Abstract
Background
Coenzyme Q10 (CoQ10) is a popular nutritional supplement that is available in both the oxidized and reduced form. The marketing of CoQ10 to physicians often asserts that one form is superior to the other. This study was designed to compare and contrast the stability, absorption and claims made for the reduced form of CoQ10 (ubiquinol) compared with the oxidized form (ubiquinone). There is a need for studies that examine the contents of commercially available ubiquinol products microscopically at room, body and 50°C temperatures. There is also a need for studies of the state of the ubiquinol contents when exposed to a 2.2 pH solution that simulates stomach acidity and an 8.2 pH solution that simulates acidity in the duodenum.
Methods
An investigation of the instability of ubiquinol supplements was conducted via an in vitro study of 13 ubiquinol products marketed in the United States that measured the extent of the conversion of the ubiquinol content to ubiquinone, when the ubiquinol was squeezed out of the capsule at room temperature and when the ubiquinol contents were exposed to a 2.2 pH solution and an 8.2 pH solution.
Results
In the in vitro study, the percentage of ubiquinol converted to ubiquinone at body temperature was greatest in the 8.2 pH simulated small intestinal juice: 76%. The percentage of ubiquinol converted to ubiquinone at body temperature in the 2.2 pH gastric juice that simulated conditions in the stomach was 54%.
Conclusions
Ubiquinol in commercial nutritional supplements is fairly stable inside the gelatin capsule but unstable in gastric and small intestine digestive fluids. Based on the data from the lab studies, most of the ubiquinol from the capsule will be converted to ubiquinone prior to reaching the absorption cells in the small intestines. Animal studies are needed to test this hypothesis.
Introduction
Coenzyme Q10 (CoQ10) is a redox molecule with 2 bioactive states, an oxidized state (ubiquinone) and a reduced state (ubiquinol), as well as an ephemeral intermediate state, semiquinone (ubisemiquinone), which may have as yet unclarified biologic functions.1 In the literature, the fully oxidized form, ubiquinone, is commonly referred to as Q10 (European usage) or CoQ10 (American usage); the fully reduced form, ubiquinol, is, correspondingly, referred to as QH2 or CoQH2. Both redox forms of CoQ10 are bioactive. CoQ10 is essential for cellular adenosine phosphate (ATP) energy production; CoQH2 is an important lipid-soluble antioxidant preventing peroxidation of the low-density lipoproteins in the blood circulation.1,2
To assert that one form of CoQ10 is more important than the other would be misleading.3,4 Both forms are important. Ubiquinol is the inherently unstable form; it is ubiquinol’s property of donating electrons that makes it useful as an antioxidant. When it donates 2 electrons, it is oxidized to the ubiquinone form.
CoQ10 is a vitamin-like substance that has vital biologic functions in human cells:2
acting as an essential cofactor in the cellular process of ATP energy generation
acting as a lipid-soluble antioxidant protecting cells against oxidative damage
protecting against atherosclerosis by improving endothelial function
acting as a factor in the expression of genes that code for proteins involved in cell signaling
having anti-inflammatory effects
It is ubiquinone that is synthesized in the human cells, not ubiquinol.3 Adequate intake of CoQ10 in the ubiquinone form should ensure an adequate supply of the antioxidant form ubiquinol. First of all, Mantle and Dybring3 report that the human body contains at least 5 enzyme systems that facilitate the conversion of ubiquinone to ubiquinol: cytochrome b5 reductase, lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase and NAD(P)H dehydrogenase quinone 1 (NQO1).
