Abstract
Rationale: Randomized controlled trials have been unable to detect a cardiovascular benefit of continuous positive airway pressure in unselected patients with obstructive sleep apnea (OSA). We hypothesize that deleterious cardiovascular outcomes are concentrated in a subgroup of patients with a heightened pulse-rate response to apneas and hypopneas (ΔHR).
Methods: We measured the ΔHR in the MESA (Multi-Ethnic Study of Atherosclerosis) (N = 1,395) and the SHHS (Sleep Heart Health Study) (N = 4,575). MESA data were used to determine the functional form of the association between the ΔHR and subclinical cardiovascular biomarkers, whereas primary analyses tested the association of the ΔHR with nonfatal or fatal cardiovascular disease (CVD) and all-cause mortality in longitudinal data from the SHHS.
Measurements and Main Results: In the MESA, U-shaped relationships were observed between subclinical CVD biomarkers (coronary artery calcium, NT-proBNP [N-terminal prohormone BNP], and Framingham risk score) and the ΔHR; notably, a high ΔHR (upper quartile) was associated with elevated biomarker scores compared with a midrange ΔHR (25th–75th centiles). In the SHHS, individuals with a high ΔHR compared with a midrange ΔHR were at increased risk of nonfatal or fatal CVD and all-cause mortality (nonfatal adjusted hazard ratio [95% confidence interval (CI)], 1.60 [1.28–2.00]; fatal adjusted hazard ratio [95% CI], 1.68 [1.22–2.30]; all-cause adjusted hazard ratio [95% CI], 1.29 [1.07–1.55]). The risk associated with a high ΔHR was particularly high in those with a substantial hypoxic burden (nonfatal, 1.93 [1.36–2.73]; fatal, 3.50 [2.15–5.71]; all-cause, 1.84 [1.40–2.40]) and was exclusively observed in nonsleepy individuals.
Conclusions: Individuals with OSA who demonstrate an elevated ΔHR are at increased risk of cardiovascular morbidity and mortality. This study identifies a prognostic biomarker for OSA that appears useful for risk stratification and patient selection for future clinical trials.
Keywords: heart-rate response, postevent tachycardia, sleep apnea, mortality, hypoxic burden
At a Glance Commentary
Scientific Knowledge on the Subject
Randomized controlled trials have been unable to demonstrate a benefit of continuous positive airway pressure therapy in cardiovascular risk reduction for all-comers with obstructive sleep apnea (OSA). A precision-medicine approach to prioritizing patients for treatment who are actually at risk of adverse cardiovascular consequences of OSA is needed.
What This Study Adds to the Field
In this study, we propose a simple and novel metric that captures the sleep apnea–specific heart-rate response (pulse-rate response to apneas and hypopneas [ΔHR]). Using two of the largest and well-defined cohort studies in the United States, we have identified a subgroup of individuals with OSA (exaggerated ΔHR) who are at elevated, OSA-dependent risk of cardiovascular morbidity and mortality. This study identifies a prognostic biomarker for OSA that could potentially facilitate cardiovascular risk stratification and patient selection for future clinical trials.
Obstructive sleep apnea (OSA) is a common chronic disorder (1) associated with hypertension, cardiovascular disease (CVD), metabolic dysfunction (2), and increased mortality (3). However, the cardiovascular and metabolic benefits of OSA treatment with continuous positive airway pressure (CPAP) remain uncertain (3–5), with recent randomized clinical trials failing to demonstrate a reduction in CVD events or mortality (4, 6, 7), posing a significant challenge to therapeutic decision-making in individuals with OSA (8).
A potential source of these discrepant findings is the substantial heterogeneity of cardiovascular risk across different subgroups of people with OSA (9, 10). This heterogeneity may be partly related to differences in the acute physiological consequences of the upper-airway obstructions during sleep (11), including intermittent hypoxia/hypercapnia, arousals, and intrathoracic pressure swings, all of which can activate the autonomic nervous system (12, 13). These frequent surges in autonomic activity are associated with an increase in the heart rate, which reflects the severity of preceding respiratory events (14), the presence (15) and the intensity of cortical arousal (16), and autonomic system responsiveness (17). Therefore, it is plausible that the OSA-specific heart-rate response encapsulates key aspects of the autonomic response to respiratory events, making it a potentially useful prognostic marker of OSA-related CVD risk.
We sought to assess how the OSA-related heart-rate response, quantified by using the pulse-rate response to apneas and hypopneas (ΔHR), identifies subgroups of individuals who are at particular risk of the cardiovascular consequences of OSA. The ΔHR was defined as the difference between the maximum pulse rate after airway opening (i.e., during a subject-specific search window) (14, 16) and an event-related minimum pulse rate (i.e., minimum pulse rate during respiratory events). The associations between the ΔHR and cardiovascular outcomes and all-cause mortality were assessed using two community-based prospective cohort studies, the MESA (Multi-Ethnic Study of Atherosclerosis) and the SHHS (Sleep Heart Health Study). We also assessed whether associations between the ΔHR and CVD morbidity and mortality varied on the basis of OSA severity or the severity of OSA-related hypoxemia. Given the inability of prior studies to detect a CPAP benefit in nonsleepy individuals (4, 6, 7), associations were also examined in relation to the level of sleepiness. Finally, given the previously reported sex-specific associations between OSA (as quantified by the apnea–hypopnea index [AHI]) and cardiovascular outcomes, mortality (3, 18), and known differences in respiratory-event features in men and women (19), sex-stratified analysis was performed. Sensitivity analyses were done to explore whether the associations varied according to the sleep stage (REM vs. non-REM [nREM]), use of β-blockers, presence of atrial fibrillation, or presence of a cardiac pacemaker.
Methods
Two independent samples were used: 1) the MESA sample was used to conduct preliminary cross-sectional analysis to understand the functional form of the relationship between the ΔHR and subclinical CVD measures, and 2) the SHHS was used to extend the observed associations in the MESA by longitudinal analysis of the clinical endpoints of nonfatal and fatal CVD and all-cause mortality.
MESA
Study population
The MESA is a community-based cohort of middle-aged and older adults initially recruited without clinically evident CVD designed to investigate the risk factors for developing subclinical CVD. Participants underwent follow-up visits including polysomnography (PSG) (2010–2013). Institutional review board approval was obtained at all study sites, and all participants provided written informed consent. Respiratory events were identified if amplitude reduction on the nasal pressure or respiratory inductance channels (if nasal pressure was not available) exceeded 30% for hypopneas and 90% for apneas for at least 10 seconds. The AHI was calculated on the basis of the number of all apneas and hypopneas associated with a ⩾3% desaturation or electroencephalogram-based arousal per hour of sleep (see Figure 1 for study ascertainment and the online supplement for more details on polysomnographic data).
Figure 1.
