Abstract
The gene (aroA) of Actinobacillus pleuropneumoniae, serotype 2, encoding 5-enolpyruvylshikimate-3-phosphate synthase was cloned by complementation of the aroA mutation in Escherichia coli K-12 strain AB2829, and the nucleotide sequence was determined. A pair of primers from the 5′ and 3′ termini were selected to be the basis for development of a specific PCR assay. A DNA fragment of 1,025 bp was amplified from lysed A. pleuropneumoniae serotypes 1 to 12 of biovar 1 or from isolated DNA. No PCR products were detected when chromosomal DNAs from other genera were used as target DNAs; however, a 1,025-bp DNA fragment was amplified when Actinobacillus equuli chromosomal DNA was used as a target, which could be easily differentiated by its NAD independence. The PCR assay developed was very sensitive, with lower detection limits of 12 CFU with A. pleuropneumoniae cells and 0.8 pg with extracted DNA. Specificity and sensitivity make this PCR assay a useful method for the rapid identification and diagnosis of A. pleuropneumoniae infections.
Actinobacillus pleuropneumoniae causes a highly contagious respiratory disease in pigs, entailing considerable economic losses for the pig-raising industry world-wide. Detailed studies of the clinical symptoms of the disease and its characteristic lung lesions, its experimental induction in pigs with viable and sonicated A. pleuropneumoniae, and the endobronchial inoculation of Apx toxins exist (7, 16, 20, 25). The virulence of A. pleuropneumoniae may be considered multifactorial, as is the case with most pathogenic bacteria; the factors involved in pathogenesis include capsular polysaccharides (13), lipopolysaccharides (2), membrane proteins (9, 10), adhesion factors (5), exotoxins (16, 23), and urease (3). Epidemiological data suggest, however, that virulence is strongly correlated with the presence of Apx toxins, which may produce lung lesions similar to those caused by natural infection. A. pleuropneumoniae strains are grouped into two biovars, biovar 1 (β-NAD dependent) and biovar 2 (β-NAD independent), the former generally being the more virulent (15, 21). Biovar 1 includes 12 serotypes based on capsular polysaccharide structure, although there may be considerable variation in prevalence, presence, and virulence. Indeed, serotypes 1 and 5 are prevalent in North America, where they are responsible for major outbreaks of A. pleuropneumoniae-associated disease with high rates of mortality, whereas serotypes 2 and 9, which have been isolated in European countries, are less virulent and cause less mortality, although they produce lung lesions similar to those produced by serotypes 1 and 5 (8, 18). A. pleuropneumoniae has been detected in vivo by serological tests such as enzyme-linked immunosorbent assays and complement fixation. Conventional cultivation of A. pleuropneumoniae has also been improved by the development of selective media (14, 26). However, detection of this bacterium by DNA PCR amplification has proved to be more sensitive than cultivation. While in some cases specificity was incomplete, in others it allowed for unambiguous detection and identification (12, 27). In this study, we describe the molecular cloning and sequence of the A. pleuropneumoniae aroA gene and its use as a target DNA to be amplified by PCR assay, which results in a specific, rapid, simple, and sensitive nucleic acid-based procedure for identifying A. pleuropneumoniae.
Cloning and sequencing of the aroA gene of A. pleuropneumoniae serotype 2.
