Abstract
Background
Leprosy is a chronic infectious disease caused by Mycobacterium leprae, the annual new case detection in 2019 was 202,189 globally. Measuring endemicity levels and burden in leprosy lacks a uniform approach. As a result, the assessment of leprosy endemicity or burden are not comparable over time and across countries and regions. This can make program planning and evaluation difficult. This study aims to identify relevant metrics and methods for measuring and classifying leprosy endemicity and burden at (sub)national level.
Methods
We used a mixed-method approach combining findings from a systematic literature review and a Delphi survey. The literature search was conducted in seven databases, searching for endemicity, burden and leprosy. We reviewed the available evidence on the usage of indicators, classification levels, and scoring methods to measure and classify endemicity and burden. A two round Delphi survey was conducted to ask experts to rank and weigh indicators, classification levels, and scoring methods.
Results
The literature review showed variation of indicators, levels, and cut-off values to measure leprosy endemicity and/or burden. The most used indicators for endemicity include new case detection rate (NCDR), new cases among children and new cases with grade 2 disability. For burden these include NCDR, MB cases, and prevalence. The classification levels ‘high’ and ‘low’ were most important. It was considered most relevant to use separate scoring methods for endemicity and burden. The scores would be derived by use of multiple indicators.
Conclusion
There is great variation in the existing method for measuring endemicity and burden across countries and regions. Our findings contribute to establishing a standardized uniform approach to measure and classify leprosy endemicity and burden at (sub)national level, which would allow effective communication and planning of intervention strategies.
Author summary
Leprosy is a chronic infectious disease caused by Mycobacterium leprae. If left untreated, it could lead to deformity and resultant disabilities. The number of new cases has remained stagnant for the past decade. Additional interventions are required tailored to the level of endemicity and burden of an area. Currently, measuring and classifying endemicity and burden in leprosy lacks a standardized uniform approach, making program planning and evaluation difficult. We did a systematic literature search and Delphi survey, a method to elicit (group) opinion from a panel of experts, to take an inventory of indicators, classification levels and cut-offs that were used to measure and classify endemicity and burden of leprosy. This study highlights the variation in usage of indicators, levels, and cut-off values to determine the endemicity and burden of leprosy across regions and countries. There was agreement that endemicity and burden should be measured separately using multiple indicators, and that the classification levels ‘high’ and ‘low’ were most important. Our findings contribute to establishing a standardized uniform approach to measure and classify leprosy endemicity and burden at (sub)national level, which would allow effective communication and planning of intervention strategies.
Introduction
Leprosy is a chronic, infectious disease caused by the bacterium Mycobacterium leprae, which primarily affects the skin and peripheral nerves. [1] The incubation period is long and variable, it is assumed to be five years on the average but could be up to 20 years before occurrence of symptoms. [2] If left untreated, it could lead to deformity and resultant disabilities. [3]
Over the past decade, global leprosy control and elimination strategies have been based on specific indicators. The WHO target for global elimination of leprosy as a public health problem by the year 2000 was described in terms of the reduction of prevalence to a level below one case per 10 000 population. [4] The same target was later applied to national level to ensure elimination goal was attained by the end of 2005. In recognition of importance of timely detection of cases, the target was later changed to reduction of the rate of new cases with grade 2 disability (G2D) by at least 35% by the end of 2015 (‘Enhanced global strategy to further reduce the disease burden due to leprosy 2011–2015’).[5] The targets of London Declaration on Neglected Tropical disease (NTDs) for leprosy included global interruption of transmission and reduction of G2D in newly detected cases to less than one per million.[6] The Global Leprosy Strategy 2016–2020 aimed at achieving zero disability among children, reduction in global G2D to less than one per million and repealing of discriminatory laws.[7] A set of 13 core programmatic indicators was defined, viz. annual new case detection, annual new case detection rate, prevalence, prevalence rate, proportion of G2D, paediatric cases, MB cases, females and foreign-born cases among new cases, number of relapses reported in a year, treatment completion/cure rate of MB and PB cases and percentage of contacts screened among the household contacts registered. The indicators were related to case detection and ability to detect early, ongoing transmission and clinical presentations.
The global annual number of new cases detected in 2019 was 202,189. [8] There is optimism that scaling up of campaigns for early case detection in several countries and initiatives to cover populations at risk with chemoprophylaxis will accelerate attainment of a world without leprosy. [9] The new global leprosy strategy 2021–2030 will include prevention of leprosy by preventive chemotherapy of contacts and other high-risk groups. Many of these interventions require an accurate definition of endemicity. Leprosy is unevenly distributed globally, with India, Brazil, and Indonesia accounting for 80% of the total number of new cases. Furthermore, within countries, it is focalised, with pockets of high endemicity existing often at sub-district level, despite relatively low country estimates. To optimise the outcome of new/additional interventions, there is the need to appropriately channel effective and feasible strategies to the respective high and low endemic situations.
Currently, measuring and classifying endemicity and burden in leprosy lack a uniform approach. In some areas the metrics used may even change over the years, making program evaluation difficult. As a result, the assessment of leprosy endemicity or disease burden is not comparable over time and across countries and regions. Establishing a uniform approach to measure and classify of leprosy endemicity and burden at a subnational level is needed to understand the leprosy situation as accurately as possible so that leprosy control activities can be tailored accordingly. A uniform approach is important for allowing effective communication, creating a monitoring framework, and ultimately assessing elimination of leprosy. Moreover, it can assist in policy decisions regarding intervention strategies and resource allocation within in a country.
This study aimed 1) to systematically review the indicators, the classification levels and scoring methods used to measure and classify endemicity and/or burden at subnational level using routine programmatic data, and 2) to obtain a balanced account of their (relative) importance for measuring endemicity and burden of leprosy through a Delphi expert study.
Methods
Systematic review
A systematic literature search was conducted in January 31st, 2018 (and updated in July 13th, 2020) using Embase, Medline Ovid, Web of science, Cochrane central, Lilacs, Scielo and Google Scholar. The following search terms were used: ‘leprosy’, ‘Hansen’s disease’, ‘Mycobacterium leprae’, ‘endemicity’, ‘hyperendemic’, ‘burden’. The search strategy was adapted for each database. In addition, records were included via cross-references from published papers and reports. The complete search strategy can be found in S1 Table.
All studies that met the inclusion criteria were included with no restriction to study designs except for systematic reviews from 1990 onwards retrieved by this search. There were no restrictions on the language of the articles. Each article needed to meet the following inclusion criteria: 1) it described endemicity and/or burden of leprosy using leprosy epidemiologic indicators, and/or 2) it classified levels of endemicity and/or burden based on indicators cut-off values. We excluded studies on: 1) pathogenesis and histopathological lesion of leprosy, (ii) serological and molecular evaluation, (iii) symptoms and clinical manifestations (case reports), (iv) diagnostics and immunological reactions, (vi) drug/treatment regimens, and (vii) systematic reviews. The selection of studies was carried out by two independent investigators. Disagreements between investigators were resolved by consensus. We registered our systematic review protocol at PROSPERO (Registration number: CRD42019104933). [10]
We extracted and assessed the following data for each article: first author and year of publication, country, study setting, the indicators used to measure endemicity and/or burden, the classification levels used, and the scoring method used to classify endemicity and/or burden. The scoring method was further assessed based on: 1) use of any existing published scoring method, 2) indicator cut-off values to define endemicity and/or burden levels, and 3) whether a single, multiple or composite indicator scoring method was used. Data obtained from separate studies using the same indicators and scoring method were combined. The data was summarized in tables and frequency plots.
Delphi survey
A two round survey was conducted to collect and prioritise expert’s opinions about methods and indicators for classifying endemicity and burden of leprosy. Sampling of experts was purposeful, and this included professionals with a proven track record in the field of leprosy, including academic researchers (known experts in the field of leprosy), clinician national program managers/NGO staff, policy makers, and independent consultants. The surveys were sent out by email through Lime survey, an online survey tool (limesurvey.org). Experts were provided with a link and unique access code, which allowed blinding of the moderators when processing responses. In total, forty experts were approached. The first round was sent out in December 2018, and the second round in June 2019. The study did not need IRB approval since it did not concern medical (patient) research nor affected participants. The responses were analysed anonymously.