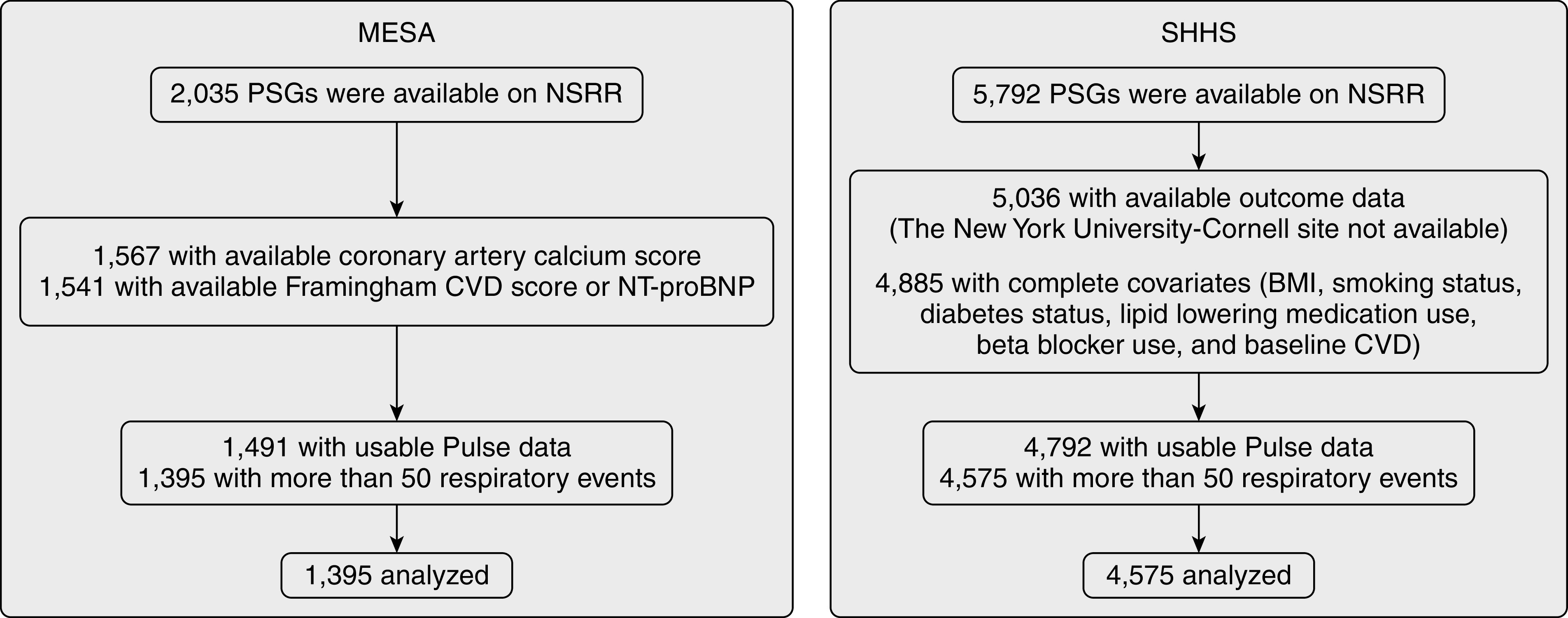
Flow diagram presenting ascertainment of the study samples. BMI = body mass index; CVD = cardiovascular disease; MESA = Multi-Ethnic Study of Atherosclerosis; NSRR = National Sleep Research Resource; NT-proBNP = N-terminal prohormone BNP; PSG = polysomnogram; SHHS = Sleep Heart Health Study.
Subclinical CVD
Because of the novelty of the ΔHR metric and uncertainty over the linearity of the associations, continuous subclinical CVD measures in the MESA were used to inform the model structure and determine thresholds. Both sleep studies and subclinical CVD assessments were obtained in conjunction with MESA examination 5 (2010–2013). Outcomes for these analyses were continuously measured indices of subclinical CVD, chosen on the basis of their strong ability to predict long-term cardiovascular endpoints (such as coronary heart disease, arterial hypertension, and heart failure) (20, 21): 1) the coronary arterial calcium (CAC) score, measured with either electron-beam computed tomography at three field centers or with multidetector computed tomography at three field centers (22); 2) the Framingham global CVD risk score (23); and 3) the NT-proBNP (N-terminal prohormone BNP) level, measured using the standard methods described previously (20). The cross-sectional associations identified in the MESA cohort were then further tested in the SHHS sample.
SHHS
Study population
The SHHS is a community-based prospective cohort study designed to examine the cardiovascular outcomes of sleep-disordered breathing. This sample included middle-aged and older adults who completed baseline PSG (1995–1998) and standardized questionnaires and were followed for a mean of 10.7 years, thus allowing assessment of associations with clinically relevant longitudinal outcomes. Similar to its calculation in the MESA, the AHI was calculated using all apneas and hypopneas associated with desaturations of ⩾3% or arousal (see Figure 1 and the online supplement for a detailed description of PSG data).
Outcomes
Nonfatal CVD was defined as the occurrence of myocardial infarction, heart failure, or stroke. Cardiovascularly related and all-cause mortality events were identified and confirmed for the SHHS using follow-up interviews, written annual questionnaires, or telephone contacts with study participants or the next of kin, surveillance of local hospital records and community obituaries, and linkage with the Social Security Administration Death Master File (3).
The ΔHR
In both studies, the pulse rate was derived from the pulse-oximetry sensor and was used to estimate the heart rate. This was chosen over the use of electrocardiogram for the following reasons: 1) photoplethysmography is commonly used in home sleep-apnea testing, making broad future application of this new prognostic biomarker possible, and 2) the photoplethysmographic “pulse” signal provides a time-averaged heart-rate signal, which, by filtering out beat-to-beat and breath-to-breath variation, conveniently allows assessment of peak event-related changes in the heart rate, which occur on a time scale of approximately 10–30 seconds. Consistent with definitions in previous studies (14, 16), the ΔHR was defined as the difference between a maximum pulse rate during a subject-specific search window (a search window extended from the preevent minimum to the postevent minimum of the event-related, ensemble-averaged pulse rate; this method has been previously used in hypoxic-burden calculation) (24) and an event-related minimum pulse rate (the minimum pulse rate during apneas and hypopneas; Figure 2; see additional sensitivity analysis in the online supplement, including the local mean pulse rate as the baseline). Finally, the individual-level ΔHR was defined as the mean of all event-specific responses.
Figure 2.
