Journal of the American Geriatrics Society, 55, 1031–1037. doi:10.1111/j.1532-5415.2007.01225.x [DOI] [PubMed] [Google Scholar]
- Mahoney F. I., Barthel D. W. (1965). Functional evaluation: The Barthel Index. Maryland State Medical Journal, 14, 61–65. [PubMed] [Google Scholar]
- Marin R. S., Biedrzycki R. C., Firinciogullari S. (1991). Reliability and validity of the Apathy Evaluation Scale. Psychiatry Research, 38, 143–162. [DOI] [PubMed] [Google Scholar]
- Maust D. T., Onyike C. U., Sheppard J. M., Mayer L. S., Samus Q. M., Brandt J., … Rosenblatt A. (2006). Predictors of caregiver unawareness and nontreatment of dementia among residents of assisted living facilities: The Maryland Assisted Living Study. The American Journal of Geriatric Psychiatry, 14, 668–675. doi:10.1097/01.JGP.0000209214.28172.45 [DOI] [PubMed] [Google Scholar]
- Moore D. H., Algase D. L., Powell-Cope G., Applegarth S., Beattie E. R. (2009). A framework for managing wandering and preventing elopement. American journal of Alzheimer’s Disease and Other Dementias, 24, 208–219. doi:10.1177/1533317509332625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor D. W., Ames D., Gardner B., King M. (2009). Psychosocial treatments of psychological symptoms in dementia: A systematic review of reports meeting quality standards. International Psychogeriatrics, 21, 241–251. doi:10.1017/S1041610208008223 [DOI] [PubMed] [Google Scholar]
- O’Neil M. E.Freeman M.Christensen V.Telerant R.Addleman A., & Kansagara D. (2011). A systematic evidence review of non-pharmacological interventions for behavioral symptoms of dementia. Washington, DC: Department of Veterans Affairs. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK54971/ [PubMed] [Google Scholar]
- Ouslander J. G., Griffiths P. C., McConnell E., Riolo L., Kutner M., Schnelle J. (2005). Functional incidental training: A randomized, controlled, crossover trial in Veterans Affairs nursing homes. Journal of the American Geriatrics Society, 53, 1091–1100. doi:10.1111/j.1532-5415.2005.53359.x [DOI] [PubMed] [Google Scholar]
- Park-Lee E.Sengupta M., & Harris-Kojetin L. D. (2013). Dementia special care units in residential care communities: United States, 2010. NCHS Data Brief, 134, 1–8. [PubMed] [Google Scholar]
- Penrod J., Yu F., Kolanowski A., Fick D. M., Loeb S. J., Hupcey J. E. (2007). Reframing person-centered nursing care for persons with dementia. Research and Theory for Nursing Practice, 21, 57–72 doi:10.1111/j.1547-5069.2008.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. J., Flesner M. (2013). Perspectives and experiences related to physical activity of elders in long-term-care settings. Journal of Aging and Physical Activity, 21, 33–50. [DOI] [PubMed] [Google Scholar]
- Ranhoff A. H. (1997). Reliability of nursing assistants’ observations of functioning and clinical symptoms and signs. Aging, 9, 378–380. [DOI] [PubMed] [Google Scholar]
- Resnick B. (Ed). (2011). Restorative care nursing for older adults: A guide for all care settings. New York: Spring Publishing. [Google Scholar]
- Resnick B., Daly M. P. (1998). Predictors of functional ability in geriatric rehabilitation patients. Rehabilitation Nursing, 23, 21–29. [DOI] [PubMed] [Google Scholar]
- Resnick B., Galik E. (2007). The reliability and validity of the physical activity survey in long-term care. Journal of Aging and Physical Activity, 15, 439–458. [DOI] [PubMed] [Google Scholar]
- Resnick B., Galik E., Gruber-Baldini A. L., Zimmerman S. (2010). Perceptions and performance of function and physical activity in assisted living communities. Journal of the American Medical Directors Association, 11, 406–414. doi:10.1016/j.jamda.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Resnick B., Galik E., Gruber-Baldini A. L., Zimmerman S. (2010). Satisfaction with assisted living: The unexplored role of physical activity. Geriatric Nursing, 31, 197–205 doi:10.1016/j.gerinurse.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Resnick, B., Galik, E., Gruber-Baldini, A., & Zimmerman, S. (2011). Testing the effect of Function-Focused Care in Assisted Living. Journal of the American Geriatrics Society, 59, 2233–2240. doi:10.1111/j.1532-5415.2011.03699.x [DOI] [PubMed] [Google Scholar]