The first-round survey contained open questions regarding concepts of endemicity and burden of leprosy, indicators to measure endemicity and burden, levels for classification of endemicity and burden, and indicator scoring methods. It also covered general items including occupation, country at work, and years of experience in the field of leprosy (See S1 File).
For second-round survey, results of systematic review and first-round survey were used. Experts were asked to rank indicators (rank 1 to 10), and to score different usage of indicator values (five-point scale), the importance of indicator cut-off values (five-point scale), the relevance of proposed classification levels and scoring methods (three-point scale) (See S2 File).
Delphi questionnaire items were analysed individually. For the first-round, a simple content analysis was conducted, i.e., the essential details from each response were noted and then the frequencies of common expressions were recorded. The answers most frequently suggested were then used to form definitions/concepts for the second-round survey.
For the second-round, we identified the most important indicators based on two criteria: 1) mean ranking scores (rank 1 to 5 were given 5 to 1 point, respectively), and 2) the percentage of respondents that ranked a particular indicator in the Top-3. Statements about the usage of indicator values and indicator cut-offs were evaluated based on: 1) mean scores (strongly agree = 4, agree = 3, neutral = 2, disagree = 1, strongly disagree = 0), and 2) the percentage that agreed with the statement. The relevance of various classification levels and methods of indicator scoring were assessed based on: 1) mean scores (highly relevant = 2, relevant = 1, not relevant = 0), and the percentage that scores at least “relevant” for a particular item.
Results
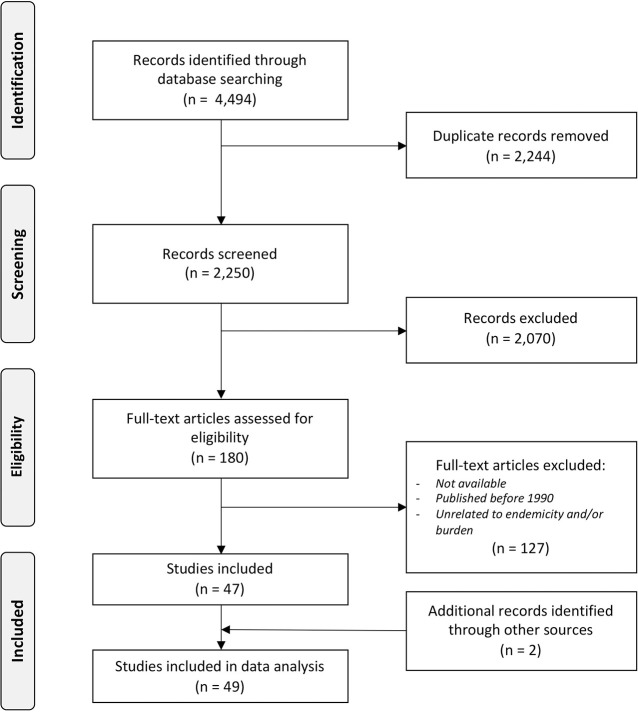
The systematic literature search in all databases yielded 4,594 articles, of which 2,244 were duplicates. The remaining 2,250 articles were screened by title and abstract, of which 180 articles were eligible. After the full-text analysis, we included 47 articles. 23 from Brazil [11–33], 6 from India [34–39], 3 from China [40–42], 2 from Bangladesh [2,43] and Comoros [44,45]. One from Cambodia [46], Sri-Lanka [47], Cameroon [48], Venezuela [49], Bangladesh and Thailand [50], Indonesia [51], Argentina [52], and Uganda [53]. Three publications focussed on the global leprosy burden. [54–56] In addition, two reports (grey literature) were identified through other sources (Fig 1). [57,58]
Fig 1. PRISMA Flow Diagram of systematic literature review.
The response rate of the Delphi survey was 65% (26 out of 40) in the first round, and 69% (18 out of 26) in the second round. Participants of the Delphi survey included researchers (n = 6; 23%), clinician (n = 7; 27%), NGO staff (n = 7; 27%), policy makers (n = 1; 4%), and others such as consultants (n = 5; 19%). Twenty seven percent of the participants worked between 1 and 14 years in the field of leprosy, 50% between 15 and 30 years, and 23% more than 30 years. Together they worked in more than 40 endemic countries. Detailed results of round 1 and 2 can be found in S1 and S2 Files.
Endemicity and burden of leprosy
In the systematic review, endemicity and burden of leprosy were described by the same indicators, indicating that there was no clear/sharp distinction between both concepts. After one round of Delphi survey, a great majority agreed that endemicity and burden of leprosy were two different concepts (96%) (See S1 File). Endemicity was associated with indicators reflecting the number of cases in an area, while burden of leprosy has a broader interpretation that primarily focused on the number of people living with disability and societal consequences, such as stigma. Besides, the concept of endemicity and burden, other concepts were suggested, including hidden endemicity, economic burden or cost of leprosy, and quality of leprosy services.
Existing methods for measuring and classifying endemicity and burden of leprosy
Two existing classification methods for endemicity were identified in the systematic review. The first method is the Leprosy Burden Scale (LBS) developed by the WHO Regional Office for Africa. This method provided cut-off scores for nine indicators into three endemicity levels: high, medium, and low (See Table 1). The endemicity level of each indicator was then combined into a composite score reflecting the overall endemicity in an area. The cut-off value for each indicator ranges from 0 = low endemicity level, 1 = medium level, and 2 = high level. Total sum of these values and endemicity level for the nine indicators is used to determine the overall endemicity level of an area. This method was used by one study from Cameroon. [48]
Table 1. Existing frameworks for classifying endemicity and burden using pre-defined levels, indicators, and cut-offs.
| WHO-AFRO Leprosy burden scale | Brazilian method | |||||||
|---|---|---|---|---|---|---|---|---|
| Indicators | High | Medium | Low | Hyper-endemic | Very High | High | Medium | Low |
| NCDR per 100,000 population | >20 | 10–20 | <10 | >40.00 | 20.00–39.99 | 10.00–19.99 | 2.00–9.99 | <2.00 |
| NCDR in children 100,000 population | >20 | 10–20 | <10 | >10.00 | 5.00–9.99 | 2.50–4.99 | 0.50–2.49 | <0.50 |
| Prevalence rate per 10,000 | >2 | 1–2 | <1 | 20.0 | 10.0–19.99 | 5.0–9.9 | 1.0–4.9 | <1.0 |
| Proportion G2D among new cases per 100,000 population | >20 | 10–20 | <10 | NS | NS | ≥10 | 5–9.9 | <5 |
| Proportion MB among new cases | <50 | 50–75 | 76–100 | Indicator not included | ||||
| Proportion of females among new | <40 | >60 | 40–60 | Indicator not included | ||||
| Proportion of PB / MB cases cured in the year with degree of incapacity II* | Indicator not included | - | - | ≥10 | 5–9.9 | <5 | ||
| Proportion of G2D cases cured at time of discharge | Indicator not included | - | - | ≥10 | 5–9.9 | <5 | ||
| Detection (new cases) in regions | >100 | 21–100 | 0–20 | Indicator not included | ||||
| Detection (new cases) in health district | >20 | 11–20 | 0–10 | Indicator not included | ||||
| Prevalence/ detection (p/d) | >2 | 1–2 | <1 | Indicator not included | ||||
| G2D rate per 100,000 population | >1 | 0.5–1 | <0.5 | Indicator not included | ||||
NCDR = new case detection rate; G2D = grade-2 disability; MB = multibacillary leprosy; PB = paucibacillary leprosy
The second classification method was published by the Brazilian Ministry of Health (Brazilian method) in 2002, and updated in 2009. [58] The updated classification method proposed six epidemiological indicators to classify endemicity, and in addition five operational indicators to monitor the quality of actions and services (See Table 1). Each epidemiologic indicator had cut-off values for five endemicity levels: hyper-endemic, very high, high, medium, and low. The endemicity level of an area was based on one or more of the proposed epidemiological indicators, and endemicity level was determined separately per indicator. In total, 22 studies from Brazil followed the Brazilian guidelines for classifying endemicity (See Table 2).
