Abstract
Formation of complexes between soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) proteins on opposing membranes is the minimal requirement for intracellular membrane fusion. The SNARE, syntaxin 2, is found on the sperm plasma membrane and a second SNARE, vesicle associated membrane protein 2 (VAMP2, also known as synaptobrevin 2, SYB2), is on the apposing outer acrosomal membrane. During the acrosome reaction, the outer acrosomal membrane fuses at hundreds of points with the plasma membrane. We hypothesized that syntaxin 2 and VAMP2 redistribute within their respective membranes prior to the acrosome reaction to form trans-SNARE complexes and promote membrane fusion. Immunofluorescence and superresolution structured illumination microscopy were used to localize syntaxin 2 and VAMP2 in mouse sperm during capacitation. Initially, syntaxin 2 was found in puncta throughout the acrosomal region. At 60 and 120 min of capacitation, syntaxin 2 was localized in puncta primarily in the apical ridge. Although deletion of bicarbonate during incubation had no effect, syntaxin 2 puncta were relocated in the restricted region in less than 20% of sperm incubated without albumin. In contrast, VAMP2 was already found in puncta within the apical ridge prior to capacitation. The puncta containing syntaxin 2 and VAMP2 did not precisely co-localize at 0 or 60 min of capacitation time. In summary, syntaxin 2 shifted its location to the apical ridge on the plasma membrane during capacitation in an albumin-dependent manner but VAMP2 was already localized to the apical ridge. Puncta containing VAMP2 did not co-localize with those containing syntaxin 2 during capacitation; therefore, formation of trans-SNARE complexes containing these SNAREs does not occur until after capacitation, immediately prior to acrosomal exocytosis.
Keywords: SNAREs, sperm, acrosome reaction, exocytosis, capacitation, syntaxin 2 (STX2), vesicle associated membrane protein 2 (VAMP2), membrane fusion, plasma membrane
Summary Sentence
Although the sperm plasma membrane SNARE protein syntaxin 2 moves laterally, trans-SNARE complexes are not formed during capacitation.
Introduction
Fertilization requires that sperm in the female tract go through physiological changes known as capacitation. Capacitation can be induced in vitro by using a medium containing HCO3−, Ca2+, and serum albumin [1]. HCO3− regulates various cellular signaling pathways in the presence of calcium Ca2+ [1–3]. Additionally, cholesterol efflux from the plasma membrane of sperm allows the destabilization of the plasma membrane and induction of capacitation [4]. Removal of sperm membrane cholesterol can be accomplished by albumin [4–6]. Cholesterol is also an important part of membrane microdomains such as lipid rafts and caveolae, which are rich in sphingolipids and saturated phospholipids [7]. Disruption of these microdomains containing cholesterol activates a number of signaling pathways that affect protein sorting, signal transduction, and membrane trafficking [7]. Therefore, the cholesterol-binding agent albumin plays a major role during capacitation of sperm [1–6, 8].
All these changes during capacitation help sperm undergo the acrosome reaction, a step necessary for sperm to penetrate the zona pellucida [1–3, 9]. Membrane fusion during the acrosome reaction is mediated by SNARE (soluble N-ethylmaleimide-sensitive-factor attachment protein receptor) complex formation, which provides the force behind most intracellular membrane fusion [9–12]. SNAREs are believed to promote fusion between the outer acrosomal membrane and the plasma membrane, allowing the acrosome reaction to proceed [11, 21].
Membrane fusion during acrosomal exocytosis is notably similar to neural exocytosis [13–16]. It has been found by various research groups that the proteins involved in exocytotic events of somatic cells are analogous to those involved in acrosomal exocytosis in sperm [13–18]. In neurons, secretory vesicles translocate to the active zone and dock there. After docking, vesicles are then primed for Ca2+ signaling. Trans-SNARE complexes form between the vesicle membrane and the target cell membrane. Calcium influx into the cell is likely the activator of membrane fusion [17]. The contents of the vesicle are then released. In neurons, exocytosis is rapid and vesicles are recycled after the release of their contents; neither of these is true for acrosomal exocytosis [16, 18].
The SNARE family includes a large number of proteins that play a major role in intracellular membrane fusion [19]. This role is conserved from yeast to mammals and from somatic cells to germ cells [20]. In sperm, three proteins form the SNARE core complex: syntaxin, synaptosome associated protein 25 (SNAP25), and vesicle associated membrane protein (VAMP) [14, 15, 19]. Assembly of the SNARE core complex in sperm is triggered by cues that are only partially known [16]. Once the tripartite core complex is assembled, it promotes fusion of the outer acrosomal membrane with plasma membranes to aid in exocytosis [16–21]. Problems with SNARE complex formation could be one of the causes of idiopathic infertility. There is evidence in stallions that semen samples with more sperm staining positively for SNAREs had higher fertility [22].
Herein, we focused on determining where vesicle and target SNARE proteins localize precisely in sperm during capacitation and how SNARE complex formation is regulated. Superresolution structured illumination fluorescence microscopy (SR-SIM), a technique with twice the resolution of traditional confocal microscopy [23], was used to assess SNARE localization during sperm capacitation. Co-localization between acrosomal vesicle and plasma membrane (target) SNAREs was also assessed.
Materials and methods
Reagents and animals
Mice used were CD-1, purchased from Harlan Sprague-Dawley (Carlsbad, CA). Syntaxin 2 (rabbit polyclonal against aa 1–265) and VAMP2 (mouse monoclonal against synthetic peptide SATAATVPPAAPAGEG) antibodies were purchased from Synaptic Systems (Goettingen, Germany). Normal IgG control (rabbit) was purchased from R&D Systems (Minneapolis, MN). Secondary antibodies coupled to Alexa Fluor-488 and Alexa Fluor-568 were purchased from Life Technologies (Carlsbad, CA). Prolong Gold antifade mount was purchased from Life Technologies (Carlsbad, CA). Antibody for actin made in a rabbit and protease inhibitor cocktail were purchased from Sigma-Aldrich (St. Louis, MO). HRP-conjugated anti-rabbit antibodies made in a goat and zona pellucida 3 receptor (ZP3R) antibodies made in mouse were purchased from Pierce Thermo Scientific (Carlsbad, CA). All antibodies are listed in Table 1. Protein markers and 4%–20% precast gradient gels were purchased from Bio-Rad (Hercules, CA). The BCA protein assay kit was purchased from Pierce Thermo Scientific. Bovine serum albumin (BSA) was used as a standard for the BCA assays.
Table 1.
| Antibody | Host/TYPE | Concentration/Assay | Source |
|---|---|---|---|
| STX2 (Syntaxin2) | Rabbit/ Polyclonal | 1 μg/ml/IF, 0.2 μg/ml/WB | Synaptic Systems, Goettingen, Germany |
| VAMP2 (Vesicle associated membrane protein2) | Mouse/Monoclonal | 1 μg/ml/IF | Synaptic Systems, Goettingen, Germany |
| ZP3R (Zona pellucida 3 receptor) | Mouse/Monoclonal | 1 μg/ml/IF | Pierce Thermo Scientific, Waltham, MA |
| Normal IgG, Control | Rabbit/ Polyclonal | 1 μg/ml/IF | R&D Systems, Minneapolis, MN |
| Alexa Fluor 488, Secondary antibody | Rabbit/ Polyclonal | 0.3 μg/ml/IF | Life Technologies, Carlsbad,CA |
| Alexa Fluor 568, Secondary antibody | Mouse/ Polyclonal | 0.3 μg/ml/IF | Life Technologies, Carlsbad,CA |
| HRP, Secondary Antibody | Rabbit/ Polyclonal | 0.16 μg/ml/WB | Pierce Thermo Scientific, Waltham, MA |
| Actin | Rabbit/ Polyclonal | 2.5 μg/ml/WB | Sigma-Aldrich, St.Louis, MO |
IF = Immunofluorescence WB = Western Blot.