Second, Mohr, et al5 demonstrated that supplementation with ubiquinone results in significantly increased concentrations of ubiquinol in the blood plasma and the circulating lipoproteins and leads to a significantly increased resistance of human low-density lipoproteins to lipid peroxidation.5
Human biosynthesis of CoQ10 generally reaches a peak in a person’s 20s and then gradually but steadily declines with increasing age.6 The decline in the endogenous production of CoQ10 is associated with the ageing process and the development of ageing-related diseases.7 It is not practical to attempt to compensate for this loss of endogenous CoQ10 by eating greater quantities of food containing CoQ10 or by choosing food more carefully. In one estimate, it would take about 3 pounds of beefsteak or 2 pounds of liver to meet the human adult need for 3.5 mg of exogenous CoQ10 daily.8
Supplementation with a CoQ10 supplement with documented absorption and bioavailability is necessary. There is significant variation in the absorption and bioavailability of commercially available CoQ10 supplements, depending on the formulation—variation in the solubilization of the CoQ10 active ingredient itself, the choice of the carrier oils for the CoQ1, and the heating and cooling process used during the manufacture of the CoQ10 capsules. CoQ10 supplements are not equally potent, not even if they are manufactured using the same CoQ10 raw material.9
Further complicating the supply of adequate CoQ10 to the body’s cells and tissues is the issue of the inhibition of CoQ10 biosynthesis by medications. For example, the use of statin medications, biophosphonates, the antidepressant drug amitriptyline and the chemotherapy drug doxorubicin (Adriamycin) will cause secondary CoQ10 deficiency.10,11
In particular, CoQ10 and cholesterol are synthesized in the same biologic pathway. It is possible that the use of statin medications may cause coronary artery calcification and may impair heart muscle function by depleting the supply of CoQ10.12
The Cleveland Clinic indicates that the population reference values for plasma or serum CoQ10 concentrations range from 0.36 to 1.59 micrograms per milliliter (mcg/mL).8 Unsupplemented normal healthy individuals who are not elderly should have a plasma CoQ10 concentration of approximately 0.8 mcg/mL.13 The current consensus among CoQ10 researchers is that a plasma CoQ10 concentration of at least 2.5 mcg/mL is required for significant benefit of CoQ10 in the adjuvant treatment of patients with heart failure. In patients with neurodegenerative diseases, plasma CoQ10 concentrations >3.5 mcg/mL are required for therapeutic effect.13
Ubiquinol Is Easily Oxidized Back to Ubiquinone
The manufacture of ubiquinol supplements is difficult and requires extra care. When exposed to room air, ubiquinol will be oxidized back to ubiquinone. It seems that many ubiquinol supplement manufacturers have taken to encapsulating ubiquinol under nitrogen to prevent oxidation of the ubiquinol to ubiquinone already in the capsule.
The ubiquinol in a commercially available product may be partially or wholly oxidized to ubiquinone by the time of ingestion. An easy way to test the contents of the ubiquinol product is to prick the capsule and squeeze out the contents. If the contents are orange, the ubiquinol has already oxidized to ubiquinone. If the contents are a milky white, then the CoQ10 is still in its reduced form, ubiquinol.14
A further question is whether the ubiquinol from the capsule remains in its reduced form as it passes through the stomach and the duodenum on its way to the small intestine absorption cells.
SIBR Research has investigated this question in 2 studies:
An in vitro study of the contents of 13 ubiquinol products marketed in the United States (Part 1 of this report)
An in vivo study of ubiquinol absorption in large dogs (Part 2 of this report)
These studies constitute 2 approaches to answering the question of whether the ubiquinol from supplements is oxidized to ubiquinone before CoQ10 reaches the intestinal absorption cells, ie, whether the ubiquinol in supplements is absorbed as ubiquinol or converted to ubiquinone and absorbed as ubiquinone before being converted back to ubiquinol in the lymph nodes.
Materials and Methods
In Vitro Study
The predominant marketing of CoQ10 to physicians and patients is characterized by assertions that one form of CoQ10 (ubiquinol) is better absorbed than the other (ubiquinone). This study was designed to investigate the stability and absorption of ubiquinol and claims that are made for ubiquinol supplements.
In the in vitro study,13 ubiquinol products marketed in the United States were randomly selected from a total of 136. A description of the ubiquinol supplements tested is available upon request. The statistical group ordered and blinded the samples to the study laboratory and analytical laboratory personnel. Each ubiquinol product sample was photographed, showing the capsule, the capsule fill and a microphotograph of the capsule fill.
Each sample capsule was cut open and squeezed into 250 ml simulated gastric juice, warmed to body temperature and with a pH of 2.2 for 60 minutes. The empty capsule content was washed into the gastric juice with 5 ml gastric juice. The gastric juice was maintained at body temperature using a water bath and the solution was slowly stirred at 2 rpm. The juices were blanketed with nitrogen and covered with plastic wrap to prevent exposure to room air. Photographs and microphotographs of the solution were taken. A 5-ml sample was obtained from the center of the gastric juice solution after 60 minutes, frozen and stored at -82°C.
The contents of each sample capsule were then exposed to 250 mL of small intestinal juice at a pH of 8.2 for 60 minutes. Again, 5-ml samples were taken from the solution, frozen and stored. Samples were sent overnight to the analytical lab for analysis of total CoQ10, ubiquinol and ubiquinone content. High-performance liquid chromatography with an electrical chemical detector was used to make the measurements. The product stability of each of the 13 ubiquinol supplements was measured in the capsule in the 2.2 pH simulated gastric juice and 8.2 pH small intestinal juice.
For each of the 13 samples, the percentage of CoQ10 in the ubiquinol form and in the ubiquinone form in the 2.2 pH juice and the 8.2 pH juice, both at body temperature for 60 minutes, was calculated. A description of the CoQ10 in the gastric juices and the simulated duodenal fluids is available upon request.
Hypothesis Tested in the In Vitro Studies
For the in vitro studies, SIBR Research assumed that the percentage of ubiquinol in the capsule should be 100%, as declared on the label, and 0% ubiquinone. In a similar fashion, we assumed that the percentage of ubiquinol in the 2.2 pH gastric juice and 8.2 simulated duodenal fluids should be 100% ubiquinol and 0% ubiquinone.
Accordingly, we wanted to test the null hypothesis that μ = 0% ubiquinone and the alternative hypothesis that μ > 0%, where μ is the expected mean group percentage of ubiquinone.
We used a one-sample t test to calculate the test statistics and then calculated the P value to see whether the deviation from zero in the group mean percentages of ubiquinone in the 3 tests—capsule, 2.2 pH gastric juice and 8.2 simulated small intestinal fluids — was statistically significant. We report the P values in the Table. P = .05 was considered significant.
Table.
Percentage of Ubiquinol Converted to Ubiquinone in the Ubiquinol Capsule in the 2.2 pH Gastric Juices and the 8.2 pH Small Intestinal Fluids
| Study | Group Mean of the 13 Percentages | Standard Deviation of the 13 Percentages | P Value of the Observed Difference From the H |
|---|---|---|---|
| Ubiquinol converted to ubiquinone in the capsule | 8.52% | 3.66% | <.0001 |
| Ubiquinol converted to ubiquinone in the 2.2pH gastric juice | 53.87% | 37.61% | .000115 |
| Ubiquinol converted to ubiquinone in the 8.2pH intestinal fluid | 75.84% | 36.42% | <.0001 |
| Ubiquinol converted to ubiquinone in the 8.2pH intestinal fluid minus 1 outlier | 81.98% | 30.20% | <.0001 |
Results
In Vitro Studies
The data from the in vitro studies showed that the ubiquinol was relatively stable in the soft-gel capsules and that unexpected percentages of ubiquinol were converted to ubiquinone in the 2.2 pH gastric juices and in the 8.2 pH small intestinal fluids.
The Ubiquinol Capsule Study
In the in vitro study, 13 ubiquinol products marketed in the United States were evaluated. Of these, 11 ubiquinol products were encapsulated in bovine gelatin, 1 in fish gelatin, and 1 in a veggie cap; 9 had glycerol in the capsule.
Of the capsules, 8 had fill material that was yellow to orange in color indicating that there was ubiquinone present inside the ubiquinol product capsule; some of the ubiquinol content had already oxidized to ubiquinone in the capsule. The capsule contents were brown in color in 2 capsules, and white in 2 capsules. The white color indicates little to no ubiquinone in those 2 products.
All of the ubiquinol products contained crystals of ubiquinol or ubiquinone, indicating the likelihood of reduced absorption as the human body cannot absorb CoQ10 crystals, only single CoQ10 molecules. One key to the absorption of CoQ10 supplements is the manufacturer’s ability to make the CoQ10 crystals dissociate to single CoQ10 molecules at body temperature.
The 2.2 pH Gastric Juice Study
In the 2.2 pH gastric juice, the ubiquinol in the capsules was oxidized at body temperature in 60 minutes to 54% ubiquinone and 46% ubiquinol, on average. The expectation of the purchaser of a ubiquinol supplement is, presumably, that the ubiquinol in the capsule will stay in the ubiquinol state as it passes through the gastrointestinal tract and is absorbed in the enterocytes. Instead, the results of this study show that more than half of the ubiquinol is oxidized to ubiquinone in 2.2 pH gastric juice.
Admittedly, there is considerable variation from product to product in the percentage of ubiquinol that is oxidized to ubiquinone. In 11 of the 13 products tested, the ubiquinone from the oxidized ubiquinol in the product floated undissolved on the surface of the gastric juice. The 2 ubiquinol products that appeared to be more soluble in the 2.2 pH gastric juice both contained polysorbate, an emulsifier used in some pharmaceutical preparations and food products.
In a similar fashion, variation in the extent of the solubilization of the individual products explains the considerable sample-to-sample variation in total CoQ10 quantity. How much CoQ10 was retrieved with each drawing of a 5-mL sample depended upon how well the ubiquinol was dissolved.
The 8.2 pH Intestinal Fluid Study
On average, only 24.16% of the ubiquinol in the 13 ubiquinol supplements remained in the ubiquinol state after 60 minutes at body temperature in the 8.2 pH simulated small intestinal juice. Fully 75.84% of the ubiquinol had been oxidized to ubiquinone.
If the data for the outlier, the number 12 sample, are removed from the calculation, the corresponding mean percentage values are 18.02% ubiquinol and 81.98% ubiquinone.
Discussion
The results of the in vitro study of the ubiquinol content in the capsule in 2.2 pH gastric juice and 8.2 pH simulated small intestinal juice suggest that the ubiquinol in ubiquinol supplements is fairly stable in the capsule but much less stable in the stomach and the duodenum. Given a typical ubiquinol supplement, the user should expect as much as three-fourths or more of the ubiquinol content to be oxidized to the ubiquinone state of CoQ10 before the ubiquinol from the capsule reaches the enterocytes. These results are not surprising; being an electron donor, ubiquinol is expected to be oxidized.
Implications for Further Research
The data from the SIBR Research lab studies presented here show that individuals purchasing a ubiquinol supplement can have a reasonable expectation of swallowing a capsule that still contains mostly unoxidized ubiquinol.
However, in the acid profile between the stomach and the small intestines, there is a wide range of acidity; therefor there are many free hydrogen and alkaline radicals in solution. Because ubiquinol is an antioxidant and electron donor, and because antioxidants are inherently unstable, the ingested ubiquinol can be expected to give up hydrogen ions to quench the acid and alkalinity of the digestive juices.
Based on the data from the lab studies done by SIBR Research, the ingested ubiquinol can be expected to be converted to ubiquinone. This hypothesis needed to be tested in a large animal study. Part 2 of this report contains the data from a large dog study of the ingestion and absorption of ubiquinol.
Conclusions
The in vitro studies reported here showed that the ubiquinol in commercially available ubiquinol supplements is relatively stable; only 8% of the ubiquinol in the capsules was oxidized, on average, to ubiquinone. A surprisingly high percentage of the ubiquinol was oxidized to ubiquinone in the 2.2 pH gastric juice; the group mean percentage of ubiquinone was nearly 54%. In the 8.2 pH of the simulated duodenal fluids, the group mean percentage of ubiquinol oxidized to ubiquinone was nearly 76%. If 1 outlier is removed from the 8.2 pH study, the group mean percentage of ubiquinol oxidized to ubiquinone increases to 84%.
The low pH in the gastric juice is characterized by the presence of many free hydrogen ions, and the elevated pH in the small intestines is characterized by the presence of many free hydroxyl ions. Both are destructive to the cell membranes. The ubiquinol, acting as an antioxidant in the gastric and small intestine juices, is converted to ubiquinone, the oxidized form of CoQ10. That the ubiquinol from supplements is converted to ubiquinone in the stomach and small intestines before absorption is a new finding.
Figure 1.
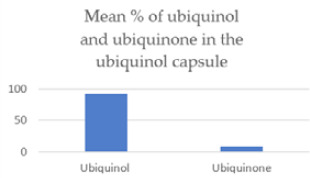
The ubiquinone content in the ubiquinol capsule was 91.5% ubiquinol and 8.5% ubiquinone, on average.
Figure 2.
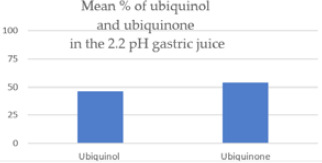
In an acidic environment simulating conditions in the stomach, 54% of the ubiquinol was oxidized to ubiquinone after 60 minutes at body temperature.
Figure 3.
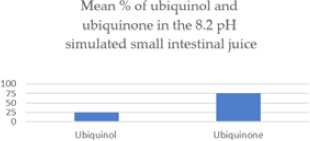
In a somewhat alkaline environment simulating conditions in the duodenum, 76% of the ubiquinol was oxidized to ubiquinone after 60 minutes at body temperature.
Figure 4.
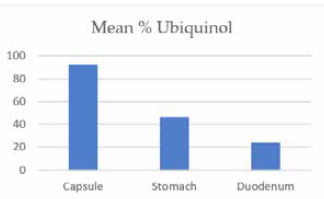
Ubiquinol as a percentage of total CoQ10 declines as the ingested ubiquinol passes through the gastrointestinal tract.
Acknowledgements
The author wishes to thank Richard Morrill, editor, q10facts.com, for assistance in the writing of this paper.
Footnotes
Conflict of Interest
None.
References
- 1.Linnane AW, Kios M, Vitetta L. Coenzyme Q(10)—its role as a prooxidant in the formation of superoxide anion/hydrogen peroxide and the regulation of the metabolome. Mitochondrion. 2007;7Suppl:S51-S61. [DOI] [PubMed] [Google Scholar]
- 2.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37:31-37. [DOI] [PubMed] [Google Scholar]
- 3.Judy WV, Stogsdill WW, Judy DS, Judy JS. Coenzyme Q10 Facts or Fabrications. NovaQ10. www.medicatrix.be/download/ubiquinone_ubiquinol_biodisponibilite.pdf. Accessed 11 August 2021.
- 4.Mantle D, Dybring A. Bioavailability of coenzyme Q10: an overview of the absorption process and subsequent metabolism. Antioxidants. 2020;9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992;1126(3):247-254. [DOI] [PubMed] [Google Scholar]
- 6.Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579-584. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Camacho JD, Bernier M, López-Lluch G, Navas P. Coenzyme Q10 supplementation in aging and disease. Front Physiol. 2018;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judy WV. Coenzyme Q10: The Substances That Powers Life, An Insider’s Guide. Haderslev, Denmark: Ny Videnskab. 2018; pp. 35-37. ISBN: 978-87-7776-186-7. [Google Scholar]
- 9.López-Lluch G, Del Pozo-Cruz J, Sánchez-Cuesta A, Cortés-Rodríguez AB, Navas P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition. 2019;57:133-140. [DOI] [PubMed] [Google Scholar]
- 10.Ayer A, Macdonald P, Stocker R. CoQ10 function and role in heart failure and ischemic heart disease. Annu Rev Nutr. 2015;35:175-213. [DOI] [PubMed] [Google Scholar]
- 11.Judy WV, Hall JH, Dugan W, Toth PD, Folkers K. Coenzyme Q10 reduction of Adriamycin cardiotoxicity. Folkers K, Yamamura Y. (eds.) Biomedical and Clinical Aspects of Coenzyme Q. Vol 4. Amsterdam, The Netherlands: Elsevier/North-Holland Biomedical Press. 1984; pp 231-241. [Google Scholar]
- 12.Okuyama H, Langsjoen PH, Hamazaki T, Ogushi Y, Hama R, Uchino H. Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Rev Clin Pharmacol. 2015;8:189-199. [DOI] [PubMed] [Google Scholar]
- 13.Langsjoen PH, Langsjoen AM. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin Pharmacol Drug Dev. 2014;3:13-17. [DOI] [PubMed] [Google Scholar]
- 14.Freye E, Strobel HP. The whole truth about Coenzyme Q10 you may not find elsewhere. Adv Complement Alt Med. 2018;2:107-116. [Google Scholar]


