Abstract
Tuta absoluta is one of the most damaging pests of tomato crops worldwide. Damage due to larvae may cause up to 100% loss of tomato production. Use of natural enemies to control the pest, notably predatory mirids such as Nesidiocoris tenuis and Macrolophus pygmaeus, is increasingly being promoted. However, considering the potential damage caused to tomatoes by these omnivorous predators in the absence of T. absoluta, an alternative solution could be required to reduce tomato damage and improve the predators’ performance. The use of companion plants can be an innovative solution to cope with these issues. The present study aimed to determine the influence of companion plants and alternative preys on the predators’ performance in controlling T. absoluta and protecting tomato plants. We evaluated the effect of predators (alone or combined) and a companion plant (sesame (Sesamum indicum)) on T. absoluta egg predation and crop damage caused by N. tenuis. The influence of an alternative prey (Ephestia kuehniella eggs) on the spatial distribution of predators was also evaluated by caging them in the prey presence or absence, either on tomato or sesame plants or on both. We found that the presence of sesame did not reduce the efficacy of N. tenuis or M. pygmaeus in consuming T. absoluta eggs; hatched egg proportion decreased when N. tenuis, M. pygmaeus, or both predators were present. More specifically, this proportion was more strongly reduced when both predators were combined. Sesame presence also reduced necrotic rings caused by N. tenuis on tomato plants. Nesidiocoris tenuis preferred sesame over tomato plants (except when food was provided only on the tomato plant) and the upper part of the plants, whereas M. pygmaeus preferred tomato to sesame plants (except when food was provided only on the sesame plant) and had no preference for a plant part. Combination of predators N. tenuis and M. pygmaeus allows for better coverage of cultivated plants in terms of occupation of different plant parts and better regulation of T. absoluta populations. Sesamum indicum is a potential companion plant that can be used to significantly reduce N. tenuis damage to tomatoes.
Introduction
The South American tomato pinworm, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae), is a key pest of tomato crops (Solanum lycopersicum L.) worldwide [1–7]. In the absence of effective management methods, serious damage owing to larval feeding activity [8–10] can lead to up 80–100% production loss [1]. In general, the spraying of chemical insecticides in high quantities remains a preferred pest management method and ensures better tomato production [11, 12]. Unfortunately, this practice promotes selection of resistant pest populations [13, 14], can generate detrimental side effects on the environment, affects beneficial arthropods [15–20] and is not always effective against T. absoluta because of resistance developed to several active substances [21, 22]. The development of more appropriate methods for better management of T. absoluta is increasingly becoming a priority [1, 2].
Integrated management programs against T. absoluta seek to keep damage below the level of economic damage to tomatoes. A key component of such programs is biological control [1, 23]. Among the possibilities of biological control, a number of natural enemies proved to reduce pest damage in glasshouse tomato crops [24–26]. Biological control practices have long emphasized the role of specialized natural enemies, whose dynamics are closely related to those of a target pest [27, 28]. However, the presence of pest complexes in the agroecosystems limits the effectiveness of specialized predators. For this reason, there has been a growing interest for generalist predators because of their high adaptation and widespread success compared to other more specialized natural enemies [29].
Generalist predators, e.g. Macrolophus pygmaeus Rambur, Nesidiocoris tenuis Reuter (Hemiptera: Miridae), Dicyphus tamaninii Wagner, and D. errans Wolff, are considered important biological control agents against several tomato crop pests such as mites, whiteflies, thrips, aphids, and pinworms [2, 8, 30, 31]. These species often coexist in agroecosystems (e.g., M. pygmaeus and N. tenuis) [32] and are able to switch successfully to the predation of the eggs of T. absoluta, an invasive pest, shortly after its introduction in the Mediterranean region [31–35]. However, M. pygmaeus is not always effective when used alone against T. absoluta because it seems to have other preferences (for example whiteflies) [33, 34]. As for N. tenuis, it causes necrotic rings due to its repeated feeding around stems and flowers often requiring the use of insecticide to reduce population density [36, 37].
In such a context, the combination of companion or service plants with predators has become a newly adopted option to optimize the efficacy of the latter and reduce N. tenuis damage on cultivated plants. Companion or service plants can serve as an appropriate refuge when the crop environment is unfavorable, for example, in the absence of prey or pesticide use [38, 39]. They could act as a mini-breeding system in fields [38], diversify habitats, reduce the frequency of encounters between predators and thus reduce antagonistic effects [40]. Therefore, special attention should be paid to companion plants such as the sesame plant Sesamum indicum L. (Pedaliaceae) in pest management systems. With or without prey, N. tenuis prefers this plant for its reproduction rather than tomato, cucumber, eggplant, or pepper [41]. In the presence of sesame, N. tenuis causes much less damage to tomato plants while preying on T. absoluta eggs [42]. The combined action of natural enemies can have synergistic or additive effects that enhance pest control [43–47]. Antagonistic effects can also be observed when natural enemies share the same prey [48]. Sesame as a companion plant is a potential option to be evaluated with N. tenuis and M. pygmaeus both in the laboratory and in agricultural systems in order to limit the phytophagy of N. tenuis on tomato crops. If confirmed, such a system’s effectiveness could enhance integrated pest management programs. Some authors [49] still believe that an effective biocontrol agent must be species-specific to the prey or host. However, an assemblage of generalist predators can be effective in decreasing populations densities of both native and exotic pest species [50–52].
In this assemblage, the impact of the alternative prey should be considered, as this factor can negatively or positively influence predator behavior and pest control [34, 53, 54]. Alternative prey may divert predation pressure by reducing the risk of predation on the focal prey [55, 56] depending on predator preferences [53, 54]. Conversely, predation on the alternative prey may stimulate predator populations to respond numerically and consume more individuals of the target prey [54, 57]. Thus, the presence of alternative prey allows generalist predators to rapidly establish themselves in agroecosystems before the arrival of pests [54, 58]. This limits the growth of pest populations after they have colonized the crop [59].
Evaluation of such a system involves monitoring the behavior of associated individuals (i.e., N. tenuis and M. pygmaeus), including monitoring their distribution within plants, their preference, intraguild predation (IGP), etc.
In this study, the objectives were to assess the effect of a companion plant (sesame) and the use of two predators (i) on the predation rates of T. absoluta eggs, (ii) on N. tenuis damage to tomato plants and (iii) to determine the influence of an alternative prey (lepidopteran eggs) on the spatial distribution of the predators.
We expected that (1) the combination of both predators in the presence of sesame would increase T. absoluta egg predation, (2) the presence of companion plants would diversify habitats and reduce the phytophagy of N. tenuis on tomato plants and (3) the presence of alternative prey would not affect the behavior of predators in relation to their distribution within plants.
Materials and methods
Biological material
Tomato (cv. Nano) and sesame (cv. T-85 Humera) plants used for insect rearing and experiments were planted in plastic pots (9 x 9 x 10 cm) in the laboratory. They were maintained in controlled conditions (24 ± 2°C, 40 ± 10% R.H. and 16:8 L:D) until they reached a height of 15 to 20 cm (4- to 5-weeks-old).