Preparation of mouse sperm for immunofluorescence assay
To obtain mouse sperm, three to five mature male mice were euthanized by carbon dioxide asphyxiation, according to the guidelines given by the Institutional Animal Care and Use Committee, University of Illinois. Cauda epididymides were collected from the mice using an approved experimental protocol. Epididymides were washed in PBS for 30 s to remove the extracellular debris. Piercing the cauda with a 23-g needle after washing released sperm. The pierced cauda epididymides were incubated for 10 min at 37°C, allowing the sperm to swim out. This was done in a petri dish containing 1 ml of media per cauda, according to the respective treatment: capacitating medium (dmKRBT; 120 mM NaCl, 2 mM KCl, 2 mM CaCl2, 10 mM NaHCO3, 1.2 mM MgSO4, 0.36 mM NaH2PO4, 5.6 mM glucose, 1.1 mM pyruvic acid, 25 mM 3-[[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]amino]-2-hydroxypropane-1-sulfonic acid (TAPSO), 18.5 mM sucrose, 0.6% BSA, 10 units /ml penicillin, 10 units/ml streptomycin (pH 7.3)) and noncapacitating medium (dmKRBT in which HCO3− was replaced with an equimolar amount of TAPSO, dmKRBT in which BSA was replaced with an equal mass of PVP or dmKRBT in which both BSA and HCO3− were replaced with TAPSO and PVP, respectively). TAPSO has a pKa of 7.635 and buffers in the range of 7.0–8.2 [24]. Sperm concentration was measured using a hemocytometer and motility using a light microscope. Only samples with at least 65% motility were used for the experiments. The sperm were incubated according to the respective treatments and were collected at 0,10, 30, 60, and 120 min of incubation at 37°C in air and a drop of 50 μl was spread on a coverslip to air dry. Air-dried sperm were fixed with 4% paraformaldehyde for 20 min. Permeabilization was done using 0.05% Triton X-100 for 10 min. Sperm on the cover slips were blocked with 3% BSA for 30 min before incubation with primary antibodies. Coverslips were incubated with primary antibodies (1:200) overnight at 4°C. Secondary antibody (1:150) incubation was done at 37°C in air for 1 h. The coverslips were washed with PBS three times between each step and five times between primary and secondary antibody incubation. Cover slips were mounted on slides with a 10 μl drop of Prolong Gold anti-fade. Slides were cured for at least 12 h at 4°C before imaging.
Confocal microscopy
Confocal microscopy was done using a seven-laser system (Zeiss LSM 710 NLO) with 405, 458, 488, 514, 561, 594, and 633 nm lasers. The appropriate laser was used to detect the fluor on the secondary antibody. Slides were focused using a 100× oil objective. All the images obtained were of intact morphologically normal sperm.
Superresolution structured illumination immunofluorescence microscopy
SR-SIM was performed using a four-laser system (Zeiss Elyra S1) with 405, 488, 561, and 633 nm lasers. The laser used was appropriate for the fluor on the secondary antibody. For co-localization of both syntaxin 2 and VAMP2 or syntaxin 2 and ZP3R, two lasers were used simultaneously. Slides were focused using a 100× oil objective. All the images obtained were of intact, morphologically normal sperm. Z-stack was adjusted with reference to the amount of fluorescence present. All the Z-sections showing fluorescence were captured. The final image was cropped in a way that only the sperm head remained a part of the image.
Immunofluorescence data analysis
Multiple images per Z-stack, collected from SR-SIM microscopy, were combined and processed to form a 3D picture using the ZEN-2011 Structured Illumination tool. Later, IMARIS was used to create 3D surfaces from the SR-SIM images. IMARIS is a software system to manage, analyze, and interpret 3D and 4D microscopy images (http://www.bitplane.com/imaris/imaris). To determine if the change in syntaxin 2 localization was accompanied by any change in the amount of syntaxin 2 that might explain the apparent syntaxin 2 movement, the “Surfaces” tool was used. This tool uses multiple volume-rendering algorithms to render 3D volume to fluorescent puncta, providing an overall volume of fluorescence as an indication of the amount of protein of interest.
Images collected using SR-SIM microscopy were analyzed on the basis of fluorescence pattern. This analysis was done without knowledge of the sample being evaluated. The number of sperm showing relocalization or no relocalization was tabulated and a percentage calculated for each treatment group from three experiments.
For images with both syntaxin 2 and VAMP2 localized, the “COLOC” tool in IMARIS was used to determine the area where both proteins were co-localizing. The data analysis tool built in to COLOC was used to calculate the Pearson correlation coefficient to assess the relationship between syntaxin 2 and VAMP2 localization.
Protein extraction and quantification
To obtain mouse sperm protein for western blots, six to eight mature male mice were euthanized by the procedure described previously. Sperm were collected and capacitated as described above, and aliquots were removed at 0, 10, 30, 60, and 120 min. Sperm samples were then centrifuged at 12,000 × g for 10 min, and supernatant was removed before resuspending in PBS. The pellet was gently mixed before centrifuging again for 5 min at 8000 × g. After removal of supernatant, the pellet was suspended in RIPA buffer (50 mM Tris-HCL, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitors, pH 7.3) and vortexed for 30 s before placing on ice for 20 min. The samples were centrifuged at 8000 × g for 5 min, and the supernatant was collected. The BCA assay was used to quantify the protein using BSA as a standard.
Western blot analysis
To verify the amount of syntaxin 2 at different treatments and time points, protein collected from the mouse sperm was diluted with Laemmli sample buffer [25] and denatured at 100°C for 5 min. Sperm protein (20 μg) was loaded in precast linear gradient 4%–20% gels (Bio-Rad, Hercules, CA). Proteins in the gel were transferred to a nitrocellulose membrane for western blotting. The membrane was blocked with 5% nonfat milk in TBST (50 mM Tris, 150 mM NaCl, 0.05% Tween 20) for 1 h at room temperature or overnight at 4°C with gentle agitation. The membrane was incubated with syntaxin 2 primary antibody (0.2 μg/ml, final concentration) for 1 h at 37°C or overnight at 4°C with gentle agitation. After this, the membrane was incubated with HRP-conjugated secondary antibody (0.16 μg/ml, final concentration) for 1 h at 37°C. HRP activity of the secondary antibodies was detected using ECL chemiluminescence peroxidase substrate (Thermo Fisher Scientific Inc., Waltham, MA) and an ImageQuant 4010 imaging system (GE Healthcare, Chicago, IL). After imaging, the membrane was stripped and an actin antibody (2.5 μg/ml, final concentration) was used to detect actin as a loading control. The membrane was washed three times for 10 min between every step. Three biological replicates were done for each treatment and time point. Band intensities of the western blots were measured using ImageQuant TL software (GE Healthcare, Chicago, IL) and normalized to actin. Statistical analysis was performed using two-way ANOVA, Tukey and post hoc tests, and P < 0.05 was considered significant.
Results
Plasma membrane syntaxin 2 relocalizes during sperm capacitation
Fusion of the plasma membrane and outer acrosomal membrane during the acrosome reaction allows the release of acrosomal contents and exposes the inner acrosomal membrane (Figure 1). Vesicle SNAREs (i.e. syntaxin 2) and target SNAREs (i.e. VAMP2) are believed to form trans-SNARE complexes at specific points to promote membrane fusion. Herein, we investigated how sperm prepare to form trans-SNARE complexes during capacitation.
