Abstract
Background
The aim of this study was to assess the association between endothelial function and early‐onset cryptogenic ischemic stroke (CIS), with subgroup analyses stratified by sex and age groups.
Methods and Results
We prospectively enrolled 136 consecutive patients aged 18 to 49 years (median age, 41 years; 44% women) with a recent CIS and 136 age‐ and sex‐matched (±5 years) stroke‐free controls. Endothelial function was measured with an EndoPAT 2000 device and analyzed as tertiles of natural logarithm of reactive hyperemia index with lower values reflecting dysfunction. We used conditional logistic regression adjusting for age, education, hypertension, diabetes mellitus, dyslipidemia, current smoking, heavy drinking, obesity, and diet score to assess the independent association between endothelial function and CIS. Patients in the lowest tertile of natural logarithm of reactive hyperemia index were more often men and they more frequently had a history of dyslipidemia; they were also more often obese, had a lower diet score, and lower high‐density lipoprotein cholesterol. In the entire cohort, we found no association in patients with endothelial function and CIS compared with stroke‐free controls. In sex‐ and age‐specific analyses, endothelial dysfunction was associated with CIS in men (adjusted odds ratio [OR], 3.50 for lowest versus highest natural logarithm of reactive hyperemia index tertile; 95% CI, 1.22–10.07) and in patients ≥41 years (OR, 5.78; 95% CI, 1.52–21.95). These associations remained significant when dyslipidemia was replaced with the ratio of total to high‐density lipoprotein cholesterol.
Conclusions
Endothelial dysfunction appears to be an independent player in early‐onset CIS in men and patients approaching middle age.
Keywords: brain infarction, cryptogenic stroke, endothelial function, ischemic stroke, microcirculation, risk factors, young adults
Subject Categories: Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- CIS
cryptogenic ischemic stroke
- FMD
flow‐mediated dilation
- GA
glycated albumin
- LnRHI
natural logarithm of reactive hyperemia index
- NIHSS
National Institutes of Health Stroke Scale
- PAT
peripheral arterial tonometry
- SECRETO
Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Etiology, Triggers, and Outcome
- WHO
World Health Organization
Clinical Perspective
What Is New?
Patients with early‐onset cryptogenic ischemic stroke and endothelial dysfunction more often have risk factors including smoking, obesity, sedentary lifestyle, and lower high‐density lipoprotein cholesterol levels than those without endothelial dysfunction.
Endothelial dysfunction is associated with early‐onset cryptogenic ischemic stroke in men and slightly older individuals independent of cardiovascular risk factors.
What Are the Clinical Implications?
Although the results give a clue regarding disease mechanism, more research is warranted to explore whether targeting secondary prevention on endothelial dysfunction also improves long‐term outcomes in this patient group.
The impact of stroke in young individuals is more multidimensional than in the elderly, affecting family, social life, and working ability for years after the event. Because of yet largely unstudied reasons, the incidence of ischemic stroke at younger ages has been increasing for several decades.1 Furthermore, young patients need more extensive pathogenic evaluation because of the manifold causes of their ischemic stroke.2 However, a European study of patients with first‐ever early‐onset ischemic stroke showed that strokes with a cryptogenic cause are more common the younger the patients, with an average proportion of ≈50%.3 Several studies have shown high rates of recurrence in the young, even in patients with cryptogenic ischemic stroke (CIS), suggesting an active underlying mechanism and unmet needs in secondary prevention.4, 5 Better knowledge of pathways leading to CIS could lead to more optimal secondary prevention, thus improving long‐term outcome after CIS.
Endothelium plays a key role in various actions, such as regulation of vascular tone, modulation of inflammation, platelet aggregation, and coagulation. There are various methods to investigate endothelial function, of which flow‐mediated dilation (FMD) test in the brachial artery and peripheral arterial tonometry (PAT) are the most commonly used.6 Previous studies have found good correlation of repeated measurements of endothelial function with PAT and the method has been well validated.7, 8, 9, 10
Based on studies in vascular disease in other arterial beds, it is plausible to hypothesize that endothelial dysfunction also contributes to cerebrovascular disease and stroke.6, 11 However, these associations have gone virtually uninvestigated in young patients with ischemic stroke. One study including young patients with spontaneous cervical artery dissection showed an impaired FMD that was not the result of stroke.12 Another study with middle‐aged patients with ischemic stroke also showed an impairment of FMD but no significant differences between stroke subtypes.13 A few studies focusing on young stroke‐free individuals showed that male sex, increasing age, body mass, binge drinking, smoking, diabetes mellitus, and ratio of total to high‐density lipoprotein cholesterol (HDL‐C) were associated with endothelial dysfunction.14, 15
We hypothesized that endothelial dysfunction may also contribute to thrombus formation in early‐onset CIS.16 Therefore, we aimed to: (1) characterize clinical and biochemical parameters associated with endothelial function in patients with CIS, and (2) assess the association between endothelial function and CIS with stratification by demographic subgroups by comparing patients with age‐ and sex‐matched stroke‐free controls.
Methods
Study Population
SECRETO (Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Etiology, Triggers, and Outcome) (NCT01934725) is a prospective multicenter case‐control study, aiming to study risk factors and causal pathways of CIS in young adults. The study has been approved by the ethics committee of the Helsinki and Uusimaa Hospital District (362/13/03/00/2012). Written informed consent was obtained from all participants. The present study is a substudy of SECRETO and conducted in the Helsinki University Hospital. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The methods of the main study have been previously described in detail.17 In this substudy, we included consecutive patients aged 18 to 49 years with a first‐ever recent CIS between October 2013 and February 2019. The National Institutes of Health Stroke Scale (NIHSS) was used to assess stroke severity in patients on admission. All patients underwent standardized and timely diagnostic workup to exclude definite causes of stroke. Investigations included brain magnetic resonance imaging, imaging of intracranial and extracranial vessels with either computed tomography angiography or magnetic resonance angiography, routine laboratory testing, 12‐lead ECG, continuous ECG for at least 24 hours, and both transthoracic and transesophageal echocardiography. Echocardiography studies were performed according to a standardized protocol.18 Ancillary testing was performed upon the discretion of the physician in charge. All patients underwent a repeat study visit at 3 months.
ASCO (atherothrombosis [A], small vessel disease [S], cardioembolism [C], and other causes [O]) classification was used to define CIS after initial diagnostic workup: absence of disease (grade 0); any of grade II (causality uncertain); and grade III (unlikely a direct cause) pathology using diagnostic testing with the highest grade of evidence.19 Thus, patients with any grade of I were excluded from this study as such patients had an evident cause for their stroke. Patients with patent foramen ovale–related strokes with any likelihood that patent foramen ovale would be potentially causal were, however, included in the study. None of the patients underwent patent foramen ovale closure before the study assessments.