Sau3A partial digestion on A. pleuropneumoniae serotype 2 chromosomal DNA, prepared as reported previously (22), generated fragments which were fractionated by agarose gel electrophoresis. Fragments of 3 to 9 kbp were selected to construct a genomic library in BamHI-digested dephosphorylated plasmid vector pUC18 (Pharmacia), and the recombinant plasmids were used to transform the electroporated Escherichia coli aroA mutant AB2829 as described elsewhere (6, 24, 28). A library of 7,000 Apr colonies was obtained when these bacteria were plated on Luria agar (LA) medium supplemented with ampicillin and incubated at 37°C for 24 h. Two recombinant clones which complemented the E. coli aroA defect were isolated by replica plating of transformants onto minimal medium (24) supplemented with ampicillin and incubation at 37°C for 48 h. Recombinant plasmid DNA from both well-grown clones was isolated with a Wizard Plus Minipreps DNA purification system (Promega Corp.) and used to retransform E. coli AB2829 to confirm the ability of the plasmids to complement the E. coli AB2829 defect when plated on defined minimal medium. Both recombinant plasmids (designated pAP1 and pAP2) were able to complement the growth of E. coli AB2829. A restriction map of pAP2 is shown in Fig. 1. The nucleotide sequence of the 2.3-kb HindIII-EcoRI fragment of pAP2 (Fig. 2), determined by the dideoxynucleotide chain termination method with double-stranded templates by means of the fmol DNA sequencing system (Promega Corp.), revealed an open reading frame downstream of a HindIII site of 1,296 nucleotides, which encodes a protein of 432 amino acids (Fig. 2). The deduced molecular weight is 47,028, and the G+C content of the aroA coding region product is 43.65%. The predicted amino acid sequence of A. pleuropneumoniae AroA (5-enolpyruvylshikimate-3-phosphate [EPSP] synthase; EC 2.5.1.19) showed a high degree of homology to AroA proteins of Haemophilus influenzae and Pasteurella haemolytica (84.95% and 85.42%, respectively), the other two genera of the Pasteurellaceae family. Furthermore, a high degree of amino acid sequence conservation was detected when A. pleuropneumoniae EPSP synthase was aligned with other bacterial EPSP synthases by means of the CLUSTAL multiple-alignment program (Fig. 3).
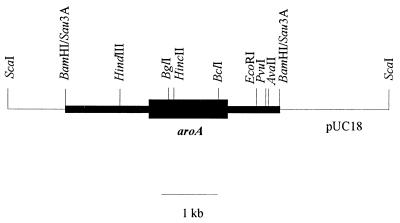
FIG. 1.
Restriction endonuclease map of the aroA locus obtained with plasmid pAP2. Solid boxes represent A. pleuropneumoniae-cloned DNA. The thicker box is the A. pleuropneumoniae aroA gene, which was oriented from 5′ (left) to 3′ (right). The thin line represents pUC18 plasmid DNA.
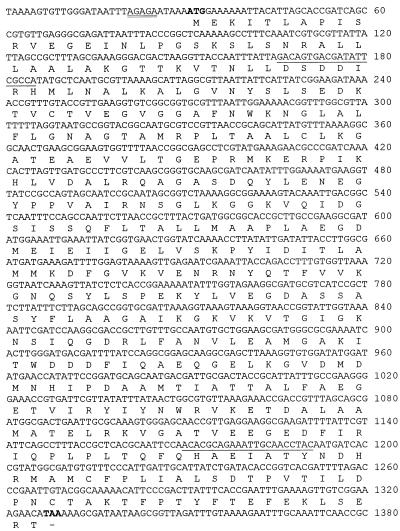
FIG. 2.
Nucleotide sequence of the aroA gene and the amino acid sequence deduced from the open reading frame of the aroA gene. DNA bases (top line) and amino acids (one-letter code) (below) are shown, and nucleotides are numbered to the right of the sequences. The ATG initiation code (boldface) is preceded by a potential Shine-Dalgarno sequence (double underlining). Primers which have been used for PCR analysis are underlined. The dash indicates the TGA termination codon (boldface).
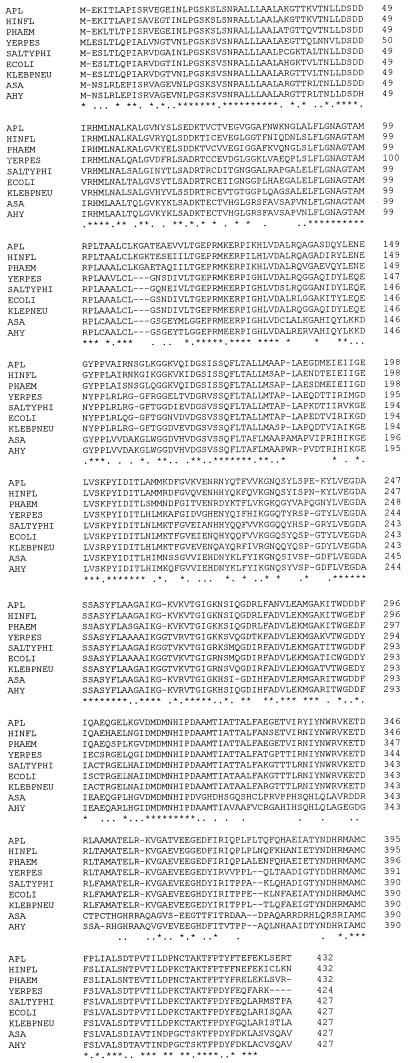
FIG. 3.