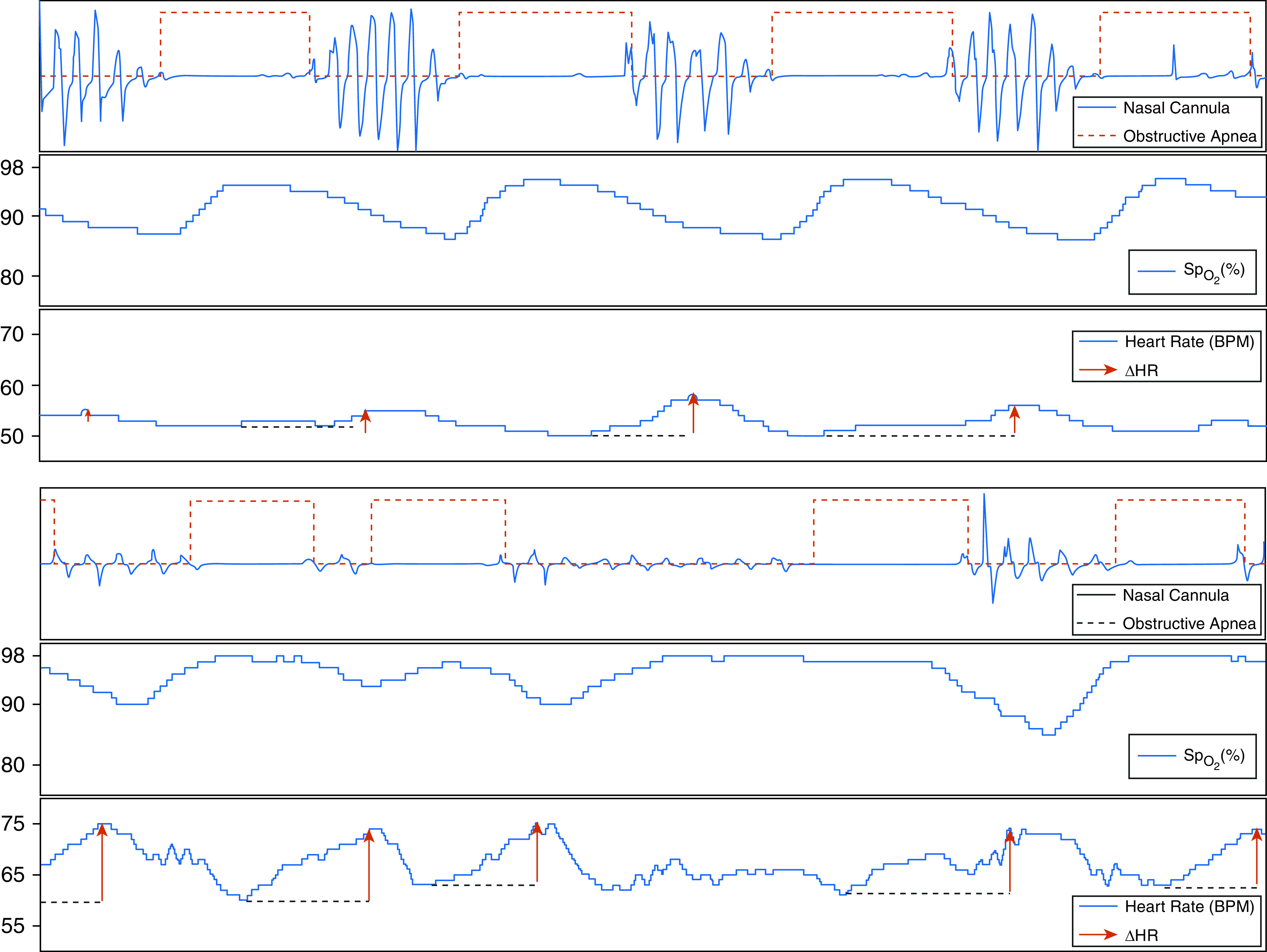
Examples of 200-second tracings of two individuals, one with a low pulse-rate response to apneas and hypopneas (ΔHR) (top) and one with a high ΔHR (bottom). Both individuals had similar events in terms of duration, depth, and associated desaturation. BPM = beats per minute; SpO2 = oxygen saturation as measured by pulse oximetry.
Statistical Analysis
Distributions of covariates, sleep measures, and the ΔHR were summarized for each study cohort. All statistical analyses were conducted using the R statistical package (R Foundation for Statistical Computing) (http://www.r-project.org), with P < 0.05 considered to indicate statistical significance.
MESA
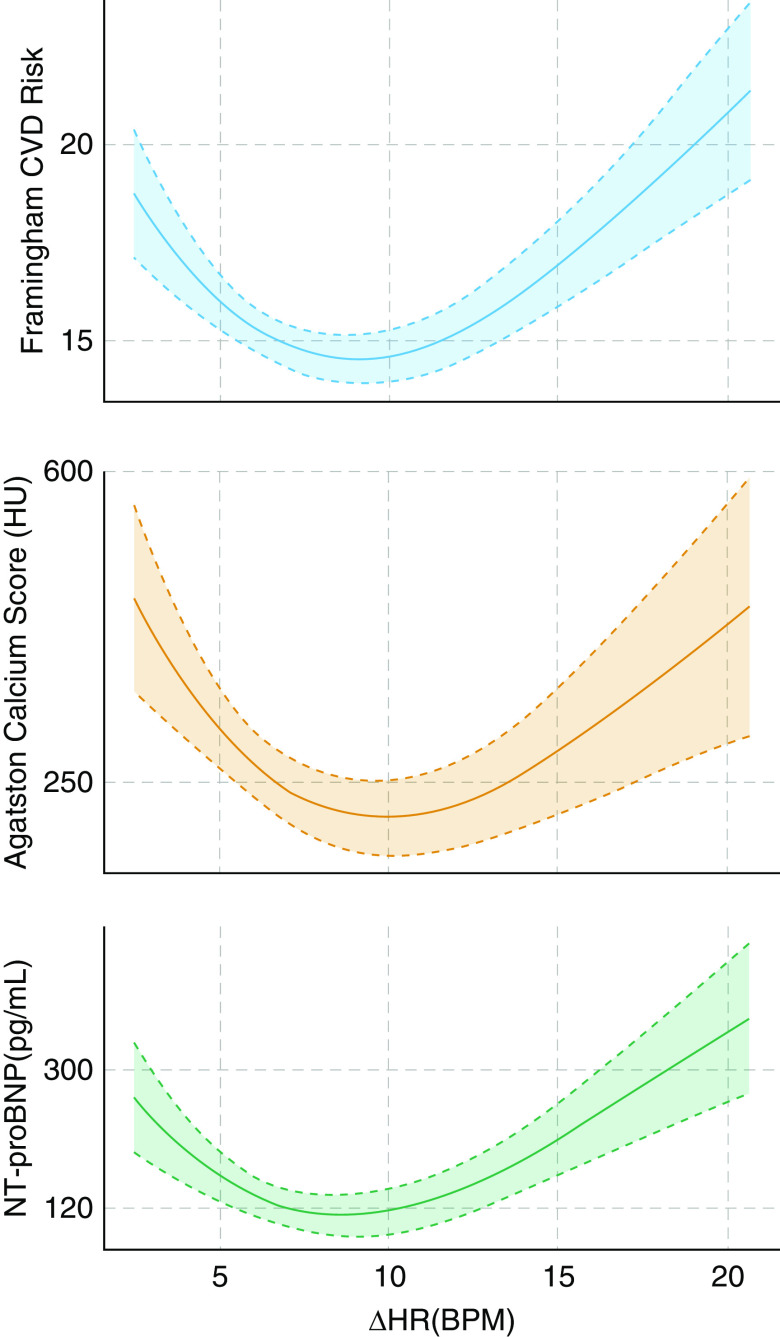
Polynomial regression models were used to determine the functional forms of the relationship between the ΔHR and subclinical CVD outcomes in the MESA by allowing higher-order polynomial terms in the model. The best model was selected on the basis of Akaike’s information criteria. This analysis revealed a U-shaped association between the ΔHR and the CAC score, as well as between the Framingham CVD risk score and NT-proBNP (Figure 3). On the basis of the observed relationship and to facilitate interpretation, in addition to polynomial models using continuously measured ΔHR values, the ΔHR was further categorized into low (lower quartile), midrange (middle two quartiles; reference group), and high (upper quartile) groups.