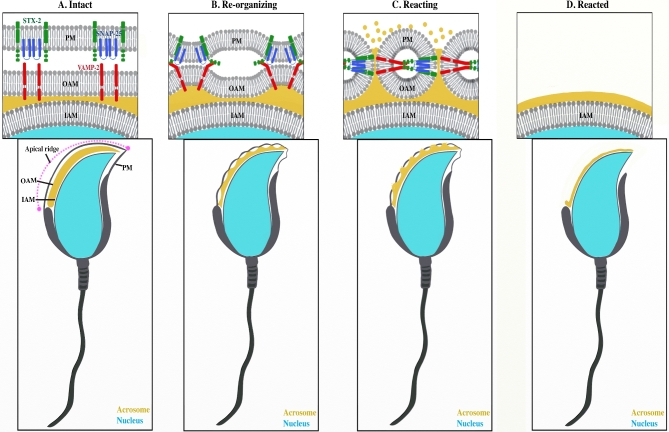
Figure 1.
Model of SNARE function in membrane fusion leading to completion of the acrosome reacion. (A) Acrosome intact mouse sperm with plasma membrane (PM), outer acrosomal membrane (OAM), and inner acrosomal membrane (IAM) immediately prior to membrane interaction. Syntaxin 2 (STX2) and SNAP25 are present on the plasma membrane, while VAMP2 is apposed to them on the outer acrosomal membrane. (B) Trans-SNARE complex formation is promoting interaction of the plasma membrane and outer acrosomal membrane. (C) Membrane fusion is leading to formation of fusion pores, leading to release of acrosomal contents. (D) Sperm after the release of the plasma and outer acrosomal membranes.
Previous reports using confocal microscopy have suggested that the distribution of syntaxin 2 in porcine sperm changes slightly during capacitation, presumably in preparation for the acrosome reaction [11]. To examine this with higher resolution, we used immunofluorescence and both confocal and SR-SIM to detect syntaxin 2. SR-SIM provides twice the resolution of confocal microscopy [23].
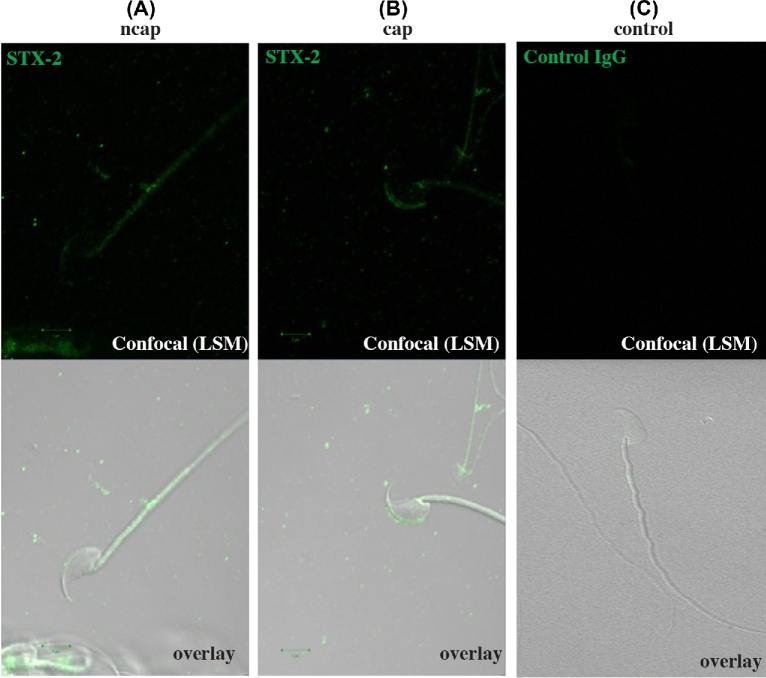
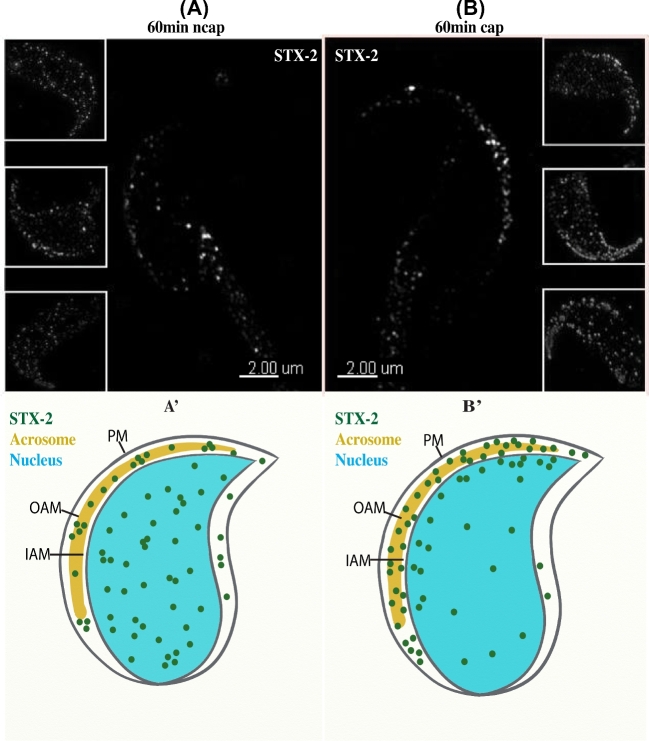
Immunofluorescence analysis showed that syntaxin 2 was localized in a punctate pattern on the sperm head on cells incubated for 60 min in medium lacking BSA and HCO3−, conditions that do not lead to capacitation (confocal microscopy, Figure 2A; SR-SIM, Figure 3A). In contrast, sperm that were incubated 60 min under capacitation conditions (same medium except that BSA and HCO3− were included) had syntaxin 2 most heavily localized to the apical ridge of the sperm head (confocal microscopy, Figure 2B; SR-SIM, Figure 3B). Heterogeneity among the sperm examined was noted. For example, two of the inset images in Figure 3B show more puncta outside of the apical ridge than the other inset or the large image. When DIC images were merged with fluorescence images, it was clear that syntaxin 2 localization in capacitated sperm was primarily over the apical ridge (Figure 2). Control experiments in which in which nonimmune IgG was used (confocal microscopy, Figure 2C) or in which primary antibody was not included (data not shown) showed no fluorescence.
Figure 2.
Location of syntaxin 2 (STX2) in capacitated and noncapacitated sperm using confocal microscopy. Sperm were collected and incubated under noncapacitating (ncap, A) and capacitating (cap, B) conditions for 60 min. Sperm were fixed, permeabilized, and STX2 (green) was detected with an antibody. (A) Sperm that were incubated in noncapacitating medium displayed STX2 in a punctate pattern over much of the sperm head. (B) Sperm that were incubated in capacitating medium displayed STX2 mostly at the apical ridge of the sperm head. (C) If sperm were incubated with nonimmune IgG rather than syntaxin antibody, no staining was observed.
Figure 3.
SR-SIM detects syntaxin 2 (STX2) change in distribution in sperm during capacitation. Sperm were collected and incubated under capacitating conditions for 60 min. Sperm were fixed, permeabilized, and STX2 was detected with an antibody and SR-SIM. (A) Images of sperm incubated in noncapacitating medium (ncap). A punctate pattern of STX2 was observed scattered over the sperm head in the large image as well as three insets. (A’) Schematic representation of (A). (B) Images of sperm incubated in capacitating media (cap). Puncta-containing STX2 were observed primarily at the apical ridge of the sperm head. This is observed in the large image and three insets. (B’) Schematic representation of (B).
We considered the possibility that the location of syntaxin 2 was altered due to the acrosome reaction. To confirm that the acrosome was present in sperm that were positive for syntaxin 2, sperm were co-labeled with a ZP3R antibody, which identifies a protein in the acrosomal matrix [26]. Both noncapacitated (Figure 4A) and capacitated sperm (Figure 4B) that labeled with syntaxin 2 were also positive for ZP3R, demonstrating that the syntaxin 2-positive sperm prior to or following capacitation were acrosome intact.
Figure 4.