A T. absoluta colony was maintained on young tomato plants in laboratory (24 ± 2°C, 40 ± 10% R.H. and 16:8 L:D). It originated from 65 individuals collected in 2009 on greenhouse tomato plants in the South of France, and at least 50 individuals collected in tomato fields were added yearly. Tuta absoluta eggs were obtained by introducing eight tomato plants in cages and adding five adult pairs of the pinworm for 48 hours in the cages. The eggs laid were then counted, and 40 were kept per plant. Plants with eggs were moved to new cages holding predators and/or companion plants.
The predators N. tenuis and M. pygmaeus were provided by Koppert Biological Systems, France and reared on a tomato plant in cages covered with a fine nylon mesh (30 x 30 x 60 cm). Predators were fed with eggs of E. kuehniella Zeller (Lepidoptera: Pyralidae) and diluted honey. The E. kuehniella eggs were replaced every two days and provided as libitum. All the experiments were carried out at the French National Institute of Agronomic, Food and Environment Research (INRAE), Sophia Antipolis in the same laboratory conditions (24 ± 2°C, 40 ± 10% R.H. and 16:8 L:D).
Influence of a companion plant and predators on Tuta absoluta predation and tomato protection
The predator activity was determined by the ability of N. tenuis and M. pygmaeus to reduce the T. absoluta egg hatching rate after 9 days in the presence or absence of a sesame plant (S). In this experiment, tomato plants with T. absoluta eggs were used in all treatments. The proportion of hatched eggs on tomato plants was therefore assessed according to the presence of both predators combined (N-M) or individually (N or M) and with or without the companion plant (S). Thus 7 combinations were made with tomato plants containing T. absoluta eggs in different cages (30 x 30 x 60 cm covered with fine nylon mesh). These treatments were composed of (1) both predators with sesame (N-M&S); (2) both predators without sesame (N-M); (3) N. tenuis with sesame (N&S); (4) M. pygmaeus with sesame (M&S); (5) N. tenuis without sesame (N); (6) M. pygmaeus without sesame (M) and (7) sesame (S).
Each treatment involved 6 repetitions with one control at each repetition (the sesame plant only). A total of 48 tomato plants and 32 sesame plants were used. For treatments with both predator species, one couple of each species was released in the cages. In contrast, treatments with only one species included two couples of that species. Forty-eight hours later, predators were removed from the different cages. Egg hatching was monitored during 9 days and eggs that did not hatch during this period were considered consumed by the predators [42]. The number of T. absoluta larvae was counted to determine the hatching reduction. The hatching reduction rate was calculated by comparing the number of larvae that emerged with the initial number of eggs (40 eggs), corrected by the average egg mortality observed in the control according to the formula: RE = 100 × [1-(Ex/Et)] where Ex is the average number of eggs hatched during the treatment and Et is the average number of eggs hatched in the control.
Nesidiocoris tenuis phytophagy
The phytophagy on the tomato plants was assessed by counting the number of necrotic rings on the main stem, young shoots, leaves and leaf petioles [37, 42] induced by N. tenuis. The number of necrotic rings was assessed in the presence or absence of the second predator M. pygmaeus (M) and with or without the sesame plant (S) as a companion plant. The necrotic rings counts were made 3 days after the predators were removed from the cages. Six replicates with a control at each replicate (sesame plant only) were performed for each treatment. A total of 48 tomato plants and 32 sesame plants were used to assess the phytophagy. The experiments were carried out at 24 ± 2°C, 40 ± 10% R.H. and 16:8 L:D.
Effect of food presence on spatial distribution of predators
In this study we evaluated the impact of the presence of food on the spatial distribution of N. tenuis and M. pygmaeus developing on tomato (T) and sesame (S) plants with or without E. kuehniella eggs (E). Four combinations were made in different cages (30 x 30 x 60 cm covered with fine nylon mesh) including (1) Eggs on both plants (T&S-E); (2) Eggs only on the tomato plant (T-E); (3) Eggs only on the sesame plant (S-E) and (4) Both plants without eggs (T&S).
For all treatments, both predators and plants were simultaneously present in the cages and only the position of food (E. kuehniella eggs) varied (on the tomato and/or on the sesame plant). An average of 100 eggs of E. kuehniella were deposited on the leaves with a fine brush. At each observation, the distribution of predators on the different plants (lower, medium and upper parts of the plant) in the cage was noted. The upper, medium and lower parts were represented respectively by the first, second and third foliar stage of the plants and the proportion of each predator was monitored per day over 48 hours. A first observation was made in the morning (7:00 to 11:00 a.m.) and the second in the afternoon (4:00 to 7:00 p.m.), reported as the periods of high activity of M. pygmaeus [60]. For each treatment (T&S-E; T-E; S-E and T&S), 6 replicates were performed with 10 adult individuals per replicate (5 adults of N. tenuis and 5 adults of M. pygmaeus). These experiments were conducted under the same laboratory conditions described previously.
Statistical analyses
All statistical analyses were carried out using R software (R Development Core Team, version 3.3.3). A separate Generalized Linear Model (GLM) with a binomial error distribution was used to test for the effects of the presence of M. pygmaeus (absence or presence), the presence of N. tenuis (absence or presence) and the presence of sesame as a companion plant (absence or presence) on the proportion of hatched T. absoluta eggs. The effects of the presence of M. pygmaeus (absence or presence and the presence of sesame as a companion plant (absence or presence)) on the number of necrotic rings caused by N. tenuis was analyzed using GLM with a Poisson error distribution. A series of linear models (LMs) were used to test for the effects of predatory species, prey availability on the crop plant (tomato plant alone or supplemented with alternative prey), prey availability on the companion plant (sesame plant alone or supplemented with alternative prey), and/or the plant part (lower, medium, upper) on (i) the proportion of predators found on the tomato plants (vs. the sesame plant), and (ii) the number of predators found on the different parts of the tomato and sesame plants. The use of LMs for these tests was appropriate since the dependent variables and the model residuals followed a normal distribution when using a Shapiro–Wilk test and a visual interpretation of quantile–quantile plots. Since at least 2 factors had a significant effect on each response variable, and multi-comparison tests were performed considering each treatment independently (’multcomp’ package, Tukey method).
