To the Editor:
The goals of managing chronic obstructive pulmonary disease (COPD) include optimizing lung function, reducing symptoms, improving health status, and reducing exacerbations. As COPD is multidimensional in nature, composite scores have been proposed to better predict risk of death and assess effects of pharmacological therapies. Regarding the latter, the concept of clinically important deterioration (CID) was proposed using the purported minimum clinically important differences (MCIDs) of health status (St. George’s Respiratory Questionnaire ⩾4), moderate–severe exacerbation incidence, and lung function (FEV1 ⩾ 100 ml) (1). This concept of CID has since been used in subsequent clinical trials. However, the lung function criterion of an MCID of 100 ml was initially posited from a literature review (2), which proffered that although a change of 100 ml can be perceived by a patient, several limitations exist, including placebo effect, reproducibility, and variability, leading to the conclusion that “The MCID in FEV1 remains an important but still undetermined issue for patients with COPD” (2). This is important when considering the American Thoracic Society (ATS)/European Respiratory Society (ERS) Task Force statement on interpretive strategies for lung function testing that suggests, for an individual patient, only changes that fall outside the coefficient of repeatability (CR) should be considered significant (3). CR determination is recommended because it takes into consideration the measurement variability over a specified interval (i.e., measurement noise) and represents the value below which the absolute difference between two measurements would lie with a 95% probability.
To further explore the robustness and, hence, utility of a 100-ml threshold as gleaned from clinical trials, as the CID was initially proposed using such data, we reviewed data from two clinical studies in patients with COPD to assess the short-term variability of change-from-baseline FEV1. We hypothesized that the FEV1 CR observed in multicenter trials would exceed the 100-ml threshold currently adopted as the MCID.
Methods
We examined data from two COPD trials (HZC102871, NCT01009463; HZC102970, NCT01017952) (4). These were registration studies designed to replicate the efficacy and safety of the inhaled corticosteroid/long-acting β2-agonist combination fluticasone furoate/vilanterol 100/25 μl. Enrolled patients were ⩾40 years old with ATS/ERS-defined COPD history (5), a smoking history of ⩾10 pack-years, a post-bronchodilator FEV1/FVC ratio of ⩽0.70, a post-bronchodilator FEV1 ⩽0.70 predicted, and ⩾1 documented moderate or severe COPD exacerbation in the year before screening. These trials were selected for post hoc analysis as they measured spirometry at baseline and 4 weeks later, during which the patients could not have had an exacerbation, a respiratory tract infection, or a change in their inhaled therapy. The patient subgroup selected for this analysis (HZC102871, N = 427; HZC102970, N = 421) was already receiving an inhaled fluticasone propionate/salmeterol 250/50 μg combination product. At baseline, patients received open-label inhaled fluticasone propionate/salmeterol for 4 weeks prior to randomization. The spirometry equipment had to meet or exceed the minimum ATS performance recommendations (6), and external spirometric tracing readings were made to assure acceptability and reproducibility. Variability of FEV1 measurements over the 4-week interval was assessed using Bland-Altman plots (7).
Results
The study design and baseline characteristics of the entire COPD cohort are completely described in the primary publication (4). Patients in this subgroup had a mean age of ∼63 years. In studies HZC102970 and HZC102871, respectively, ∼45% and 55% were male; mean (±SD) FEV1 values were 1.26 L (±0.42) and 1.22 L (±0.46); and mean (±SD) predicted post-bronchodilator FEV1 percentages were 45.8% (±12.8) and 43.9% (±13.6). The Bland-Altman plots are shown in Figures 1 and 2; the CR for both COPD trials exceeded the putative MCID of 100 ml (370 ml and 422 ml, respectively).
Figure 1.