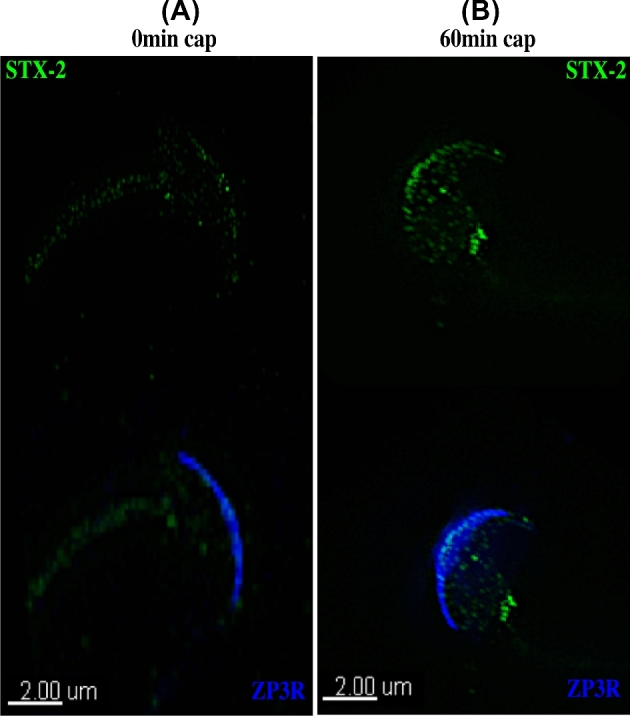
Syntaxin 2 (STX2) change in location is not due to acrosomal exocytosis. Sperm were stained with antibodies to STX2 (green) and ZP3R (blue). Images are of sperm incubated in capacitating media for 0 min (A) or 60 min (B) and examined by SR-SIM. Upper images show STX2 localization and lower images show STX2 and ZP3R overlay. At 60 min, STX2 was localized to the apical ridge in sperm that were stained by the ZP3R antibody and therefore had an intact acrosome.
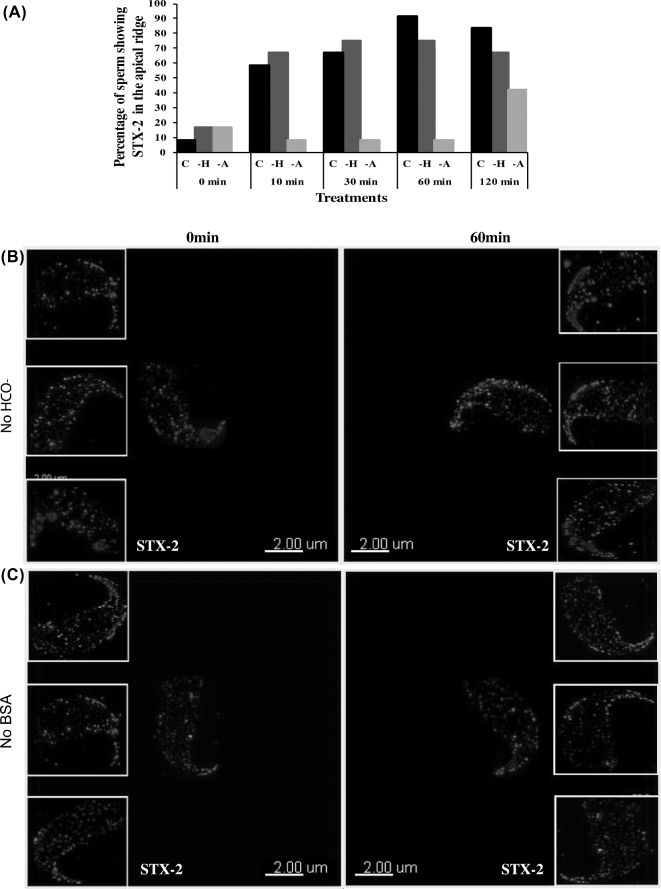
To assess the potential change in location of syntaxin 2 during capacitation, sperm were fixed at various time points during the incubation under capacitating conditions (n = 16 sperm for each time point and treatment). At 0 min, less than 10% sperm showed syntaxin 2 puncta localized in the apical ridge. Within only 10 min of capacitation time, syntaxin 2 puncta shifted into the apical ridge in nearly 60% of the sperm. After 30 min of capacitation, syntaxin 2 shifted toward the apical ridge of the sperm head in 66% of the sperm observed. At 60 min of capacitation, syntaxin 2 was localized in puncta that were mostly restricted to the apical ridge of the plasma membrane overlying the acrosome in over 90% of sperm (Figure 5A). The relocalization of syntaxin 2 puncta occurred quickly. Thus, during sperm capacitation, the location of syntaxin 2 on the plasma membrane changed quickly; after capacitation, it was found primarily on the apical ridge of sperm.
Figure 5.
The location of syntaxin 2 (STX2) in sperm incubated in albumin- or bicarbonate-free medium. (A) The pattern of STX2 localization was analyzed from the images collected at different time points (0, 10, 30, 60, or 120 min) from sperm incubated in capacitating medium (C), medium without BSA (-A), and medium without HCO3− (–H). (B) Sperm incubated in medium with no HCO3− for 0 min showed punctate STX2 localization over the sperm head in the large as well as three insets. Sperm incubated in media with no HCO3− for 60 min showed punctate labeling more abundantly at the apical ridge of the sperm head in the large image and three insets. (C) Sperm incubated in medium with no albumin for 0 min showed punctate STX2 localization over the sperm head in the large as well as three insets. Sperm incubated in media with no albumin for 60 min showed punctate labeling scattered over the sperm head in the large image and three insets, much like sperm at 0 min. A total of 16 sperm were analyzed for each treatment and time point.
Syntaxin 2 location during capacitation in mouse sperm in the absence of bicarbonate (HCO3−)
Bicarbonate activates sperm soluble adenylate cyclase and the protein kinase A pathway, leading to hyperactivated motility and capacitation [1, 27]. Because the location of syntaxin 2 was altered during capacitation, we hypothesized that this change might be regulated by HCO3−. To address this possibility, sperm were incubated in medium in which HCO3− was replaced by an equimolar amount of the buffer TAPSO.
Syntaxin 2 was localized in the same punctate pattern in the apical ridge of the sperm head region at 60 min of incubation in HCO3−-free medium (Figure 5B) as it was when HCO3− was present (Figure 3). Therefore, withdrawal of HCO3− during the incubation did not affect syntaxin location.
Syntaxin 2 location during mouse sperm capacitation in the absence of bovine serum albumin
Removal of cholesterol from the sperm plasma membrane modulates sperm capacitation [4, 28]. Bovine serum albumin in the capacitating medium is a cholesterol acceptor and thereby could change the function of membrane rafts [11, 29] and, perhaps, syntaxin location. Sperm for immunofluorescence were incubated in medium in which BSA was replaced with PVP. The location of syntaxin 2 at 0 min incubation was consistent with the location at 0 min in capacitating medium and medium with no HCO3− (Figure 5C). However, at 60 min of incubation in the absence of albumin in >90% of these cells, syntaxin 2 remained located in the same punctate pattern scattered over the entire sperm head as it was in the beginning of the incubation (Figure 5C). In summary, these results showed that albumin was necessary for the location of syntaxin 2 to the apical ridge after capacitation.