Results
Influence of companion plant and predators on Tuta absoluta predation and tomato protection
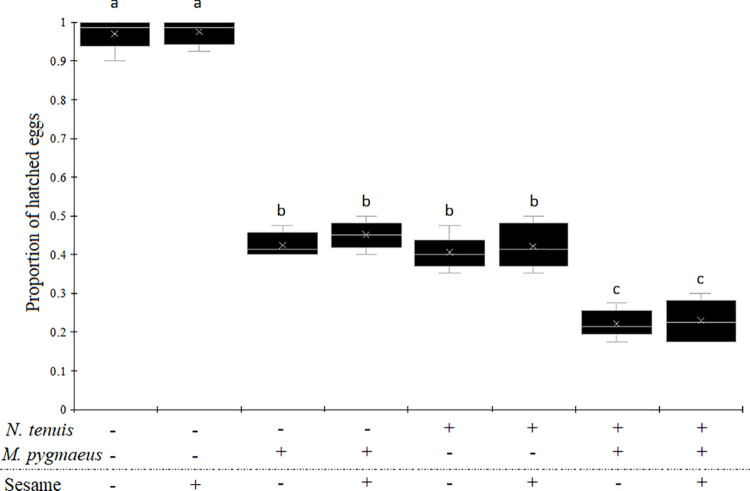
The proportion of hatched eggs of T. absoluta varied significantly depending on the simultaneous presence or not of N. tenuis and M. pygmaeus (Table 1, Fig 1). It was twice lower in the presence of N. tenuis or M. pygmaeus compared to the condition without predators (Fig 1). When both predators were present simultaneously, this proportion was four times lower compared to the treatment without predators. The presence of sesame did not modulate the egg hatching (Table 1).
Table 1. Results of the linear (F value) and Generalized Linear Model (χ2 deviance).
| Variable to explain | Explanatory variables | F value or χ2 deviance | P value |
|---|---|---|---|
| Presence of N. tenuis | 294.6 | < 0.001 | |
| Proportion of hatched eggs | Presence of M. pygmaeus | 310.0 | < 0.001 |
| Presence of sesame | 0.5 | 0.484 | |
| Number of necrotic rings | Presence of M. pygmaeus | 5.0 | 0.025 |
| Presence of sesame | 15.4 | < 0.001 | |
| Proportion of predators | Predator species | 74.4 | < 0.001 |
| on tomato plant | Prey availability on crop plant | 37.4 | < 0.001 |
| Prey availability on companion plant | 29.0 | < 0.001 | |
| Predator species | 19.7 / 20.5 | < 0.001 / < 0.001 | |
| Number of predators | Prey availability on crop plant | 8.8 / 11.2 | 0.004 / 0.001 |
| (on tomato / on sesame) | Prey availability on companion plant | 8.8 / 8.7 | 0.004 / 0.004 |
| Plant part | 18.1 / 41.9 | < 0.001 / < 0.001 |
Fig 1. Proportion of hatched eggs of Tuta absoluta according to the presence or absence of Nesidiocoris tenuis and/or Macrolophus pygmaeus and the presence or absence of sesame plant.
The ‘+’ indicates the presence and the ‘-’ indicates the absence. Boxplot followed by the same lower case letter did not differ significantly.
Nesidiocoris tenuis phytophagy
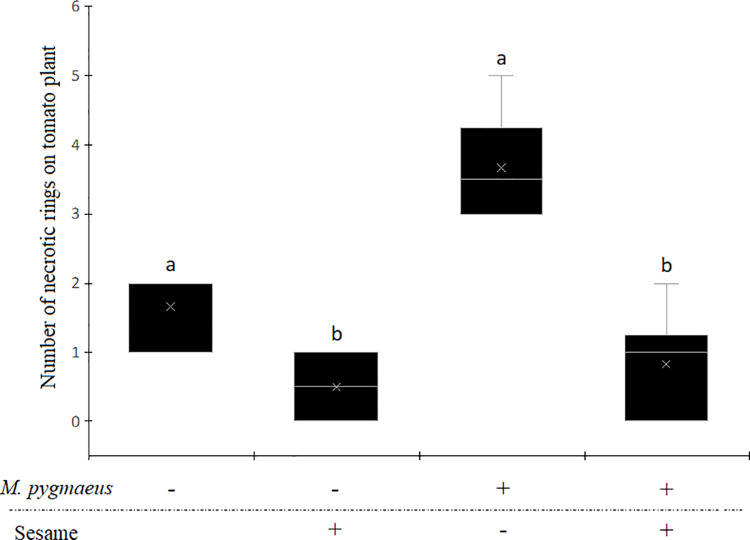
The number of necrotic rings caused by N. tenuis on tomato plants significantly depended on the presence of either M. pygmaeus or sesame (Table 1, Fig 2). Without M. pygmaeus, the number of necrotic rings was 3.3 times lower in presence of sesame than in absence of the companion plant. When both predators were present simultaneously, the number of necrotic rings was 4.4 lower in presence of sesame than in absence of the companion plant. The number of necrotic rings was 2 times higher in presence of both predators compared to the treatment when M. pygmaeus was absent.
Fig 2. Number of necrotic rings caused by Nesidiocoris tenuis on the tomato plant according to the presence or absence of Macrolophus pygmaeus and the presence or absence of sesame plant.
The ‘+’ indicates the presence and the ‘-’ indicates the absence. Boxplot followed by the same lower case letter did not differ significantly.
Effect of food presence on spatial distribution of predators
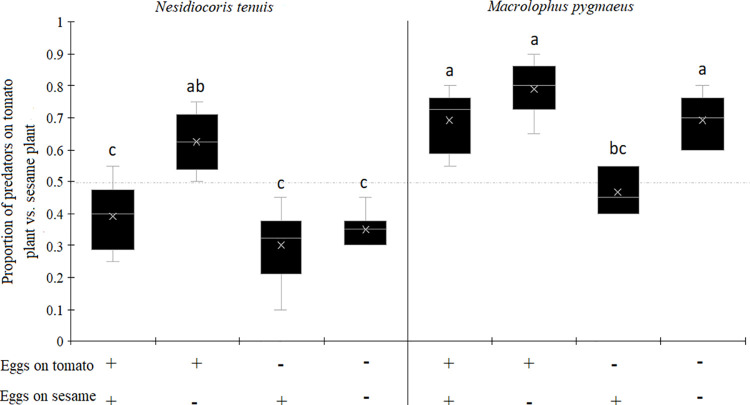
The proportion of predators on the tomato plant vs. the sesame plant varied significantly according to the predator species, the prey availability on the host crop plant and the prey availability on the companion plant (Table 1, Fig 3). Nesidiocoris tenuis preferred tomato when the prey was only on tomato (proportion of N. tenuis on tomato = 69%). In contrast, the proportion of N. tenuis was lower when the prey was on both plants (39%), only on sesame (30%) or without prey on both plants (35%). Macrolophus pygmaeus preferred tomato than sesame plant when prey was present on both plants, only on tomato or in absence of prey on both plants with the proportions of 69, 80 and 69%, respectively. This proportion was low (less than 50%) when the prey was only on sesame plant.
Fig 3. Proportion of predators (Nesidiocoris tenuis or Macrolophus pygmaeus) on tomato vs sesame plants according to the presence or absence of Ephestia kuehniella eggs on the tomato or the sesame plant.
The ‘+’ indicates the presence and the ‘-’ indicates the absence. Boxplot followed by the same lower case letter did not differ significantly.
