Abstract
Background
Although beta‐blockers are recommended following myocardial infarction (MI), the benefits of long‐term treatment have not been established. The study's aim was to evaluate beta‐blocker efficacy by dose in 1‐year post‐MI survivors.
Methods and Results
The OBTAIN (Outcomes of Beta‐Blocker Therapy After Myocardial Infarction) registry included 7057 patients with acute MI, with 6077 one‐year survivors. For this landmark analysis, beta‐blocker dose status was available in 3004 patients and analyzed by use (binary) and dose at 1 year after MI. Doses were classified as no beta‐blocker and >0% to 12.5%, >12.5% to 25%, >25% to 50%, and >50% of target doses used in randomized clinical trials. Age was 63 to 64 years, and approximately two thirds were men. Median follow‐up duration was 1.05 years (interquartile range, 0.98–1.22). When analyzed dichotomously, beta‐blocker therapy was not associated with improved survival. When analyzed by dose, propensity score analysis showed significantly increased mortality in the no–beta‐blocker group (hazard ratio,1.997; 95% CI, 1.118–3.568; P<0.02), the >0% to 12.5% group (hazard ratio, 1.817; 95% CI, 1.094–3.016; P<0.02), and the >25% to 50% group (hazard ratio, 1.764; 95% CI, 1.105–2.815; P<0.02), compared with the >12.5% to 25% dose group. The mortality in the full‐dose group was not significantly higher (hazard ratio, 1.196; 95% CI, 0.687–2.083). In subgroup analyses, only history of congestive heart failure demonstrated significant interaction with beta‐blocker effects on survival.
Conclusions
This analysis suggests that patients treated with >12.5% to 25% of the target dose used in prior randomized clinical trials beyond 1 year after MI may have enhanced survival compared with no beta‐blocker and other beta‐blocker doses. A new paradigm for post‐MI beta‐blocker therapy is needed that addresses which patients should be treated, for how long, and at what dose.
Keywords: beta‐blocker, landmark analysis, myocardial infarction, survival
Subject Categories: Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- CAPRICORN
Carvedilol Post‐Infarct Survival Control in LV Dysfunction
- COMMIT
Clopidogrel and Metoprolol in Myocardial Infarction Trial
- CRUSADE
Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines
- OBTAIN
Outcomes of Beta‐Blocker Therapy After Myocardial Infarction
- VALIANT
Valsartan in Acute Myocardial Infarction Trial
Clinical Perspective
What Is New?
This 1‐year landmark analysis of the effects of beta‐blocker therapy on survival after myocardial infarction did not demonstrate overall improvement when analyzed dichotomously, on (all doses) versus off beta‐blocker therapy.
As noted in the original OBTAIN (Outcomes of Beta‐Blocker Therapy After Myocardial Infarction) report, there appeared to be a dose dependence of the benefit of beta‐blocker therapy on survival, even beyond 1 year after myocardial infarction, with benefit noted at one quarter the dose targeted in clinical trials demonstrating the benefit of beta‐blocker therapy.
What Are the Clinical Implications?
Even in the modern era of management of myocardial infarction, with all of the newer therapies that have been implemented since the original beta‐blocker trials were performed, there is a signal for continued benefit of beta‐blocker therapy that requires further elucidation in prospective trials.
The most recent guidelines1, 2, 3, 4 recommend beta‐blocker therapy after acute myocardial infarction (MI). These guidelines do not generally address duration of treatment, but treatment has been recommended for at least 3 years.5 The predominance of data supporting beta‐blocker use after MI derives from multiple randomized clinical trials performed in the 1970s and 1980s, before the standard use of some of the currently available therapies that also improve survival including primary percutaneous coronary intervention/thrombolysis, aspirin, statins, and angiotensin‐converting enzyme inhibitors. A meta‐analysis6 suggested that beta‐blockers are not effective in the postthrombolysis era, though this was driven largely by the findings of the COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) trial.7 Although early trials, such as the Norwegian timolol trial,8 demonstrated preservation of the improved outcome associated with beta‐blocker use for as long as 6 years, the survival curves continue to diverge only slightly beyond 1 year and for a limited period of time, raising the question whether beta‐blocker use should be continued beyond this time period. None of the randomized clinical trials performed a landmark analysis of the efficacy of beta‐blocker therapy beyond 1 year after MI.
The OBTAIN (Outcomes of Beta‐Blocker Therapy After Myocardial Infarction) study9 was a multicenter observational registry designed to evaluate the effect of beta‐blocker dose on outcome after MI. Although the study hypothesized that greater benefit would be observed with the use of beta‐blocker doses that were targeted in the randomized clinical trials that demonstrated the efficacy of beta‐blocker therapy after MI compared with lower doses, no such benefit was observed, though there was an overall benefit to beta‐blocker therapy. The present analysis evaluates the efficacy of continued beta‐blocker therapy and the effect of dose among patients who survived 1 year after myocardial infarction. Based upon the primary OBTAIN analysis,9 we postulated that the optimum survival would be noted in patients treated with 25% of the target doses of beta‐blockers used in the randomized clinical trials.