Table 2. Overview of usage of indicator, cut-offs, and levels for endemicity classification.
| Source | Study setting | Endemicity/ Burden | Scoring method | Indicators | Classification levels | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyperendemic | Very high | High | Medium | Low | Undefined endemic | Non-endemic | |||||
| Ajalla et al. 2016 [17] | Brazil, State-level | Endemicity | Scoring single indicator Brazil method | New case detection rate | - | 20–39 per 100,000 (data: 29.5 per 100,000) | - | - | - | - | - |
| Alencar et al. 2012a [24] | Brazil, state-level | Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | >40.00 per 100,000 | - | - | - | - | - | - |
| New case detection rate in children under 15 years | - | - | - | <2.5 per 100,000 | - | - | - | ||||
| Alencar et al. 2012b [22] | Brazil, municipality-level | Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | - | - | 95 per 100,000b | - | - | - | - |
| New case detection rate in children under 15 years | - | - | 28.4 per 100,000b | - | - | - | - | ||||
| Rate with grade 2 disability | - | - | 4.4 per 100,000b | - | - | - | - | ||||
| Anchieta et al. 2016 [29] | Brazil, State-level | Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | 51 per 100,000 | - | - | - | - | - | - |
| New case detection rate under 15 years | 17.5 per 100,000 | - | - | - | - | - | - | ||||
| Barbosa et al. 2018 [30] | Brazil, Muncipality | Endemicity & burden | Scoring multiple indicators separately Brazil method | New case detection rate | >40.00 per 100,000 | 20.00–39.99 per 100,000 | 10.00–19.99 per 100,000 | 2.00–9.99 per 100,000 | <2.00 per 100,000 | - | - |
| New case detection rate in children under 15 years | >10.00 per 100,000 | 5.00–9.99 per 100,000 | 2.50–4.99 per 100,000 | 0.50–2.49 per 100,000 | <0.50 per 100,000 | - | - | ||||
| New case with grade 2 disability at time of diagnosis | - | >10.00 per 100,000 | 5.00–9.99 per 100,000 | 2.00–4.99 per 100,000 | 0.1–1.99 per 100,000 | - | - | ||||
| Bernardes et al. 2017 [14] | Brazil, state-level |
Endemicity | Scoring multiple indicators separately | Prevalence rate | - | - | - | 4.4 per 10,000 | 0.73 per 10,000 | - | - |
| New case detection rate | - | - | - | 4.76 per 100,000 | - | - | - | ||||
| Brito et al. 2015 [20] | Brazil, state-level | Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | - | 20.00–39.99 per 100,000 | - | - | - | - | - |
| Proportion of leprosy cases with G2D at the time of diagnosis | - | - | >10%; | 5%– 9.9% | <5% | - | - | ||||
| Cunha et al. 2015 [19] | Brazil, State level | Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | >40.00 per 100,000 | 20.00–40 per 100,000 | 10.00–20 per 100,000 | - | - | - | - |
| New case detection rate in children under 15 years | >10.00 per 100,000 | 5.00–10 per 100,000 | - | - | - | - | - | ||||
| Da Silva et al. 2010 [26] | Brazil, town | Endemicity | Scoring single indicator Brazil method | New case detection rate | 40 per 10,000 | - | - | - | - | - | - |
| De Oliveira et al. 2012 [23] | Brazil, municipality | Endemicity | Scoring single indicator Brazil method | New case detection rate | >40 per 100,000 | - | - | - | - | - | - |
| De Sousa et al. 2020 [31] | Brazil, district | Endemicity | Scoring single indicator Brazil method | New case detection rate | >40.00 per 100,000 | 20.00–39.99 per 100,000 | 10.00–19.99 per 100,000 | 2.00–9.99 per 100,000 | <2.00 per 100,000 | - | - |
| De Souza et al. 2018 [33] | Brazil, State-level | Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | >40 per 100,000 | 20–40 per 100,000 | 10–20 per 100,000 | 2–10 per 100,000 | <2 per 100,000 | - | - |
| New case detection rate in children under 15 years | >10.00 per 100,000 | 5.00–9.99 per 100,000 | 2.50–4.99 per 100,000 | 0.50–2.49 per 100,000 | <0.50 per 100,000 | - | - | ||||
| Rate with grade 2 disability at time of diagnosis | > 8 per 100,000 | 4–8 per 100,000 | 2–4 per 100,000 | 1–2 per 100,000 | < 1 per 100,000 | - | - | ||||
| Fontes et al. 2017 [11] | Brazil, state-level | Endemicity | Scoring single indicatorBrazil method | New case detection rate | >4.0 per 10,000 | - | - | - | - | - | - |
| New case detection rate in children under 15 years | - | 0.5–1.0 per 10,000 | - | - | - | - | - | ||||
| Freitas et al. 2016 [18] |
Brazil, state-level |
Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | >40.00 per 100,000 | 20.00–39.99 per 100,000 | 10.00–19.99 per 100,000 | 2.00–9.99 per 100,000 | <2.00 per 100,000 | - | - |
| New case detection rate in children under 15 years | >10.00 per 100,000 | 5.00–9.99 per 100,000 | 2.50–4.99 per 100,000 | 0.50–2.49 per 100,000 | <0.50 per 100,000 | - | - | ||||
| Rate of new cases with G2D at time of diagnosis | - | ≥4 cases | >0–4 cases | - | 0 cases | - | - | ||||
| Freitas et al. 2017a [15] | Brazil, state-level | Endemicity | Scoring single indicator Brazil method | New case detection rate in children under 15 years | >10.00 per 100,000 (data: 22.7 per 100,000) | 5.00–9.99 per 100,000 | 2.50–4.99 per 100,000 | 0.50–2.49 per 100,000 | <0.50 per 100,000 | - | - |
| Freitas et al. 2017b [13] | Brazil, municipality level | Endemicity | Scoring single indicator Brazil method | New case detection rate | > 40 per 100,000 | - | - | - | - | - | - |
| Ignotti et al. 2007 [28] | Brazil, State-level | Endemicity | Scoring single indicator | Trend in proportion of new cases with a single lesion at time of diagnosis | 20.3–49.1% (time series) | - | - | - | - | - | - |
| Imbiriba et al. 2008 [27] | Brazil, state-level | Endemicity |
Scoring multiple indicators separately Brazil method | Prevalence rate | >20.0 per 10.000 | 10.0–20.0 per 10.000 | 5.0–10.0 per 10.000 | 1.0–5.0 per 10.000 | <1.0 per 10,000 | - | - |
| New case detection rate | >4.0 per 10,000 | 2.0–4.0 per 10,000 | 1.0–2.0 per 10,000 | 0.2–1.0 per 10,000 | <0.2 per 10,000 | - | - | ||||
| New case detection rate in children under 15 years | 1.0 per 10,000 | 0.5–1.0 per 10,000 | 0.25–0.5 per 10,000 | 0.05–0.25 per 10,000 | <0.05 per 10,000 | - | - | ||||
| Moreira et al. 2014 [21] | Brazil, State level | Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | >40.00 per 100,000 | 20.00–39.99 per 100,000 | 10.00–19.99 per 100,000 | 2.00–9.99 per 100,000 | <2.00 per 100,000 | - | - |
| New case detection rate in children under 15 years | >10.00 per 100,000 | 5.00–9.99 per 100,000 | 2.50–4.99 per 100,000 | 0.50–2.49 per 100,000 | <0.50 per 100,000 | - | - | ||||
| Proportion of leprosy cases with grade 2 disability at diagnosis | - | - | >10% | 5–9.9% | <5% | - | - | ||||
| Prevalence rate | - | - | - | 2.5 per 10,000 | - | - | - | ||||
| Pereira et al. 2011 [25] | Brazil, municipality | Endemicity | Scoring multiple indicators separatelyBrazil method | New case detection rate | >40.00 per 100,000 | 20.00–39.99 per 100,000 | 10.00–19.99 per 100,000 | 2.00–9.99 per 100,000 | <2.00 per 100,000 | - | - |