Figure 3.
Third-degree polynomial regression (fill shows 95% confidence interval) between subclinical cardiovascular markers, including the Framingham cardiovascular disease (CVD) risk, coronary arterial calcium score, and NT-proBNP (N-terminal prohormone BNP), and the pulse-rate response to apneas/hypopneas (ΔHR) revealed a consistent U-shaped functional form, suggesting increased risk of CVD in both low and high ΔHRs. This analysis used data from the MESA (Multi-Ethnic Study of Atherosclerosis) (N = 1,395). BPM = beats per minute; HU = Hounsfield units.
SHHS
The U-shaped relationship, observed in the MESA, was tested in the SHHS using Cox regression analysis to predict nonfatal and fatal CVD and all-cause mortality. The same polynomial function for continuously expressed ΔHR values from the MESA was examined. In addition, categories of the ΔHR using cutoff values determined in the MESA sample were modeled separately. Models were adjusted for age, sex, race, body mass index, smoking status, prevalent diabetes, hypertension, baseline CVD, lipid-lowering medication use, β-blocker use, event-related minimum pulse rate (as defined above), and AHI.
In additional analyses, we tested whether associations between the ΔHR and CVD morbidity and mortality are stronger in those with more frequent respiratory events (quantified by using the AHI) or more severe hypoxemia (quantified by using the hypoxic burden). To assess this, the same models previously described were separately constructed in those with moderate-to-severe OSA (AHI ⩾ 15 events/h), those with severe OSA (AHI ⩾ 30 events/h), and those with substantial OSA-specific hypoxemia (those with a hypoxic burden ⩾ the 75th percentile of the sample). The hypoxic burden was defined as the total area under the desaturation curve from a pre–respiratory-event baseline (24). Additional sensitivity analyses further adjusted for the hypoxic burden as a covariate (see online supplement).
Further analyses were done to assess whether the risk in individuals reporting excessive daytime sleepiness was different from the risk in those reporting less sleepiness. Excessive daytime sleepiness was quantified using two different approaches: 1) an Epworth Sleepiness Scale (ESS) score > 10 and 2) frequency of excessive daytime sleepiness measured using the question “How often do you feel excessively (overly) sleepy during the day?” Those who responded with “often (5–15 times/mo)” or “almost always (16–30 times/mo)” were considered sleepy. Sex differences in the associations were further explored with stratified analyses in men and women. The effect of REM sleep on nonfatal CVD and CVD mortality was examined (see Table E4 in the online supplement). A sensitivity analysis assessed the association between the ΔHR and the new incidence of nonfatal or fatal CVD after excluding those with baseline CVD. A final sensitivity analysis excluded those with atrial fibrillation, those on β-blockers, and those with a cardiac pacemaker.
Results
The baseline characteristics of participants in the MESA and SHHS are shown in Table 1. In the baseline sleep study, MESA participants were older and more ethnically diverse, and they had more severe OSA (quantified by using the AHI and hypoxemia; Table 1) than SHHS participants. In addition, SHHS participants had a higher prevalence of CVD at baseline than those in the MESA (Table 1). The baseline characteristics of participants per ΔHR are shown in Table E1.
Table 1.
Baseline Characteristics of Cohort Studies, Including the MESA and the SHHS
| MESA (N = 1,395) | SHHS (N = 4,575) | |
|---|---|---|
| Age, yr | 68.5 (9.12) | 64.2 (11.0) |
| Race | ||
| White | 519 (37.2%) | 4,029 (88.1%) |
| Black | 355 (25.4%) | 272 (5.95%) |
| Chinese | 172 (12.3%) | — |
| Hispanic | 349 (25.0%) | — |
| Other | — | 274 (5.99) |
| Sex, M | 663 (47.5%) | 2,183 (47.7%) |
| BMI, kg/m2 | 28.8 (5.49) | 28.3 (5.03) |
| Smoking | ||
| Never | 645 (46.4%) | 2,121 (46.4%) |
| Current | 96 (6.90%) | 420 (9.18%) |
| Former | 650 (46.7%) | 2,034 (44.5%) |
| Diabetes | 159 (11.4%) | 346 (7.56%) |
| Hypertension | 794 (56.9%) | 1,867 (40.8%) |
| Lipid-lowering medications | 536 (38.4%) | 583 (12.7%) |
| Baseline CVD | 91 (6.55%) | 751 (16.4%) |
| β-Adrenergic blockers | 250 (17.9%) | 564 (12.3%) |
| Systolic blood pressure, mm Hg* | 123 (20.2) | 125 (18.4) |
| Diastolic blood pressure, mm Hg* | 68.5 (9.73) | 72.0 (10.9) |
| Total sleep time, min | 365 (78.1) | 364 (62.7) |
| AHI, events/h | 19.3 (10.7–33.1) | 14.1 (7.65–24.4) |
| SpO2 < 90%, % of total sleep time | 0.76 (0.07–3.44) | 0.24 (0.00–2.06) |
| Event-related minimum HR, BPM | 60.4 (8.89) | 61.9 (8.74) |
| ΔHR categories† | ||
| Midrange | 697 (50.0%) | 2,288 (50.0%) |
| Low | 349 (25.0%) | 1,736 (37.9%) |
| High | 349 (25.0%) | 551 (12.0%) |
Definition of abbreviations: AHI = apnea–hypopnea index (all apneas and hypopneas associated with ⩾3% desaturation or arousal); BMI = body mass index; BPM = beats per minute; CVD = cardiovascular disease; ΔHR = HR response to apneas/hypopneas; HR = pulse rate; MESA = Multi-Ethnic Study of Atherosclerosis; SHHS = Sleep Heart Health Study; SpO2 = oxygen saturation as measured by pulse oximetry.
Average values are the mean (SD), n (%), or median (interquartile range).
Mean of two measurements in the MESA and SHHS.
Cutoffs in the MESA and SHHS are based on quartiles of the ΔHR in the MESA cohort.
Subclinical CVD and ΔHR (MESA)
As shown in Figure 3, there was a consistent U-shaped relationship between all three subclinical CVD measures and the ΔHR. Third-degree polynomial functions provided the best fit for these relationships, indicating that those with low (first quartile, <5.8 beats/min [BPM]; mean [SD], 4.6 [1.0] BPM) and high (fourth quartile, >10.1 BPM; mean [SD], 13.5 [3.4] BPM) ΔHRs have higher Framingham CVD risk score as well as higher CAC scores and higher levels of NT-proBNP than those with midrange ΔHRs (middle two quartiles, 5.8–10.1 BPM; mean [SD], 7.7 [1.2] BPM).