CLUSTAL computer alignment of the deduced amino acid sequences encoded by the aroA gene from A. pleuropneumoniae (APL), H. influenzae (HINFL), P. haemolytica (PHAEM), Yersinia pestis (YERPES), Salmonella typhimurium (SALTYPHI), E. coli (ECOLI), K. pneumoniae (KLEBPNEU), Aeromonas salmonicida (ASA), and A. hydrophila (AHY). Identical amino acids in all species are indicated by an asterisk; conservative substitutions are indicated by a dot.
PCR amplification of the aroA gene from A. pleuropneumoniae.
Samples to be analyzed by PCR were either cultured bacteria or bacterium-extracted DNA. Actinobacillus strains were grown on brain heart infusion agar or broth (Biolife) supplemented with 0.1% NAD (wt/vol) when needed. Aeromonas hydrophila SO2/2 (4), used as a negative control in PCR assays, was grown on Luria broth or LA (19). E. coli strains were grown on Luria broth or LA. Haemophilus parasuis (field isolate) was grown on chocolate agar containing 1% IsoVitaleX (BBL Microbiology Systems, Cockeysville, Md.). P. haemolytica ATCC 33396, Pasteurella multocida ATCC 12048, and Klebsiella pneumoniae (patient isolate) were grown on blood agar plates (tryptic soy agar with 5% sheep erythrocytes). All strains were routinely cultivated at 37°C except for A. hydrophila, which was incubated at 28°C. PCR amplification was carried out with a DNA thermal cycler (Perkin-Elmer Cetus) and a PCR kit (Boehringer) with some modification of the manufacturer’s instructions. Briefly, the reaction mixture consisted of 1 μl of DNA-containing sample, 1.25 U of Taq DNA polymerase, 5 μl of 10× PCR buffer (100 mM Tris-HCl, 20 mM MgCl2, 500 mM KCl [pH 8.3]), a 1 μM concentration of each primer, 0.5 mM concentrations of deoxynucleoside triphosphates, and double-distilled water to a final volume of 50 μl. To minimize evaporation, 50 μl of mineral oil was added to the mixture. DNA denaturation was carried out at 94°C for 2 min, and then a total of 40 cycles were run under the following conditions: DNA denaturation at 92°C for 1 min, primer annealing at 58°C for 30 s, and DNA extension at 72°C for 40 s. After the final cycle, reactions were terminated by an extra run at 72°C for 5 min. Reactions were kept at 4°C until analyzed by endonuclease digestion and agarose gel electrophoresis (2.5% agarose gels with a Tris-borate-EDTA buffer). The pair of primers used in this study, FAP (23-nucleotide-long forward primer, 5′-GCCGCTTTAGCGAAAGGGACGAC-3′, corresponding to positions 94 to 116 of the aroA gene nucleotide sequence) and RAP (22-nucleotide-long reverse primer, 5′-GTAGGTTGCAATTTCTGCGTGT-3′, which corresponds to positions 1,140 to 1,161 of the aroA gene nucleotide sequence), successfully primed the synthesis of an expected 1,025-bp DNA fragment, which represents most of the aroA gene sequence (Fig. 2) of all 12 serotypes of A. pleuropneumoniae currently recognized and also tested for this study (Fig. 4). No PCR amplification product was obtained when E. coli C600-1, A. hydrophila, P. haemolytica, P. multocida, K. pneumoniae, and H. influenzae cells were used as sources of target DNA, with the exception of Actinobacillus equuli NCTC 8529, which rendered an identical band after PCR amplification (Fig. 4). Negative results were obtained when cells from other Actinobacillus species (A. lignieresii NCTC 4189, A. ureae NCTC 10219, A. capsulatus P1364, A. suis CCM 5586, and A. rossii NCTC 10801) were used as target DNAs for PCR amplification of the aroA gene (data not shown). A single 1,025-bp band was obtained with the 500-bp Sau3A fragment of the aroA gene cloned from A. pleuropneumoniae serotype 1 (data not shown). PCR assay sensitivity was evaluated by making a serial dilution of A. pleuropneumoniae cells in suspension, as detailed in reference 4. Amplification resulting in detectable levels of PCR product was achieved when a minimum of 12 CFU of A. pleuropneumoniae was lysed or 0.8 pg of extracted DNA was used.