The altered location of syntaxin 2 during capacitation does not change the amount of syntaxin 2
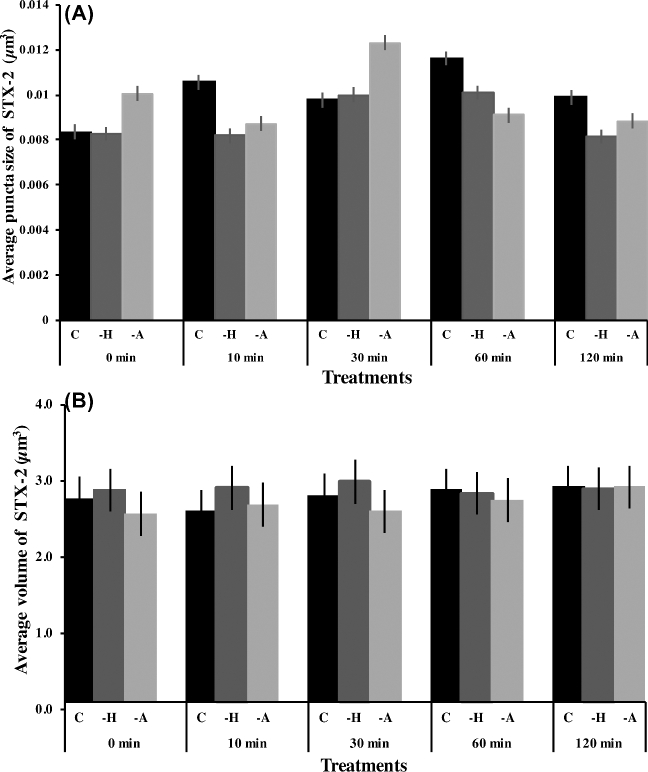
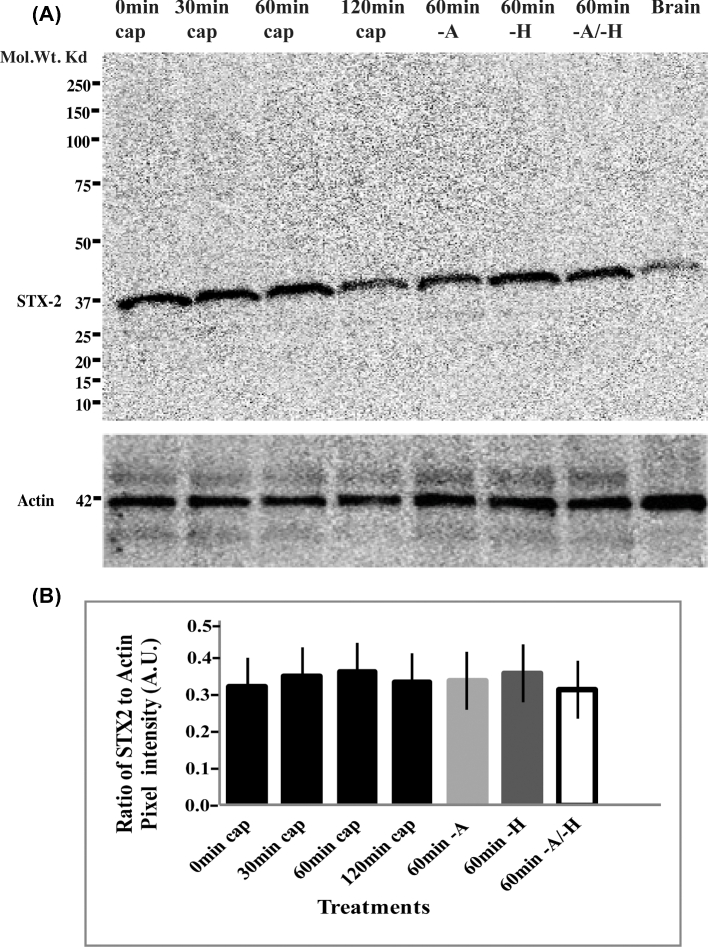
Although sperm are not active in protein synthesis [30], we determined if the change in syntaxin 2 location was due to new synthesis of syntaxin 2 protein or previously cryptic epitopes appearing during capacitation. First, we measured the overall volume of syntaxin 2-positive fluorescence and average puncta size on sperm. The median size of individual puncta was 0.008–0.014 μm3, which was not different between capacitation times (Figure 6A). Furthermore, the total syntaxin 2-positive volume in sperm remained constant during capacitation (Figure 6B). Another possible explanation for the location change is that some cryptic syntaxin 2 epitopes might have become accessible during capacitation. To examine this possibility, we collected sperm protein at different time points (0, 30, 60, or 120 min) of capacitation and performed western blots with syntaxin 2 antibody (Figure 7). There was also no change in the amount of syntaxin 2 by western blotting, indicating that it was highly unlikely that the change in distribution of syntaxin 2 was due to synthesis of new protein or exposure of previously cryptic epitopes. Rather, it was due to relocation of existing syntaxin 2 protein during capacitation.
Figure 6.
The change in location of syntaxin 2 (STX2) during capacitation does not change the average size of STX2 positive puncta and STX2 positive volume. (A) Quantification of images collected using SR-SIM. The average size of STX2 positive puncta on sperm head was calculated at different time points (0, 10, 30, 60, or 120 min) from sperm incubated in capacitating medium (C), media without BSA (–A), and medium without HCO3− (–H). A total of 16 sperm were analyzed for each treatment and time point. No statistically significant difference was found. (B) The STX2 positive fluorescence volume on the sperm head was calculated. Images were collected at 0, 10, 30, 60, or 120 min from sperm incubated in capacitating medium (C), medium without BSA (-A), and medium without HCO3− (-H). A total of 16 sperm were analyzed for each treatment and time point. No statistically significant difference was observed.
Figure 7.
Relocalization of syntaxin 2 (STX2) during capacitation does not change the abundance of STX2. Sperm were incubated for 0, 30, 60, or 120 min in capacitating media or for 60 min in medium without BSA (–A), without HCO3− (–H), and without both HCO3− and BSA (–A/–H). Protein lysates were characterized by immunoblotting with a STX2 antibody. Actin was used as a loading control and mouse brain was used as a positive control. (A) Syntaxin 2 immunoblotting. There was no change in the amount of STX2 collected from mouse sperm. (B) Graphical representation of the STX2 immunoblotting data. Syntaxin 2 was normalized against actin band intensities. No significant difference was observed; n = 3.
Along with normal capacitating medium, we also tested whether there was an increase or decrease in the amount of syntaxin 2 under noncapacitating conditions, medium without either BSA or HCO3−. The amount of syntaxin 2 did not change regardless of whether it was quantitated by immunofluorescence (Figure 7) or western blots (Figure 7). Furthermore, the median volume of individual syntaxin 2-positive puncta was not affected by the withdrawal of BSA or HCO3− (Figure 6). Thus, incubation under conditions that promote or do not promote capacitation did not affect the abundance of syntaxin 2 protein on sperm.