- Resnick B., Galik E., Pretzer-Aboff I., Rogers V., Gruber-Baldini A. L. (2008). Testing the reliability and validity of self-efficacy and outcome expectations of restorative care performed by nursing assistants. Journal of Nursing Care Quality, 23, 162–169. doi:10.1097/01.NCQ.0000313766.09891.43 [DOI] [PubMed] [Google Scholar]
- Resnick B., Gruber-Baldini A. L., Pretzer-Aboff I., Galik E., Buie V. C., Russ K., Zimmerman S. (2007). Reliability and validity of the evaluation to sign consent measure. The Gerontologist, 47, 69–77. [DOI] [PubMed] [Google Scholar]
- Resnick B., Petzer-Aboff I., Galik E., Russ K., Cayo J., Simpson M., Zimmerman S. (2008). Barriers and benefits to implementing a restorative care intervention in nursing homes. Journal of the American Medical Directors Association, 9, 102–108. doi:10.1016/j.jamda.2007.08.011 [DOI] [PubMed] [Google Scholar]
- Resnick B., Rogers V., Galik E., Gruber-Baldini A. (2007). Measuring restorative care provided by nursing assistants: Reliability and validity of the Restorative Care Behavior Checklist. Nursing Research, 56, 387–398. [DOI] [PubMed] [Google Scholar]
- Resnick B., Simpson M. (2003). Restorative care nursing activities: Pilot testing self-efficacy and outcome expectation measures. Geriatric Nursing (New York), 24, 82–89. [DOI] [PubMed] [Google Scholar]
- Robert P. H., Clairet S., Benoit M., Koutaich J., Bertogliati C., Tible O., … Bedoucha P. (2002). The apathy inventory: Assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. International Journal of Geriatric Psychiatry, 17, 1099–1105. doi:10.1002/gps.755 [DOI] [PubMed] [Google Scholar]
- Sallis J. F., Cervero R. B., Ascher W., Henderson K. A., Kraft M. K., Kerr J. (2006). An ecological approach to creating active living communities. Annual Review of Public Health, 27, 297–322. doi:10.1146/annurev.publhealth.27.021405.102100 [DOI] [PubMed] [Google Scholar]
- Santos J. M., Kowatsch I., Tsutsui J. M., Negrão C. E., Canavesi N., Carvalho Frimm C., … MathiasW.,Jr. (2010). Effects of exercise training on myocardial blood flow reserve in patients with heart failure and left ventricular systolic dysfunction. The American Journal of Cardiology, 105, 243–248. doi:10.1016/j.amjcard.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Schutzer K. A., Graves B. S. (2004). Barriers and motivations to exercise in older adults. Preventive Medicine, 39, 1056–1061. doi:10.1016/j.ypmed.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Sloane P. D., Zimmerman S., Gruber-Baldini A. L., Hebel J. R., Magaziner J., Konrad T. R. (2005). Health and functional outcomes and health care utilization of persons with dementia in residential care and assisted living facilities: Comparison with nursing homes. The Gerontologist, 45, 124–132. [DOI] [PubMed] [Google Scholar]
- Teri L., Huda P., Gibbons L., Young H., van Leynseele J. (2005). STAR: A dementia-specific training program for staff in assisted living residences. The Gerontologist, 45, 686–693. [DOI] [PubMed] [Google Scholar]
- Tighe S. K., Leoutsakos J. M., Carlson M. C., Onyike C. U., Samus Q., Baker A., … Lyketsos C. G. (2008). The association between activity participation and time to discharge in the assisted living setting. International Journal of Geriatric Psychiatry, 23, 586–591. doi:10.1002/gps.1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Lee C. (2010). Site and neighborhood environments for walking among older adults. Health Place, 16, 1268–1279. [DOI] [PubMed] [Google Scholar]
- Zimmerman S., Mitchell C. M., Chen C. K., Morgan L. A., Gruber-Baldini A. L., Sloane P. D., … Munn J. (2007). An observation of assisted living environments: Space use and behavior. Journal of Gerontological Social Work, 49, 185–203. doi:10.1300/J083v49n03_11 [DOI] [PubMed] [Google Scholar]
- Zimmerman S., Sloane P. D., Reed D. (2014). Dementia prevalence and care in assisted living. Health Affairs (Project Hope), 33, 658–666. doi:10.1377/hlthaff.2013.1255 [DOI] [PubMed] [Google Scholar]