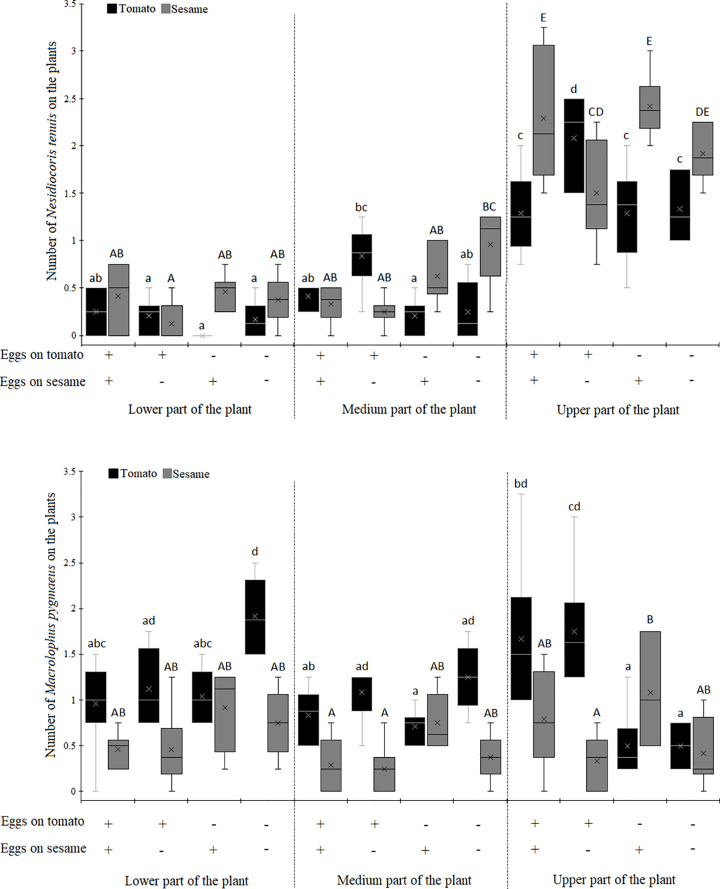
The number of predators established on the tomato and sesame plants varied significantly according to the predator species, the prey availability on the host crop plant, the prey availability on the companion plant and the part of plant (Table 1, Fig 4A and 4B). Nesidiocoris tenuis significantly preferred the upper part of the tomato and sesame plants compared to the medium and lower parts, whereas no difference was observed between the medium and the lower parts (Fig 4A). The presence of N. tenuis on the lower part of the tomato or sesame plant did not vary according to prey availability on the tomato or sesame plant. The number of N. tenuis individuals observed on the medium part of the tomato plant was higher when the alternative prey was provided only on the tomato plant than when the alternative prey was present only on the sesame plant. By contrast, the number of N. tenuis individuals on the medium part of the sesame plant did not vary according to availability of the alternative prey. Finally, the presence of N. tenuis on the upper part of the tomato varied according to prey availability as a higher number of individuals were observed on tomato plants when the alternative prey was provided only on the tomato plant compared to when alternative food was provided on the sesame plant, on both plants or was not provided. When considering the upper part of the sesame plant, the number of individuals observed was higher when the alternative prey was provided only on the sesame plant or on both plant species than when alternative food was provided only on the tomato plant.
Fig 4.
A. Number of Nesidiocoris tenuis on the different parts of the tomato or sesame plants depending on the presence or absence of Ephestia kuehniella eggs on the tomato or the sesame plant. The ‘+’ indicates the presence and the ‘-’ indicates the absence. Boxplot followed by the same lower or upper case letter did not differ significantly with respect to plant species (tomato and sesame, respectively). B. Number of predators Macrolophus pygmaeus on the different parts of the tomato or sesame plants depending on the presence or absence of Ephestia kuehniella eggs on the tomato or the sesame plant. The ‘+’ indicates the presence and the ‘-’ indicates the absence. Boxplot followed by the same lower or upper case letter did not differ significantly with respect to plant species (tomato and sesame, respectively).
The number of M. pygmaeus did not vary among the different tomato or sesame plant parts (Fig 4B). The number of individuals in the lower part of the tomato plant was higher when no alternative prey was provided compared to when alternative prey was provided on the sesame plant only and on both plant species. By contrast, the number of M. pygmaeus individuals did not vary when they were developed on the lower part of sesame. The number of individuals was similar in the different treatments (when alternative prey was provided on the tomato plant, on the sesame plant, on both plants or was not provided) for predators in medium part of the plants. Finally, the number of individuals in the upper part of the tomato plant was higher when the alternative prey was provided on the tomato plant only or on both plant species than when the alternative prey was provided on the sesame plant only or was not provided at all. By contrast, the number of individuals in the upper part of the sesame plant was higher when the alternative prey was provided on the sesame plant than when the alternative prey was provided on the tomato plant only.
Discussion
Competition for the same prey in coexisting predators is expected to increase phytophagy of omnivorous predators, induce intraguild predation, and increase their effectiveness as biological control agents. However, the combined action of coexisting predators was also reported to be beneficial in controlling pests. In such a context, we evaluated the impact of the presence of a companion plant on predator localization, effectiveness in controlling pests and reducing damage to cultivated plants. Intraguild predation occurs when two species that share a common prey resource also engage in trophic interaction with each other (e.g., predation) [61–63]. The results of our study did not provide evidence of intraguild predation between the predators N. tenuis et M. pygmaeus. This could be due to several factors such as the stage (adult) of the individuals in competition, the presence of other food sources (plants), the duration of the competition (2 days) or the complexity of the habitat (sesame + tomato). Indeed, the relative size and higher mobility of individuals is an important determinant of predator-predator interaction [64, 65]. For example, adult females of N. tenuis are known to inflict a high mortality rate only on young nymphs but not on adults of M. pygmaeus in the absence of alternative prey [66]. In addition, the ability of predators to consume food resources (plants) other than the shared resource (prey) can reduce competitive interaction and promote coexistence [32, 65, 67].
In our study we observed a much lower hatching rate of T. absoluta eggs when both predators were present together than when only one predator species was released. This could be due to competition for the same prey (T. absoluta eggs), the voracity of predators or the distribution of predators on all plant parts. Such behaviors could initially reduce egg hatching but also improve crop protection against this pest. Walzer et al. [68] indicated that bean protection against Tetranycus urticae Koch (Acari: Tetranychidae) was improved when the predators Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae) and Neoseiulus californicus McGregor (Acari: Phytoseiidae) coexisted. Also, the complementary occupation by both predators of different parts of the plant i.e., N. tenuis in the upper part and M. pygmaeus on all parts could increase their efficacy on the whole plant. Moreover, according to Lucas and Alomar [69], the level of whitefly predation was higher in the lower part of the plant when D. tamaninii and M. pygmaeus coexisted than when there was only one species. Moreno-Ripoll et al. [70] recommended the combined use of Eretmocerus mundus Mercet (Hymenoptera: Aphelinidae), M. pygmaeus and N. tenuis in order to improve Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) biological control. In a review study on generalist predators, Symondson et al. [29] showed a significant reduction of pests (Mollusca, Diptera, Lepidoptera, Coleoptera, Acari, Hemiptera and others) under experimental conditions by these predators in 79% of 52 studies consulted and yield increased significantly for 65% of the 26 cases where effects on plants were measured. Our results also provide evidence that the presence of a companion plant such as S. indicum does not influence mirid predation on T. absoluta eggs (consistent with Biondi et al. [42]). Sesame could therefore have a positive impact on the biological control of T. absoluta by N. tenuis and M pygmaeus. The positive influence of this plant in improving the biocontrol performance of predatory mirid Cyrtorhinus lividipennis Reuter (Hemiptera: Miridae) was already demonstrated on rice pests including Nilaparvata lugens Stål (Hemiptera: Delphacidae), Marasmia Patnalis Bradley (Lepidoptera: Pyralidae) and Cnaphalocrocis medinalis Guenée (Lepidoptera: Crambidae) [71].