Vesicle associated membrane protein 2 does not relocalize during capacitation in mouse sperm
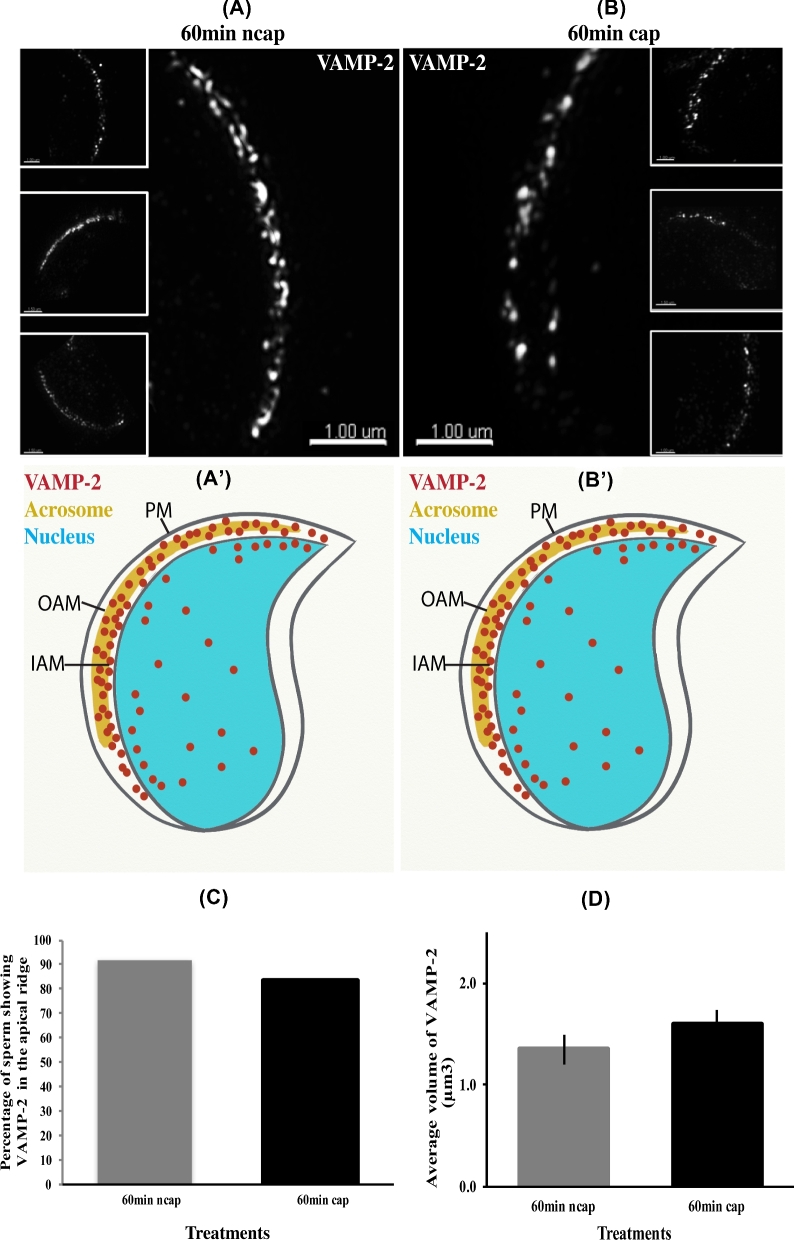
To form a trans-SNARE complex, VAMP2 in the outer acrosomal membrane must localize at fusion points apposing syntaxin 2 on the plasma membrane [32, 33]. As an internal membrane, the outer acrosomal membrane is inaccessible to albumin, rendering the direct removal of cholesterol by BSA improbable. We examined whether VAMP2 would, like syntaxin 2, relocalizes during capacitation. Using immunofluorescence, VAMP2 was localized at the apical ridge of the sperm in the head region in both capacitating and noncapacitating conditions at 60-min incubation time (Figure 8). Negative control experiments were also conducted in which primary antibody was not included; without primary antibody, no fluorescence was detected (data not shown). Thus, no change in VAMP2 localization during capacitation was observed.
Figure 8.
VAMP2 detected by SR-SIM does not redistribute in sperm during capacitation. (A) Images of sperm incubated in noncapacitating medium (ncap) for 60 min. A punctate pattern of VAMP2 can be observed at the apical ridge of the sperm in the large image as well as three insets. (A΄) Schematic representation of (A). (B) Images of sperm incubated in capacitating medium (cap) for 60 min. A similar pattern of VAMP2 can be observed at the apical ridge of the sperm head when compared with (A). (B΄) Schematic representation of (B). The larger image and three insets show a similar result. (C) Images sperm at 60 in of incubation in capacitating medium and noncapacitating conditions collected using SR-SIM microscopy were quantified. The percentage of sperm with VAMP2 at the apical ridge was plotted. (D) The volume of VAMP2-positive fluorescence on sperm head was calculated. Images were collected at 60 min from sperm incubated in capacitating and noncapacitating medium. A total of 16 sperm were analyzed for each treatment and time point. No statistical difference was found.
Vesicle associated membrane protein 2 does not change its volume during capacitation
To determine if the amount of VAMP2 changes during capacitation, we estimated the volume of sperm stained with the VAMP2 antibody. The results showed that the amount of VAMP2 in the sperm, assessed by fluorescence volume, did not change during a 60-min capacitation time (Figure 8D).
Vesicle associated membrane protein 2 and syntaxin 2 do not co-localize during capacitation in mouse sperm
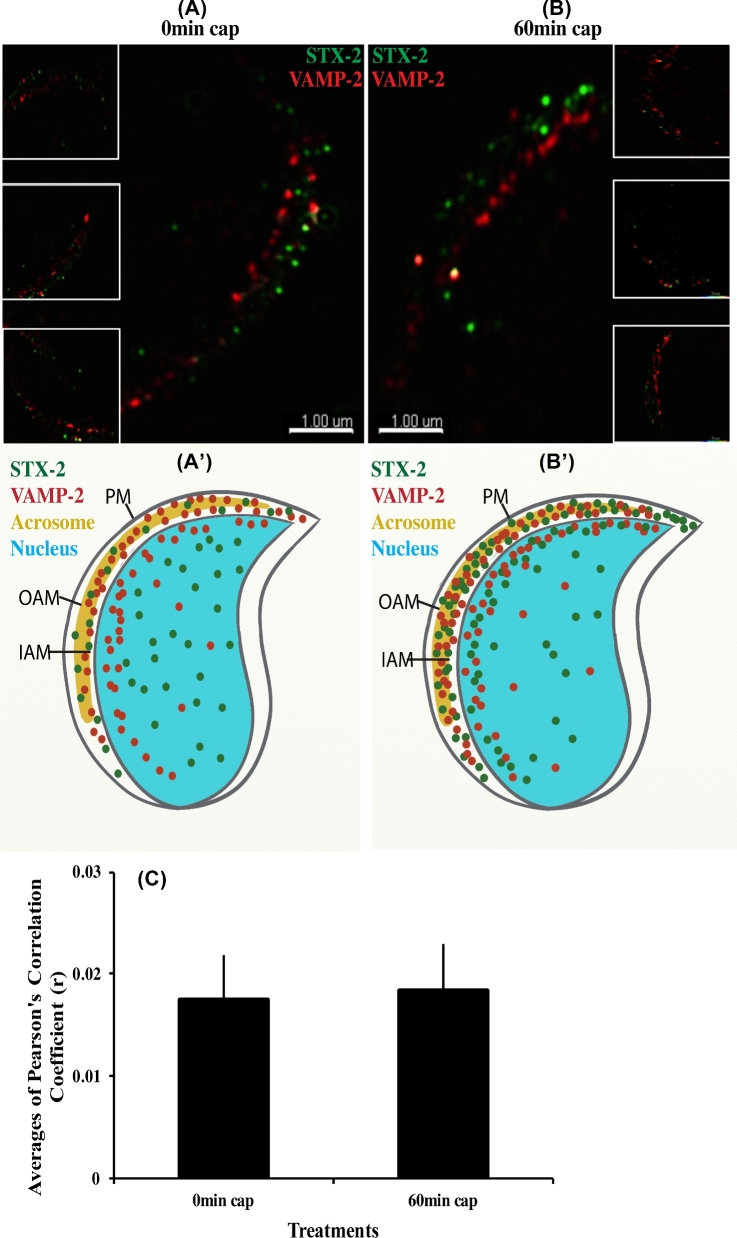
To better understand when trans-SNARE complexes form during fertilization, plasma membrane syntaxin 2 and acrosomal membrane VAMP2 co-localization were assessed during capacitation. Both proteins were identified using immunofluorescence with different secondary antibodies to distinguish VAMP2 and syntaxin 2. Control experiments were also conducted in which primary antibody was not included; no fluorescence was detected in controls (data not shown). But in samples in which both syntaxin 2 and VAMP2 antibodies were used, there was no apparent co-localization at 0 or 60 min (Figure 9A and B).
Figure 9.