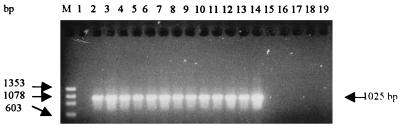
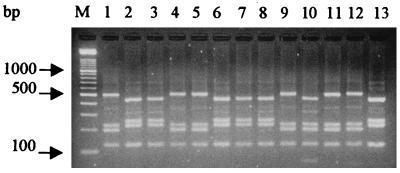
FIG. 4.
Agarose gel electrophoresis of PCR amplification products from total DNA from E. coli (lane 1), A. pleuropneumoniae serotypes 1 to 12 (ATCC 27088, ATCC 27089, ATCC 27090, NCTC 11384, NCTC 11383, ATCC 33590, WF 83, 405, CVJ 13261, D 13039, 16153, and 8329, respectively) (lanes 2 to 13), A. equuli NCTC 8529 (lane 14), H. influenzae ATCC 35056 (lane 15), P. haemolytica ATCC 33396 (lane 16), P. multocida ATCC 12048 (lane 17), K. pneumoniae (patient isolate) (lane 18), and A. hydrophila SO2/2 (lane 19). M, standard DNA size markers (HaeIII-digested φX174).
RFLP study.
The 1,025-bp PCR-amplified products, which represent most of the aroA gene sequence, from all of the A. pleuropneumoniae serotypes and the A. equuli cells used in this study, were analyzed with restriction endonucleases. From the restriction enzyme digestion of these PCR products, we found three different restriction fragment length polymorphism (RFLP) patterns with Sau3A (Fig. 5). Serotypes 1, 4, 5, 9, 11, and 12 presented RFLP pattern 1, which rendered fragments of 500, 220, 190, and 115 bp when PCR products were digested with Sau3A. Serotypes 2, 3, 6, 7, and 8, as well as A. equuli, presented RFLP pattern 2, with fragment sizes of 450, 240, 220, and 115 bp after Sau3A digestion. RFLP pattern 3 was presented by serotype 10, which rendered fragments of 450, 220, 190, 115, and 50 bp. Identical grouping was obtained when aroA-PCR-amplified products from all A. pleuropneumoniae serotypes were restricted with HpaII endonuclease, with the exception of serotype 7, which presented a clearly different RFLP pattern. In this case, serotype 10 was grouped within RFLP 2 serotypes (data not shown).
FIG. 5.
Agarose gel electrophoresis of fragments produced by Sau3A digestion of A. pleuropneumoniae aroA genes amplified by PCR. Lanes 1 to 12, serotypes 1 to 12; lane 13, A. equuli. M, standard DNA size markers (HaeIII-digested φX174).
Discussion.