One sex‐ and age‐matched (±5 years) stroke‐free control subjects from the same region was searched and matched to each patient. A list of 20 potential control subjects per 1 patient was randomly generated by the population registry and an invitation letter was sent to these individuals one by one. If a fit, willing control subject was not found by this strategy, patients’ nonrelated proxies, hospital staff, or their proxies were recruited.
Risk Factors and Cardiovascular Comorbidities
Clinical history was obtained from all participants with medical records and a structured interview during a study visit. Level of education was dichotomized as: (1) low, ie, primary or lower secondary education, or upper secondary education; and (2) high, ie, post‐secondary nontertiary or tertiary education. Risk factors registered were history of hypertension (mean of 2 office blood pressure measurements >140/90 mm Hg at study visit, prior diagnosis of hypertension, or prior antihypertensive medication), diabetes mellitus (prior diagnosis of any diabetes mellitus and/or prior antidiabetic medication), cardiovascular disease (any prior diagnosis of coronary heart disease, congestive heart failure, peripheral arterial disease, or atrial fibrillation), current tobacco smoking (smoking at least 1 cigarette during the preceding year before index stroke). Obesity was defined according to the World Health Organization (WHO) based on waist/hip ratio for women >0.85 and for men >0.90.20 We assessed physical activity with the short version of the International Physical Activity Questionnaire21 and estimated total metabolic equivalents per week. Adaptation of the WHO’s Alcohol, Smoking and Substance Involvement Screening Test22 was applied to assess alcohol use. Heavy alcohol use was defined as at least 5 doses of alcohol per day or 16 doses per week for women and at least 7 doses of alcohol per day or 24 doses per week for men.23 Dietary patterns were assessed using the Mediterranean Diet Score, which scores 10 main diet components including nonrefined cereals, fruits, vegetables, potatoes, legumes, olive oil, fish, red meat, poultry, and full‐fat dairy products.24 Consumption close to a healthy diet pattern was assigned scores 0, 1, 2, 3, 4, and 5 when the participant reported no, rare, frequent, very frequent, weekly, and daily consumption, respectively. Consumption away from the health diet pattern was scored on a reverse scale.
Blood Biomarkers
We assessed blood markers that are known to associate with endothelial function, using both samples taken as part of routine care and samples obtained at study visit. All samples were analyzed in a central accredited laboratory (HUSLAB in Helsinki, Finland) with an automated analyzer. Total cholesterol, plasma low‐density lipoprotein cholesterol (LDL‐C), HDL‐C, creatinine, and C‐reactive protein were obtained from patient samples taken on the next working day after admission (admission samples). Glomerular filtration rate was estimated with the Chronic Kidney Disease Epidemiology Collaboration equation.25
At study visit, investigational nonfasting plasma and serum samples were obtained from all participants, preprocessed according to standardized methods, and stored in cryogens at −180°C in liquid nitrogen at the Finnish Institute for Molecular Medicine. Plasma total cholesterol, LDL‐C, HDL‐C, creatinine, and high‐sensitivity C‐reactive protein were analyzed at HUSLAB with an automated analyzer. To assess the glycemic state on the stored investigational samples, serum concentration of glycated albumin (GA) was determined by competitive ELISA (human glycated albumin enzyme‐linked immunosorbent assay Kit, CSB‐E09599h; Cusabio) according to the manufacturers' instructions.
Endothelial Function by PAT
Endothelial function was measured with an EndoPAT 2000 device (Itamar Medical Ltd.) twice in patients (both at baseline and at 3‐month study visit) and once in healthy controls. EndoPAT is a noninvasive, validated, and previously described technique used to evaluate peripheral endothelial function by measuring digital pulse amplitude.7, 26 Specially designed biosensors, which are composed of inflatable latex air cuffs connected by pneumatic tubes to an inflating device controlled by a computerized algorithm, were placed on the tip of each index finger. Briefly, in EndoPAT, after 5 minutes of baseline measurement of finger‐tip arterial blood volume, a temporary occlusion of blood flow in the test arm is applied through air cuffs. After 5 minutes of arterial occlusion, the cuff is deflated and PAT tracing is recorded for another 5 minutes. The EndoPAT device provides a ratio of the PAT comparing the signal after cuff release with baseline recording in the test arm indexed to the contralateral arm. This ratio reflects a reactive hyperemia index, and natural logarithm of reactive hyperemia index (LnRHI) was used to estimate endothelial function.27
Statistical Analysis
Clinical and biochemical parameters were reported for all participants and stratified by sex and age groups, dichotomized at median. McNemar test was used for univariable comparisons of dichotomized variables, paired t test was used to compare normally distributed continuous variables, and Wilcoxon signed rank test was used for non‐normally distributed continuous variables. LnRHI was divided in tertiles based on the control subjects’ distribution. Clinical and biochemical parameters were characterized in LnRHI tertiles in the study patients.
The independent association between endothelial function and CIS was assessed with conditional logistic regression analysis adapted for a matched case‐control study, reported as odds ratios (ORs) and 95% CIs. We constructed 4 models to study the association: (1) adjusted for demographics and clinical vascular risk factors (hypertension, diabetes mellitus, dyslipidemia, current smoking, heavy drinking, physical activity, obesity, and diet score); (2) adjusted for demographics, clinical vascular risk factors except dyslipidemia but including total cholesterol/HDL‐C ratio based on admission samples in patients; (3) adjusted for demographics, clinical vascular risk factors except dyslipidemia but including total cholesterol/HDL‐C ratio based on study visit samples; and (4) similar to model 3 but diabetes mellitus replaced with GA based on study visit samples.
We reported variables with missing data. Statistical analyses were performed with SPSS Statistics for Windows, version 25.0 (IBM). Statistical significance was set at P<0.05.
Results
Characteristics of Patients and Matched Controls
Of the 142 consecutive patients with CIS, we excluded 1 because of an uncertain primary diagnosis of ischemic stroke (multiple sclerosis diagnosis during follow‐up), 2 because of unwillingness to participate in this substudy, and 3 because of contraindications for EndoPAT measurement. Thus, 136 patients with CIS were left for analysis (median age, 41 years; interquartile range [IQR], 35–46 years [44% women]) and compared with 136 age‐ and sex‐matched controls (median age, 42 years; IQR, 35–47 years).
In the study patients, the median delay from index stroke to hospital admission and obtaining first routine blood samples was 0 days (IQR, 0–1 days). The median delay from index stroke to study visit including EndoPAT assessment was 10 days (IQR, 8–15 days) and to first study blood samples was 10 days (IQR, 7–15 days). Median NIHSS on admission was 2 (IQR, 0–3; range, 0–13).
Compared with controls, patients had a lower level of education, were more often current smokers and obese, and had a lower diet score. Patients were more often using antihypertensive treatments before stroke compared with controls (11.0% versus 7.4%, respectively) but less frequently using statins (2.9% versus 7.4%). After hospital admission, antihypertensive medication had been commenced in 47.1% and statins in 81.6% of patients. At study visit, patients had lower LDL‐C, HDL‐C, and GA levels, as well as total cholesterol/HDL‐C ratio, compared with controls. In patients, LDL‐C, HDL‐C, and total cholesterol/HDL‐C ratio decreased significantly between admission and study visit (P<0.001 for all pairwise comparisons) (Table 1).
Table 1.
Characteristics and Comorbidities in Patients With CIS and Stroke‐Free Controls in the Entire Sample and Stratified By Sex
| Clinical Variables (No. With Missing Data) | All | Women | Men | |||
|---|---|---|---|---|---|---|
| Patients (n=136) | Controls (n=136) | Patients (n=60) | Controls (n=60) | Patients (n=76) | Controls (n=76) | |
| Low level of education (3) | 69 (50.7) | 48 (36.1)* | 26 (43.3) | 18 (31.0) | 43 (56.6) | 30 (40.0)* |
| Hypertension | 41 (30.1) | 33 (24.3) | 21 (35.0) | 14 (23.3) | 20 (26.3) | 19 (25.0) |
| Dyslipidemia | 75 (55.1) | 78 (57.4) | 26 (43.3) | 22 (36.7) | 49 (64.5) | 56 (73.7) |
| Diabetes mellitus | 4 (2.9) | 4 (2.9) | 1 (1.7) | 0 | 3 (3.9) | 4 (5.3) |
| Cardiovascular disease | 0 | 1 (0.7) | 0 | 0 | 0 | 1 (1.3) |
| Current smoking | 42 (30.9) | 26 (19.1)* | 13 (21.7) | 11 (18.3) | 29 (38.2) | 15 (19.7)* |
| Heavy alcohol use | 33 (24.3) | 21 (15.4) | 11 (18.3) | 11 (18.3) | 22 (28.9) | 10 (13.2)* |
| Physical activity, 1000 MET per wk | 3.4 (1.6–4.5) | 2.8 (1.6–4.2) | 3.3 (1.4–4.0) | 2.6 (1.5–4.1) | 3.1 (1.9–6.0) | 2.8 (1.8–4.2) |
| Obesity | 76 (55.9) | 57 (41.9)† | 26 (43.3) | 13 (21.7)† | 50 (65.8) | 44 (57.9) |
| Diet score (10) | 25.0 (5.2) | 27.0 (4.6)† | 25.7 (5.9) | 28.2 (4.7)* | 24.4 (4.5) | 26.1 (4.2)* |
| Prestroke medication | ||||||
| Antihypertensive | 15 (11.0) | NA | 8 (13.3) | NA | 7 (9.2) | NA |
| Statin | 4 (2.9) | NA | 1 (1.7) | NA | 3 (3.9) | NA |
| Medication at baseline visit | ||||||
| Antihypertensive | 64 (47.1) | 10 (7.4)† | 23 (38.3) | 4 (6.7)† | 41 (53.9) | 6 (7.9)† |
| Statin | 111 (81.6) | 10 (7.4)† | 48 (80.0) | 0† | 63 (82.9) | 10 (13.2)† |
| Routine laboratory assessment on admission | ||||||
| CRP, mg/L | 1.5 (1.5–3.0) | NA | 1.5 (1.5–1.5) | NA | 1.5 (1.5–4.0) | NA |
| eGFR, mL/min per 1.73 m2 | 102 (16) | NA | 101 (18) | NA | 103 (15) | NA |
| LDL‐C, mmol/L | 3.1 (0.9) | NA | 2.9 (0.9) | NA | 3.2 (0.9) | NA |
| HDL‐C, mmol/L (1) | 1.3 (0.4) | NA | 1.5 (0.5) | NA | 1.2 (0.3) | NA |
| Total cholesterol/HDL‐C ratio (1) | 3.8 (1.2) | NA | 3.5 (1.2) | NA | 4.2 (1.2) | NA |
| Triglycerides, mmol/L | 1.4 (1.2) | NA | 1.2 (0.7) | NA | 1.6 (1.4) | NA |
| HbA1c, mmol/mol | 34 (32–37) | NA | 34 (32–36) | NA | 34 (33–38) | NA |
| Study visit laboratory assessment | ||||||
| hs‐CRP, mg/L | 0.9 (0.4–3.2) | 0.8 (0.3–2.2) | 1.5 (0.4–4.0) | 1.2 (0.5–3.8) | 0.8 (0.4–1.6) | 0.7 (0.3–1.7) |
| eGFR, mL/min per 1.73 m2 | 66 (62–71) | 66 (63–69) | 70 (66–74) | 69 (66–72) | 64 (60–66) | 64 (59–66) |
| LDL‐C, mmol/L | 2.3 (0.7) | 3.1 (0.9)† | 2.2 (0.8) | 2.9 (0.8)† | 2.3 (0.6) | 3.2 (1.0)† |
| HDL‐C, mmol/L | 1.1 (0.3) | 1.3 (0.4)† | 1.2 (0.3) | 1.5 (0.4)† | 1.0 (0.2) | 1.2 (0.3)† |
| Total cholesterol/HDL‐C ratio | 3.5 (0.8) | 3.7 (1.1)* | 3.1 (0.7) | 3.2 (0.8) | 3.7 (0.7) | 4.2 (1.1)† |
| Triglycerides, mmol/L | 1.5 (0.8) | 1.5 (1.1) | 1.4 (0.7) | 1.2 (0.9) | 1.6 (0.8) | 1.8 (1.1) |
| GA (1), nmol/mL | 87.8 (29.1) | 98.0 (30.7)† | 94.7 (32.6) | 102.8 (34.4) | 82.4 (24.9) | 94.2 (27.0)† |
Data are expressed as number (percentage), median (interquartile range), or mean (SD).
CIS indicates cryptogenic ischemic stroke; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; GA, glycated albumin; HDL‐C, high‐density lipoprotein cholesterol; HbA1c, glycated hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MET, metabolic equivalent; and NA, not available.
P<0.05.
P<0.01.
Compared with male controls, male patients had a lower level of education and a lower diet score, were more often current smokers, and used alcohol more frequently. Female patients were more frequently obese and had a lower diet score compared with female controls. Female patients more frequently used antihypertensive treatment before stroke compared with female controls (13.3% versus 6.7%). Male controls were more frequently users of statins than male patients before stroke (13.2% versus 3.9%), whereas there was no significant difference among women. At the study visit, both male and female patients had lower LDL‐C and HDL‐C levels, and male patients also had lower GA levels and total cholesterol/HDL‐C ratio compared with controls.
In age‐specific comparison, younger patients with CIS were more often heavy alcohol users and had a lower diet score compared with controls, whereas older patients had a lower level of education, were more often smokers and obese, and had a lower diet score compared with controls. There were no relevant differences in prior medication either between younger or older patients and controls. In both age groups, patients had lower LDL‐C levels, but only older patients also had lower HDL‐C and GA levels, as well as total cholesterol/HDL‐C ratio, compared with older controls (Table 2).
Table 2.
Characteristics and Comorbidities in Patients With CIS and Stroke‐Free Controls Stratified By Age (Dichotomized at a Median of 41 y)
| Clinical Variables (No. With Missing Data) | Younger Participants | Older Participants | ||
|---|---|---|---|---|
| Patients (n=69) | Controls (n=69) | Patients (n=67) | Controls (n=67) | |
| Low level of education (3) | 36 (52.2) | 29 (42.0) | 32 (48.5) | 19 (28.8)* |
| Hypertension | 12 (17.4) | 13 (18.8) | 29 (43.3) | 20 (29.9) |
| Dyslipidemia | 34 (49.3) | 31 (44.9) | 41 (61.2) | 47 (70.1) |
| Diabetes mellitus | 1 (1.4) | 0 (0) | 3 (4.5) | 4 (6.0) |
| Cardiovascular disease | 0 (0) | 1 (1.4) | 0 (0) | 0 0) |
| Current smoking | 17 (24.6) | 15 (21.7) | 25 (37.3) | 11 (16.4)* |
| Heavy alcohol use | 22 (31.9) | 12 (17.4)* | 11 (16.4) | 9 (13.4) |
| Physical activity, 1000 MET per wk | 3.5 (1.7–4.8) | 2.6 (1.6–3.9) | 3.3 (1.4–4.3) | 2.9 (1.7–4.3) |
| Obesity | 28 (40.6) | 19 (27.5) | 48 (71.6) | 38 (56.7) |
| Diet score (10) | 25.3 (5.4) | 27.3 (4.5)* | 24.6 (5.0) | 26.7 (4.6)† |
| Prestroke medication | ||||
| Antihypertensive | 3 (4.3) | NA | 12 (17.9) | NA |
| Statin | 0 (0) | NA | 4 (6.0) | NA |
| Medication at study visit | ||||
| Antihypertensive | 21 (30.4) | 4 (5.8)† | 43 (64.2)† | 6 (9.0) |
| Statin | 47 (68.1) | 2 (2.9)† | 64 (95.5)† | 8 (11.9) |
| Admission laboratory assessment | ||||
| CRP, mg/L | 1.5 (1.5–3.0) | NA | 1.5 (1.5–3.0) | NA |
| eGFR, mL/min per 1.73 m2 | 105 (18) | NA | 99 (14) | NA |
| LDL‐C, mmol/L | 2.9 (0.9) | NA | 3.2 (1.0) | NA |
| HDL‐C, mmol/L (1) | 1.4 (0.4) | NA | 1.3 (0.3) | NA |
| Total cholesterol/HDL‐C ratio | 3.6 (1.1) | NA | 4.1 (1.3) | NA |
| Triglycerides, mmol/L | 1.2 (0.7) | NA | 1.6 (1.5) | NA |
| HbA1c, mmol/mol | 34 (32–36) | NA | 35 (33–38) | NA |
| Study visit laboratory assessment | ||||
| hs‐CRP, mg/L | 0.9 (0.4–3.2) | 0.7 (0.3–2.1) | 0.9 (0.4–3.9) | 0.9 (0.5–2.4) |
| eGFR, mL/min per 1.73 m2 | 67 (64–72) | 67 (64–71) | 64 (62–70) | 65 (60–67) |
| LDL‐C, mmol/L | 2.4 (0.8) | 2.8 (1.0)* | 2.2 (0.7) | 3.3 (0.8)† |
| HDL‐C, mmol/L | 1.2 (0.3) | 1.3 (0.3) | 1.0 (0.3) | 1.3 (0.4)† |
| Total cholesterol/HDL‐C ratio | 3.4 (0.8) | 3.5 (1.1) | 3.5 (0.7) | 4.0 (1.0)† |
| Triglycerides, mmol/L | 1.4 (0.7) | 1.3 (0.9) | 1.6 (0.9) | 1.7 (1.2) |
| GA (1), nmol/mL | 86.9 (31.8) | 92.3 (24.4) | 88.8 (26.3) | 103.9 (35.2)† |
Data are expressed as number (percentage), median (interquartile range), or mean (SD).
CIS indicates cryptogenic ischemic stroke; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; GA, glycated albumin; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MET, metabolic equivalent; and NA, not available.
P<0.05.
P<0.01.
Characteristics Associated With Endothelial Function in Patients
The proportion of men and dyslipidemia decreased by increasing LnRHI. Patients in the second tertile tended to have more hypertension and had the lowest diet score. Obesity was less frequent and total cholesterol/HDL‐C ratio on admission decreased and HDL‐C concentration increased by increasing LnRHI (Table 3).
Table 3.
Clinical and Laboratory Characteristics in Patients With CIS by Tertiles of LnRHI
| LnRHI | ||||
|---|---|---|---|---|
| Low LnRHI | Medium LnRHI | High LnRHI | P Value | |
| Age, median (IQR), y | 43.8 (36.1–47.1) | 40.6 (36.1–46.2) | 40.2 (29.6–44.1) | 0.157 |
| Men | 33 (67.3) | 32 (56.1) | 11 (36.7) | 0.009 |
| Low level of education | 28 (57.1) | 25 (43.9) | 16 (53.3) | 0.590 |
| Hypertension | 15 (30.6) | 23 (40.4) | 3 (10.0) | 0.019 |
| Dyslipidemia | 34 (69.4) | 27 (47.4) | 14 (46.7) | 0.029 |
| Diabetes mellitus | 3 (6.1) | 0 (0) | 1 (3.3) | 0.331 |
| Current smoking | 17 (34.7) | 21 (36.8) | 4 (13.3) | 0.098 |
| Heavy alcohol use | 13 (26.5) | 15 (26.3) | 5 (16.7) | 0.367 |
| Physical activity, 1000 MET per wk | 3.14 (1.1–5.4) | 3.31 (1.7–4.1) | 3.59 (1.9–7.5) | 0.549 |
| Obesity | 35 (71.4) | 34 (59.6) | 7 (23.3) | <0.001 |
| Diet score (10) | 25 (21–29) | 22 (20–28) | 26 (24–31) | 0.028 |
| Prestroke medication | ||||
| Antihypertensive | 3 (6.1) | 12 (21.1) | 0 (0) | 0.742 |
| Statin | 3 (6.1) | 1 (1.8) | 0 (0) | 0.099 |
| Medication at study visit | ||||
| Antihypertensive | 25 (51.0) | 32 (56.1) | 7 (23.3) | 0.039 |
| Statin | 40 (81.6) | 48 (84.2) | 23 (76.7) | 0.660 |
| Admission laboratory assessment | ||||
| CRP, mg/L | 1.5 (1.5–4.5) | 1.5 (1.5–2.3) | 1.5 (1.5–1.5) | 0.093 |
| eGFR, mL/min per 1.73 m2 | 103 (17) | 102 (15) | 101 (18) | 0.343 |
| LDL‐C, mmol/L | 3.1 (1.0) | 3.1 (1.0) | 3.1 (0.9) | 0.997 |
| HDL‐C, mmol/L | 1.2 (0.4) | 1.3 (0.4) | 1.5 (0.4) | 0.027 |
| Total cholesterol/HDL‐C ratio | 4.1 (1.3) | 3.8 (1.3) | 3.4 (0.9) | 0.032 |
| Triglycerides, mmol/L | 1.6 (1.8) | 1.4 (0.7) | 1.1 (0.5) | 0.138 |
| HbA1c, mmol/mol | 36 (32–38) | 34 (33–37) | 34 (32–35) | 0.246 |
| Study visit laboratory assessment | ||||
| hs‐CRP, mg/L | 1.14 (0.56–4.20) | 0.77 (0.41–3.16) | 0.98 (0.37–1.69) | 0.387 |
| eGFR, mL/min per 1.73 m2 | 65 (63–70) | 67 (63–71) | 65 (62–71) | 0.782 |
| LDL‐C, mmol/L | 2.3 (0.7) | 2.2 (0.7) | 2.4 (0.7) | 0.650 |
| HDL C, mmol/L | 1.1 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 0.047 |
| Total cholesterol/HDL‐C ratio | 3.6 (0.8) | 3.4 (0.8) | 3.3 (0.7) | 0.115 |
| Triglycerides, mmol/L | 1.7 (1.0) | 1.4 (0.6) | 1.3 (0.7) | 0.126 |
| GA (1), nmol/mL | 87.5 (27.7) | 88.9 (26.9) | 91.5 (23.2) | 0.913 |
Data are expressed as number (percentage), median (interquartile range), or mean (SD).
CIS indicates cryptogenic ischemic stroke; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; GA, glycated albumin; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; LnRHI, natural logarithm of reactive hyperemia index; MET, metabolic equivalent; and NA, not applicable.
Univariate Comparison of Endothelial Function Between Patients and Controls
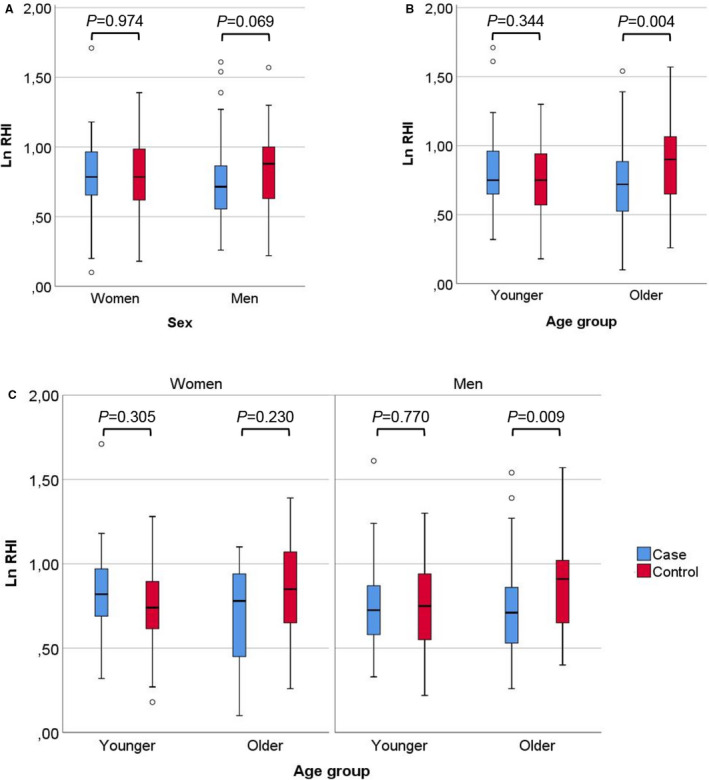
In the entire cohort, there was no difference in LnRHI as a continuous variable between patients and controls (median LnRHI 0.75 [95% CI, 0.59–0.92] versus 0.82 [95% CI, 0.63–1.00]; P=0.177). Older patients had significantly lower LnRHI compared with older controls (0.72 [95% CI, 0.52–0.89] versus 0.90 [95% CI, 0.65–1.07]; P=0.004). In men alone, LnRHI tended to be lower in patients compared with controls (0.72 [95% CI, 0.55–0.87] versus 0.88 [95% CI, 0.64–0.97]; P=0.069). In other subgroups, there was no difference in LnRHI between the patients and the controls (Figure). Moreover, there was no difference between LnRHI at baseline and 3‐month measurements in patients (0.75 [95% CI, 0.59–0.92] versus 0.75 [95% CI, 0.53–0.89]; P=0.143).
Figure 1. Case‐control comparison of natural logarithm of reactive hyperemia index (LnRHI) between (A) women and men, (B) age groups (dichotomized at median), and (C) age groups (dichotomized at median) stratified by sex.
Conditional Logistic Regression on Association Between Endothelial Function and CIS
In multivariate analyses, the lowest tertile of LnRHI was associated with CIS in men (OR, 3.50 for lowest versus highest tertile; 95% CI, 1.22–10.07) and in participants aged ≥41 years (OR, 5.78; 95% CI, 1.52–21.95) when adjusted for demographics and vascular risk factors. These associations remained significant when dyslipidemia was replaced with admission total cholesterol/HDL‐C ratio. However, when using study visit total cholesterol/HDL‐C ratio, the association between LnRHI and CIS in men dampened to nonsignificant, whereas the significant association in the older group persisted. When GA was used instead of history of diabetes mellitus with study visit total cholesterol/HDL‐C ratio, the only significant association between LnRHI and CIS remained in participants aged ≥41 years. Finally, there was no association between endothelial dysfunction and CIS in the entire study population, in women, or in patients aged <41 years alone after adjustment (Table 4).
Table 4.
Conditional Logistic Regression on the Association Between Endothelial Function (Tertiles of LnRHI) and CIS Stratified By Sex and Age (Dichotomized At Median)
| All Participants | Prevalence in Patients | Model 1: Adjusted for Demographics and Vascular Risk Factors* | Model 2: Adjusted for Demographics, Vascular Risk Factors, and Admission Total Cholesterol/HDL‐C Ratio† | Model 3: Adjusted for Demographics, Vascular Risk Factors, and Study Visit Total Cholesterol/HDL‐C Ratioǂ | Model 4: Adjusted for Demographics, Vascular Risk Factors, and Study Visit Total Cholesterol/HDL‐C Ratio and GA |
|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Low LnRHI | 49 (36.0) | 1.38 (0.74–2.56) | 1.39 (0.75–2.56) | 1.22 (0.64–2.33) | 1.08 (0.54–2.15) |
| Medium LnRHI | 57 (41.9) | 1.54 (0.76–3.12) | 1.67 (0.82–3.35) | 1.36 (0.65–2.84) | 1.33 (0.61–2.87) |
| High LnRHI | 30 (22.1) | Reference | Reference | Reference | Reference |
| Women | |||||
| Low LnRHI | 16 (26.7) | 0.47 (0.18–1.28) | 0.48 (0.18–1.29) | 0.49 (0.17–1.40) | 0.34 (0.10–1.17) |
| Medium LnRHI | 22 (36.7) | 1.05 (0.33–3.34) | 0.95 (0.29–3.12) | 0.95 (0.29–3.19) | 1.06 (0.30–3.78) |
| High LnRHI | 22 (36.7) | Reference | Reference | Reference | Reference |
| Men | |||||
| Low LnRHI | 33 (43.4) | 3.50 (1.22–10.07) | 3.52 (1.24–10.03) | 2.89 (0.98–8.54) | 2.78 (0.82–9.44) |
| Medium LnRHI | 32 (42.1) | 1.97 (0.68–5.73) | 2.29 (0.81–6.52) | 1.88 (0.63–5.61) | 1.86 (0.58–6.02) |
| High LnRHI | 11 (14.5) | Reference | Reference | Reference | Reference |
| Younger participants | |||||
| Low LnRHI | 19 (27.5) | 0.69 (0.27–1.81) | 0.68 (0.27–1.72) | 0.54 (0.20–1.50) | 0.50 (0.17–1.52) |
| Medium LnRHI | 21 (30.4) | 0.98 (0.36–2.67) | 0.90 (0.32–2.55) | 0.91 (0.33–2.56) | 0.99 (0.35–2.82) |
| High LnRHI | 29 (42.0) | Reference | Reference | Reference | Reference |
| Older participants | |||||
| Low LnRHI | 41 (61.2) | 5.78 (1.52–21.95) | 4.16 (1.32–13.14) | 3.72 (1.02–13.62) | 5.74 (1.04–31.63) |
| Medium LnRHI | 18 (26.9) | 2.62 (0.63–10.83) | 1.94 (0.54–6.90) | 2.01 (0.03–7.51) | 2.70 (0.52–14.12) |
| High LnRHI | 8 (11.9) | Reference | Reference | Reference | Reference |
CIS indicates cryptogenic ischemic stroke; GA, glycated albumin; LnRHI, natural logarithm of reactive hyperemia index; and OR, odds ratio.
Vascular risk factors included hypertension, dyslipidemia, current smoking, heavy drinking, obesity, diet score, and diabetes mellitus in men and older patients.
Vascular risk factors included hypertension, current smoking, heavy drinking, obesity, diet score, and diabetes mellitus in men and older patients. From patients, total cholesterol/high‐density lipoprotein cholesterol (HDL‐C) ratio was obtained on admission; from controls, total cholesterol/HDL‐C ratio was obtained at study visit.
Vascular risk factors included hypertension, current smoking, heavy drinking, obesity, diet score, and diabetes mellitus in men and older patients. Total cholesterol/HDL‐C ratio was obtained at study visit.
Discussion
Our case‐control study suggests an association between endothelial dysfunction and early‐onset CIS in men and a subgroup of patients aged >41 years. The association remained significant after adjusting for demographic factors and a range of comorbidities, lifestyle habits, and laboratory measurements.
Only a few studies have focused on endothelial dysfunction in young patients with stroke. One study included 25 consecutive patients with spontaneous internal carotid artery dissection (mean age, 41.8 years) and 39 individuals with vertebral artery dissection (mean age, 45.6 years), and compared them with 23 patients with CIS (43.6 years).12 Patients with internal carotid artery dissection had significantly lower FMD index compared with young patients with CIS (5.7%±6.2% in internal carotid artery dissection, 5.0%±9.3% in vertebral artery dissection, and 13.2%±6.5% in CIS; P<0.0005). No difference between patients with carotid and vertebral dissection was observed. Another study with 50 middle‐aged patients with stroke (mean age, 57.5 years) found patients with stroke to have a lower FMD index compared with 50 healthy controls. In that same study, FMD index in patients with ischemic stroke was lower than in patients with hemorrhagic stroke (3.8%±0.9% versus 6.0%±1.6%, respectively).13 No difference in FMD index between ischemic stroke subtypes was observed. It needs to be recognized that the number of individuals in these studies was small, allowing only limited subgroup analyses.
In young stroke‐free individuals, male sex, increasing age, body mass, binge drinking, smoking, diabetes mellitus, and total cholesterol/HDL‐C ratio were associated with endothelial dysfunction.14, 15 This is in accordance with our results showing that our patients with stroke in the lowest LnRHI tertile were also slightly older, more often men and obese, and had a higher total cholesterol/HDL‐C ratio and a lower HDL‐C level. Moreover, our finding suggesting that endothelial dysfunction associated with CIS particularly in men and older young adults is reasonable since features associated with endothelial dysfunction tend to cluster in these demographic patient subgroups. However, when LnRHI was analyzed as a continuous variable, a significant difference between cases and controls was shown only in older patients.
Endothelial function can be improved with several cardiovascular drugs, such as angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and statins.11 Statins have several beneficial pleiotropic effects, including anti‐inflammatory and antioxidant activities, thus improving endothelial function both in primary and secondary prevention of cardiovascular events, including stroke. For statins, this beneficial effect is also distinct and independent of their lipid‐lowering effect.28, 29 The effects of statins may also be present in our patients since statins were initiated in 4 of 5 patients at admission. Studies have shown that even relatively short exposure to statins may have beneficial effects on endothelial function.28 Accordingly, adjustment for admission lipid status did not affect the association of endothelial dysfunction with CIS, but when we adjusted for study visit lipid status, we found no association with LnRHI and CIS in men. The change in lipid status upon the study visit likely serves as a surrogate for statin initiation, but it is unlikely that short‐term statin use would have had a strong effect on our results. Nevertheless, it is possible that our findings could have been more profound without the wide initiation of statins particularly among our male and older patients.
In our study, men and older patients had lower GA levels compared with matched controls. Furthermore, replacing history of diabetes mellitus with GA did not affect the magnitude of the association of LnRHI with CIS in older individuals in our model adjusted for demographics, vascular risk factors, and study visit total cholesterol/HDL‐C ratio. Earlier studies have shown that GA enhances platelet activation and aggregation contributing to endothelial dysfunction30, 31 and has been shown to have a different impact on stroke events as it only associates with large‐artery atherosclerosis but not small‐vessel occlusion and cardioembolism in patients with diabetes mellitus and acute ischemic stroke.32 Furthermore, GA was associated with increased early neurological deterioration in patients with prediabetes mellitus who had acute ischemic stroke,33 which reflects its variability in a disease‐specific manner. Regarding short‐term glycemia, GA levels can be affected by acute blood glucose change, albumin turnover or metabolism, and proteolysis degradation,34, 35, 36 factors that could not be adjusted for in our analysis. Nevertheless, in light of the existing data on GA in ischemic stroke, the low number of patients with preexisting diabetes mellitus and the absence of clinically significant atherosclerosis and findings on GA in our study, it seems unlikely that glycemic state has a major contribution on endothelial dysfunction in young patients with CIS.
Strengths of our study include a robust study protocol, prospective design, timely and extensive diagnostic workup, and structured data collection. Furthermore, stroke diagnoses were verified with magnetic resonance imaging. Extensive data collection enabled multiple confounders to be considered in logistic regression analyses. Moreover, there were only few cases with missing data. Some limitations need to be acknowledged, however. As our study was cross‐sectional in nature, it cannot prove causality between endothelial function and CIS. There was a delay of 10 days from stroke onset to EndoPAT measurement and, because of this delay, we were not able to measure endothelial function without secondary prevention medication including statins already initiated. However, this delay was inevitable, since recruitment and EndoPAT measurement was possible only after baseline diagnostic workup was completed and hence the stroke classified as cryptogenic. It seems unlikely that stroke itself would have affected peripheral endothelial function measured with EndoPAT. To assess endothelial function in our study we used EndoPAT, which is less invasive and likely less operator‐dependent compared with assessment using FMD.37 However, some studies have shown that PAT and FMD are not interchangeable and FMD might demonstrate better reliability for the between‐day measurements.38, 39 Since measuring LnRHI is an indirect method for assessing endothelial function, our results should be confirmed with FMD assessment as well. Furthermore, patients in our study presented with mainly mild to moderate strokes, with NIHSS on admission ranging from 0 to 13. It has been previously suggested that young patients with stroke with undetermined cause might have milder strokes compared with well‐established causes.40 Nevertheless, some selection bias might be present despite aiming to enroll consecutive patients.
Conclusions
In this case‐control study, we showed an association between endothelial dysfunction and CIS in men and patients aged 41 to 49 years. This association emerged independent of several cardiovascular risk factors.
Sources of Funding
This work was supported by the Helsinki and Uusimaa Hospital District research fund (TYH2014407, TYH2018318); Academy of Finland (286246, 318075, 322656); Doctoral School in Health Sciences, University of Helsinki, and Sahlgrenska University Hospital. D.G. was supported by the Academy of Finland (1219001), University of Helsinki (Clinical Researcher stint), Wilhelm and Else Stockmann Foundation, Finska Läkaresällskapet (Medical Society of Finland), Liv och Hälsa Society, Sigrid Juselius Foundation, Päivikki and Sakari Sohlberg Foundation, and Perklén Foundation.
Disclosures
D.G. has received lecture or advisory honoraria from AstraZeneca, Boehringer Ingelheim, Fresenius, GE Healthcare, and Novo Nordisk; and support to attend medical meetings from CVRx and Sanofi Aventis. T.T. reports academic grants from European Union, Finnish Academy of Sciences, University of Gothenburg, Sahlgrenska University Hospital, Sigrid Juselius Foundation, and Wennerström’s Foundation; research contracts with Bayer, Boehringer Ingelheim, and Bristol Myers Squibb; and advisory board memberships with Bayer, Boehringer Ingelheim, Bristol Myers Squibb, and Portola Pharm. The remaining authors have no disclosures to report.
Acknowledgments
We are indebted to Laura‐Leena Kupari, RN, and Anu Eräkanto, research secretary, for their invaluable assistance in conducting the study.
For Sources of Funding and Disclosures, see page 11.
References
- 1.Bejot Y, Delpont B, Giroud M. Rising stroke incidence in young adults: more epidemiological evidence, more questions to be answered. J Am Heart Assoc. 2016;11:5:e003661. DOI: 10.1161/JAHA.116.003661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085–1096. DOI: 10.1016/S1474-4422(10)70251-9 [DOI] [PubMed] [Google Scholar]
- 3.Yesilot Barlas N, Putaala J, Waje‐Andreassen U, Vassilopoulou S, Nardi K, Odier C, Hofgart G, Engelter S, Burow A, Mihalka L, et al. Etiology of first‐ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol. 2013;20:1431–1439. DOI: 10.1111/ene.12228 [DOI] [PubMed] [Google Scholar]
- 4.Rutten‐Jacobs LC, Maaijwee NA, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, van der Vlugt MJ , van Dijk EJ , de Leeuw FE . Long‐term risk of recurrent vascular events after young stroke: the FUTURE study. Ann Neurol. 2013;74:592–601. DOI: 10.1002/ana.23953 [DOI] [PubMed] [Google Scholar]
- 5.Putaala J, Haapaniemi E, Metso AJ, Metso TM, Artto V, Kaste M, Tatlisumak T. Recurrent ischemic events in young adults after first‐ever ischemic stroke. Ann Neurol. 2010;68:661–671. DOI: 10.1002/ana.22091 [DOI] [PubMed] [Google Scholar]
- 6.Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. DOI: 10.1080/07853890701854702 [DOI] [PubMed] [Google Scholar]