Damage induced by N. tenuis on tomato plants was also mitigated by sesame plant presence: a higher number of necrotic rings was observed when predators were combined on the tomato plant in the absence of a sesame plant. This could be due to competition for the same prey causing a rapid drop in the availability of this prey and encouraging the repeated feeding of mirids on the only food source present, i.e., the tomato plant. When the level of prey in the crop is low, the phytophagy activity of N. tenuis may be of critical importance [36, 37]. According to Sanchez [72], the damage caused by N. tenuis to the tomato plant is inversely related to the abundance of prey. Damage can also be explained by the predators’ need for plant-based nutrients in order to optimize digestion and/or assimilation of its prey [73]. The intensity of damage varies according to the availability of prey and the presence of a plant resource other than a cultivated plant [65, 67]. This could explain our observation that the number of necrotic rings was reduced when the sesame plant was added. This also implies that the presence of sesame reduces the damage caused by N. tenuis to tomato plants. Indeed, N. tenuis prefers sesame to tomato plants for its nutrition and development [41, 42, 74]. In other words, this predator prefers to feed on sesame rather than on tomato plants when offered the choice, as was the case in our study conditions. Sesame could be a better source of plant nutrients than tomato. A study by Naselli et al. [75] showed that sesame plants emitted lower amounts of hydrocarbon monoterpenes but higher levels of oxygenated terpenes than tomato plants. While hydrocarbon terpenes are known to have insect pest repellent properties [76], oxygenated monoterpenes and Green Leaf Volatiles (GLV) compounds have been shown to play a role in attracting predatory mirids [75, 77]. Also, in addition to having no influence on egg predation, sesame considerably reduces N. tenuis damage to tomato plants. According to Gillespie and McGregor [73] and Biondi et al. [42], this companion plant is a good food source that is effective in disrupting the phytophagy activity of this mirid on the cultivated plant without influencing predation. In this study, N. tenuis preferred the sesame to the tomato plant (except when food was provided only on the tomato plant) and preferred the upper part of the plants, whereas M. pygmaeus preferred the tomato to the sesame plant (except when food was provided only on the sesame plant) and did not prefer a plant part. The presence of sesame did not reduce the efficacy of N. tenuis or M. pygmaeus in consuming T. absoluta eggs (the proportion of hatched eggs decreased when N. tenuis, M. pygmaeus or both predators were present) and clearly reduced the number of necrotic rings when N. tenuis or both predators were present simultaneously.
In addition, the high proportion of N. tenuis on the sesame plant and of M. pygmaeus on the tomato plant indicates that both predators have opposite preferences for plant species. This is not a surprise because N. tenuis is considered an important pest in sesame cultivation in India and Japan [41]. Nesidiocoris tenuis was more attracted to this plant than Dittrichia viscosa for its reproduction [42]. According to Nakahishi et al. [41], sesame can be an insectary plant for this predator. Macrolophus pygmaeus was more present on tomato than sesame. Tomato plants could be a better food supplement than sesame for this mirid. In crops, this bug is mainly observed on solanaceous plants, more particularly on tomatoes and eggplant. Macrolophus pygmaeus develops well on tomato plants even when preys are absent [78]. The high proportion of N. tenuis on the upper part of the plant and of M. pygmaeus on the entire plant also indicates different preferences in terms of occupation of different parts of plants. Nesidiocoris tenuis is usually present in the upper part of plants while M. pygmaeus explores the lower leaves [32, 79]. The difference between our results and those of Perdikis et al. [32] on the distribution of M. pygmaeus could be due to the relative size of the individuals and the number of individuals combined (16 nymphs) in their experience. Predators were distributed in our experimental conditions such that both species encountered each other more often and could not avoid each other completely. Moreover, in a similar experiment, Perdikis et al. [32] observed that one N. tenuis and one M. pygmaeus could meet 5 times in 30 minutes for more than 3 seconds. Although avoidance mechanisms are often not clearly identified [80], the avoidance of hetero specific competitors is not always systematic [81]. Our results hint that by occupying different parts of the plant neither predator was in competition. Generally, avoidance behavior occurs between closely related species with a marked overlap in diet i.e., between species of specialized predators [82]. Moreno-Ripoll et al. [66] showed that the distribution of these predators was not modified when they were associated or not. The occupation of different strata on plants by each species allows for better coverage of the plant by predators. This could therefore have important implications for biological control as the potential activity of the predators thus distributed may be complementary [32, 69, 83, 84]. The results of the present study could enable optimizing biological control methods against T. absoluta, although multiple other factors should be considered to achieve fully effective and sustainable Integrated Pest Management (IPM) strategies [18, 85, 86].
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper.
Funding Statement
Hortinet project funded by PRESED-CI C2D, 2016-2020 AMRUGE-CI 2 to TM. Campus France TO KAJK. National Center of Agricultural Research (CNRA) to KAJK. Project ACOR (Casdar) to ND.