| New case detection rate in children under 15 years | >10.00 per 100,000 | - | - | - | - | - | - | ||||
| Pereira et al. 2019 [32] | Brazil, municipality | Endemicity | Scoring multiple indicators separately Brazil method | New case detection rate | >40.00 per 100,000 | - | - | - | - | - | - |
| Proportion of children under 15 years | 7.2% | - | - | - | - | - | - | ||||
| Proportion of leprosy cases with grade 2 disability | - | - | 7.0% | - | - | - | - | ||||
| Santos et al. 2016 [16] | Brazil, municipality-level | Endemicity | Scoring single indicator Brazil method | New case detection rate in children under 15 years | - | 5.00–9.99 per 100,000 |
- | - | - | - | - |
| Aggarwal et al. 2010 [36] | India, district- & community-level | Burden | Scoring single indicator | New case detection rate (district-level) | - | - | 24 per 10,000 | - | - | - | - |
| New case detection rate (community-level) | - | - | 5 per 10,000 | - | 2 per 10,000 | - | - | ||||
| Govindharaj et al. 2019 [39] | India, district-level | Endemicity | Scoring multiple indicators separately | Prevalence rate | - | - | 3.52 per 10,000 | - | - | - | - |
| New case detection rate | - | - | 47.20 per 100,000 | - | - | - | - | ||||
| Kumar et al. 2007 [37] | India, district-level |
Endemicity | Scoring multiple indicators separately | Prevalence rate | - | - | - | - | - | 16.4 per 10,000 |
- |
| Incidence rate | - | - | - | - | - | 6.2 per 10,000 person years |
- |
||||
| Kumar et al. 2018 [38] | India, state-level | Endemicity | Scoring single indicator | New case detection rate | - | - | 1.28 per 10,000 | - | 0.21 per 10,000. | - | - |
| Mohite et al. 2013 [35] | India, district-level | Endemicity | Scoring single indicator | Prevalence rate | - | - | - | - | - | >1 per 10,000 | - |
| Murugaiyan et al. 2017 [34] | India, district level | Burden | Scoring single indicator | New case detection rate | - | - | >10 per 100,000 | - | - | - | - |
| Dharmshaktu 2020 [56] | Global, country-level | Endemicity | Scoring multiple indicators separately | Prevalence rate | - | - | >1 per 10,000 | - | - | - | - |
| New case detection rate | - | - | >9 per 100,000 | - | - | - | - | ||||
| Penna et al. 2012 [54] |
Global, country-level |
Endemicity | Scoring multiple indicators separately | Prevalence rate | - | - | >1 per 10,000 | - | - | - | - |
| New case detection rate | - | - | >9 per 100,000 | - | - | - | - | ||||
| WER, WHO 1998 [55] | Global, country-level | Endemicity | Scoring single indicator | Prevalence rate | 5–15 per 10,000 | - | - | - | - | 3–5 per 10,000 1–3 per 10,000 |
- |
| Basel et al. 2014 [2] | Bangladesh, district-level | Burden | Scoring single indicator | Incidence rate | - | - | 3.7 per 10,000 person years at risk | - | - | - | - |
| Blok et al. 2018 [43] | Bangladesh, regional-level | Endemicity | Scoring single indicator | New case detection rate | - | - | 25 per 100,000 | 5 per 100,000 | 1 per 100,000 | - | - |
| Richardus et al. 2005 [50] | Bangladesh and Thailand, district- & province-level | Endemicity | Scoring single indicator | New case detection rate (average over 10 years) | - | - | 50 per 100,000 | - | 1.5 per 100,000 | - | - |
| Chen et al. 2007 [41] | China, province-level | Endemicity | Scoring single indicator | Prevalence rate per 100,000 | - | - | - | - | - | - | <1/100,000 (referred as: dying-out) |
| Chen et al. 2018 [42] | China, province-level | Endemicity | Scoring single indicator | New case detection rate | - | - | 1.13 per 100,000 | - | - | - | - |
| Shen et al. 2010 [40] | China, province-level | Endemicity | Scoring single indicator | Case detection rate | - | - | - | - | - | >0.2 per 100,000 | <0.2 per 100,000 |
| Hasker et al. 2017 [44] | Comoros, island | Endemicity | Scoring single indicator | Incidence rate | - | - | 7.4 per 10,000 | - | - | - | - |
| Ortuno-Gutierrez et al. 2019 [45] | Comoros, island | Endemicity | Scoring single indicator | Incidence rate | - | - | 5–10 per 10,000 | - | - | - | - |
| Odriozola et al. 2017 [52] | Argentina, province-level | Burden | Scoring single indicator | New case detection | - | - | >100 cases | - | - | - | - |
| Furst et al. 2018 [46] | Cambodia, country-level | Endemicity | Scoring single indicator | New case detection rate | - | - | - | - | 0.1 per 100,000 | - | - |
| Tabah et al. 2016 [48] | Cameroon, regional-level | Endemicity & burden | Composite score with multiple indicators WHO-AFRO LBS method | Prevalence rate | - | - | >2 per 10,000 | 1–2 per 10,000 | <1 per 10,000 | - | - |
| New case detection rate | - | - | >20 per 100,000 | 10–20 per 100,000 | <10 per 100,000 | - | - | ||||
| % MB among new cases | - | - | <50% | 50–75% | 76–100% | - | - | ||||
| % children among new cases | - | - | >20% | 10–20% | <10% | - | - | ||||
| % G2D among new cases | - | - | >20% | 10–20% | <10% | - | - | ||||
| % females among new cases | - | - | <40% | >60% | 40–60% | - | - | ||||
| Prevalence/detection ratio | - | - | >2 | 1–2 | <1 | - | - | ||||
| Rate with grade 2 disability | - | - | >1 per 100,000 | 0.5–1 per 100,000 | <0.5 per 100,000 | - | - | ||||
| Bakker et al. 2002 [51] |
Indonesia, islands | Endemicity | Scoring multiple indicators separately | Prevalence rate | - | - | 195 per 10,000 | - | - | - | - |
| Dabrera et al. 2016 [47] |
Sri Lanka, district-level |
Endemicity | Scoring multiple indicators separately | New case detection rate | - | - | 205 per 10,000 | - | - | - | - |
| Prevalence rate | - | - | 511 per 10,000 | - | - | - | - | ||||
| Child prevalence rate | - | - | 1832.4 per 100,000 | - | - | - | - | ||||
| Aranzazu et al. 2012 [49] | Venezuela, community-level | Endemicity | Scoring multiple indicators separately | Prevalence rate | 3.4 per 10,000 | - | - | - | - | - | - |
| Case detection rate | > 4 per 100 | ||||||||||
a Table provides an overview of scoring method and indicators used to describe endemicity and/or burden. Only indicators with cut-off values are presented. Note: 2 out of 47 articles did not provide classification cut-off: Araujo et al. 2017 [12] and Aceng et al 2019 [53]
b classified as high transmission areas.
Indicators used for measuring endemicity and burden
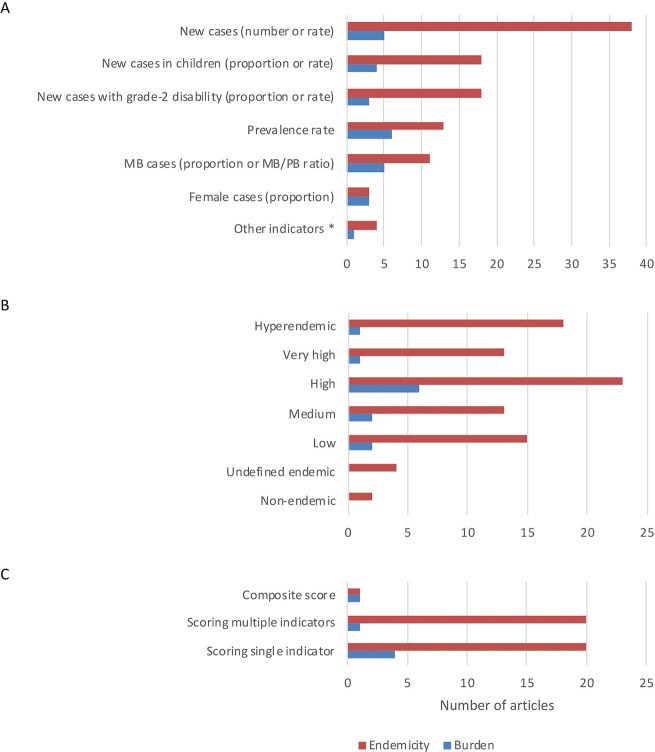
In the literature, nine leprosy epidemiological indicators were used to determine endemicity and burden of leprosy in an area (See Fig 2A). The NCD (number and/or rate) was the most used indicator to measure endemicity (n = 38), followed by child cases (n = 18) and new cases with G2D (n = 18). To measure burden, the prevalence rate was the most used indicator (n = 6), followed by NCD (n = 5), MB cases (n = 5), child cases (n = 4) and new cases with G2D (n = 3).