Syntaxin 2 (STX2) and VAMP2 do not co-localize in capacitated sperm. Sperm were incubated for 60 min in capacitating conditions and stained with antibodies and appropriate fluorescent secondary antibodies to STX2 (green) and VAMP2 (red). No co-localization was observed between STX2 and VAMP2 when images collected at either 0 min (A) or 60 min (B) of capacitation time. (A΄ and B΄) are schematic representations of (A and B). (C) Graphical representation of co-localization data obtained from the SR-SIM images. A total of 16 sperm were analyzed for each treatment and time point. There was no significant correlation in localization observed at either 0 or 60 min sperm capacitation time.
To test objectively whether syntaxin 2 and VAMP2 were co-localized, the COLOC tool in IMARIS and Pearson's correlation were used. Areas that were syntaxin 2 positive were not the same as areas that were VAMP2 positive. Hence, there was no significant correlation in localization observed at either 0 or 60 min sperm capacitation time (Figure 9C). Thus, trans-SNARE complexes were not formed when capacitation was completed.
Discussion
It has been proposed that SNARE complex formation is promoted during sperm capacitation, preparing sperm for acrosomal exocytosis [31]. But it is not clear how and when acrosomal vesicle SNAREs and plasma membrane SNAREs form the minimal tripartite complex [31]. Although there are reports that other syntaxins and VAMPs are found on sperm [12], we used antibodies against syntaxin 2 and VAMP2 as proxies for SNARES of these families. Using superresolution microscopy, we observed that, during capacitation, puncta containing syntaxin 2, a plasma membrane SNARE, move laterally to a region overlying the acrosomal ridge, the region of the plasma membrane that fuses with the outer acrosomal membrane during the acrosome reaction. Syntaxin 2 puncta had median volumes ranging from 0.008 to 0.014 μm3. It is not certain if these puncta are membrane rafts or some other structures. Sperm rafts are heterogeneous and syntaxin 2 may be in a subset of rafts [34]. Nevertheless, the lateral movement of syntaxin 2 puncta demonstrates that protein and lipids present in the sperm membrane shift and re-model in response to capacitation [8]. This is a dramatic example of the dynamic nature of membrane proteins and lipids [8, 35]. These changes in the sperm apical plasma membrane are the result of lipid, cholesterol, and protein modification, which happen during capacitation [29, 36, 37]. Thus, spatial regulation of SNARE localization is a way in which acrosomal exocytosis is controlled.
Capacitation is contingent upon the action of HCO3−, albumin, and Ca2+ [38–41]. These components activate different stimuli that, in total, lead to surface molecule removal, migration, or re-arrangement as part of capacitation [1, 9]. We found that albumin but not HCO3− was required for syntaxin 2 relocalization. Thus, conditions necessary for the completion of capacitation (inclusion of HCO3−) are not necessary for syntaxin redistribution. Because albumin can remove cholesterol from the sperm plasma membrane resulting in reorganization of lipid rafts [1, 28–30] and syntaxin 2 is found in lipid rafts, we propose that, in the absence of a cholesterol acceptor, membrane rafts are not re-organized and syntaxin 2 does not relocalize. The amount of total syntaxin 2 in sperm did not change during capacitation, an expected outcome because mature sperm do not synthesize protein. Furthermore, cryptic epitopes were not exposed during capacitation. This is consistent with the proposal that there is no turnover of syntaxin 2 but instead, it is simply shifted laterally in the plasma membrane.
VAMP2 is a v-SNARE (vesicle-associated SNARE protein), which is present on the outer acrosomal membrane [11, 21, 32, 42, 43]. It was also found in puncta but, in contrast to syntaxin 2, VAMP2 was already located at the apical ridge of sperm and its location did not change during capacitation. These results were found to be at odds with a previous report, which suggested that VAMP2 also shifts in response to capacitation in porcine sperm [11]. This may be a species difference or may be due to the higher resolution of SR-SIM. Regardless, co-localization data indicated that syntaxin 2 and VAMP2 did not become apposed to each other during capacitation. To form a trans-SNARE complex for exocytosis, VAMP2 and syntaxin 2 must co-localize. Thus, even after capacitation, either VAMP2 or syntaxin 2 or both must still move laterally just before membrane fusion. For clarity, we are defining capacitation as the time-dependent events that occur before acrosomal exocytosis, although some researchers include the acrosome reaction as a part of capacitation [30, 44].
It is also possible that other SNAREs not recognized by the antibodies to syntaxin 2 and VAMP2 have different localization patterns. If so, the other SNARE isoforms may co-localize at the end of capacitation. To address this possibility requires a more exhaustive investigation of the other syntaxins and VAMPs found in sperm.
The observation that syntaxin 2 and VAMP2 do not co-localize during capacitation and that syntaxin 2 but not VAMP2 can shift in the membrane means their location is separately regulated. Final acrosomal exocytosis occurs in response to a stimulus that is Ca2+ dependent [2, 45]. Ca2+ is the main trigger for the fusion of sperm's plasma membrane with the outer acrosomal membrane [45]. Although there is much evidence that Ca2+ affects synaptotagmin, there is little evidence Ca2+ affects syntaxin 2 or VAMP2 function directly [46–48].
SR-SIM observation of SNAREs provided the high resolution necessary to make these observations. SR-SIM increases lateral resolution two-fold (resolution is 100–120 nm) and volumetric resolution eight-fold compared to optimal resolution by confocal microscopy. It improves resolution by assessing the interference of sample fluorescence variation in a modulated linear wave illumination pattern, captured using a wide field camera. Algorithms are used that can reconstruct the image, considering information above and below the focal plane, improving both lateral and axial resolution. This technique has only recently been applied to sperm [49].
The result that syntaxin 2 moved laterally on the plasma membrane in the absence of HCO3− means that syntaxin 2 location can change even if sperm have not completed capacitation, a process that requires HCO3−. Rather, the location of syntaxin 2 is linked to the presence of albumin and possibly to cholesterol depletion. Furthermore, syntaxin 2 and VAMP2 do not co-localize in sperm during capacitation but instead delay their co-localization and trans-SNARE complex formation until acrosomal exocytosis. How and why plasma membrane and acrosomal membrane SNAREs are regulated independently is an intriguing and important question, the answers to which will provide a better understanding of how this unusual membrane fusion in sperm is regulated.
Acknowledgments
We thank Dr Sivaguru Mayandi and the staff at the Core Facilities at the Carl R. Woese Institute for Genomic Biology at the University of Illinois at Urbana-Champaign for assistance with microscopy. We also thank members of the Miller laboratory for the critique of the manuscript. We also appreciate greatly the assistance of Katyana Palafox for drawing the schematics.