References
- 1.Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S. 2010. Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. Journal of Pest Science 83: 197–215. [Google Scholar]
- 2.Biondi A, Guedes RNC, Wan F-H, Desneux N. 2018. Ecology, worldwide spread and management of the invasive South American tomato pinworm, Tuta absoluta: past, present and future. Annual Review of Entomology 63: 239–58. doi: 10.1146/annurev-ento-031616-034933 [DOI] [PubMed] [Google Scholar]
- 3.Desneux N, Luna MG, Guillemaud T, Urbaneja A. 2011. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. Journal of Pest Science 84: 403–408. 10.1007/s10340-011-0398-6 [DOI] [Google Scholar]
- 4.Campos MR, Biondi A, Adiga A, Guedes RNC, Desneux N. 2017. From the Western Palearctic region to beyond: Tuta absoluta ten years after invading Europe. Journal of Pest Science 90: 787–9. [Google Scholar]
- 5.Han P, Zhang YN, Lu ZZ, Wang S et al. 2018. Are we ready for the invasion of Tuta absoluta? Unanswered key questions for elaborating an integrated pest management package in Xinjiang, China. Entomologia Generalis 38: 113–125. [Google Scholar]
- 6.Han P, Bayram Y, Shaltiel-Harpaz L, Sohrabi F et al. 2019. Tuta absoluta continues to disperse in Asia: damage, ongoing management and future challenges. Journal of Pest Science 92: 1317–1327. [Google Scholar]
- 7.Verheggen F, Fontus RB. 2019. First record of Tuta absoluta in Haiti. Entomologia Generalis 38 (4): 349–353. 10.1127/entomologia/2019/0778 [DOI] [Google Scholar]
- 8.Urbaneja A, Desneux N, Gabarra R, Arnó J, González-Cabrera J, Mafra-Neto A, et al. 2013. Biology, ecology and management of the tomato borer, Tuta absoluta. Peña JE(ed) Potential invasive pests of agricultural crops, CABI; series 98–125. https://doi.org/10.1079%2F9781845938291.0098 [Google Scholar]
- 9.Cherif A, Attia-Barhoumi S, Mansour R, Zappalà L, Grissa-Lebdi K. 2019. Elucidating key biological parameters of Tuta absoluta on different host plants and under various temperature and relative humidity regimes. Entomologia Generalis 39: 1–7. doi: 10.1127/entomologia/2019/0685 [DOI] [Google Scholar]
- 10.Rostami E, Madadi H, Abbasipour H, Allahyari H, Cuthbertson AGS. 2020. Pest density influences on tomato pigment contents: the South American tomato pinworm scenario Entomologia Generalis 40 (2): 195–205. 10.1127/entomologia/2020/0788 [DOI] [Google Scholar]
- 11.Teixeira CA, Lacerda Filho AF, Pereira F, Souza LH, Rousso JR. 2005. Balanço energético de uma cultura de tomate. Revista Brasileira de Engenharia Agrícola e Ambiental 9: 429–432. [Google Scholar]
- 12.Latorraca A, Marques GJG, Sousa KV, Fornés NS. 2008. Agrotóxicos utilizados na produção do tomate em Goiânia e Goianápolis e efeitos na saúde humana. Com. Ciências Saúde 19: 419–423. [Google Scholar]
- 13.Siqueira HAA, Guedes RNC, Picanço MC. 2000. Insecticide resistance in population of Tuta absoluta (Lepidoptera: gelechiidae). Agric For Entomol 2: 147–153. [Google Scholar]
- 14.Silva JE, Ribeiro LMS, Vinasco N. et al. 2019. Field-evolved resistance to chlorantraniliprole in the tomato pinworm Tuta absoluta: inheritance, cross-resistance profile, and metabolism. Journal of Pest Science 92: 1421–1431. 10.1007/s10340-018-1064-z [DOI] [Google Scholar]
- 15.Weisenburger DD. 1993. Human health-effects of agrichemicals use. Human Pathology 24 (6): 571–576. doi: 10.1016/0046-8177(93)90234-8 [DOI] [PubMed] [Google Scholar]
- 16.Desneux N, Rafalimanana H, Kaiser L. 2004. Dose-response relationship in lethal and behavioural effects of different insecticides on the parasitic wasp Aphidius ervi. Chemosphere 54: 619–627. doi: 10.1016/j.chemosphere.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 17.Desneux N, Ramirez- Romero R, Kaiser L. 2006. Multistep bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environmental Toxicology and Chemistry 25: 2675–2682. doi: 10.1897/05-562r.1 [DOI] [PubMed] [Google Scholar]
- 18.Desneux N, Decourtye A, Delpuech JM. 2007. The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology 52: 81–106. doi: 10.1146/annurev.ento.52.110405.091440 [DOI] [PubMed] [Google Scholar]
- 19.Soares MA, Campos MR, Passos LC, Carvalho GA, Haro MM, Lavoir AV, et al. 2019. Botanical insecticide and natural enemies: a potential combination for pest management against Tuta absoluta. Journal of Pest Science 92: 1433–1443. doi: 10.1007/s10340-018-01074-5 [DOI] [Google Scholar]
- 20.Menail AH, Boutefnouchet-Bouchema, Wided F, Haddad N, Taning CNT, Smagghe G, et al. 2020. Effects of thiamethoxam and spinosad on the survival and hypopharyngeal glands of the African honey bee (Apis mellifera intermissa). Entomologia Generalis 40 (2): 207–215. doi: 10.1127/entomologia/2020/0796 [DOI] [Google Scholar]
- 21.Mansour R, Brévault T, Chailleux A, Cherif A, Grissa-Lebdi K, Haddi K, et al. 2018. Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomologia Generalis 38(2): 83–112. doi: 10.1127/entomologia/2018/0749 [DOI] [Google Scholar]
- 22.Guedes RNC, Roditakis E, Campos MR, Haddi K, Bielza P, Siqueira HA A, et al. 2019. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. Journal of Pest Science 92(4): 1329–1342. doi: 10.1007/s10340-019-01086-9 [DOI] [Google Scholar]
- 23.Desneux N, Han P, Mansour R, Arnó J, Brévault T et al. 2021. Integrated Pest Management of Tuta absoluta: practical implementations across different regions around the world. Journal of Pest science. Accepted. [Google Scholar]
- 24.Chailleux A, Biondi A, Han P, Desneux N. 2013a. Suitability of the pest-plant system Tuta absoluta (Lepidoptera: Gelechiidae)-tomato for Trichogramma (Hymenoptera: Trichogrammatidae) parasitoids and insights for biological control. J Econ Entomol. 106: 2310–2321. [DOI] [PubMed] [Google Scholar]
- 25.Zappalà L, Biondi A, Alma A, Al-Jboory IJ, Arnò J, Bayram A, et al. 2013. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle-East, and their potential use in pest control strategies. Journal of Pest Sciences 86: 635–647. doi: 10.1007/s10340-013-0531-9 [DOI] [Google Scholar]
- 26.Zang LS, Wang S, Zhang F, Desneux N. 2021. Biological Control with Trichogramma in China: History, Present Status, and Perspectives Annual Review of Entomology 66: 463–484. doi: 10.1146/annurev-ento-060120-091620 [DOI] [PubMed] [Google Scholar]
- 27.Beddington JR, Free CA, and Lawton JH. 1978. Characteristics of successful enemies in models of biological control of insect pests. Nature 273: 513–519. doi: 10.1038/273513a0 [DOI] [PubMed] [Google Scholar]