Fig 2.
Indicators (A), classification levels (B) and scoring methods (C) used to classify endemicity and burden of leprosy. The bars represent the number of articles that used a particular indicator, classification level, or scoring method to classify endemicity (red) or burden (blue). An article could mention multiple indicators and classification levels. Other indicators include cure rate (2; endemicity), new cases with dimorphic clinical form (2; endemicity), trend in proportion of new cases with a single lesion at time of diagnosis (1; endemicity), and prevalence/detection ratio (1; burden).
The second round of the Delphi survey showed that most respondents (rank score = 4.11) ranked NCD (number and/or rate) as the most relevant indicator to classify endemicity, which was followed by NCDR in children (rank score = 2.00), and G2D among new cases (1.17) (See Table 3). To measure burden of leprosy, the top 3 ranked indicators include: prevalence of people with disabilities due to leprosy (score = 1.63), NCD (number and/or rate) (1.59), and number of reactions, neuritis, and lasting disabilities (1.13). Generally, the ranking scores of all indicators are below 2, indicating that there is no clear agreement about the choice of indicators for burden. Other indicators, such as quality of control programme and economic measures and treatment cost, but were not considered a priority for scoring endemicity and burden. A full list of all indicators can be found in S2 File.
Table 3. Results of Delphi survey.
| Endemicity (N = 18) | Score | Burden (N = 16) | Score | ||
|---|---|---|---|---|---|
| Top indicators | Range 0–5 * | % Rank Top 3 | Top indicators | Range 0–5 * | % Rank Top 3 |
| New case detection (number and/or rate) | 4.11 | 88.9 | Prevalence of people with disabilities due to leprosy | 1.63 | 37.5 |
| New cases detected among children (number and/or rate) | 2.00 | 44.4 | New case detection (number and/or rate) | 1.63 | 31.3 |
| Proportion of child cases among total new cases detected | 1.22 | 27.8 | Number of reactions, neuritis & lasting disabilities | 1.50 | 31.3 |
| Proportion of G2D cases among total new cases detected | 1.17 | 22.2 | New cases detected with G2D (number and/or rate) | 1.13 | 18.8 |
| New cases detected with G2D (number and/or rate) | 0.89 | 16.7 | Prevalence (number and/or rate) | 1.00 | 18.8 |
| New case detection trend | 0.78 | 16.7 | Proportion of G2D cases among total new cases detected | 0.94 | 18.8 |
| Disability-adjusted life years | 0.94 | 18.8 | |||
| Usage of Indicator value | Range: 0–4** | % Agree | Usage of Indicator value | Range: 0–4 | % Agree |
| Single year value and average value of past three/five/ten years | 3.3 | 83.3 | Single year value and average value of past three or five years | 3.1 | 75.0 |
| Average of past three years | 2.4 | 44.4 | Average of past three or five years | 3.1 | 87.5 |
| Average of past five years | 2.4 | 44.4 | Single-year value | 2.0 | 31.3 |
| Average of past ten years | 2.2 | 50.0 | |||
| Single-year value | 1.8 | 38.9 | |||
| Classification levels | Range: 0–2*** | % Relevant | Classification levels | Range: 0–2** | % Relevant |
| High | 1.39 | 94.4 | High | 1.50 | 93.8 |
| Low | 1.39 | 94.4 | Low | 1.38 | 87.5 |
| Non-endemic | 1.33 | 83.3 | No burden | 1.25 | 75.0 |
| Medium | 1.17 | 77.8 | Very High | 1.06 | 68.8 |
| Hyper | 0.89 | 61.1 | Medium | 0.94 | 62.5 |
| No specific level (i.e. endemic/ non-endemic) | 0.72 | 50.0 | |||
| Indicator cut-offs | Range: 0–4** | % Agree | Indicator cut-offs | Range: 0–4 | % Agree |
| Usage of indicator cut-off values is essential | 2.7 | 57.1 | Usage of indicator cut-off values is essential | 2.8 | 73.3 |
| Indicator cut-off values should be standardized | 2.9 | 78.6 | Indicator cut-off values should be standardized | 2.3 | 60.0 |
| Preferred Method of Indicator Scoring | Range: 0–2*** | % Relevant | Preferred Method of Indicator Scoring | Range: 0–2** | % Relevant |
| Score of multiple relevant indicators ^ | 1.24 | 88.2 | Score of multiple relevant indicators ^ | 1.3 | 86.7 |
| Composite score ^^ | 1.18 | 82.4 | Composite score ^^ | 1.0 | 80.0 |
| Score of a single (most relevant) indicator ^^^ | 0.94 | 70.6 | Score of a single (most relevant) indicator ^^^ | 0.8 | 66.7 |
* based on ranking scores: rank 1 to 10; rank 1 (5 pts), 2 (4 pts), 3 (3pts), 4 (2pts), 5 (1pt), 6–10 (0 pts)
** scoring based on five categories: strongly agree (4), agree (3), neutral (2), disagree (1), strongly disagree (0)
*** scoring based on three categories: highly relevant (2); relevant (1), and not relevant (0)
^ i.e. multiple classification level: one for each indicator
^^ i.e. one overall classification level based on multiple relevant indicators
^^^ i.e. one overall classification level
Usage of indicator values
Only single-year indicator values have been solely used to define endemicity and burden of leprosy in published literature. The Delphi survey indicated that single year value was least favoured while trends of indicator and values representing multiple years was preferred to measure endemicity and burden (See Table 3). There was no consensus about the number of years to be considered for indicator values. Moreover, trend of indicators was preferred over average of years.
Classification levels of endemicity and burden
The classification level high is the most used in the literature for endemicity (n = 23) and burden (n = 6), followed by the levels: hyperendemic, low, medium, very high, undefined endemic, and non-endemic (See Fig 2B). Twenty-two studies use a predefined level taken from the Brazilian method, and one study followed the LBS classification. Studies without using an existing method used varying levels, including high, low, undefined endemic and non-endemic (See Table 2).
Based on the results of the second Delphi survey, the levels high, low, non-endemic and medium were considered most relevant for classifying endemicity (See Table 3). Hyperendemic and the use of no specific level were least relevant (i.e., score < 1). For classifying the burden of leprosy, high, low, no burden and very high were considered most relevant. In contrast to endemicity, the medium level was least relevant for classifying burden of leprosy.
Scoring endemicity and burden
There was variability in the usage of indicators, levels of endemicity, and cut-off values in majority of the studies. Overall, 45 out of 47 studies classified endemicity and/or burden-based indicator cut-off values, of which 29 studies used known pre-defined cut-off values from the WHO-Afro LBS, the Brazilian method or self-defined, and 16 studies classified endemicity and/or burden based on the actual score of the indicator i.e., data-driven scores. One study used a composite score, while the other studies scored single (endemicity n = 20; burden n = 4) or multiple (endemicity n = 20; burden n = 1) indicators separately (See Fig 2C and Table 2). Two studies only mentioned the indicators used to classify endemicity/burden of the country of study but did not provide a classification cut-off. [22,52]
Respondents of the Delphi survey indicated that indicator cut-off values for scoring endemicity and burden are essential, and that these cut-off values should be standardized worldwide (See Table 3). There was no agreement about the actual proposed cut-off values of indicators to classify endemicity or burden of leprosy (See S1 File). Scoring of multiple indicators separately to classify endemicity and burden was considered most relevant, followed by using a composite score. Classification based on a single most relevant indicator was considered least relevant for both endemicity and burden. Respondents also highlighted that trend values of indicators are most useful for a scoring method, and that the total burden is generally unknown. For a burden scoring method, it was suggested that the prevalence of disability is more informative than incidence, and the discrimination and/or social exclusion and financial measures. Generally, it is important to ensure the reliability and quality of the control programme.
Discussion
This study shows that there is great variation in the existing method for classification of endemicity and burden across countries and regions and in classification levels and indicator cut-off values. We also observed this variation among studies that do not adhere to an existing method. Using a two-round Delphi survey, we identified relevant indicators, usage of indicators, classification levels, and scoring methods to inform a standardized method to classify endemicity and burden of leprosy.
The NCDR, child case rate and G2D rate were the most important indicators to classify endemicity at national and subnational level. Prevalence did not fall into the top three of indicators both in systematic review and Delphi survey. There have been remarkable changes in the use of leprosy indicators since the 1990s. [20] During the 1990s, prevalence was the indicator of choice for monitoring the trend of leprosy as the elimination as a public health problem was defined in terms of registered prevalence. Later in the 2000s, the indicator preference shifted from prevalence rate to NCDR [54] due to the criticism that prevalence was affected by changes in case definitions or treatment duration and other operational factors. [18,20,59] Also, with the WHO target focusing on the reduction of new child cases with G2D and G2D (new case) rate, we observe a consensus of using indicators that indicate recent transmission and potential delays in diagnosis, respectively.
The top indicators for classifying burden of leprosy were new case detection, prevalence of disability, number of patients with reactions, neuritis and lasting disabilities, cases with G2D, prevalence and disability-adjusted life years. Although the systematic review and Delphi survey to an extent agreed, there was no clear consensus in the Delphi panel on which of these indicators is most relevant to reflect burden. Our findings also show a lot of overlap with indicators used to measure endemicity, which was both evident form the systematic review and survey. One explanation is the lack of full conceptual clarity of endemicity and burden. Although there was consensus that endemicity and burden are different concepts, the definitions provided by the respondents did not cover all aspects of the concept burden. Endemicity has been defined to describe frequency of disease events in which new cases of disease can arise without importation of infection from outside.[60] Burden of disease considers the total consequences of a disease in a community, including health (e.g. disabilities), social and environmental aspects and cost to an individual and society.[61]
Another possible explanation is that most burden indicators are not routinely collected. Only the number of new cases and cases with grade 2 disability at diagnosis are available in most countries. These indicators are not sufficient to reflect the full notion of burden, as leprosy is known to have a burden beyond physical impairment, including poor mental health, stigma and social participation restrictions of patients. [62] These consequences can be life-long and are likely to remain even after elimination of transmission. To understand the burden, more comprehensive data are needed including grade-1 disability, stigma, mental health, and social participation (e.g., social capital, education, and employment). These data are also necessary to improve calculations of common-used burden measures, such as Disability-Adjusted Life Years (DALYs), and cost. Currently, the disability weights for leprosy to calculate DALYs only cover physical impairment (grade-1 and 2) and do not include other consequences such as mental health. Current weights have therefore been argued to underestimate the burden of leprosy. [63,64]
Our Delphi panel favoured using the value of multiple years or trends over the use of single-year values only to measure endemicity and burden. Single year values may vary on account of random fluctuation in transmission, local migration, operational changes/factors. The moving average was also suggested as a potential solution to overcome these variations. Using trends was considered to be more informative. This is especially true for monitoring changes over time, which cannot be done based on single-year figures.
Regarding classification levels of endemicity and burden, high and low were generally most used or considered most relevant. These levels were considered most relevant for operational purposes, i.e., targeting interventions. The level non-endemic or no burden were also considered useful because it may indicate that effort should be focused on surveillance rather than services. However, this level can only be reached when for a certain period (e.g., 5 years) no new cases have been detected. There is great variability in the use of cut-off values to classify endemicity and burden. For example, the range of cut-off values used for high and low endemicity based on the NCDR were set as: ≥10 to >100 per 100,000 population for a high level, and <1 to <20 per 100,000 for a low level (See Table 2 and S1 File). This is partly influenced by the setting context, as the rates may vary greatly across countries and regions. A certain rate may be considered high in one country or region but low in another, making interpretation of cut-off values more complex. There is clearly room to standardize cut-off values for relevant indicators, which may be addressed by future research.
Nevertheless, respondents to our survey indicated to have a preference for a standardized approach worldwide, since this would support monitoring and policy decision-making. The discrepancies between countries and regions are mainly due to the population size. For example, small populations may lead to high rates, while the absolute number of cases might be low. Therefore, a uniform approach should also consider meaningful population sizes and absolute number of new cases.
There are currently two existing scoring methods for classification of endemicity and burden (WHO-AFRO LBS and the Brazilian method). One of these two proposes a composite score, while the other method suggest scoring of multiple indicators independently. Scoring multiple indicators were also considered most relevant from the Delphi study. A single indicator is not sufficient to reflect the overall endemicity and burden unless it is a composite score. A composite score was considered relevant only if data for all indicators needed for composite scoring are available from routine data collection. Another consideration is the practical utility as a composite score is more complex. The LBS composite score has been only been used once to date, which may be due to these reasons. [48] An example of a composite indicator currently part of the WHO Road map 2021–2030 is ‘the number of people requiring interventions for NTDs’. To calculate this for a disease like leprosy, data would need to include people requiring preventive chemotherapy, leprosy medicines, treatment of reactions and nerve damage, surgical treatment, and interventions to improve mental wellbeing, social participation, and inclusion. While this presents a major challenge, it shows that the discussion on measuring endemicity and burden more relevant than ever.
To the best of our knowledge, this was the first systematic review and Delphi survey that focused on measuring and classifying endemicity and burden of leprosy. The literature search was performed in seven databases, and a fair amount of experts representing most important leprosy endemic countries participated in the Delphi survey. However, this study has limitations that may affect the generalizability of our findings. First, the systematic review consist predominantly of studies from Brazil. The main reason for this is that Brazil is the only country that has a formal published scoring method for endemicity and burden. Second, as treatment outcomes are not always referred as endemicity and/or burden, we might have missed some relevant publications in our literature search, especially regarding indicators. Another limitation is the decline in response rate from 65% in the first to 45% in the second round of the Delphi survey, which may infringe the generalizability of the ranking agreement in the second round. However, the results of the survey were validated by triangulation of the findings from the systematic review which served as a complementing research approach.
Conclusion
There is great variation in the existing method for measuring endemicity and burden across countries and regions. Creating a standardized and uniform scoring framework for endemicity and burden of leprosy is important to allow effective communication, planning and monitoring of intervention strategies, as well as comparability across countries and regions. This study highlights key findings around relevant indicators, classification levels, and scoring methods, which could guide the development of a (new) standardized uniform approach to measure and classify leprosy endemicity and burden at (sub)national level.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Wichor M. Bramer from the Erasmus MC Medical Library for developing the search strategies for the bibliographic databases, and all experts who participated in the Delphi survey.