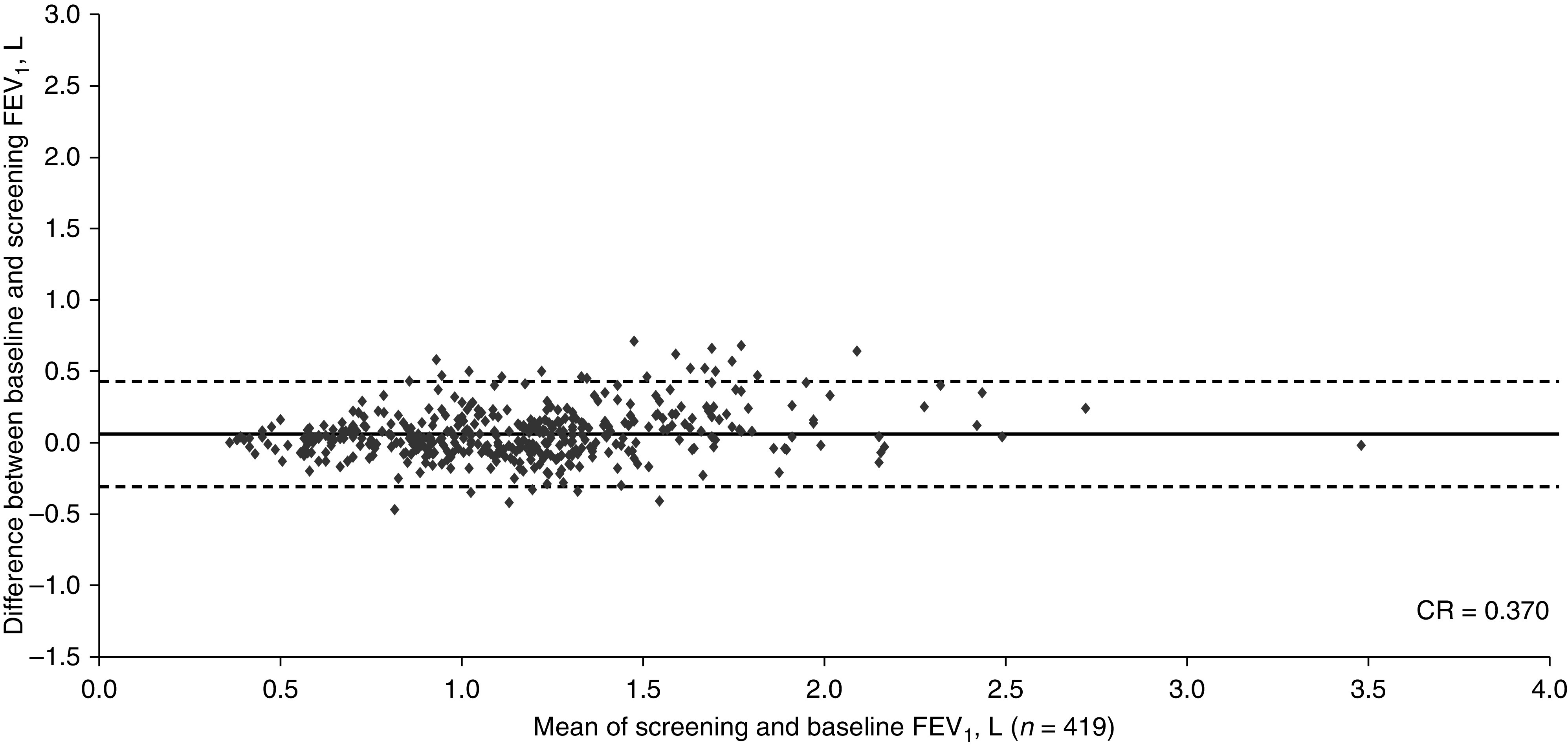
Bland-Altman plot depicting the reproducibility between the screening values and measurements at 4 weeks (baseline) for FEV1 from study HCZ102970 in subjects with COPD (ClinicalTrials.gov NCT01017952). The central horizontal line shows the mean of differences, and the dashed lines represent the 95% limits of agreement. Of the total N = 421, data were plotted for n = 419 (2 patients had missing data). CR = coefficient of repeatability.
Figure 2.
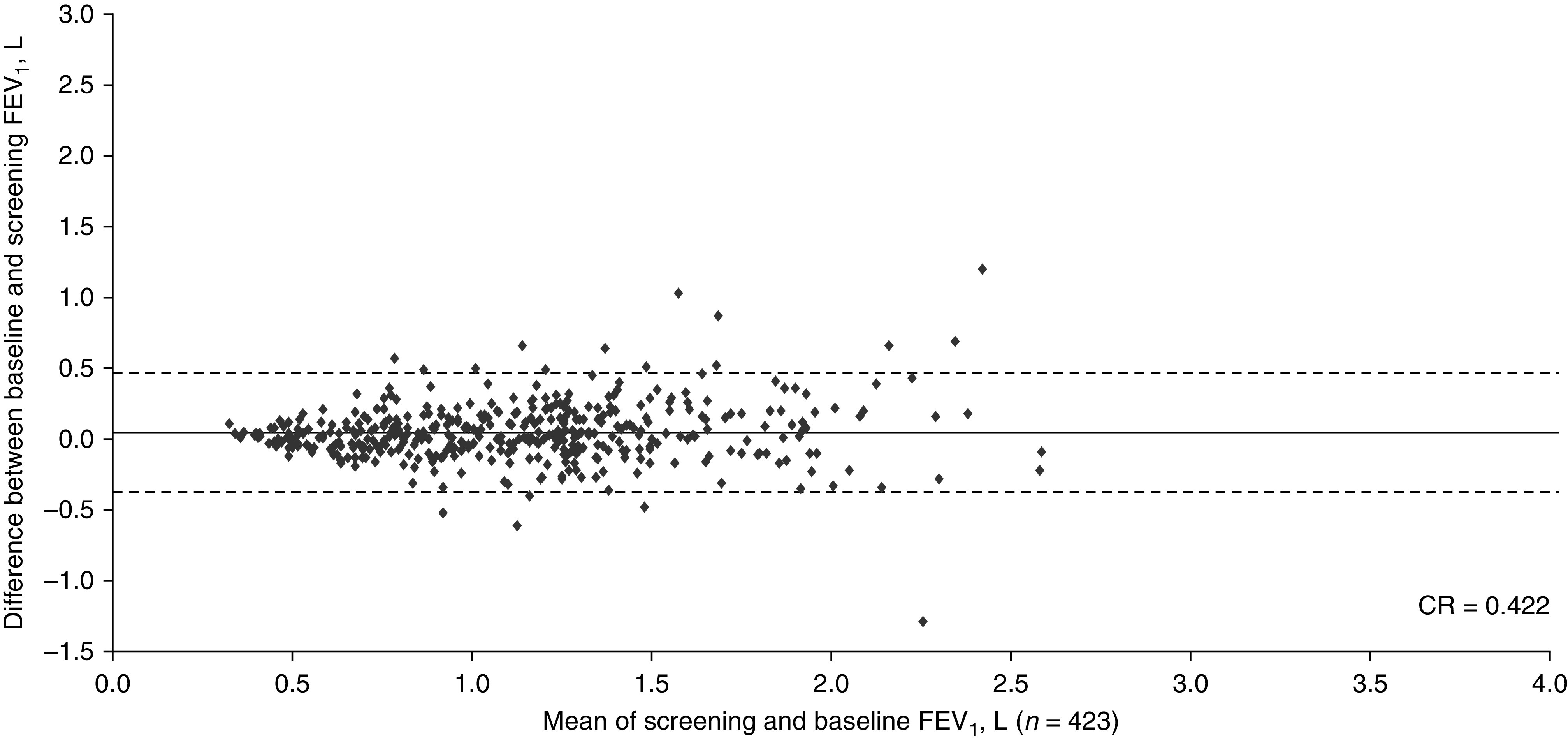
Bland-Altman plot depicting the reproducibility between the screening values and measurements at 4 weeks (baseline) for FEV1 from study HZC102871 in subjects with COPD (ClinicalTrials.gov NCT01009463). The central horizontal line shows the mean of differences, and the dashed lines represent the 95% limits of agreement. Of the total N = 427, data were plotted for n = 423 (4 patients had missing data). CR = coefficient of repeatability.
Discussion
The 4-week CR attained in patients with COPD in multicenter clinical trials exceeds the putative MCID of 100 ml. CR values obtained in both trials are similar to the 3-month CR of 400 ml reported in the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points) study (8). This is notable because in the ECLIPSE report, 37% of the patients with COPD either had an exacerbation or changed their medication during that 3-month window, and the severity of airflow limitation ranged from moderate to very severe. Patients with more severe airflow limitation, and hence lower baseline values, were more likely to demonstrate less variability compared with patients with less impaired airflow and perhaps more demonstrable reversibility. However, the percentage change would likely be of similar magnitude. Conversely, the Figures clearly illustrate the differences in individual variability. According to the ATS/ERS Task Force on interpreting lung function studies, week-to-week FEV1 variability should exceed 20% (and year-to-year exceed 15%) before considering it as a significant change in patients with COPD (3).
An MCID should not only represent a change in an endpoint detectable to the patient but also one that the patient or clinician deems as clinically beneficial. A patient with severe/very severe airflow limitation may not only perceive but benefit from a specified improvement in FEV1 compared with a patient with moderate airflow obstruction. For example, when measured on a seven-category scale (“much worse . . . about the same . . . much better”), a correlation of FEV1 with dyspnea at r = 0.29 (95% confidence interval, 0.22–0.35) and a correlation of FEV1 with overall health of only 0.10 (95% confidence interval, 0.03–0.17) was reported (9). The rating change from “about the same” to a “little bit better/worse” related to an average FEV1 change of 112 ml (10). Patients or clinicians could debate whether “little bit better/worse” is clinically meaningful. In the noninterventional ECLIPSE trial, a 100-ml FEV1 decrease was associated with an increased odds ratio of a subsequent exacerbation (9). However, there are no robust data purporting that an FEV1 increase of 100 ml decreases exacerbation risk (6), and a recent study demonstrated minimal overlap of FEV1 “responders” with those of several patient-reported outcome instruments (11).
Clearly, identifying an MCID is important in clinical practice. As with the unicorn, it was often sought but difficult to capture. From this analysis, one cannot conclude that 100 ml is not the MCID but rather that FEV1 data gleaned from multicenter clinical trials do not support the validity of this adopted value. It is probable that spirometry performed at a single center, with highly trained personnel, allows for determination of “abnormality” by demonstrating a CR for FEV1 with a value <100 ml. However, although such an abnormality might be detectable to patients, it remains to be proven that it is clinically or biologically important. Therefore, we propose that COPD therapy should be guided to improve FEV1 above the CR determined in one’s pulmonary function laboratory rather than focusing on some “universal” number, while reducing symptoms and exacerbations.
Footnotes
Supported by GlaxoSmithKline plc.
Author Contributions: C.C. and J.F.D. contributed to the conception/design of the study; all authors contributed to the data analysis/interpretation and approved the final manuscript for submission.
Originally Published in Press as DOI: 10.1164/rccm.202012-4322LE on March 22, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Singh D, Maleki-Yazdi MR, Tombs L, Iqbal A, Fahy WA, Naya I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int J Chron Obstruct Pulmon Dis. 2016;11:1413–1424. doi: 10.2147/COPD.S101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 3. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretive strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 4. Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1:210–223. doi: 10.1016/S2213-2600(13)70040-7. [DOI] [PubMed] [Google Scholar]
- 5. Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 6. Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun-Niggli G, Worthy G. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12:40. doi: 10.1186/1465-9921-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 8. Crim C, Celli B, Edwards LD, Wouters E, Coxson HO, Tal-Singer R, et al. ECLIPSE investigators. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med. 2011;105:1069–1078. doi: 10.1016/j.rmed.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 9. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 10. Redelmeier DA, Goldstein RS, Min ST, Hyland RH. Spirometry and dyspnea in patients with COPD. When small differences mean little. Chest. 1996;109:1163–1168. doi: 10.1378/chest.109.5.1163. [DOI] [PubMed] [Google Scholar]
- 11. Kostikas K, Greulich T, Mackay AJ, Lossi NS, Aalamian-Mattheis M, Nunez X, et al. Treatment response in COPD: does FEV1 say it all? A post hoc analysis of the CRYSTAL study. ERJ Open Res. 2019;5:00243–02018. doi: 10.1183/23120541.00243-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