Nonfatal or Fatal CVD and All-Cause Mortality and ΔHR in the SHHS Cohort
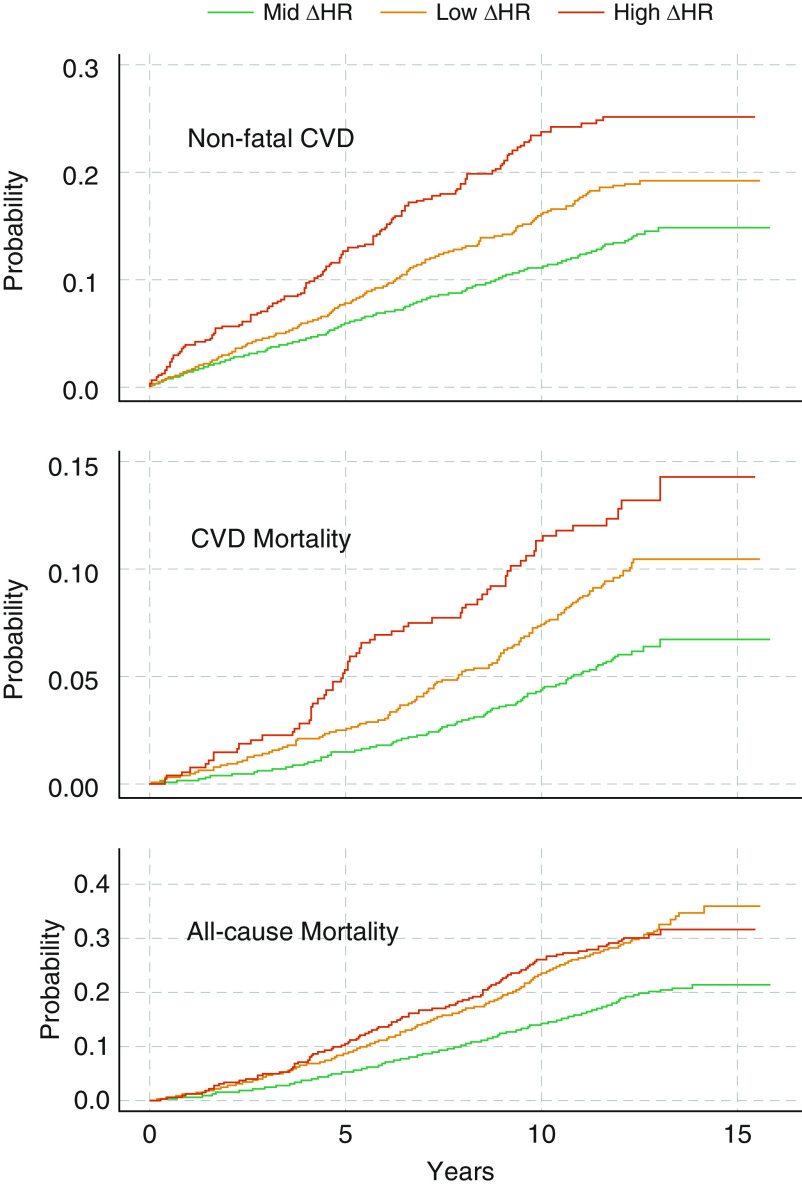
In the SHHS cohort, over a mean follow-up period of 10.7 ± 3.0 years, there were 331 cardiovascular deaths and 1,067 all-cause deaths. There were 658 nonfatal CVD events, with the mean time to nonfatal events being 5.6 ± 3.4 years. Figure 4 demonstrates the unadjusted Kaplan-Meier cumulative incidence of nonfatal CVD as well as CVD mortality and all-cause mortality per category of ΔHR. In the adjusted third-degree polynomial model, the ΔHR was significantly associated with an increased hazard ratio for nonfatal CVD (P < 0.001) and cardiovascular (P < 0.001) and all-cause mortality (P < 0.0001). As shown in Table 2, compared with individuals in the midrange-ΔHR group, those with a high ΔHR had nonfatal and fatal CVD hazard ratios of 1.60 (95% confidence interval [CI], 1.28–2.00) and 1.68 (95% CI, 1.22–2.30), respectively. In contrast, those in low-ΔHR group had hazard ratios of 1.07 (95% CI, 0.90–1.27) and 1.21 (95% CI, 0.94–1.54), respectively. After excluding individuals with baseline CVD, only individuals with a high ΔHR had a significantly increased incidence of nonfatal or fatal CVD (N = 3,824; high- vs. midrange-ΔHR hazard ratio, 1.60 [95% CI, 1.26–2.04]; low- vs. midrange-ΔHR hazard ratio, 0.92 [95% CI, 0.77–1.10]). Both high and low ΔHRs were significantly associated with all-cause mortality (high- vs. midrange-ΔHR hazard ratio, 1.29 [95% CI, 1.07–1.55]; low- vs. midrange-ΔHR hazard ratio, 1.26 [95% CI, 1.10–1.44]; Table 2). These associations persisted after adjusting for the AHI, event-related minimum pulse rate, baseline-prevalent CVD, and hypoxic burden (Table E2).
Figure 4.
Unadjusted cumulative incidence Kaplan-Meier curves for categories of the ΔHR in the SHHS (Sleep Heart Health Study). Only one study site followed participant and provided mortality data after Year 13 (no CVD death reported from this study during this period). CVD = cardiovascular disease; ΔHR = pulse-rate response to apneas/hypopneas; Mid = midrange.
Table 2.
