The success of highly effective modulator therapy (HEMT) in cystic fibrosis (CF) now illustrates two areas of deficiency: the lack of HEMT for younger children and for approximately 10% of the CF population without a qualifying mutation.
Inflammation, infection, and structural changes in the CF lung start in infancy or the early preschool years (1). Computed tomography scans of the chest and lung clearance index measurements are abnormal early and are not clearly associated with infection (1). The cardinal pulmonary lesion in CF, bronchiectasis, can be detected on chest computed tomography in up to 30–40% of children with CF aged between 3 and 4 years old with airway dilatation and thickening reported as early as the first few months of life (1). Linked to early inflammation are poor growth and nutrition. Restriction of early lung growth worsens CF outcomes, but improvement in nutrition can ameliorate this (2). This evidence has led to an effort to bring effective therapies to infants and preschoolers with CF in an attempt to slow or stop progression. Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) targets the basic defect in CF by improving function of the native CFTR. It is hoped that early treatment with HEMT will mitigate this early disease progression, improve nutrition with a resultant improvement in lung growth, and change the course of CF over the lifetime. The urgency for early effective treatments for CF is real. Until we are able to address the basic defect in CF in the infant, we will likely continue to see significant morbidity because of this disease.
In this issue of the Journal, Zemanick and colleagues (pp. 1522–1532) share the results of a phase III clinical trial to prove safety and tolerability of ELX/TEZ/IVA in children aged 6 to 11 years (3). In this study, the open-label use of ELX/TEZ/IVA was studied in 66 children. The primary endpoint of the study was safety and tolerability, and the current article shows that the safety profile for ELX/TEZ/IVA in 6- to 11-year-old children was similar to the safety profile in older individuals (4, 5). The secondary endpoints were efficacy in terms of FEV1 as well as reduction in sweat chloride, improvement in body mass index, and improvement in symptom scores. The coronavirus disease (COVID-19) worldwide epidemic occurred during the performance of this study. This epidemic had a global effect on clinical research, as the safety of participants in clinical trials took precedent over study visits and unnecessary exposures. Although adverse events were continuously collected for the entire study for participants, fewer participants had a full complement of efficacy measures. The intriguing data from those participants with efficacy endpoints at 24 weeks show a significant effect on both lung function (+10.2%) and sweat chloride levels (−60.9%). As is true for most pediatric studies, the starting lung function on average was normal. The lung function improvement was present despite this normalcy, even in the absence of a control group, suggesting significant efficacy, not just safety, in this age group. The 2-year open-label follow-up study will give more data on lung growth and disease progression. In addition, the weight-for-age z-score improved significantly from baseline, 0.37 versus −0.16. The authors contend that the reduction in sweat chloride deserves extra attention. All of the participants with two F508del alleles reduced their sweat chloride levels below the diagnostic level of 60 mmol/L, with 42.9% achieving normal levels <30 mmol/L. The authors suggest that this finding alone may indicate a true change in the paradigm of the future of CF in these children. Although individuals with mild CF mutations who have lower sweat chloride values have better outcomes, this may or may not be applicable to patients with pharmaceutically induced lower sweat chloride values. It is, however, intriguing and should spur the community to bring ELX/TEZ/IVA to the youngest individuals with CF as soon as possible. It is advantageous that the a priori design of this study was to have the primary outcome of safety. A study based on efficacy would have been significantly prolonged or not completed because of the COVID-19 crisis. Again, the 2-year follow-up study for these participants will be important to clarify both the impact and side effects of this therapy.
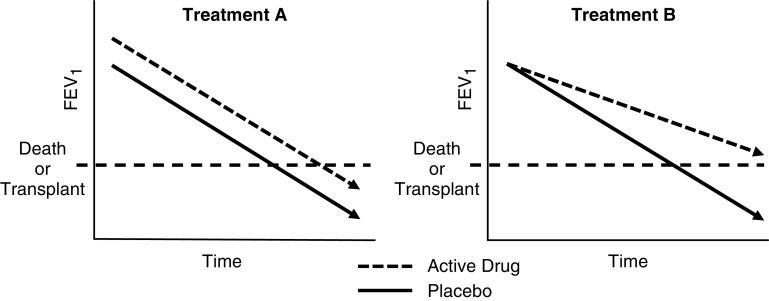
HEMF, such as IVA in individuals with gating mutations and ELX/TEZ/IVA in those with F508del, has been proven in pivotal phase III trials to significantly improve lung function, nutritional status, symptoms scores, and quality-of-life measures (4–6). It also significantly decreases sweat tests. Most importantly, however, HEMT decreases the incidence of pulmonary exacerbation (4, 5). Longer-term studies of modulators prove that there is a clear reduction in the rate of lung decline in individuals treated with these therapies. This reduction in the lung function decline for IVA is dramatic, from −8.3% to −0.7% using U.S. and U.K. registry data (7, 8). Although a therapy that improves lung function alone may improve longevity by a matter of years, a therapy that slows the rate of decline has an ongoing effect over the lifetime with a greater extension of life (Figure 1). ELX/TEZ/IVA appears to be a therapy that will do both and have the greatest long-term impact.
Figure 1.
Schematic representation of two potential treatment responses. Treatment A acutely improves FEV1 but does not slow rate of decline in FEV1, resulting in a minor improvement in the long-term outcome. Treatment B does not acutely improve FEV1 but does slow the rate of decline in FEV1, resulting in sustained benefit.