References
- 1.Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, Visconti PE. Signaling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl, 2007: 65:245–259. [PubMed] [Google Scholar]
- 2.Abou-haila A, Tulsiani DRP. Signal transduction pathways that regulate sperm capacitation and the acrosome reaction. Arch Biochem Biophys, 2009, 485:72–81. [DOI] [PubMed] [Google Scholar]
- 3.De Lamirande E., Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod, 1997, 3:175–194. [DOI] [PubMed] [Google Scholar]
- 4.Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, Colenbrander B, Gadella BM. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci, 2001, 114:3543–3555. [DOI] [PubMed] [Google Scholar]
- 5.Nolan JP, Hammerstedt RH. Regulation of membrane stability and the acrosome reaction in mammalian sperm. FASEB J, 1997, 11:670–682. [DOI] [PubMed] [Google Scholar]
- 6.Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol, 1999, 214:429–443. [DOI] [PubMed] [Google Scholar]
- 7.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol, 1999, 19:7289–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedu-Addo K, Lefievre L, Moseley FLC, Barratt CLR, Publicover SJ. Bicarbonate and bovine serum albumin reversibily 'switch' capacitation-induced events in human spermatozoa. Mol Hum Reprod, 2005, 11:683–691. [DOI] [PubMed] [Google Scholar]
- 9.Boerke A, Tsai PS, Garcia-Gil N, Brewis IA, Gadella BM. Capacitation-dependent reorganization of microdomains in the apical sperm head plasma membrane: functional relationship with zona binding and the zona-induced acrosome reaction. Theriogenology, 2008, 70:1188–1196. [DOI] [PubMed] [Google Scholar]
- 10.Duman JG, Forte JG. What is the role of SNARE proteins in membrane fusion. Am J Physiol Cell Physiol, 2003, 285:C237–C249. [DOI] [PubMed] [Google Scholar]
- 11.Tsai PS, Vries KJ, De Boer-Brouwer MD, Garcia-Gil N, Van Gestel RA, Colenbrander B, Gadella B, Van Haeften T. Syntaxin-2 and VAMP association with lipid rafts depends on cholesterol depletion in capacitating sperm cells. Mol Membr Biol, 2007, 24:313–324. [DOI] [PubMed] [Google Scholar]
- 12.Tsai PS, Garcia-Gil N, van Haeften T, Gadella BM. How pig sperm prepares to fertilize: stable acrosome docking to the plasma membrane. PLoS One, 2010, 5:e11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ungar D, Hughson FM. SNARE protein structure and function. Ann Rev Cell Dev Biol, 2003, 19:493–517. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen JB., SNARE complexes prepare for membrane fusion. Trends Neurosci, 2005, 28:453–455. [DOI] [PubMed] [Google Scholar]
- 15.Van den Bogaart G, Holt MG, Bunt G, Riedel D, Wouters FS, Jahn R. One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol, 2010, 17:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Burkin HR, Shi X, Li L, Reim K, Miller DJ. Complexin I is required for mammalian sperm acrosomal exocytosis. Dev Biol, 2007, 309:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber SH, Südhof TC. Molecular determinants of regulated exocytosis. Diabetes, 2002, 51, (suppl 1):S3–S11. [DOI] [PubMed] [Google Scholar]
- 18.Meizel S. The sperm, a neuron with a tail: “neuronal” receptors in mammalian sperm. Biol Rev Camb Philos Soc, 2004, 79:713–732. [DOI] [PubMed] [Google Scholar]
- 19.Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol, 2001, 2:98–106. [DOI] [PubMed] [Google Scholar]
- 20.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA, 1998, 95:15781–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Blas GA, Roggero CM, Tomes CN, Mayorga LS. Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol, 2005, 3:e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamboa S, Ramalho-Santos J. SNARE proteins and caveolin-1 in stallion spermatozoa: possible implications for fertility. Theriogenology, 2005, 64:275–291. [DOI] [PubMed] [Google Scholar]
- 23.Allen JR, Ross ST, Davidson MW. Structured illumination microscopy for superresolution. Chemphyschem, 2014, 15:566–576. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg RN, Kishore N, Lennen RM. Thermodynamic quantities for the ionization reactions of buffers. J Phys Chem, 2002, 31:231. [Google Scholar]
- 25.Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem, 1977, 252:1102–1106. [PubMed] [Google Scholar]
- 26.Kim KS, Cha MC, Gerton LG. Mouse sperm protein sp56 is a component of the acrsomal matrix. Biol Reprod, 2001, 64:36–43 [DOI] [PubMed] [Google Scholar]
- 27.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Moss SB. The soluble adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell, 2005, 9:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest, 2002, 110:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Gestel R A, Brewis IA, Ashton PR, Helms JB, Brouwers JF, Gadella BM. Capacitation-dependent concentration of lipid rafts in the apical ridge head area of porcine sperm cells. Mol Hum Reprod, 2005, 11:583–590. [DOI] [PubMed] [Google Scholar]
- 30.Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev, 2006, 20:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buffone MG, Hirohashi N, Gerton GL. Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biol Reprod, 2014, 90:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Shen QT, Kiel A, Wang J, Wang HW, Melia TJ, Pincet F. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science, 2012, 335:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol, 2008, 15:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell L, Peterson R, Freund M. Direct evidence for formation of hybrid vesicles by fusion of plasma and outer acrosomal membranes during the acrosome reaction in boar spermatozoa. J Exp Zool, 1979, 208:41–56. [DOI] [PubMed] [Google Scholar]
- 35.Asano A, Selvaraj V, Buttke DE, Nelson JL, Green K, Evans JE, Travis AJ. Biochemical characterization of membrane fractions in murine sperm: identification of three distinct subtypes of membrane rafts. J Cell Physiol, 2009, 218:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeagle P., Lipid regulation of cell membrane structure and function. FASEB J, 1989, 3:1833–1842. [PubMed] [Google Scholar]
- 37.Tomes CN, Michaut M, De Blas G, Visconti P, Matti U, Mayorga LS. SNARE complex assembly is required for human sperm acrosome reaction. Dev Biol, 2002, 243:326–338. [DOI] [PubMed] [Google Scholar]
- 38.Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod, 2013, 19:785–793. [DOI] [PubMed] [Google Scholar]
- 39.DasGupta S, Mills CL, Fraser LR. Ca2+-related changes in the capacitation state of human spermatozoa assessed by a chlortetracycline fluorescence assay. Reproduction, 1993, 99:135–143. [DOI] [PubMed] [Google Scholar]
- 40.Florman H M, Corron ME, Kim DH, Babcock DF. Activation of voltage-dependent calcium channels of mammalian sperm is required for zona pellucida-induced acrosomal exocytosis. Dev Biol, 1992, 152:304–314. [DOI] [PubMed] [Google Scholar]
- 41.Fraser LR, Abeydeera LR, Niwa K. Ca2+-regulating mechanisms that modulate bull sperm capacitation and acrosomal exocytosis as determined by chlortetracycline analysis. Mol Reprod Dev, 1995, 40:233–241. [DOI] [PubMed] [Google Scholar]
- 42.Tsai PS, Brewis IA, van Maaren J, Gadella BM. Involvement of complexin 2 in docking, locking and unlocking of different SNARE complexes during sperm capacitation and induced acrosomal exocytosis. PLoS One, 2012, 7:e32603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossetto O. Vesicle-associated membrane protein (VAMP)/VAMP-2 is associated with dense core secretory granules in PC12 neuroendocrine cells. J Biol Chem, 1995, 270:1332–1336. [DOI] [PubMed] [Google Scholar]
- 44.Chang MC. The meaning of sperm capacitation a historical perspective. J Androl, 1984, 5:45–50. [DOI] [PubMed] [Google Scholar]
- 45.De Blas G, Michaut M, Treviño CL, Tomes CN, Yunes R, Darszon A, Mayorga LS. The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. J Biol Chem, 2002, 277:49326–49331. [DOI] [PubMed] [Google Scholar]
- 46.Castillo Bennett J, Roggero CM, Mancifesta FE, Mayorga LS. Calcineurin-mediated dephosphorylation of synaptotagmin VI is necessary for acrosomal exocytosis. J Biol Chem, 2010, 285:26269–26278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugita S, Han W, Butz S, Liu X, Fernandez-Chacn R, Lao Y, Sudhof TC. Synaptotagmin VII as a plasma membrane Ca2+ sensor in exocytosis. Neuron, 2001, 30:459–473. [DOI] [PubMed] [Google Scholar]
- 48.Ramalho-Santos J, Moreno RD, Sutovsky P, Chan AW, Hewistson L, Wessel GM, Simerly CM, Schatten G. SNAREs in mammalian sperm: possible implications for fertility. Dev Biol, 2000, 223:54–69. [DOI] [PubMed] [Google Scholar]
- 49.Al-Dossary A, Bathala P, Caplan JL, Martin-Deleon PA. Oviductosome-sperm membrane interaction in cargo delivery detection of fusion and underlying molecular players using three-dimensional super-resolution structured illumination microscopy (sr-sim). J Biol Chem, 2015, 290:17710–17723. [DOI] [PMC free article] [PubMed] [Google Scholar]