Multivariable Cox Regression Analysis for Nonfatal CVD and Cardiovascular and All-Cause Mortality in the SHHS
| ΔHR Categories | Nonfatal CVD* [Hazard Ratio (95% CI)] | CVD Mortality [Hazard Ratio (95% CI)] | All-Cause Mortality [Hazard Ratio (95% CI)] |
|---|---|---|---|
| All subjects (N = 4,575) | |||
| Midrange | 1.00 | 1.00 | 1.00 |
| Low | 1.07 (0.90–1.27) | 1.21 (0.94–1.54) | 1.26 (1.10–1.44)† |
| High | 1.60 (1.28–2.00)† | 1.68 (1.22–2.30)‡ | 1.29 (1.07–1.55)‡ |
| AHI ⩾ 15 events/h (N = 2,148) | |||
| Midrange | 1.00 | 1.00 | 1.00 |
| Low | 1.13 (0.90–1.43) | 1.39 (1.00–1.93) | 1.13 (0.94–1.36) |
| High | 1.69 (1.28–2.22)† | 1.92 (1.29–2.86)‡ | 1.36 (1.09–1.71)‡ |
| AHI < 15 events/h (N = 2,427) | |||
| Midrange | 1.00 | 1.00 | 1.00 |
| Low | 0.97 (0.75–1.25) | 0.98 (0.67–1.42) | 1.40 (1.14–1.71)‡ |
| High | 1.60 (1.06–2.39)§ | 1.28 (0.73–2.22) | 1.08 (0.75–1.55) |
| AHI ⩾ 30 events/h (N = 820) | |||
| Midrange | 1.00 | 1.00 | 1.00 |
| Low | 1.40 (0.95–2.04) | 2.09 (1.17–3.74)§ | 1.11 (0.82–1.51) |
| High | 1.90 (1.28–2.80)‡ | 2.96 (1.60–5.48)† | 1.51 (1.10–2.07)§ |
| AHI < 30 events/h (N = 3,755) | |||
| Midrange | 1.00 | 1.00 | 1.00 |
| Low | 1.01 (0.83–1.23) | 1.06 (0.81–1.39) | 1.29 (1.11–1.50)† |
| High | 1.51 (1.13–2.00)‡ | 1.38 (0.93–2.03) | 1.16 (0.91–1.48) |
| HB ⩾ 62%min/h (N = 1,138) | |||
| Midrange | 1.00 | 1.00 | 1.00 |
| Low | 1.38 (1.00–1.89)§ | 1.89 (1.19–3.00)‡ | 1.44 (1.13–1.83)‡ |
| High | 1.93 (1.36–2.73)† | 3.50 (2.15–5.71)† | 1.84 (1.40–2.40)† |
| HB < 62%min/h (N = 3,409) | |||
| Midrange | 1.00 | 1.00 | 1.00 |
| Low | 0.96 (0.78–1.17) | 0.94 (0.70–1.26) | 1.17 (1.00–1.38) |
| High | 1.35 (0.99–1.84) | 0.93 (0.58–1.50) | 0.89 (0.66–1.18) |
Definition of abbreviations: AHI = apnea–hypopnea index; CI = confidence interval; CVD = cardiovascular disease; ΔHR = pulse-rate response to apneas/hypopneas; HB = sleep-apnea–specific hypoxic burden; SHHS = Sleep Heart Health Study.
All models were adjusted for age, sex, race, body mass index, smoking, diabetes, hypertension, lipid-lowering medication, β-blockers, baseline CVD, event-related minimum pulse rate, and AHI. Bold indicates statistical significance at the level indicated by the footnote symbol.
The number of participants in nonfatal CVD analysis is lower than reported for CVD and all-cause mortality because of exclusion of fatal CVD events from the analysis. A test of interaction was only significant for the interaction between high ΔHR and HB categories.
P < 0.001.
P < 0.01.
P < 0.05.
Nonfatal/Fatal CVD and All-Cause Mortality and ΔHR in Relation to OSA Severity
Secondary models revealed that the association of a high ΔHR with nonfatal and fatal CVD and all-cause mortality was moderated by the severity of OSA, whether defined on the basis of the frequency of respiratory events or defined on the basis of the degree of hypoxemia (Table 2). In moderate-to-severe OSA (AHI ⩾ 15 events/h), those with a high ΔHR versus a midrange ΔHR had adjusted hazard ratios of 1.69 (95% CI, 1.28–2.22) and 1.92 (95% CI, 1.29–2.86) for nonfatal CVD and CVD mortality, respectively (Table 2). This association appeared stronger among individuals with an AHI ⩾ 30 events/h and was strongest among individuals with a high hypoxic burden (adjusted hazard ratio for fatal CVD, 3.50 [95% CI, 2.15–5.71]; Table 2). The associations of a high ΔHR and all-cause mortality in those with an AHI ⩾ 15 events/h, an AHI ⩾ 30 events/h, or a high hypoxic burden were in the same direction but were of smaller magnitude (Table 2). In contrast, there was no association between a high ΔHR and fatal CVD or all-cause mortality in those with an AHI < 15 events/h or a low hypoxic burden (Table 2). Finally, there was no consistent pattern of associations between a low ΔHR and mortality.
Exploratory Analyses
In analyses stratified by sleepiness, a high ΔHR was associated with an increased risk of nonfatal CVD, fatal CVD, and all-cause mortality only among individuals with an ESS score of <11 (N = 3,345; nonfatal CVD, 1.77 [95% CI, 1.34–2.33]; fatal CVD, 1.97 [95% CI, 1.37–2.83]; all-cause mortality, 1.39 [95% CI, 1.12–1.74]; Table E3). Similar findings were observed when sleepiness was quantified by using the frequency of excessive daytime sleepiness (Table E3). However, a test for the interaction between a high ΔHR and the presence of excessive sleepiness (ESS score > 10) was not significant (nonfatal CVD, P = 0.20; fatal CVD, P = 0.12; all-cause mortality, P = 0.27).
Similarly, sex-stratified analyses suggested a stronger association between a high ΔHR and cardiovascular and all-cause mortality in women than in men, although a test for the interaction between sex and a high ΔHR did not produce significant results (nonfatal CVD, P = 0.92; fatal CVD, P = 0.12; all-cause mortality, P = 0.16) (Table E3).
Excluding individuals on β-blockers, those with atrial fibrillation present, or those with cardiac pacemakers did not change the primary findings (N = 3,958; high- vs. midrange-ΔHR hazard ratios: nonfatal CVD, 1.62 [95% CI, 1.26–2.10]; cardiovascular mortality, 1.82 [95% CI, 1.26–2.63]; all-cause mortality, 1.27 [95% CI, 1.02–1.58]; Table E3). Finally, the ΔHR was modestly higher in REM sleep than in nREM sleep (median difference, 0.3 [−1.4 to 2.1] BPM; P < 0.001). When the state-specific ΔHR was used, the associations between a high ΔHR and nonfatal and fatal CVD and all-cause mortality appeared to be stronger in events analyzed in nREM sleep than in events analyzed in REM sleep (Table E4).
Discussion
In this study, we found that the sleep apnea–specific ΔHR was significantly associated cross-sectionally with markers of cardiovascular risk, with both low and high ΔHRs being associated with increased cardiovascular risk markers. In longitudinal analysis, a high ΔHR predicted nonfatal and fatal CVD and all-cause mortality in a general community sample. Moreover, as a potential prognostic biomarker in OSA, a high ΔHR predicted a high risk of CVD morbidity and mortality among individuals with moderate-to-severe OSA (AHI ⩾ 15 events/h) or severe OSA (AHI ⩾ 30 events/h), and it was highest among those with substantial OSA-related hypoxemia (sleep apnea–specific hypoxic burden ⩾ 62% of min/h) but was not predictive of mortality in those with an AHI < 15 events/h or a lower hypoxic burden. The association of a high ΔHR with CVD morbidity and mortality was somewhat stronger in women than in men, although this difference was not statistically significant. Although a prior study has shown that excessive daytime sleepiness is predictive of OSA-related CVD risk (10), we observed that a high ΔHR was associated with increased CVD morbidity and mortality risk in individuals without reported excessive sleepiness. This suggests that a high ΔHR is a novel biomarker that identifies a subgroup of patients with OSA (high hypoxic burden plus high ΔHR) at markedly elevated cardiovascular risk even in the absence of excessive sleepiness. This has important implications for both clinical practice, by allowing clinicians seeking to modify cardiovascular risk through treatment of OSA to target these individuals even in the absence of sleepiness, and the design of clinical trials, by permitting recruitment to focus on high-risk individuals.
There are several plausible mechanisms that may explain the observed U-shaped relationship between ΔHR and cardiovascular risk. Available evidence suggests that a high ΔHR reflects more severe respiratory events (14) or an overreactive autonomic response to events (25), both of which will adversely affect the cardiovascular system. Conversely, a low ΔHR likely represents more subtle respiratory events (14) or an underresponsive cardiovascular system, possibly due to existing heart disease (26), diabetes (27), or other causes of autonomic dysfunction. This is consistent with the finding that those with a low ΔHR were older, had a higher baseline pulse rate, and had a higher prevalence of CVD (Figure E1). Thus, a high ΔHR likely reflects OSA targets that are modifiable through treatment, which might be expected to reduce cardiovascular risk, whereas a low ΔHR appears to reflect factors unrelated to OSA (Figure E1), per se, that are unlikely to be ameliorated by OSA treatment and thus unlikely to lead to substantive improvement in cardiovascular outcomes.
There is a growing consensus that characterizing OSA by frequency-oriented metrics, including the AHI, arousal index, and oxygen desaturation index, does not adequately capture the acute physiologic or long-term health consequences associated with this disorder (28). To address this problem, our group and others have identified novel measures that we believe more accurately quantify the respiratory event–specific hypoxemia (24, 29, 30), arousal intensity (16, 31), and autonomic response (14, 32, 33). These metrics were shown to more consistently correlate with incident heart failure (30), cardiovascularly related deaths, and all-cause mortality (24, 34). In line with these advances in identifying robust prognostic OSA biomarkers, this study has identified a simple measure that may reflect both the parasympathetic and sympathetic responses to an event. The change from a minimum heart rate during an event to a maximum heart rate after an event may reflect effects of both the parasympathetic and sympathetic systems. Indeed, a higher ΔHR may reflect a more pronounced vagally induced bradycardia (35) during an event (larger decrease in heart rate during an event), a more pronounced sympathetic response (13) to hypoxemia/hypercapnia (larger increase in heart rate), and/or a combination of both. Further investigation is needed to assess the individual contributions of sympathetic/parasympathetic activity to the ΔHR.
As discussed above, metrics that better capture the sleep apnea–specific hypoxic burden tend to more consistently predict cardiovascular outcomes than conventional frequency-oriented metrics. In this study, the strong statistical interaction between the hypoxic burden and the ΔHR indicates that both metrics analyzed together may be particularly useful for improving cardiovascular risk stratification in OSA.
Emerging research has shown that individuals with OSA with excessive daytime sleepiness may be at a heightened risk of cardiovascular outcomes (10); it has been suggested that excluding these individuals from randomized controlled trials, such as SAVE (The Sleep Apnea Cardiovascular Endpoints Study) (6), may partly explain the lack of CPAP benefit. Exploratory findings from this study suggest that the risk associated with an elevated ΔHR may be strongest in those without excessive sleepiness. Indeed, nonsleepy individuals with OSA may have increased sympathetic nervous system activity, as observed in patients with OSA and heart failure (36).
Prior research on the sex-specific differences in OSA and its consequences remains limited and inconsistent (19, 37–41). Indeed, prior sex-specific investigations involving SHHS data were not able to detect an association between OSA and incident coronary heart disease (18), incident heart failure (18), or all-cause mortality (3) in women over a follow-up of approximately 8 years. This may reflect in part the small number of women with moderate-to-severe OSA and their generally low cardiovascular event rates. Although sex differences in the relation of high ΔHR to mortality were not statistically significant, the strong CVD morbidity and mortality risk noted in women with a high ΔHR suggests that this metric may be useful for identifying those women at high risk for OSA-related adverse outcomes. Finally, a post hoc state-specific analysis showed that the nREM-related ΔHR appeared to have consistent associations with outcomes compared with the REM-related ΔHR (see Table E4). This may be due to measurement noise that results in the REM-related ΔHR being associated with fewer respiratory events than the nREM-related ΔHR; however, further investigation is warranted.
Strengths and Limitations
This study has several strengths: 1) the ΔHR can be generated automatically in large-scale clinical and research studies, only requiring data from an oximetry channel and respiratory-event scoring, which are readily available from home sleep-apnea tests, and 2) multiple covariate adjustments and consistency across two independent studies suggest likely generalizability of the results. However, the study also had several limitations, including the underrepresentation of young individuals (age range, 40–90 yr). In addition, the impact of medications on the ΔHR in the setting of sleep apnea needs to be further investigated. For example, β-blocking agents tend to depress the heart rate. However, the findings did not change materially in a sensitivity analysis that excluded those on β-blockers.
Future Directions
Additional studies are needed to prospectively validate these findings. Nonetheless, the findings of this study strongly suggest the potential utility of a high ΔHR to identify a high-risk subgroup of patients with OSA, particularly when combined with measures of the hypoxic burden. This should inform the design of future randomized clinical trials to assess the impact of OSA treatment on major adverse cardiovascular events. Such trials have generally excluded sleepy individuals because of concerns regarding the ethics of withholding active treatment from symptomatic individuals, although this exclusion may have resulted in the selection of a generally low-risk group that was unlikely to benefit from CPAP treatment. The ability to identify a sample of high-risk, nonsleepy patients with OSA would greatly enhance the power of intervention trials to detect a cardiovascular benefit of OSA treatment.
Conclusions
This study provides evidence that individuals with a heightened ΔHR, especially those with moderate-to-severe OSA, are at elevated risk of cardiovascular morbidity/mortality. In addition, the results show consistent associations in men and women and in those who do not report excessive daytime symptoms.
Acknowledgments
Acknowledgment
The authors thank the SHHS investigators and Brigham and Women’s Reading Center team for expert sleep scoring and the National Sleep Research Resource for providing the data. The authors also thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the NHLBI and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. This publication was developed under the Science to Achieve Results research assistance agreements RD831697 (MESA Air [Multi-Ethnic Study of Atherosclerosis and Air Pollution]) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication. This work was also supported by the NIH (R01HL102321, R01HL128658, P01HL095491, UL1RR025758). The SHHS (Sleep Heart Health Study) was supported by the NHLBI through the following cooperative agreements: U01-HL53940 (University of Washington), U01-HL53941 (Boston University), U01-HL63463 (Case Western Reserve University), U01-HL53937 (Johns Hopkins University), U01-HL53938 (University of Arizona), U01-HL53916 (University of California, Davis), U01-HL53934 (University of Minnesota), U01-HL63429 (Missouri Breaks Research), and U01-HL53931 (New York University). A.A. was supported by the NIH (R01HL153874), the American Heart Association (19CDA34660137), and the American Academy of Sleep Medicine Foundation (188-SR-17). S.A.S. was supported by the American Heart Association (15SDG25890059) and the American Thoracic Society Foundation. L.T.-M. was supported by the American Heart Association (17POST33410436). A.A., S.A.S., S.R., and A.W. were partially supported by NHLBI grant R35HL135818.
Author Contributions: A.A. was responsible for study design, analysis, interpretation of data, and drafting the manuscript. S.A.S. and M.Y. contributed to the analysis, interpretation of the data, and critical revision of the manuscript. L.T.-M., T.S., D.V., R.M.A., and S.-W.K. contributed to interpretation of the data and critical revision of the manuscript. D.J.G. contributed to the acquisition and interpretation of the data and critical revision of the manuscript. D.P.W. contributed to the interpretation of the data and critical revision of the manuscript. S.R. contributed to the study design, acquisition, analysis, interpretation of the data, and critical revision of the manuscript. A.W. contributed to the study design, analysis, interpretation of the data, and critical revision of the manuscript. A.A., S.R., and A.W. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202010-3900OC on January 5, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. PubMed [DOI] [PubMed] [Google Scholar]
- 5. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. PubMed [DOI] [PubMed] [Google Scholar]
- 6. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. PubMed [DOI] [PubMed] [Google Scholar]
- 7. Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, et al. Spanish Sleep Network. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8:359–367. doi: 10.1016/S2213-2600(19)30271-1. PubMed [DOI] [PubMed] [Google Scholar]
- 8. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. PubMed [DOI] [PubMed] [Google Scholar]
- 9. Zinchuk A, Yaggi HK. Sleep apnea heterogeneity, phenotypes, and cardiovascular risk. Implications for trial design and precision sleep medicine. Am J Respir Crit Care Med. 2019;200:412–413. doi: 10.1164/rccm.201903-0545ED. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200:493–506. doi: 10.1164/rccm.201808-1509OC. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. PubMed [DOI] [PubMed] [Google Scholar]
- 12. Davies CWH, Crosby JH, Mullins RL, Barbour C, Davies RJ, Stradling JR. Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax. 2000;55:736–740. doi: 10.1136/thorax.55.9.736. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azarbarzin A, Ostrowski M, Moussavi Z, Hanly P, Younes M. Contribution of arousal from sleep to postevent tachycardia in patients with obstructive sleep apnea. Sleep. 2013;36:881–889. doi: 10.5665/sleep.2716. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schneider H, Schaub CD, Andreoni KA, Schwartz AR, Smith PL, Robotham JL, et al. Systemic and pulmonary hemodynamic responses to normal and obstructed breathing during sleep. J Appl Physiol (1985) 1997;83:1671–1680. doi: 10.1152/jappl.1997.83.5.1671. [DOI] [PubMed] [Google Scholar]
- 16. Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep. 2014;37:645–653. doi: 10.5665/sleep.3560. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. PubMed [DOI] [PubMed] [Google Scholar]
- 18. Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Won CHJ, Reid M, Sofer T, Azarbarzin A, Purcell S, White D, et al. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. Sleep. 2020;43:zsz274. doi: 10.1093/sleep/zsz274. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanchez OA, Jacobs DR, Jr, Bahrami H, Peralta CA, Daniels LB, Lima JA, et al. Increasing aminoterminal-pro-B-type natriuretic peptide precedes the development of arterial hypertension: the multiethnic study of atherosclerosis. J Hypertens. 2015;33:966–974. doi: 10.1097/HJH.0000000000000500. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panagopoulou V, Deftereos S, Kossyvakis C, Raisakis K, Giannopoulos G, Bouras G, et al. NTproBNP: an important biomarker in cardiac diseases. Curr Top Med Chem. 2013;13:82–94. doi: 10.2174/1568026611313020002. PubMed [DOI] [PubMed] [Google Scholar]
- 22. McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. PubMed [DOI] [PubMed] [Google Scholar]
- 23. D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. PubMed [DOI] [PubMed] [Google Scholar]
- 24. Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. PubMed [DOI] [PubMed] [Google Scholar]
- 26. Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, et al. Heart Rate Working Group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. PubMed [DOI] [PubMed] [Google Scholar]
- 27. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. PubMed [DOI] [PubMed] [Google Scholar]
- 28. Pevernagie DA, Gnidovec-Strazisar B, Grote L, Heinzer R, McNicholas WT, Penzel T, et al. On the rise and fall of the apnea-hypopnea index: a historical review and critical appraisal. J Sleep Res. 2020;29:e13066. doi: 10.1111/jsr.13066. PubMed [DOI] [PubMed] [Google Scholar]
- 29. Leppänen T, Töyräs J, Mervaala E, Penzel T, Kulkas A. Severity of individual obstruction events increases with age in patients with obstructive sleep apnea. Sleep Med. 2017;37:32–37. doi: 10.1016/j.sleep.2017.06.004. PubMed [DOI] [PubMed] [Google Scholar]
- 30. Azarbarzin A, Sands SA, Taranto-Montemurro L, Vena D, Sofer T, Kim SW, et al. The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest. 2020;158:739–750. doi: 10.1016/j.chest.2020.03.053. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amatoury J, Azarbarzin A, Younes M, Jordan AS, Wellman A, Eckert DJ. Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep. 2016;39:2091–2100. doi: 10.5665/sleep.6304. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lachapelle P, Cascon J, Pamidi S, Kimoff RJ. Accuracy of portable devices in sleep apnea using oximetry-derived heart rate increases as a surrogate arousal marker. Sleep Breath. 2019;23: 483–492. doi: 10.1007/s11325-018-1708-5. PubMed [DOI] [PubMed] [Google Scholar]
- 33. Gao X, Azarbarzin A, Keenan BT, Ostrowski M, Pack FM, Staley B, et al. Heritability of heart rate response to arousals in twins. Sleep. 2017;40:zsx055. doi: 10.1093/sleep/zsx055. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sankari A, Ravelo LA, Maresh S, Aljundi N, Alsabri B, Fawaz S, et al. Longitudinal effect of nocturnal R-R intervals changes on cardiovascular outcome in a community-based cohort. BMJ Open. 2019;9:e030559. doi: 10.1136/bmjopen-2019-030559. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cortelli P, Lombardi C, Montagna P, Parati G. Baroreflex modulation during sleep and in obstructive sleep apnea syndrome. Auton Neurosci. 2012;169:7–11. doi: 10.1016/j.autneu.2012.02.005. PubMed [DOI] [PubMed] [Google Scholar]
- 36. Taranto Montemurro L, Floras JS, Millar PJ, Kasai T, Gabriel JM, Spaak J, et al. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest. 2012;142:1222–1228. doi: 10.1378/chest.11-2963. PubMed [DOI] [PubMed] [Google Scholar]
- 37. Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27:1113–1120. doi: 10.1093/sleep/27.6.1113. PubMed [DOI] [PubMed] [Google Scholar]
- 39. Campos-Rodriguez F, Martínez-García MA, Reyes-Nuñez N, Selma-Ferrer MJ, Punjabi NM, Farre R. Impact of different hypopnea definitions on obstructive sleep apnea severity and cardiovascular mortality risk in women and elderly individuals. Sleep Med. 2016;27–28:54–58. doi: 10.1016/j.sleep.2016.05.020. PubMed [DOI] [PubMed] [Google Scholar]
- 40. Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156:115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. PubMed [DOI] [PubMed] [Google Scholar]
- 41. Roca GQ, Redline S, Claggett B, Bello N, Ballantyne CM, Solomon SD, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation. 2015;132:1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]