In this study, we developed a method for the rapid and easy identification of A. pleuropneumoniae. The aroA gene, encoding 5-enolpyruvylshikimate-3-phosphate synthase, contributes to aromatic amino acids and the folic acid universal pathway in bacteria and has been used successfully for taxonomic purposes before. The Aeromonas genus was analyzed by PCR-RFLP of the aroA gene (4) with good results. PCR-RFLP of the aroA gene has also been used for identifying and typing Staphylococcus aureus (29). The results obtained in each case were related to genetic diversity of strains and species. Several PCR assays have been proposed for rapid detection and identification of A. pleuropneumoniae (11, 12, 17, 27); the one proposed here is able to rapidly identify all 12 serotypes of A. pleuropneumoniae so far described by PCR amplification of an expected 1,025-bp fragment representing most of the aroA gene sequence. No other species or genera yielded positive reactions in our PCR assays, with the exception of A. equuli. PCR amplification of the A. pleuropneumoniae aroA gene is further enhanced when combined with RFLP after Sau3A PCR amplification product digestion, which allows reasonable discrimination among serotypes. This PCR-RFLP combination allowed A. pleuropneumoniae to be divided into three groups: group 1, with serotypes 1, 4, 5, 9, 11, and 12; group 2, with serotypes 2, 3, 6, 7, and 8 and A. equuli; and group 3, with serotype 10. The fact that A. equuli gave a positive PCR amplification did not present a problem, since A. equuli can be easily differentiated by its NAD-independent growth. These results accord well with those of Gram and Ahrens (11), in whose study the 12 serotypes of A. pleuropneumoniae were divided into three groups based on omlA gene nucleotide sequences of reference serotypes. Some discrepancies with Gram and Ahrens’ results could be observed; serotypes 5 and 10, for example, are closely related on the basis of omlA gene sequences (11), although our PCR-RFLP procedure assigns them to different groups. Moreover, omlA gene nucleotide sequences would place serotype 4 in our group 2 and serotype 2 in our group 1. These discrepancies have also been observed when groupings based on omlA nucleotide sequences and Apx toxin patterns are compared (1, 11). In conclusion, our PCR-RFLP assay may be considered a useful taxonomic tool for the identification of A. pleuropneumoniae because of its simplicity, specificity, and sensitivity and because it has a reasonable discriminatory power among serotypes. Furthermore, based on its high sensitivity, our PCR-RFLP test may be used to detect A. pleuropneumoniae in clinical samples.
Acknowledgments
This work was supported by a grant from Spanish Ministry of Education and Culture (Plan Nacional de I + D AGF98-0187).
REFERENCES
- 1.Beck M, Van Den Bosch J F, Jongeneleen I M C A, Loeffen P L W, Nielsen R, Nicolet J, Frey J. RTX toxin genotypes and phenotypes in Actinobacillus pleuropneumoniae field strains. J Clin Microbiol. 1994;32:2749–2754. doi: 10.1128/jcm.32.11.2749-2754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bélanger M, Bégin C, Jacques M. Lipopolysaccharides of Actinobacillus pleuropneumoniae bind pig hemoglobin. Infect Immun. 1995;63:656–662. doi: 10.1128/iai.63.2.656-662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossé J T, MacInnes J I. Genetic and biochemical analyses of Actinobacillus pleuropneumoniae urease. Infect Immun. 1997;65:4389–4394. doi: 10.1128/iai.65.11.4389-4394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cascón A, Anguita J, Hernanz C, Sánchez M, Fernández M, Naharro G. Identification of Aeromonas hydrophila hybridization group 1 by PCR assays. Appl Environ Microbiol. 1996;62:1167–1170. doi: 10.1128/aem.62.4.1167-1170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dom P, Haesebrouck F, Ducatelle R, Charlier G. In vivo association of Actinobacillus pleuropneumoniae serotype 2 with the respiratory epithelium of pigs. Infect Immun. 1994;62:1262–1267. doi: 10.1128/iai.62.4.1262-1267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;7:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenwick B, Henry S. Porcine pleuropneumonia. J Am Vet Med Assoc. 1994;204:1334–1340. [PubMed] [Google Scholar]
- 8.Frey J. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 1995;3:257–261. doi: 10.1016/s0966-842x(00)88939-8. [DOI] [PubMed] [Google Scholar]
- 9.Gerlach G F, Anderson C, Potter A A, Klashinsky S, Willson P J. Cloning and expression of a transferrin-binding protein from Actinobacillus pleuropneumoniae. Infect Immun. 1992;60:892–898. doi: 10.1128/iai.60.3.892-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González G C, Caamano D L, Schryvers A B. Identification and characterization of a porcine-specific transferrin receptor in Actinobacillus pleuropneumoniae. Mol Microbiol. 1990;4:1173–1179. doi: 10.1111/j.1365-2958.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 11.Gram T, Ahrens P. Improved diagnostic PCR assay for Actinobacillus pleuropneumoniae based on the nucleotide sequence of an outer membrane lipoprotein. J Clin Microbiol. 1998;36:443–448. doi: 10.1128/jcm.36.2.443-448.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gram T, Ahrens P, Nielsen J P. Evaluation of a PCR for detection of Actinobacillus pleuropneumoniae in mixed bacterial cultures from tonsils. Vet Microbiol. 1996;51:95–104. doi: 10.1016/0378-1135(96)00013-2. [DOI] [PubMed] [Google Scholar]
- 13.Inzana T J, Todd J, Veit H P. Safety, stability, and efficacy of noncapsulated mutants of Actinobacillus pleuropneumoniae for use in live vaccines. Infect Immun. 1993;61:1682–1686. doi: 10.1128/iai.61.5.1682-1686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen M J, Nielsen J P. Development and evaluation of a selective and indicative medium for isolation of Actinobacillus pleuropneumoniae. Vet Microbiol. 1995;47:191–197. doi: 10.1016/0378-1135(95)00062-f. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen M J, Nielsen J P, Nielsen R. Comparison of virulence of different Actinobacillus pleuropneumoniae serotypes and biotypes using an aerosol infection model. Vet Microbiol. 1996;49:159–168. doi: 10.1016/0378-1135(95)00184-0. [DOI] [PubMed] [Google Scholar]
- 16.Kamp E M, Stockhofe-Zurwieden N, Van Leengoed L A M G, Smits M A. Endobronchial inoculation with Apx toxins of Actinobacillus pleuropneumoniae leads to pleuropneumonia in pigs. Infect Immun. 1997;65:4350–4354. doi: 10.1128/iai.65.10.4350-4354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo T M, Ward C K, Inzana T J. Detection and identification of Actinobacillus pleuropneumoniae serotype 5 by multiplex PCR. J Clin Microbiol. 1998;36:1704–1710. doi: 10.1128/jcm.36.6.1704-1710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maccines J I, Smart N L. Actinobacillus and Haemophilus. In: Gyles C L, Thoen C O, editors. Pathogenesis of bacterial infections in animals. Ames: Iowa State University Press; 1993. pp. 188–200. [Google Scholar]
- 19.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 20.Nicolet J. Actinobacillus pleuropneumoniae. In: Leman A D, Straw B, Mengeling W L, D’Allaire S, Taylor D J, editors. Diseases of swine. 7th ed. Ames: Iowa State University Press; 1992. pp. 401–408. [Google Scholar]
- 21.Niven D F, O’Reilly T. Significance of V-factor dependency in the taxonomy of Haemophilus species and related organisms. Int J Syst Bacteriol. 1990;40:1–4. doi: 10.1099/00207713-40-1-1. [DOI] [PubMed] [Google Scholar]
- 22.Priefer U, Simons R, Puhler A. Cloning with cosmids. In: Timmis K N, Duhler A, editors. Advanced molecular genetics. Berlin, Germany: Springer-Verlag KG; 1986. pp. 190–201. [Google Scholar]
- 23.Reimer D, Frey J, Jansen R, Veit H P, Inzana T J. Molecular investigation of the role of ApxI and ApxII in the virulence of Actinobacillus pleuropneumoniae serotype 5. Microb Pathog. 1995;18:197–209. doi: 10.1016/s0882-4010(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 25.Sebunya T N K, Saunders J R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983;182:1331–1337. [PubMed] [Google Scholar]
- 26.Sidibé M, Messier S, Lariviére S, Gottschalk M, Mittal K R. Detection of Actinobacillus pleuropneumoniae in the porcine upper respiratory tract as a complement to serological tests. Can J Vet Res. 1993;57:204–208. [PMC free article] [PubMed] [Google Scholar]
- 27.Sirois M, Lemire E G, Levesque R C. Construction of a DNA probe and detection of Actinobacillus pleuropneumoniae by using polymerase chain reaction. J Clin Microbiol. 1991;29:1183–1187. doi: 10.1128/jcm.29.6.1183-1187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 29.Yugueros Marcos J, Cascón Soriano A, Sánchez Salazar M, Hernanz Moral C, Suárez Ramos S, Smeltzer M S, Naharro Carrasco G. Rapid identification and typing of Staphylococcus aureus by PCR-restriction fragment length polymorphism analysis of the aroA gene. J Clin Microbiol. 1999;37:570–574. doi: 10.1128/jcm.37.3.570-574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]