- 28.Hassell MP, May RM. 1986. Generalist and specialist natural enemies in insect predator–prey interactions. Journal of Animal Ecology 55: 923–940. [Google Scholar]
- 29.Symondson WOC, Sunderland KD and Greenstone MH. 2002. Can generalist predators be effective biocontrol agents? Annual Review of Entomology 47: 561–594. doi: 10.1146/annurev.ento.47.091201.145240 [DOI] [PubMed] [Google Scholar]
- 30.Calvo FJ, Lorente MJ, Stansly PA, Belda JE. 2012. Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomologia Experimentalis Applicata 143: 111–119. [Google Scholar]
- 31.Bompard A, Jaworski CC, Bearez P, Desneux N. 2013. Sharing a predator: can an invasive alien pest affect the predation on a local pest? Population Ecology 55: 433–440. [Google Scholar]
- 32.Perdikis D, Lucas E, Garantonakis N, Giatropoulos A, Kitsis P, Maselou D, et al. 2014. Intraguild predation and sublethal interactions between two zoophytophagous mirids, Macrolophus pygmaeus and Nesidiocoris tenuis. Biological Control 70: 35–41. [Google Scholar]
- 33.Jaworski CC, Bompard A, Genies L, Amiens-Desneux E, Desneux N. 2013. Preference and prey switching in a generalist predator attacking local and invasive alien pests. PLoS ONE 8 (12): e82231 doi: 10.1371/journal.pone.0082231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaworski CC, Chailleux A, Bearez P, Desneux N. 2015. Apparent competition between major pests reduces pest population densities on tomato crop, but not yield loss. Journal of Pest Science 88(4): 793–803. doi: 10.1007/s10340-015-0698-3 [DOI] [Google Scholar]
- 35.Mollà O, Biondi A, Alonso-Valiente M, Urbaneja A. 2014. A comparative life history study of two mirid bugs preying on Tuta absoluta and Ephestia kuehniella eggs on tomato crops: implications for biological control. Biological Control 59: 175–183. [Google Scholar]
- 36.Calvo FJ, Bolckmans K, Belda JE. 2009. Development of a biological control based Integrated Pest Management method for Bemisia tabaci for protected sweet pepper crops. Entomologia Experimentalis Applicata 133: 9–18. [Google Scholar]
- 37.Castañé C, Arnò J, Gabarra R, Alomar O. 2011. Plant damage to vegetable crops by zoophytophagous mirid predators. Biological Control 59: 22–29. [Google Scholar]
- 38.Parolin P, Bresch C, Poncet C, Desneux N. 2012. Functional characteristics of secondary plants for increased pest management. International Journal of Pest Management 58 (4): 369–377. doi: 10.1080/09670874.2012.734869 [DOI] [Google Scholar]
- 39.Saeed R, Razaq M, Hardy IC. 2015. The importance of alternative host plants as reservoirs of the cotton leafhopper, Amrasca devastans, and its natural enemies. Journal of Pest Science 88: 517–531. [Google Scholar]
- 40.Finke DL, Denno RF. 2002. Intraguild predation diminished in complex-structured vegetation: implications for prey suppression. Ecology, 83 (3): 643–652. [Google Scholar]
- 41.Nakaishi K, Fukui Y, Arakawa R. 2011. Reproduction of Nesidiocoris tenuis (Reuter) on Sesame. Journal of Applied Entomology and Zoology 55: 199–205. [Google Scholar]
- 42.Biondi A, Zappalà L, Di Mauro A, Tropea Garzia G, Russo A, Desneux N, et al. 2016. Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? Biological Control 61 (1): 79–90. [Google Scholar]
- 43.Lucas E, Coderre D, Brodeur J. 1998. Intraguild predation among aphid predator’s characterization and influence of intermediate prey density. Ecology 79: 1084–1092. [Google Scholar]
- 44.Chang GC. 1996. Comparison of single versus multiple species of generalist predators for biological control. Environmental Entomology 25: 207–212. [Google Scholar]
- 45.Chailleux A, Bearez P, Pizzol J, Amiens-Desneux E, Ramirez-Romero R, Desneux N. 2013. b. Potential for combined use of parasitoids and generalist predators for biological control of the key invasive tomato pest Tuta absoluta. Journal of Pest Science 86: 533–541. [Google Scholar]
- 46.Bao-Fundora L, Ramirez-Romero R, Sánchez-Hernández CV, Sánchez Martínez J, Desneux N. 2016. Intraguild predation of Geocoris punctipes on Eretmocerus eremicus and its influence on the control of the whitefly Trialeurodes vaporariorum. Pest management science 72 (6): 1110–1116. doi: 10.1002/ps.4163 [DOI] [PubMed] [Google Scholar]
- 47.Pérez-Valencia LI, Camorlinga-Cortés P, Carrillo-Arámbula LC, Palmeros-Suárez PA, Ramirez-Romero R. 2019. Why can a predator increase its consumption of prey when it is released along with a parasitoid? Entomologia Generalis 39: 205–219. doi: 10.1127/entomologia/2019/0810 [DOI] [Google Scholar]
- 48.Sánchez-Hernández J, Finstad AG, Arnekleiv JV, Kjærstad G, Amundsen P-A. 2021. Beyond ecological opportunity: Prey diversity rather than abundance shapes predator niche variation. Freshwater Biology 66: 44–61. doi: 10.1111/fwb.13606 [DOI] [Google Scholar]
- 49.DeBach P, Rosen D. 1991. Biological Control by Natural Enemies. London: Cambridge Univ. Press. 440 pp. [Google Scholar]
- 50.Van Driesche RG, Bellows TS. 1996. Biological Control. New York: Chapman & Hall/ITP. 539 pp. doi: 10.1093/jmedent/33.5.812 [DOI] [Google Scholar]
- 51.Sunderland KD, Axelsen JA, Dromph K, Freier B, Hemptinne J-L, et al. 1997. Pest control by a community of natural enemies. Acta Jutl. 72: 271–326. [Google Scholar]
- 52.Sunderland KD. 2001. Invertebrate pest control by carabids. The Agroecology of Carabid Beetles: 165–214. [Google Scholar]
- 53.Desneux N, O’Neil RJ. 2008. Potential of an alternative prey to disrupt predation of the generalist predator, Orius insidiosus, on the pest aphid, Aphis glycines, via short-term indirect interactions. Bulletin of Entomological Research 98: 631–639. doi: 10.1017/S0007485308006238 [DOI] [PubMed] [Google Scholar]
- 54.Desneux N, Kaplan I, Yoo HJS, Wang S, O’Neil RJ. 2019. Temporal synchrony mediates the outcome of indirect effects between prey via a shared predator. Entomologia Generalis 39 (2): 127–136. [Google Scholar]
- 55.Eubanks MD, Styrsky JD, Denno RF. 2003. The evolution of omnivory in heteropteran insects. Ecology 84: 2549–2556. [Google Scholar]
- 56.Vance-Chalcraft HD, Soluk DA. 2005. Multiple predator effects result in risk reduction for prey across multiple prey densities. Oecologia 144(3): 472–480. doi: 10.1007/s00442-005-0077-5 [DOI] [PubMed] [Google Scholar]
- 57.Liu C, Yan L, Li H, Wang G. 2006. Effects of predator-mediated apparent competition on the population dynamics of Tetranychus urticae on apples. Biocontrol 51: 453–463. doi: 10.1007/s10526-005-4363-z [DOI] [Google Scholar]