Data Availability
All data are in the manuscript and in supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Lastória JC, de Abreu MAMM. Leprosy: Review of the epidemiological, clinical, and etiopathogenic aspects—Part 1. An Bras Dermatol. 2014;89(2):205–18. doi: 10.1590/abd1806-4841.20142450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basel P, Pahan D, Moet FJ, Oskam L, Richardus JH. Leprosy incidence: six years follow-up of a population cohort in Bangladesh. Lepr Rev. 2014;85(3):158–69. [PubMed] [Google Scholar]
- 3.Franco-Paredes C, Rodriguez-Morales AJ. Unsolved matters in leprosy: a descriptive review and call for further research. Ann Clin Microbiol Antimicrob. 2016;15(1):33. doi: 10.1186/s12941-016-0149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinmann P, Reed SG, Mirza F, Hollingsworth TD, Richardus JH. Innovative tools and approaches to end the transmission of Mycobacterium leprae. The Lancet Infectious Diseases. 2017;17(9):e298–e305. doi: 10.1016/S1473-3099(17)30314-6 [DOI] [PubMed] [Google Scholar]
- 5.WHO. Global leprosy situation 2012. Weekly epidemiological record. 2012;34(87):317–28. [PubMed] [Google Scholar]
- 6.WHO. Accelerating work to overcome the global impact of neglected tropical diseases. 2012. [Google Scholar]
- 7.WHO. Global Leprosy Strategy 2016–2020. World Health Organization,. 2016. [Google Scholar]
- 8.Organization WH. Leprosy: Global consultation of National Leprosy Programme managers, partners and affected persons on Global Leprosy Strategy 2021–2030: Report of the virtual meeting 26–30 October 2020. New Delhi: World Health Organization, Regional Office for South-East Asia; License: CC BY-NCSA 3.0 IGO. India; 2020. [Google Scholar]
- 9.Richardus JH, Tiwari A, Barth-Jaeggi T, Arif MA, Banstola NL, Baskota R, et al. Leprosy post-exposure prophylaxis with single-dose rifampicin (LPEP): an international feasibility programme. The Lancet Global Health. 2021;9(1):e81–e90. doi: 10.1016/S2214-109X(20)30396-X [DOI] [PubMed] [Google Scholar]
- 10.Fadugba Ogunsumi D, Blok D. Classification of leprosy endemicity: a systematic review. PROSPERO CRD42019104933. 2019. [Google Scholar]
- 11.Fontes ANB, Lima LNGC, Mota RMS, Almeida RLF, Pontes MA, Gonçalves HDS, et al. Genotyping of Mycobacterium leprae for better understanding of leprosy transmission in Fortaleza, Northeastern Brazil. PLoS Negl Trop Dis. 2017;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araujo K, Leano HAD, Rodrigues RN, Bueno ID, Lana FCF. Epidemiological trends of leprosy in an endemic state. Revista Da Rede De Enfermagem Do Nordeste. 2017;18(6):771–8. [Google Scholar]
- 13.Freitas BHBM, Cortela DDCB, Ferreira SMB. Trend of leprosy in individuals under the age of 15 in Mato Grosso (Brazil), 2001–2013. Rev Saude Publica. 2017;51:28. doi: 10.1590/S1518-8787.2017051006884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardes F, Paula NA, Leite MN, Abi-Rached TLC, Vernal S, Silva MBD, et al. Evidence of hidden leprosy in a supposedly low endemic area of Brazil. Mem Inst Oswaldo Cruz. 2017;112(12):822–8. doi: 10.1590/0074-02760170173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitas LRS, Duarte EC, Garcia LP. Analysis of the epidemiological situation of leprosy in an endemic area in Brazil: spatial distribution in the periods 2001–2003 and 2010–2012 Portuguese, English. Rev bras epidemiol. 2017;20(4):702–13. doi: 10.1590/1980-5497201700040012 [DOI] [PubMed] [Google Scholar]
- 16.Santos SD, Penna GO, Costa Md.a C, Natividade MS, Teixeira MG. Leprosy in children and adolescents under 15 years old in an urban centre in Brazil. Mem Inst Oswaldo Cruz. 2016;111(6):359–64. doi: 10.1590/0074-02760160002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajalla ME, Andrade SM, Tamaki EM, Waissmann W, Diettrich SH, Silva BA. The context of leprosy in Brazil-Paraguay border. Cien Saude Colet. 2016;21(1):225–32. doi: 10.1590/1413-81232015211.20572014 [DOI] [PubMed] [Google Scholar]
- 18.Freitas LRS, Duarte EC, Garcia LP. Trends of main indicators of leprosy in Brazilian municipalities with high risk of leprosy transmission, 2001–2012. BMC Infect Dis. 2016;16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha C, Pedrosa VL, Dias LC, Braga A, Chrusciak-Talhari A, Santos M, et al. A historical overview of leprosy epidemiology and control activities in amazonas, brazil. Rev Soc Bras Med Trop. 2015;48:55–62. doi: 10.1590/0037-8682-0103-2013 [DOI] [PubMed] [Google Scholar]
- 20.Brito KKGd,Andrade SSdC, Santana EMFd, Peixoto VB, Nogueira JdA, Soares MJGO. Análise epidemiológica da hanseníase em um estado endêmico do nordeste brasileiro. Rev Gaúcha Enferm. 2015;36(spe):24–30. [DOI] [PubMed] [Google Scholar]
- 21.Moreira SC, Batos CJC, Tawil L. Epidemiological situation of leprosy in Salvador from 2001 to 2009. An Bras Dermatol. 2014;89(1):107–17. doi: 10.1590/abd1806-4841.20142175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alencar CH, Ramos AN Jr, Barbosa JC, Kerr LR, De Oliveira ML, Heukelbach J. Persisting leprosy transmission despite increased control measures in an endemic cluster in Brazil: the unfinished agenda. Lepr Rev. 2012;83(4):344–53. [PubMed] [Google Scholar]
- 23.de Oliveira DT, Bezerra MM, de Almeida JAP, Duthie M, Reed S, de Jesus AR. Neurological disability in leprosy: Incidence and gender association in Sergipe, Brazil. Geospatial Health. 2012;6(3 SUPPL.):S125–S9. doi: 10.4081/gh.2012.130 [DOI] [PubMed] [Google Scholar]
- 24.Alencar CH, Ramos AN, dos Santos ES, Richter J, Heukelbach J. Clusters of leprosy transmission and of late diagnosis in a highly endemic area in Brazil: Focus on different spatial analysis approaches. Trop Med Int Health. 2012;17(4):518–25. doi: 10.1111/j.1365-3156.2011.02945.x [DOI] [PubMed] [Google Scholar]
- 25.Pereira EVE, Machado HAS, Ramos CHM, Nogueira LT, Lima LAN. Epidemiologic profile of the leprosy of the city of Teresina, in the period of 2001–2008. An Bras Dermatol. 2011;86(2):235–40. doi: 10.1590/s0365-05962011000200005 [DOI] [PubMed] [Google Scholar]
- 26.da Silva AR, de Matos WB, Silva CCB, Goncalves EDD. Leprosy in Buriticupu, State of Maranhao: active search for cases in the adult population. Revista Da Sociedade Brasileira De Medicina Tropical. 2010;43(6):691–4. doi: 10.1590/s0037-86822010000600018 [DOI] [PubMed] [Google Scholar]
- 27.Imbiriba EB, Hurtado-Guerrero JC, Garnelo L, Levino A, da Graça Cunha M, Pedrosa V. Epidemiological profile of leprosy in children under 15 in Manaus (Northern Brazil), 1998–2005. Rev Saude Publica. 2008;42(6):1021–6. doi: 10.1590/s0034-89102008005000056 [DOI] [PubMed] [Google Scholar]
- 28.Ignotti E, Bayona M, Alvarez-Garriga C, Andrade VLG, Valente JG. Single lesion as an indicator to monitor the leprosy trend to elimination in hyperendemic areas. Rev Bras Epidemiol. 2007;10(3):421–31. [Google Scholar]
- 29.Anchieta JJS, Costa L, Campos LC, Vieira MDR, Mota OS, Morais Neto OL, et al. Trend analysis of leprosy indicators in a hyperendemic Brazilian state, 2001–2015. Rev Saude Publica. 2019;53:61. doi: 10.11606/S1518-8787.2019053000752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbosa CC, Bonfim CVD, de Brito CMG, Ferreira AT, Gregorio V, de Oliveira ALS, et al. Spatial analysis of reported new cases and local risk of leprosy in hyper-endemic situation in Northeastern Brazil. Trop Med Int Health. 2018;23(7):748–57. doi: 10.1111/tmi.13067 [DOI] [PubMed] [Google Scholar]
- 31.de Sousa DB, Souza-Santos R, Duarte da Cunha M, Sobral A. Hot spots of leprosy in the endemic area of Sao Luis, Maranhao State, Northeastern Brazil. J Infect Public Health. 2020;13(2):228–34. doi: 10.1016/j.jiph.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 32.Pereira TM, Silva L, Dias MSA, Monteiro LD, Silva M, Alencar OM. Temporal trend of leprosy in a region of high endemicity in the Brazilian Northeast. Rev Bras Enferm. 2019;72(5):1356–62. doi: 10.1590/0034-7167-2018-0682 [DOI] [PubMed] [Google Scholar]
- 33.de Souza EA, Ferreira AF, Boigny RN, Alencar CH, Heukelbach J, Martins-Melo FR, et al. Leprosy and gender in Brazil: trends in an endemic area of the Northeast region, 2001–2014. Revista De Saude Publica. 2018;52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murugaiyan R, Saravanan G, Karthikeyan K. Changing trends of leprosy in post elimination Era -A study from an endemic area. Indian J Lepr. 2017;89(1):23–7. [Google Scholar]
- 35.Mohite RV, Mohite VR, Durgawale PM. Differential trend of leprosy in rural and urban area of Western Maharashtra. Indian J Lepr. 2013;85(1):11–8. [PubMed] [Google Scholar]
- 36.Aggarwal A, Pandey A. Inverse sampling to study disease burden of leprosy. Indian J Med Res. 2010;132(10):438–41. [PubMed] [Google Scholar]
- 37.Kumar A, Girdhar A, Girdhar BK. Incidence of leprosy in Agra District. Lepr Rev. 2007;78(2):131–6. [PubMed] [Google Scholar]
- 38.Kumar A, Husain S, Murthy BN. Burden of leprosy-related disability: Results of a population survey in two states in India. Leprosy Review. 2018;89(4):353–61. [Google Scholar]
- 39.Govindharaj P, Srinivasan S, Darlong J. Social participation of persons affected by leprosy in an Endemic district, West Bengal, India. Leprosy Review. 2019;90(1):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen J, Zhou M, Xu X, Ray A, Zhang G, Yan L. A big challenge in case finding at low endemic situation: Analysis on 1462 new leprosy patients detected in China in 2007. Lepr Rev. 2010;81(3):176–83. [PubMed] [Google Scholar]
- 41.Chen S, Zheng Y, Zheng M, Wang D. Rapid survey on case detection of leprosy in a low endemic situation, Zhucheng County, Shandong Province, the People’s Republic of China. Lepr Rev. 2007;78(1):65–9. [PubMed] [Google Scholar]
- 42.Chen X, You YG, Yuan YH, Yuan LC, Zhang Y, Yan W. Evaluation of antigen-specific immune responses for leprosy diagnosis in a hyperendemic area in China. PLoS Negl Trop Dis. 2018;12(9):e0006777. doi: 10.1371/journal.pntd.0006777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blok DJ, de Vlas SJ, Geluk A, Richardus JH. Minimum requirements and optimal testing strategies of a diagnostic test for leprosy as a tool towards zero transmission: A modeling study. PLoS Negl Trop Dis. 2018;12(5):e0006529. doi: 10.1371/journal.pntd.0006529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasker E, Baco A, Younoussa A, Mzembaba A, Grillone S, Demeulenaere T, et al. Leprosy on Anjouan (Comoros): persistent hyper-endemicity despite decades of solid control efforts. Lepr Rev. 2017;88(3):334–42. [Google Scholar]
- 45.Ortuno-Gutierrez N, Baco A, Braet S, Younoussa A, Mzembaba A, Salim Z, et al. Clustering of leprosy beyond the household level in a highly endemic setting on the Comoros, an observational study. BMC Infect Dis. 2019;19(1):501. doi: 10.1186/s12879-019-4116-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furst T, Cavaliero A, Lay S, Dayer C, Chan S, Smrekar A, et al. Retrospective active case finding in Cambodia: An innovative approach to leprosy control in a low-endemic country. Acta Trop. 2018;180:26–32. doi: 10.1016/j.actatropica.2017.12.031 [DOI] [PubMed] [Google Scholar]
- 47.Dabrera TM, Tillekeratne LG, Fernando MS, Kasturiaratchi ST, Østbye T. Prevalence and Correlates of Leprosy in a High-Risk Community Setting in Sri Lanka. Asia Pac J Public Health. 2016;28(7):586–91. doi: 10.1177/1010539516666360 [DOI] [PubMed] [Google Scholar]
- 48.Tabah EN, Nsagha DS, Bissek ACZK, Bratschi MW, Njamnshi TN, Plushke G, et al. The Burden of Leprosy in Cameroon: Fifteen Years into the Post-elimination Era. PLoS Neglected Tropical Diseases. 2016;10(10). doi: 10.1371/journal.pntd.0005012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aranzazu N, Parra JJ, Cardenas M, Rada E, Zerpa O, Rivera T, et al. Cojedes: A leprosy hyperendemic state. Int J Dermatol. 2012;51(2):186–94. doi: 10.1111/j.1365-4632.2011.05080.x [DOI] [PubMed] [Google Scholar]
- 50.Richardus JH, Meima A, van Marrewijk CJ, Croft RP, Smith TC. Close contacts with leprosy in newly diagnosed leprosy patients in a high and low endemic area: comparison between Bangladesh and Thailand. Int J Lepr Other Mycobact Dis. 2005;73(4):249–57. [PubMed] [Google Scholar]
- 51.Bakker MI, Hatta M, Kwenang A, Klatser PR, Oskam L. Epidemiology of leprosy on five isolated islands in the Flores Sea, Indonesia. Trop Med Int Health. 2002;7(9):780–7. doi: 10.1046/j.1365-3156.2002.00931.x [DOI] [PubMed] [Google Scholar]
- 52.Odriozola EP, Quintana AM, González V, Pasetto RA, Utgés ME, Bruzzone OA, et al. Towards leprosy elimination by 2020: forecasts of epidemiological indicators of leprosy in Corrientes, a province of northeastern Argentina that is a pioneer in leprosy elimination. Mem Inst Oswaldo Cruz. 2017;112(6):419–27. doi: 10.1590/0074-02760160490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aceng FL, Kawuma HJ, Majwala R, Lamunu M, Ario AR, Rwabinumi FM, et al. Spatial distribution and temporal trends of leprosy in Uganda, 2012–2016: a retrospective analysis of public health surveillance data. BMC Infect Dis. 2019;19(1):1016. doi: 10.1186/s12879-019-4601-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penna MLF, Penna GO. Leprosy frequency in the world, 1999–2010. Mem Inst Oswaldo Cruz. 2012;107(supl.1):3–12. doi: 10.1590/s0074-02762012000900002 [DOI] [PubMed] [Google Scholar]
- 55.WHO. Global leprosy distribution in 1998. Wkly Epidemiol Rec. 1998;73(25):188–90. [PubMed] [Google Scholar]
- 56.Dharmshaktu N. An Exploratory Review of Magnitude of COVID19 Cases in Non-Leprosy affected Countries to Leprosy Endemic Countries. Epidemiology International (E-ISSN: 2455-7048). 2020;5(2):1–9. [Google Scholar]
- 57.Ministério da Saúde SdVeS, Secretaria de Atenção à Saúde. Guia para o Controle da Hanseníase. Brasilia: Ministério da Saúde; 2002. [Google Scholar]
- 58.Ministério da Saúde SdVeS, Secretaria de Atenção à Saúde. Define ações de controle da hanseníase Brasilia: Ministério da Saúde; 2009. [Google Scholar]
- 59.Ezz El-Dawela R, Sheneef A, Yousef F. Analysis of newly detected leprosy in Sohag Governorate, Upper Egypt, 2004–20082012. 71–9 p. [PubMed]
- 60.Swaroop S. Index of endemicity. Bull World Health Organ. 1957;16(6):1083–101. [PMC free article] [PubMed] [Google Scholar]
- 61.Hessel F. Burden of disease. In: Kirch W, editor. Encyclopedia of Public Health. Dordrecht: Springer; 2008. [Google Scholar]
- 62.Van Brakel WH, Sihombing B, Djarir H, Beise K, Kusumawardhani L, Yulihane R, et al. Disability in people affected by leprosy: the role of impairment, activity, social participation, stigma and discrimination. Global health action. 2012;5(1):18394. doi: 10.3402/gha.v5i0.18394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Idema WJ, Majer IM, Pahan D, Oskam L, Polinder S, Richardus JH. Cost-Effectiveness of a Chemoprophylactic Intervention with Single Dose Rifampicin in Contacts of New Leprosy Patients. PLOS Neglected Tropical Diseases. 2010;4(11):e874. doi: 10.1371/journal.pntd.0000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Veen NH, McNamee P, Richardus JH, Smith WCS. Cost-effectiveness of interventions to prevent disability in leprosy: a systematic review. PLoS One. 2009;4(2):e4548. doi: 10.1371/journal.pone.0004548 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All data are in the manuscript and in supporting information files.