The efficacy of HEMT points out, however, the lack of available HEMT to a subset of people living with CF. Mutations that are not amenable to HEMT are more common in individuals of minority race and ethnicity, and this contributes to the poor outcomes in these groups (9, 10). In this study, only 1 of 66 participants was a minority. Fighting for equity for all individuals living with CF has become a main emphasis for the CF Foundation in terms of research, access, and outcomes.
At this point, the previous modulators IVA, lumacaftor/IVA, and TEZ/IVA have all been approved for younger ages than the original phase III qualifying studies (11, 12). IVA has recently been approved down to 4 months, whereas lumacaftor/IVA is approved to 2 years and TEZ/IVA is approved to 6 years (13–15). Longer-term data from these extensions show ongoing safety and efficacy even in the youngest patients (16, 17). Previous modulators have been shown to be safe, both in the short and long term, in young children. This knowledge should reaffirm the safety findings in the current study and should prove the appropriateness of moving ELX/TEZ/IVA to the 6- to 11-year-old age group. The CF community has been waiting for the results of this study and to have ELX/TEZ/IVA for children. The goal and expectation will be much better long-term outcomes for people living with CF.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202104-0850ED on April 26, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ranganathan SC, Hall GL, Sly PD, Stick SM, Douglas TA. AREST-CF. Early lung disease in infants and preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2017;195:1567–1575. doi: 10.1164/rccm.201606-1107CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanders DB, Fink A, Mayer-Hamblett N, Schechter MS, Sawicki GS, Rosenfeld M, et al. Early life growth trajectories in cystic fibrosis are associated with pulmonary function at age 6 years. J Pediatr. 2015;167:1081–8.e1. doi: 10.1016/j.jpeds.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zemanick ET, Taylor-Cousar JL, Davies J, Gibson RL, Mall MA, McKone EF, et al. VX18-445-106 Study Group. A phase 3 open-label study of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis and at least one F508del allele. Am J Respir Crit Care Med. 2021;203:1522–1532. doi: 10.1164/rccm.202102-0509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heijerman HGM, KcKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. VX17-445-103 Study Group. Efficacy and safety of the elexacaftor/tezacaftor/ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomized, phase 3 trial. Lancet. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, et al. VX08-770-103 (ENVISION) Study Group. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–1225. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bessonova L, Volkova N, Higgins M, Bengtsson L, Tian S, Simard C, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax. 2018;73:731–740. doi: 10.1136/thoraxjnl-2017-210394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Volkova N, Moy K, Evans J, Campbell D, Tian S, Simard C, et al. Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J Cyst Fibros. 2020;19:68–79. doi: 10.1016/j.jcf.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 9. Rho J, Ahn C, Gao A, Sawicki GS, Keller A, Jain R. Disparities in mortality of Hispanic patients with cystic fibrosis in the United States. A national and regional cohort study. Am J Respir Crit Care Med. 2018;198:1055–1063. doi: 10.1164/rccm.201711-2357OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol. 2021 doi: 10.1002/ppul.25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Milla CE, Ratjen F, Marigowda G, Liu F, Waltz D, Rosenfeld M. VX13-809-011 Part B Investigator Group. Lumicaftor/ivacaftor in patients aged 6-11 years with cystic fibrosis and homozygous for F508del-CFTR. Am J Respir Crit Care Med. 2017;195:912–920. doi: 10.1164/rccm.201608-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walker S, Flume P, McNamara J, Solomon M, Chilvers M, Chmiel J, et al. VX15-661-113 Investigator Group. A phase 3 study of tezacaftor in combination with ivacaftor in children aged 6 through 11 years with cystic fibrosis. J Cyst Fibros. 2019;18:708–713. doi: 10.1016/j.jcf.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 13. Rosenfeld M, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, et al. ARRIVAL study group. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study. Lancet Respir Med. 2018;6:545–553. doi: 10.1016/S2213-2600(18)30202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies JC, Wainwright CE, Sawicki GS, Higgins MN, Campbell D, Harris C, et al. ARRIVAL Study Group. Ivacaftor in infants aged 4 to <12 months with cystic fibrosis and a gating mutation: Results of a two-part phase 3 clinical trial. Am J Respir Crit Care Med. 2021;203:585–593. doi: 10.1164/rccm.202008-3177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNamara JJ, McColley SA, Marigowda G, Liu F, Tian S, Owen CA, et al. Safety, pharmacokinetics, and pharmacodynamics of lumacaftor and ivacaftor combination therapy in children aged 2-5 years with cystic fibrosis homozygous for F508del-CFTR: an open-label phase 3 study. Lancet Respir Med. 2019;7:325–335. doi: 10.1016/S2213-2600(18)30460-0. [DOI] [PubMed] [Google Scholar]
- 16. Rosenfeld M, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. KLIMB study group. An open-label extension study of ivacaftor in children with CF and a CFTR gating mutation initiating treatment at age 2-5 years (KLIMB) J Cyst Fibros. 2019;18:838–843. doi: 10.1016/j.jcf.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chilvers MA, Davies JC, Milla C, Tian S, Han Z, Cornell AG, et al. Long-term safety and efficacy of lumacaftor-ivacaftor therapy in children aged 6-11 years with cystic fibrosis homozygous for the F508del-CFTR mutation: a phase 3, open-label, extension study. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(20)30517-8. [DOI] [PubMed] [Google Scholar]