- 58.Harwood JD, Desneux N, Yoo HJS, Rowley DL, Greenstone MH, Obrycki JJ, et al. 2007. Tracking the role of alternative prey in soybean aphid predation by Orius insidiosus: a molecular approach. Molecular Ecology 16 (20): 4390–4400. doi: 10.1111/j.1365-294X.2007.03482.x [DOI] [PubMed] [Google Scholar]
- 59.Settle WH, Ariawan H, Astuti ET, Cahyana W, Hakim AL, Hindayana D, et al. 1996. Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology 77: 1975–1988. doi: 10.2307/2265694 [DOI] [Google Scholar]
- 60.Perdikis D, Lykouressis D, Economou L. 2004. Influence of Light-Dark Phase, Host Plant, Temperature, and Their Interactions on the Predation Rate in an Insect Predator. Environmental Entomology 33 (5): 1137–1144. doi: 10.1603/0046-225X-33.5.1137 [DOI] [Google Scholar]
- 61.Polis G A, Myers CA. 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annual Review of Ecology and Systematics 20: 297–330. [Google Scholar]
- 62.Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA. 1995. Intraguild predation among biological control agents: theory and evidence. Biological Control 5: 303–335. [Google Scholar]
- 63.Holt RD, Huxel GR. 2007. Alternative prey and the dynamics of intraguild predation: theoretical perspectives. Ecology 88: 2706–2712. doi: 10.1890/06-1525.1 [DOI] [PubMed] [Google Scholar]
- 64.Lucas E. 2005. Intraguild predation among aphidophagous predators. European Journal of Entomology 102: 351–364. [Google Scholar]
- 65.Lucas E. 2012. Intraguild Interactions. Ecology and Behavior of the Ladybird Beetles (Coccinellidae) 343–374. doi: 10.1002/9781118223208.ch7 [DOI] [Google Scholar]
- 66.Moreno-Ripoll R, Agustí N, Berruezo R, Gabarra R. 2012. Conspecific and heterospecific interactions between two omnivorous predators on tomato. Biological Control 62: 189–196. [Google Scholar]
- 67.Lucas E, Rosenheim J. 2011. Influence of extraguild prey density on intraguild predation by heteropteran predators: A review of the evidence and a case study. Biological Control 59 (1): 61–67. doi: 10.1016/j.biocontrol.2011.05.010 [DOI] [Google Scholar]
- 68.Walzer A, Moder K, Schausberger P. 2009. Spatiotemporal within-plant distribution of the spider mite Tetranychus urticae and associated specialist and generalist predators. Bulletin of Entomological Research 99: 457–466. doi: 10.1017/S0007485308006494 [DOI] [PubMed] [Google Scholar]
- 69.Lucas E, Alomar O. 2002. Impact of the presence of Dicyphus tamaninii Wagner (Heteroptera: Miridae) on whitefly (Homoptera: Aleyrodidae) predation by Macrolophus caliginosus (Wagner) (Heteroptera: Miridae). Biological Control 25: 123–128. doi: 10.1603/0022-0493-95.6.1123 [DOI] [PubMed] [Google Scholar]
- 70.Moreno-Ripoll R, Gabarra R, Symondson WOC, King RA, Agustí N. 2014. Do the interactions among natural enemies compromise the biological control of the whitefly Bemisia tabaci? Journal of Pest Science 87: 133–141. [Google Scholar]
- 71.Zhu P, Lu Z, Heong K, Chen G, Zheng X et al. 2014. Selection of nectar plants for use in ecological engineering to promote biological control of rice pests by the predatory bug, Cyrtorhinus lividipennis, (Heteroptera: Miridae). PLoS ONE 9(9): e108669.doi: 10.1371/journal.pone.0108669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanchez JA. 2009. Density thresholds for Nesidiocoris tenuis (Heteroptera: Miridae) in tomato crops. Biological Control 51: 493–498. [Google Scholar]
- 73.Gillespie DR, McGregor RR. 2000. The functions of plant feeding in the omnivorous predator Dicyphus hesperus: water places limits on predation. Ecological Entomology 25: 380–386. [Google Scholar]
- 74.Ahirwar RM, Gupta MP, Banerjee S. 2010. Field efficacy of natural and indigenous products on sucking pests of Sesame. Indian Journal Natural Products and Resources 1: 221–226. [Google Scholar]
- 75.Naselli M, Zappala L, Gugliuzzo A, Garzia GT, Biondi A, Rapisarda C, et al. 2017. Olfactory response of the zoophytophagous mirid Nesidiocoris tenuis to tomato and alternative host plants. Arthropod-Plant Interactions 11: 121–131. [Google Scholar]
- 76.Nerio LS, Olivero-Verbel J, Stashenko E. 2010. Repellent activity of essential oils: a review. Bioresource Technologie 101: 372. doi: 10.1016/j.biortech.2009.07.048 [DOI] [PubMed] [Google Scholar]
- 77.Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. 2008. Shared signals–‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol Lett 11: 24–34. doi: 10.1111/j.1461-0248.2007.01123.x [DOI] [PubMed] [Google Scholar]
- 78.Perdikis D, Lykouressis D. 2000. Effects of various items, host plants and temperatures on the development and survival of Macrolophus pygmaeus Rambur (Heteroptera: Miridae). Biological Control 17: 55–60. [Google Scholar]
- 79.Arnó J, Castañé C, Riudavets J, Gabarra R. 2010. Risk of damage to tomato crops by the generalist zoophytophagous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). Bulletin of Entomology Research 100: 105–115. [DOI] [PubMed] [Google Scholar]
- 80.Chailleux A, Wajnberg E, Zhou Y, Amiens-Desneux E, Desneux N. 2014. New parasitoid-predator associations: female parasitoids do not avoid competition with generalist predators when sharing invasive prey. Naturwissenschaften 101: 1075–1083. doi: 10.1007/s00114-014-1246-3 [DOI] [PubMed] [Google Scholar]
- 81.Janssen A, Pallini A, Venzon M, Sabelis MW. 1999. Absence of odour-mediated avoidance of heterospecific competitors by the predatory mite Phytoseiulus persimilis. Entomolgia Experimentalis et Applicata 92: 73–82. [Google Scholar]
- 82.Vet LE. 1999. From chemical to population ecology: info chemical use in an evolutionary context. Journal of Chemical Ecology 25(1): 31–49. [Google Scholar]
- 83.Aparicio Y, Gabarra R, Arno J. 2020. Interactions among Myzus persicae, predators and parasitoids may hamper biological control in Mediterranean peach orchards. Entomologia Generalis 40: 217–228. [Google Scholar]
- 84.Chen X, Jaworski CC, Dai H. et al. 2021. Combining banker plants to achieve long-term pest control in multi-pest and multi-natural enemy cropping systems. Journal of Pest Science doi: 10.1007/s10340-021-01405-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mansour R, Biondi A. 2021. Releasing natural enemies and applying microbial and botanical pesticides for managing Tuta absoluta in the MENA region. Phytoparasitica 49: 179–194. [Google Scholar]
- 86.Siscaro G, Lo Pumo C, Tropea Garzia G, Tortorici S, Gugliuzzo A, Ricupero M, et al. 2019. Temperature and tomato variety influence the development and the plant damage induced by the zoophytophagous mirid bug Nesidiocoris tenuis. Journal of Pest Science 92: 1049–1056. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper.