Abstract
Background
Myocardial infarction is an important complication after noncardiac surgery. Therefore, perioperative troponin surveillance is recommended for patients at risk. The aim of this study was to identify patients at high risk of perioperative myocardial infarction (POMI), in order to aid appropriate selection and to omit redundant laboratory measurements in patients at low risk.
Methods and Results
This observational cohort study included patients ≥60 years of age who underwent intermediate to high risk noncardiac surgery. Routine postoperative troponin I monitoring was performed. The primary outcome was POMI. Classification and regression tree analysis was used to identify patient groups with varying risks of POMI. In each subgroup, the number needed to screen to identify 1 patient with POMI was calculated. POMI occurred in 216 (4%) patients and other myocardial injury in 842 (15%) of the 5590 included patients. Classification and regression tree analysis divided patients into 14 subgroups in which the risk of POMI ranged from 1.7% to 42%. Using a risk of POMI ≥2% to select patients for routine troponin I monitoring, this monitoring would be advocated in patients ≥60 years of age undergoing emergency surgery, or those undergoing elective surgery with a Revised Cardiac Risk Index class >2 (ie >1 risk factor). The number needed to screen to detect a patient with POMI would be 14 (95% CI 14–14) and 26% of patients with POMI would be missed.
Conclusions
To improve selection of high‐risk patients ≥60 years of age, routine postoperative troponin I monitoring could be considered in patients undergoing emergency surgery, or in patients undergoing elective surgery classified as having a revised cardiac risk index class >2.
Keywords: myocardial ischemia, noncardiac surgery, troponin
Subject Categories: Clinical Studies
Nonstandard Abbreviations and Acronyms
- CART analysis
Classification and Regression Tree Analysis
- MANAGE
Dabigatran in Patients with Myocardial Injury after Non‐Cardiac Surgery trial
- MICA
Myocardial Infarction Cardiac Arrest Calculator
- NSQIP
National Surgical Quality Improvement Program
- POMI
perioperative myocardial infarction
- RCRI
Revised Cardiac Risk Index
- VISION
Vascular Events In Noncardiac Surgery Patients Cohort Evaluation study
Clinical Perspective
What Is New?
Among patients ≥60 years undergoing noncardiac surgery, patients undergoing emergency surgery, or patients with Revised Cardiac Risk Index class >2 (ie, >1 risk factor) undergoing elective surgery are at highest risk for perioperative myocardial infarction.
What Are the Clinical Implications?
Routine perioperative troponin monitoring could be considered in these patient groups at highest risk for perioperative myocardial infarction.
Perioperative myocardial injury and myocardial infarction (POMI) are strongly associated with short‐ and long‐term mortality.1, 2, 3, 4, 5 These complications frequently occur after noncardiac surgery, with incidences of perioperative myocardial injury between 8% and 53% in patients above 45 years of age and incidences of perioperative myocardial infarction between 0.6% and 5%.1, 6, 7, 8 Presentation of perioperative myocardial ischemia is mainly silent. Identifying only those patients with ischemic signs or symptoms will therefore result in an underestimation of POMI.1, 8 As a result, current guidelines have recommended consideration of perioperative monitoring of troponin in high risk patients because this could have therapeutic consequences.9, 10, 11
Nevertheless, data on how to identify such high‐risk patients are sparse. The Revised Cardiac Risk Index (RCRI) and Myocardial Infarction Cardiac Arrest (MICA) calculator derived from the National Surgical Quality Improvement Program database are validated risk scores to assess the risk of major cardiac events such as POMI and cardiac arrest, but are not developed to predict myocardial injury as measured by troponin elevation.12, 13 It therefore is suggested to consider troponin monitoring in patients with RCRI class 3 or higher, in vascular surgery patients with RCRI class 2 or higher and in patients with impaired exercise tolerance9; or in patients aged at least 65 years, or aged at least 45 years with a documented history of coronary artery disease, peripheral vascular disease, or cerebrovascular disease.11, 14 However, none of these criteria are based on evidence that this will result in optimal selection of patients.
Consequently, in absence of established selection criteria for routine perioperative troponin monitoring, patients are selected based on their age (for example, 60 years or older), the surgical risk (intermediate to high), and expected postoperative length of hospital stay (more than 24 hours). As troponin elevation is found in ≈20% of patients if these criteria are applied, troponin measurements might be redundant in a considerable number of patients.5
In order to identify patients at highest risk for myocardial injury and infarction, ie, those who could benefit most from routine perioperative monitoring of troponin, better selection criteria are required. Therefore, the aim of this study was to identify noncardiac surgery patients who are at high risk of perioperative myocardial injury and infarction, in order to aid proper patient selection and to omit redundant laboratory measurements.
Methods
Patients
This observational cohort study included patients ≥60 years of age who underwent intermediate to high risk noncardiac surgery between January 1, 2012 and December 31, 2014 at the University Medical Center Utrecht, The Netherlands, a 1000 bed tertiary referral hospital. A part of this cohort was included in a previous study.5 High risk surgery was defined according to the RCRI criteria as intrathoracic, intraperitoneal, or supra‐inguinal vascular surgery.12 Intermediate risk surgery was defined as a procedure with an expected postoperative length of stay of at least 24 hours. In our hospital, routine troponin I monitoring was performed in these patients on the first 3 days after surgery as part of the standard postoperative protocol. For patients who underwent surgery more than once, the first surgery was included in the analyses. A reoperation was included as a novel case if this surgery took place at least 1 year after the first surgery. Patients were excluded if troponin I was not measured.
The local medical ethics committee assessed the study protocol. The need for informed consent was waived because only routinely collected patient data were used, and data were anonymized before analysis (University Medical Center Utrecht Medical Research Ethics Committee 18‐762/C). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Outcome
The primary outcome was POMI, defined according to the Fourth Universal Definition of Myocardial Infarction as troponin elevation above the 99th percentile upper reference limit and at least 1 of the following: symptoms of myocardial ischemia, new ischemic ECG changes, development of pathological Q waves, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology, or identification of a coronary thrombus by angiography or autopsy.10 Other myocardial injury was a secondary outcome, defined as troponin I elevation without angina or any evidence of ischemia being present.
Troponin (TnI) was measured once daily in the first 3 days after surgery, and was analyzed using a third‐generation enhanced AccuTnI assay (Beckman Coulter, Brea, CA). Troponin elevation was defined as TnI level above the clinical cut‐off of 60 ng/L which is the lowest measurable value with a <10% coefficient of variation above the 99th percentile. In case of postoperative TnI elevation a cardiologist was consulted. Further diagnostic procedures including an ECG, echocardiography and repeated measurements of TnI to assess the occurrence of POMI were performed at discretion of the consultant cardiologist. An independent cardiologist retrospectively evaluated all patients with elevated TnI including review of ECGs for meeting the criteria of POMI as defined above. A rise and/or fall in TnI was defined as a difference of ≥60 ng/L between two postoperative measurements, according to the 99th percentile of healthy individuals and the local clinical cut‐off value for troponin elevation.1
Data Collection
All preoperative and postoperative data were obtained from electronic medical records. Data collected included patient characteristics, comorbidities, medication used, a modified RCRI, postoperative TnI measurements, perioperative ECGs and the occurrence of POMI. Preoperative ECGs were collected until 6 months before surgery. The RCRI criteria high risk surgery, history of cerebrovascular disease and preoperative treatment with insulin were defined according to the original RCRI.12 The other criteria were adapted to be able to obtain the index from the available data. Chronic kidney disease was defined as a preoperative estimated glomerular filtration rate <45 mL/min per 1.73 m2. Ischemic heart disease was defined as previous myocardial infarction and/or coronary revascularization. Preoperative heart failure with reduced ejection fraction was defined as a left ventricular ejection fraction <40%. Emergency surgery was defined as surgery required within 72 hours after the indication was set. The municipal personal record database was consulted to obtain 1‐year mortality data.
Statistical Analysis
Baseline characteristics were compared between patients without myocardial injury, patients with POMI, and patients with other myocardial injury using the Chi‐square test or one‐way ANOVA, as appropriate.
The occurrence of POMI could not be assessed in patients with elevated TnI and a missing postoperative ECG. Omitting these patients from the analyses and conducting a complete case analysis only, is known to lead to biased effect estimates.15, 16 Alternatively, classifying these patients without postoperative ECGs as having other myocardial injury would lead to misclassification bias. Therefore, we used multiple imputation to estimate the occurrence of POMI in these patients.17 Forty data sets were imputed by the method of predictive mean matching with all known patient and procedure characteristics as predictor variables. In order to perform the following analyses, the 40 imputed data sets were stacked into 1 large data set. For comparison of the results, analyses were performed on both the imputed data and the original data. In the latter analysis, patients without postoperative ECG were classified as having other myocardial injury.
We used Classification and Regression Tree (CART) analysis to identify patient groups at increased risk of POMI and other myocardial injury.18, 19 CART analysis divides the cohort into two subgroups based on an independent variable that most optimally separates patients based on the incidence of the outcome. After each split the resulting subgroup is again divided into two subgroups by an independent variable, resulting in increasingly homogenous groups with respect to the outcome. This stepwise procedure, also referred to as recursive partitioning, continues until no further split can be made, or when a set minimum number of patients in a subgroup is reached. Finally, the analysis results in a decision tree with so‐called nodes; following a path from the root to the terminal node provides the characteristics of the patients in the terminal node. CART analysis therefore is suitable for the generation of clinical decision rules, since groups of patients are classified as "low risk" versus "high risk" of the outcome variable, instead of predicting individual patient risks as is done with common regression techniques. Therefore, this analysis can identify patient groups at highest risk of POMI.
In our analysis we used patient and procedure characteristics to identify groups of patients with varying risk on POMI or other myocardial injury. The dependent variable consisted of three categories; no myocardial injury, POMI, and other myocardial injury. Predictor variables included age, sex, emergency surgery, type of surgery, physical status classification of the American Society of Anesthesiologists (ASA class), RCRI class, high risk surgery, history of ischemic heart disease, chronic heart failure with reduced ejection fraction, hypertension, (paroxysmal) atrial fibrillation, pacemaker and/or implantable cardioverter defibrillator, history of cerebrovascular disease, peripheral vascular disease, chronic kidney disease, diabetes mellitus, and chronic obstructive pulmonary disease. Since the dependent variable had three possible outcome categories, we conducted a multinomial CART analysis. We used the Gini index to obtain maximum homogeneity, and the minimum classification improvement per node was set at 0.0001. The minimum size of parent and child nodes was set at 20. The maximum number of steps between the first node to 1 of the terminal nodes was set at 5. No pruning was applied.
After obtaining the tree, the independent variable importance of each variable in the tree was determined by assessing the normalized importance of each variable, which is defined as the predictive ability of a single variable as compared to the variable with the highest predictive ability in the model. Next, the absolute risks of POMI and other myocardial injury were calculated for the patients in each node in the tree. Furthermore, for each node the number needed to screen (NNS) to identify 1 patient with POMI was calculated, defined as the total number of patients within the node divided by the number of patients with POMI.
We validated the resulting tree using bootstrapping to obtain CIs of the patient numbers, predicted risks and NNS estimates in each node of the final tree. To perform this analysis, first, patients were allocated to the nodes which they were a member of in the final tree. Subsequently 10 000 sample data sets were created by random sampling with replacement. For each node in each sample data set, the absolute risk of POMI, the absolute risk of myocardial injury, and the NNS for POMI was calculated. Next, from the 10 000 samples, for each node we calculated the mean total number of patients, the mean numbers of patients with POMI and other myocardial injury, the mean risk of POMI and other myocardial injury, and the median NNS for POMI with 95% CIs. This bootstrapping procedure was conducted for each of the 40 imputed data sets separately. Finally, Rubin's rules were applied to pool the results from each of the 40 imputed data sets, by taking the average over the parameter estimates from all imputed data sets, and combining the within imputation variance and the between imputation variance to obtain pooled standard errors.20, 21
The analysis was performed using SPSS (release 25.0 for Windows), and RStudio (release 1.1.456 for Windows) using the mice library for multiple imputation.
Results
During the study period 6710 patients were eligible for inclusion. After exclusion of patients who died on the day of surgery and patients who were transferred on the day of surgery, 6690 patients remained (Figure 1). Of these patients, 1100 (16%) were excluded because TnI was not measured during the first three postoperative days, resulting in 5590 study patients (Table ).
Figure 1. Flow chart of patient inclusion.
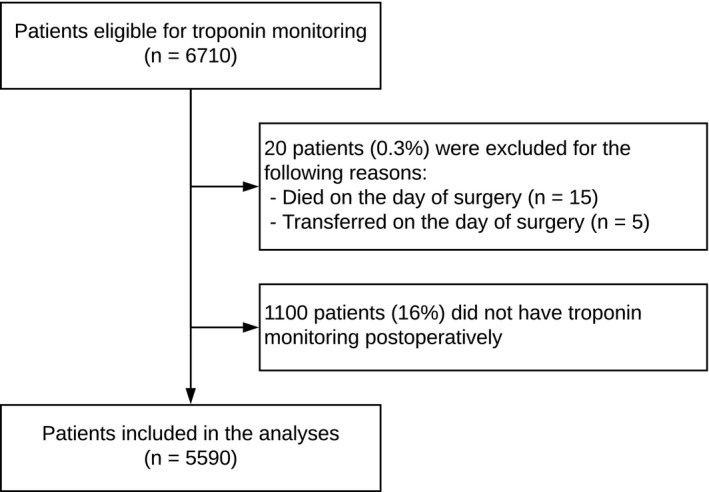
Table 1.
Baseline Characteristics
| Total (n=5590) | ||
|---|---|---|
| Male (%) | 2985 | (53.4) |
| Median age (IQR) | 70 | (65–76) |
| Hypertension (%) | 2995 | (53.6) |
| Diabetes mellitus (%) | 1020 | (18.2) |
| History of ischemic heart disease (%) | 896 | (16.0) |
| History of heart failure (%) | 148 | (2.6) |
| History of cerebrovascular disease (%) | 1084 | (19.4) |
| Chronic kidney disease (%) | 566 | (10.1) |
| Peripheral vascular disease (%) | 613 | (11.0) |
| RCRI class (%) | ||
| I | 2347 | (42.0) |
| II | 2004 | (35.8) |
| III | 870 | (15.6) |
| IV | 369 | (6.6) |
| ASA class (%) | ||
| 1 | 560 | (10.0) |
| 2 | 3405 | (60.9) |
| ≥3 | 1625 | (29.1) |
| General anesthesia (%) | 5368 | (96.0) |
| High risk surgery (%) | 1895 | (33.9) |
| Emergency surgery (%) | 1266 | (22.6) |
| Type of surgery (%) | ||
| Vascular | 884 | 15.8 |
| Orthopedic | 632 | 11.3 |
| Neuro/head/neck | 2045 | 36.6 |
| General/gynecology/urology | 2029 | 36.3 |
ASA indicates physical status classification by the American Society of Anesthesiologists; IQR, interquartile range; and RCRI, Revised Cardiac Risk Index.
POMI and Other Myocardial Injury
TnI elevation occurred in 1058 patients (19%), and 201 patients (4%) fulfilled the criteria for POMI. A rise and/or fall in TnI of ≥60 ng/L was present in 660 (62%) of these 1058 patients, and in 159 (79%) of the 201 patients with POMI. To assess the occurrence of POMI, a postoperative ECG was performed in 810 patients (77%), ie, in 23% of the patients the clinical protocol was not followed. In patients without postoperative ECG, the median TnI level was lower (90 ng/L, interquartile range [IQR] 70–127), as compared with patients in whom an postoperative ECG was available (170 ng/L, IQR 99–560, P<0.01). Comparison with a preoperative ECG was possible in 97% of patients after elective surgery, and in 71% of patients after emergency surgery. When comparison with a preoperative ECG was not possible, consultation by a cardiologist or a rise and/or fall in troponin was used to establish the diagnosis of POMI. After imputation of the missing ECG data, the mean number of patients who were classified as having POMI and other myocardial injury was 216 (4%) and 842 patients (15%), respectively.
Classification and Regression Tree Analysis
The obtained tree consisted of 14 terminal nodes (Figure 2). The maximum number of steps to reach a terminal node was 5. The first variables that were used to divide patients into groups were emergency surgery and RCRI class. However, age had the highest normalized variable importance, followed by RCRI class, emergency surgery and ASA class (Figure 3). In the different nodes, the absolute risk of POMI ranged from 1.7% to 42% and was highest among emergency patients above 81 years of age classified as RCRI class >2 and without a history of cerebrovascular disease (node 21). The absolute risk of POMI was lowest (ie 1.7%) among elective surgery patients classified as RCRI class ≤2 (node 4). The absolute risk of POMI in all elective surgery patients was 2.4% (node 2). The NNS for POMI ranged from 2 to 60 patients, was lowest (ie 2) in node 21, and was highest (ie 60) in node 4. The absolute risk of other myocardial injury ranged from 10 to 61%. Figure 2 also shows 1‐year mortality in each different node. One‐year mortality was 16.5% in the total cohort (node 1), ranging from 13.4% (node 4) to 48.5% (node 27). Table S1 represents the patient numbers and risks with their CIs for each node, as obtained from the bootstrapping procedure.
Figure 2.
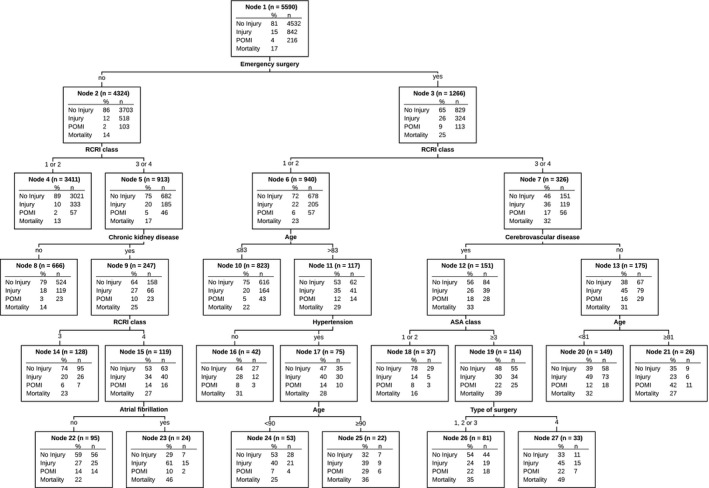
Classification and regression tree presenting the risk of perioperative myocardial infarction (POMI) and other myocardial injury (Injury) in each subgroup.
Predictor variables included age, sex, physical status classification of the American Society of Anesthesiologists (ASA class), Revised Cardiac Risk Index (RCRI class), comorbidities, type of surgery (1—vascular, 2—orthopedic, 3—neuro/head/neck, 4—general/gynecology/urology), emergency surgery, and high‐risk surgery. In each node 1‐year mortality is shown as well, but this variable was not included in the Classification and Regression Tree (CART) analysis.
Figure 3. The normalized importance of the predictor variables in the model.
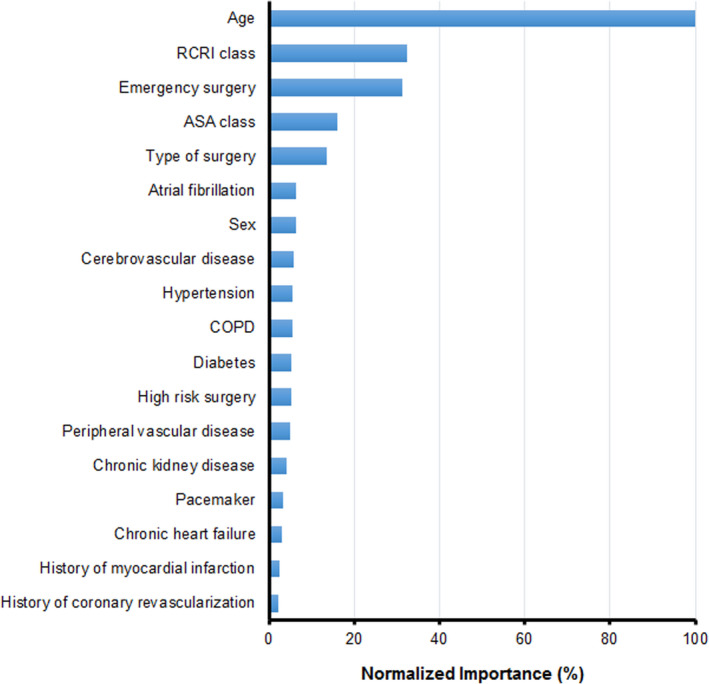
ASA indicates physical status classification of the American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; and RCRI, revised cardiac risk index.
The sensitivity analysis on the original data resulted in a comparable tree. With respect to the independent variable importance, age also had the largest predictive ability in this tree, and was followed by type of surgery, RCRI class, ASA class, and emergency surgery.
Using the Tree for Patient Selection
When using the obtained tree to select patients for TnI monitoring, the number of to be screened patients and the number of patients with missed POMI and other myocardial injury depend on the minimum risk of POMI that is used to include patients. When a risk of POMI ≥2% would be used to select patients, the patients in terminal node 4 (ie, those undergoing elective surgery with RCRI class ≤2) would be excluded from routine TnI monitoring (Figure 2). Hence, this would result in monitoring emergency surgery patients and elective surgery patients with RCRI class >2 following noncardiac surgery. In this study, this would result in 2179 (95% CI 2174–2186) patients eligible for TnI monitoring. These 2179 patients comprise 39% of the initially included 5590 patients. The overall NNS to detect 1 patient with POMI would be 14 (95% CI 14–14). The number of patients with missed POMI and other myocardial injury would be 57 (95% CI 56–59) and 333 (95% CI 329–336), respectively.
Discussion
Bottom Line and Take‐Home Message (Message Implication)
Guidelines recommend perioperative assessment of troponin in patients at high risk for myocardial injury and infarction. We aimed to improve selection of such patients by identifying groups of patients with the highest risk of POMI in whom routine perioperative troponin monitoring could provide most benefit. We found that in patients ≥60 years of age undergoing intermediate to high risk noncardiac surgery, emergency surgery and elective surgery with RCRI class >2 (ie, >1 risk factor) were corresponding with the highest risk of POMI. If these criteria would be applied, the NNS to identify 1 patient with POMI would decrease from 26 to 14, since troponin monitoring could be omitted in 61% of patients at the cost of missing 26% of the patients with POMI.
Clinical Implications
Although the etiology of postoperative troponin elevation is largely unclear and it is often unknown whether and how the prognosis in patients with such troponin elevation can be improved,5, 22, 23, 24 troponin monitoring in high risk patients after noncardiac surgery is currently advocated by experts and in several guidelines on noncardiac surgery.9, 11, 14
If the selection criteria from the 2014 European Society of Cardiology (ESC)/European Society of Anaesthesiology (ESA) guidelines on noncardiac surgery are applied to our cohort, 1505 patients would fulfil the criteria for troponin monitoring, ie, those classified RCRI class ≥3 or those scheduled for vascular surgery being RCRI class ≥2.9 Of the 5590 patients, 109 patients (1.9%) would be diagnosed with POMI. The NNS for POMI would be 14 and in 107 patients (50%) POMI would be missed. It should be noted that an impaired exercise tolerance, which is another selection criterion according to this guideline, was not taken into account in this calculation because date on exercise tolerance were not available in our study. In comparison, when the criteria as determined in our study are applied to select patients for troponin monitoring, the NNS for POMI would be 14, and 57 patients (26%) with POMI would be missed. Hence, according to the criteria as determined in our study less POMI will be missed as compared with the criteria from the ESC/ESA guidelines, and less patients need to be screened as compared to when a simple age criterion is used, as advised in other reports and as used in previous studies.4, 5, 7, 11, 14
Because we aimed at preoperative selection of patients, perioperative and postoperative factors were not studied. However, in selecting patients for troponin monitoring, factors that may be associated with POMI and other myocardial injury such as hypotension, ST segment changes, tachycardia and anemia could also be taken into account.10, 25, 26, 27 Hence, apart from the proposed selection criteria, troponin monitoring may also be considered in patients in whom the risk of POMI is high because of a complicated intra‐ or postoperative course.
Still, an important problem with respect to perioperative troponin monitoring is the unresolved relevance of troponin elevation in absence of POMI. It is a known predictor of poor outcome, but it is unknown whether we can improve prognosis and from what intervention patients could benefit. It is therefore conceivable that the current guidelines on perioperative management do not concur in this respect. As compared with the ESC/ESA guidelines on noncardiac surgery,9 the 2014 American College of Cardiology (ACC)/ American Heart Association (AHA) Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery is more hesitant by stating that the usefulness of routine postoperative troponin monitoring in unselected patients without signs or symptoms suggestive of myocardial ischemia or myocardial infarction is uncertain in absence of established risks and benefits of a defined management strategy.28 In contrast, the 2017 Canadian Cardiovascular Society Guidelines on Perioperative Cardiac Risk Assessment and Management for Patients Who Undergo Noncardiac Surgery recommends the initiation of acetylsalicylic acid and a statin in patients with myocardial injury or infarction based on the results of a cohort study.11, 29 More recently, the MANAGE (Dabigatran in Patients with Myocardial Injury after Non‐Cardiac Surgery) trial suggested a potential of dabigatran to prevent major vascular complications in patients with ischemic myocardial injury after noncardiac surgery.23 Additional research is essential to confirm these results, and to further investigate the etiology of myocardial injury and the possibility of interventions.
Therefore importantly, as long as routine troponin monitoring has not been shown to have beneficial effects, we believe that this monitoring should be limited to those patients at highest risk. We believe that the CART analysis as performed in this study is a way to select groups of high‐risk patients, in order to appoint selection criteria that can be easily used in clinical practice. Of course, other models may serve the same purpose as well.
Considering the costs of troponin monitoring, and when applying the proposed selection criteria resulting in a decrease in NNS from 26 to 14, the costs of these measurements (assuming that three troponin measurements cost about 20 euros) would be reduced from 520 to 280 euro to identify 1 patient with POMI, while missing a quarter of patients with POMI. Whether this is justified from a cost‐effectiveness perspective is not known at this moment. Because the benefit of identifying patients with silent POMI was not known in this study, neither in preventing cardiovascular events and death nor in terms of quality of life, it was not possible to estimate cost‐effectiveness.
Literature
The Revised Cardiac Risk Index is currently the most widely used index to estimate perioperative cardiac risk, and therefore was included as a predictor variable in our study.12 It was derived from a cohort of 4315 patients aged ≥50 years undergoing elective noncardiac surgery by using routine creatine kinase‐MB measurements and ECGs after surgery to assess the occurrence of cardiac complications. High risk surgery, ischemic heart disease, heart failure, cerebrovascular disease, serum creatinine >2.0 mg/dL and insulin use were identified as independent predictors of POMI and other major cardiac complications.
Several other studies determined single predictors of adverse cardiac events by using routine measurements of cardiac biomarkers.8, 13, 30, 31, 32, 33, 34 Gupta and colleagues derived a cardiac risk calculator from a multicenter prospective database, the American College of Surgeons' 2007 NSQIP database, including 211 410 patients. Type of surgery, dependent functional status, abnormal creatinine, ASA class, and increasing age were identified as cardiac risk factors, and were incorporated in the Myocardial Infarction and Cardiac Arrest (MICA) risk calculator.13 The VISION (Vascular Events In Noncardiac Surgery Patients Cohort Evaluation) study included 15 065 patients in a prospective cohort. The authors reported that age ≥75 years, male sex, atrial fibrillation, diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, peripheral vascular disease, stroke, impaired glomerular filtration rate, and urgent/emergent surgery were independent predictors of myocardial injury.8 Furthermore, in patients with 1 or more cardiac risk factors preoperative troponin values were prognostic for postoperative myocardial infarction and mortality.30, 31 Preoperative insulin therapy, heart rate and elevated pulse pressure were reported to predict cardiac complications as well.32, 33, 34 In contrast to our study, all these studies determined predictors and risk calculators suitable for assessing the absolute risk in individual patients, which is less useful in selecting groups of high‐risk patients.
Limitations
We were able to include a large cohort of consecutive patients in whom troponin monitoring after surgery is part of routine care. This is a major strength, as often troponin is only measured at the discretion of the attending physician based on the clinical course of a certain case. However, several limitations must be addressed. First, troponin was not measured according to protocol in 1100 patients (16%), therefore some selection bias may have been present. After start of the implementation of monitoring in 2011, the protocol was not immediately followed as routine. This explains most missing measurements. Second, since troponin assessment was not routinely performed before surgery, patients with preexisting elevation may have been incorrectly assigned to have acute myocardial injury. However importantly, although preoperative measurements were not available, a clear rise and/or fall in troponin was seen postoperatively in the majority (79%) of patients with POMI. Third, because troponin was only measured on the first 3 days after surgery, outcomes that may have occurred after the third postoperative day were missed. However, troponin elevation occurs mainly in the first 24 to 48 hours after surgery according to previous research.8, 25, 29 Fourth, a postoperative ECG was performed in only 77% of patients with troponin elevation. As a consequence, POMI may have been missed in the patients without ECG. Because ECGs were performed only if clinically indicated by the consultant cardiologist, it may not have been performed in particular patients with a low suspicion of POMI. This is supported by our finding that the troponin level in patients without ECG was significantly lower as compared with patients with ECG. However, we considered classifying patients without postoperative ECG as having other myocardial injury not appropriate, therefore we used multiple imputation to estimate the occurrence of POMI in these patients. Moreover, CART analysis with the original data, classifying patients without ECG as having other myocardial injury, resulted in a comparable tree and recommendation. Finally, a contemporary troponin I assay was used in this study. If a high sensitivity assay was used, myocardial injury may have been found more frequently, and even patients with very small troponin elevation (below the cut off value of the assay used in this study) may have fulfilled the criteria for POMI.
Conclusions
We identified patient groups at highest risk for POMI, in order to define selection criteria for routine postoperative troponin I monitoring in patients aged ≥60 years. Based on our findings, routine troponin I monitoring could be considered in patients undergoing emergency surgery, or in patients undergoing elective surgery classified as RCRI class >2.
Sources of Funding
None.
Disclosures
None.
Supporting information
Table S1
(J Am Heart Assoc. 2021;10:e019912. DOI: 10.1161/JAHA.120.019912.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019912
For Sources of Funding and Disclosures, see page 8.
References
- 1.Puelacher C, Lurati Buse G, Seeberger D, Sazgary L, Marbot S, Lampart A, Espinola J, Kindler C, Hammerer A, Seeberger E, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137:1221–1232. DOI: 10.1161/CIRCULATIONAHA.117.030114. [DOI] [PubMed] [Google Scholar]
- 2.Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373:2258–2269. DOI: 10.1056/NEJMra1502824. [DOI] [PubMed] [Google Scholar]
- 3.Levy M, Heels‐Ansdell D, Hiralal R, Bhandari M, Guyatt G, Yusuf S, Cook D, Villar JC, McQueen M, McFalls E, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology. 2011;114:796–806. DOI: 10.1097/ALN.0b013e31820ad503. [DOI] [PubMed] [Google Scholar]
- 4.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators , Devereaux PJ, Chan MTV, Alonso‐Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, et al. Association between postoperative troponin levels and 30‐day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. DOI: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 5.Van Waes JAR, Grobben RB, Nathoe HM, Kemperman H, de Borst GJ, Peelen LM, van Klei WA, Buhre WF, de Graaff JC, Kalkman CJ, et al. One‐year mortality, causes of death, and cardiac interventions in patients with postoperative myocardial injury. Anesth Analg. 2016;123:29–37. DOI: 10.1213/ANE.0000000000001313. [DOI] [PubMed] [Google Scholar]
- 6.Levy M, Heels‐Ansdell D, Hiralal R, Bhandari M, Guyatt G, Yusuf S, Cook D, Villar JC, McQueen M, McFalls E, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery. Anesthesiology. 2011;114:796–806. DOI: 10.1097/ALN.0b013e31820ad503. [DOI] [PubMed] [Google Scholar]
- 7.van Waes JAR, Nathoe HM, de Graaff JC, Kemperman H, de Borst GJ, Peelen LM, van Klei WA, Buhre WF, de Graaff JC, Kalkman CJ, et al. Myocardial injury after noncardiac surgery and its association with short‐term mortality. Circulation. 2013;127:2264–2271. DOI: 10.1161/CIRCULATIONAHA.113.002128. [DOI] [PubMed] [Google Scholar]
- 8.Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group, on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators , Botto F, Alonso‐Coello P, Chan MTV, Villar JC, Xavier D, Srinathan S, Guyatt G, Cruz P, Graham M, Wang CY, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120:564–578. DOI: 10.1097/ALN.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 9.Authors/Task Force Members , Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, De Hert S, Ford I, Juanatey JRG, Gorenek B, Heyndrickx GR, et al. 2014 ESC/ESA guidelines on non‐cardiac surgery. Eur J Anaesthesiol. 2014;31:517–573. DOI: 10.1097/EJA.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth Universal Definition Of Myocardial Infarction (2018). Circulation. 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 11.Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, Graham M, Tandon V, Styles K, Bessissow A, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33:17–32. DOI: 10.1016/j.cjca.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KKL, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. DOI: 10.1161/01.CIR.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 13.Gupta PK, Gupta H, Sundaram A, Kaushik M, Fang X, Miller WJ, Esterbrooks DJ, Hunter CB, Pipinos II, Johanning JM, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387. DOI: 10.1161/CIRCULATIONAHA.110.015701. [DOI] [PubMed] [Google Scholar]
- 14.Mauermann E, Puelacher C, Buse GL. Myocardial injury after noncardiac surgery: an underappreciated problem and current challenges. Curr Opin Anaesthesiol. 2016;29:403–412. DOI: 10.1097/ACO.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 15.Donders ART, van der Heijden GJMG, Stijnen T, Moons KGM. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. DOI: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Groenwold RHH, Donders ART, Roes KCB, Harrell FE, Moons KGM. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175:210–217. DOI: 10.1093/aje/kwr302. [DOI] [PubMed] [Google Scholar]
- 17.van Buuren S, Groothuis‐Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 18.Breiman L, Friedman J, Olshen RA, Stone CJ. Classification and Regression Trees. 1st ed. New York: Chapman & Hall; 1984. [Google Scholar]
- 19.Lewis RJ, Ph D, Street WC. An Introduction to Classification and Regression Tree (CART) Analysis. 2000. [Google Scholar]
- 20.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. DOI: 10.1186/1471-2288-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 2004. [Google Scholar]
- 22.Landesberg G, London MJ. The enigma of postoperative troponin elevation. Anesth Analg. 2016;123:5–7. DOI: 10.1213/ANE.0000000000001336. [DOI] [PubMed] [Google Scholar]
- 23.Devereaux PJ, Duceppe E, Guyatt G, Tandon V, Rodseth R, Biccard BM, Xavier D, Szczeklik W, Meyhoff CS, Vincent J, et al. Dabigatran in patients with myocardial injury after non‐cardiac surgery (MANAGE): an international, randomised, placebo‐controlled trial. Lancet. 2018;391:2325–2334. DOI: 10.1016/S0140-6736(18)30832-8. [DOI] [PubMed] [Google Scholar]
- 24.Smilowitz NR, Redel‐Traub G, Hausvater A, Armanious A, Nicholson J, Puelacher C, Berger JS. Myocardial injury after non‐cardiac surgery: a systematic review and meta‐analysis. Cardiol Rev. 2019;27:267–273. DOI: 10.1097/CRD.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009;119:2936–2944. DOI: 10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 26.Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121:706–721. DOI: 10.1016/j.bja.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Landesberg G, Mosseri M, Zahger D, Wolf Y, Perouansky M, Anner H, Drenger B, Hasin Y, Berlatzky Y, Weissman C. Myocardial infarction after vascular surgery: the role of prolonged, stress‐induced, ST depression‐type ischemia. J Am Coll Cardiol. 2001;37:1839–1845. DOI: 10.1016/S0735-1097(01)01265-7. [DOI] [PubMed] [Google Scholar]
- 28.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila‐Roman VG, Gerhard‐Herman MD, Holly TA, Kane GC, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary. Circulation. 2014;130:2215–2245. DOI: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 29.Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, Leslie K, Rao‐Melacini P, Chrolavicius S, Yang H, et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154:523–528. DOI: 10.7326/0003-4819-154-8-201104190-00003. [DOI] [PubMed] [Google Scholar]
- 30.Weber M, Luchner A, Manfred S, Mueller C, Liebetrau C, Schlitt A, Apostolovic S, Jankovic R, Bankovic D, Jovic M, et al. Incremental value of high‐sensitive troponin T in addition to the revised cardiac index for peri‐operative risk stratification in non‐cardiac surgery. Eur Heart J. 2013;34:853–862. DOI: 10.1093/eurheartj/ehs445. [DOI] [PubMed] [Google Scholar]
- 31.Nagele P, Brown F, Gage BF, Gibson DW, Miller JP, Jaffe AS, Apple FS, Scott MG. High‐sensitivity cardiac troponin T in prediction and diagnosis of myocardial infarction and long‐term mortality after noncardiac surgery. Am Heart J. 2013;166:325–332. DOI: 10.1016/j.ahj.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott T, Pearse RM, Archbold RA, Wragg A, Kam E, Ahmad T, Khan AW, Niebrzegowska E, Rodseth RN, Devereaux PJ, et al. Association between preoperative pulse pressure and perioperative myocardial injury: an international observational cohort study of patients undergoing non‐cardiac surgery. Br J Anaesth. 2017;119:78–86. DOI: 10.1093/bja/aex165. [DOI] [PubMed] [Google Scholar]
- 33.Sousa G, Lopes A, Reis P, Carvalho V, Santos A, Abelha FJ. Major cardiac events after non‐cardiac surgery. World J Surg. 2016;40:1802–1808. DOI: 10.1007/s00268-016-3476-3. [DOI] [PubMed] [Google Scholar]
- 34.Ladha KS, Beattie WS, Tait G, Wijeysundera DN. Association between preoperative ambulatory heart rate and postoperative myocardial injury: a retrospective cohort study. Br J Anaesth. 2018;121:722–729. DOI: 10.1016/j.bja.2018.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