- 7.Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens. 2013;31:1984–1990. DOI: 10.1097/HJH.0b013e328362d913 [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Wang J, Jin Y, Roethig HJ, Unverdorben M. Variability of peripheral arterial tonometry in the measurement of endothelial function in healthy men. Clin Cardiol. 2009;32:700–704. DOI: 10.1002/clc.20668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCrea CE, Skulas‐Ray AC, Chow M, West SG. Test‐retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17:29–36. DOI: 10.1177/1358863X11433188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen AS, Butt JH, Holm‐Yildiz S, Karlsson W, Kruuse C. Validation of repeated endothelial function measurements using EndoPAT in stroke. Front Neurol. 2017;8:178. DOI: 10.3389/fneur.2017.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(suppl 2):S314–S321. DOI: 10.2337/dc09-S330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas C, Lecroart JL, Gautier C, Leclerc X, Dauzat M, Leys D, Deklunder G. Impairment of endothelial function in patients with spontaneous cervical artery dissection: evidence for a general arterial wall disease. Cerebrovasc Dis. 2004;17:170–174. DOI: 10.1159/000075787 [DOI] [PubMed] [Google Scholar]
- 13.Omisore AD, Ayoola OO, Ibitoye BO, Fawale MB, Adetiloye VA. Sonographic evaluation of endothelial function in brachial arteries of adult stroke patients. J Ultrasound Med. 2017;36:345–351. DOI: 10.7863/ultra.16.03100 [DOI] [PubMed] [Google Scholar]
- 14.Goslawski M, Piano MR, Bian JT, Church EC, Szczurek M, Phillips SA. Binge drinking impairs vascular function in young adults. J Am Coll Cardiol. 2013;62:201–207. DOI: 10.1016/j.jacc.2013.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart study. Circulation. 2008;117:2467–2474. DOI: 10.1161/CIRCULATIONAHA.107.748574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. DOI: 10.1186/s12872-015-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putaala J, Martinez‐Majander N, Saeed S, Yesilot N, Jäkälä P, Nerg O, Tsivgoulis G, Numminen H, Gordin D, von Sarnowski B, et al. Searching for explanations for cryptogenic stroke in the young: revealing the triggers, causes, and outcome (SECRETO): rationale and design. Eur Stroke J. 2017;2:116–125. DOI: 10.1177/2396987317703210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeed S, Gerdts E, Waje‐Andreassen U, Sinisalo J, Putaala J. Searching for explanations for cryptogenic stroke in the young: revealing the etiology, triggers, and outcome (SECRETO): echocardiography performance protocol. Echo Res Pract. 2019;6:53–61. DOI: 10.1530/ERP-19-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A‐S‐C‐O (phenotypic) classification of stroke. Cerebrovasc Dis. 2009;27:502–508. DOI: 10.1159/000210433 [DOI] [PubMed] [Google Scholar]
- 20.Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist‐Hip Ratio. Eur J Clin Nutr. 2010;64:2–5. DOI: 10.1038/ejcn.2009.139 [DOI] [PubMed] [Google Scholar]
- 21.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. DOI: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 22.WHO Assist Working Group . The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–1194. DOI: 10.1046/j.1360-0443.2002.00185.x [DOI] [PubMed] [Google Scholar]
- 23.Niemela O, Niemela M, Bloigu R, Aalto M, Laatikainen T. Where should the safe limits of alcohol consumption stand in light of liver enzyme abnormalities in alcohol consumers? PLoS One. 2017;12.e0188574. DOI: 10.1371/journal.pone.0188574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2006;16:559–568. DOI: 10.1016/j.numecd.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. DOI: 10.1016/S0002-8703(03)00094-2 [DOI] [PubMed] [Google Scholar]
- 27.Axtell AL, Gomari FA, Cooke JP. Assessing endothelial vasodilator function with the Endo‐PAT 2000. J Vis Exp. 2010;44:e2167. DOI: 10.3791/2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23_suppl_1):III‐39–III‐43. DOI: 10.1161/01.CIR.0000131517.20177.5a [DOI] [PubMed] [Google Scholar]
- 29.Wan Ahmad WN, Sakri F, Mokhsin A, Rahman T, Mohd Nasir N, Abdul‐Razak S, Md Yasin M, Mohd Ismail A, Ismail Z, Nawawi H. Low serum high density lipoprotein cholesterol concentration is an independent predictor for enhanced inflammation and endothelial activation. PLoS One. 2015;10.e0116867. DOI: 10.1371/journal.pone.0116867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubenstein DA, Maria Z, Yin W. Glycated albumin modulates endothelial cell thrombogenic and inflammatory responses. J Diabetes Sci Technol. 2011;5:703–713. DOI: 10.1177/193229681100500325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenstein DA, Yin W. Glycated albumin modulates platelet susceptibility to flow induced activation and aggregation. Platelets. 2009;20:206–215. DOI: 10.1080/09537100902795492 [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Jang MU, Kim Y, Park SY, Kim C, Kim YJ, Sohn JH. Effect of prestroke glycemic variability estimated glycated albumin on stroke severity and infarct volume in diabetic patients presenting with acute ischemic stroke. Front Endocrinol (Lausanne). 2020;11:230. DOI: 10.3389/fendo.2020.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, Kim Y, Park SY, Kim C, Kim YJ, Sohn JH. Pre‐stroke glycemic variability estimated by glycated albumin is associated with early neurological deterioration and poor functional outcome in prediabetic patients with acute ischemic stroke. Cerebrovasc Dis. 2021;50:26–33. DOI: 10.1159/000511938 [DOI] [PubMed] [Google Scholar]
- 34.Rabbani N, Thornalley PJ. Protein glycation ‐ biomarkers of metabolic dysfunction and early‐stage decline in health in the era of precision medicine. Redox Biol. 2021;42:101920. DOI: 10.1016/j.redox.2021.101920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Xing G, Zhang L. Glycated albumin level is significantly decreased in patients suffering nephrotic syndrome. Prog Mol Biol Transl Sci. 2019;162:307–319. DOI: 10.1016/bs.pmbts.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Johnson RN, Easdale RW, Tatnell M, Baker JR. Significance of variation in turnover of glycated albumin on indices of diabetic control. Clin Chim Acta. 1991;198:229–238. DOI: 10.1016/0009-8981(91)90356-H [DOI] [PubMed] [Google Scholar]
- 37.Woo JS, Jang WS, Kim HS, Lee JH, Choi EY, Kim JB, Kim WS, Kim KS, Kim W. Comparison of peripheral arterial tonometry and flow‐mediated vasodilation for assessment of the severity and complexity of coronary artery disease. Coron Artery Dis. 2014;25:421–426. DOI: 10.1097/MCA.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 38.Allan RB, Delaney CL, Miller MD, Spark JI. A comparison of flow‐mediated dilatation and peripheral artery tonometry for measurement of endothelial function in healthy individuals and patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2013;45:263–279. DOI: 10.1016/j.ejvs.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 39.Onkelinx S, Cornelissen V, Goetschalckx K, Thomaes T, Verhamme P, Vanhees L. Reproducibility of different methods to measure the endothelial function. Vasc Med. 2012;17:79–84. DOI: 10.1177/1358863X12436708 [DOI] [PubMed] [Google Scholar]
- 40.Martinez‐Majander N, Aarnio K, Pirinen J, Lumikari T, Nieminen T, Lehto M, Sinisalo J, Kaste M, Tatlisumak T, Putaala J. Embolic strokes of undetermined source in young adults: baseline characteristics and long‐term outcome. Eur J Neurol. 2018;25:535–541. DOI: 10.1111/ene.13540 [DOI] [PubMed] [Google Scholar]