Methods
Patient Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. Detailed information about the OBTAIN study design has been published.9 Briefly, the OBTAIN registry enrolled 7057 patients with acute MI from 26 participating centers (25 in the United States and 1 in Canada). Acute MI was diagnosed by (1) either creatine kinase elevation >2 times or troponin elevation >3 times the upper limit of normal and (2) either chest pain (or equivalent symptoms suggestive of MI) or electrocardiographic changes consistent with MI. The study was initiated in 2007 and enrolled patients through 2011. All data were collected at the site, and deidentified patient information was entered in a web‐based electronic data capture system. Vital status was assessed by chart review, the Social Security Administration Death Master File, or direct communication with the patient/family. Follow‐up beta‐blocker dosing was recorded when available. Particularly for sites that participated in the original version of the registry, longer term follow‐up (3+ years) was available. The study was funded by the National Heart, Lung and Blood Institute. The study was approved by each site's institutional review board with a waiver of consent for registry enrollment. Participating centers and study committees and personnel are listed in the original report.9
The present analysis focuses on the 6077 patients who survived 1 year after their acute MI. One‐year beta‐blocker dose information was available in 3004 patients. Table 1 shows the characteristics of these patients compared with the 3073 patients in whom beta‐blocker dosing information was not available.
Table 1.
Characteristics of the Study Population and Mortality According to Beta‐Blocker Dose at 1 Year
| 1‐y Follow‐Up Information | P Value No vs Yes | Not on Beta‐Blocker | Beta‐Blocker Dose, % of Target Dose | P Value Among 5 Doses | |||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | >0%–12.5% | >12.5%–25% | >25%–50% | >50% | ||||
| n=3073 | n=3004 | n=319 | n=597 | n=906 | n=728 | n=454 | |||
| Patient characteristics | |||||||||
| Age, y | 62.8±13.6 | 63.0±13.1 | 0.46 | 62.9±14 | 64.3±13.3 | 62.4±13.1 | 63±12.8 | 62.7±12.5 | 0.09 |
| Men | 2099 (68.3) | 2053 (68.3) | 0.98 | 195 (61.1) | 389 (65.2) | 624 (68.9) | 514 (70.6) | 331 (72.9) | 0.002 |
| Race | |||||||||
| White | 2312 (75.2) | 2525 (84.1) | <0.0001 | 265 (83.1) | 521 (87.3) | 758 (83.7) | 612 (84.1) | 369 (81.3) | 0.11 |
| Black | 375 (12.2) | 254 (8.5) | <0.0001 | 30 (9.4) | 38 (6.4) | 78 (8.6) | 61 (8.4) | 47 (10.4) | 0.21 |
| Asian | 65 (2.1) | 80 (2.7) | 0.16 | 5 (1.6) | 12 (2) | 22 (2.4) | 24 (3.3) | 17 (3.7) | 0.21 |
| American Indian | 12 (0.4) | 17 (0.6) | 0.32 | 2 (0.6) | 2 (0.3) | 8 (0.9) | 4 (0.5) | 1 (0.2) | 0.53 |
| Pacific Islander | 11 (0.4) | 4 (0.1) | 0.12 | 0 (0) | 2 (0.3) | 0 (0) | 2 (0.3) | 0 (0) | 0.27 |
| Unknown | 303 (9.9) | 127 (4.2) | <0.0001 | 17 (5.3) | 24 (4) | 40 (4.4) | 25 (3.4) | 21 (4.6) | 0.66 |
| Other | 5 (0.2) | 3 (0.1) | 0.73 | 0 (0) | 2 (0.3) | 0 (0) | 0 (0) | 1 (0.2) | 0.20 |
| Hispanic | 295 (10.4) | 150 (5.3) | <0.0001 | 21 (7.2) | 30 (5.4) | 45 (5.3) | 30 (4.3) | 24 (5.5) | 0.50 |
| BMI, kg/m2 | 29.1±6.4 | 29.4±6.5 | 0.16 | 28.8±6.1 | 28.2±6.3 | 29.3±6.2 | 29.8±6.8 | 30.8±6.9 | <0.0001 |
| Medical history | |||||||||
| Diabetes mellitus | 982 (32) | 889 (29.6) | 0.04 | 81 (25.4) | 143 (24) | 249 (27.5) | 227 (31.2) | 189 (41.6) | <0.0001 |
| Hypertension | 2047 (66.7) | 2003 (66.7) | 0.97 | 187 (58.6) | 339 (56.8) | 602 (66.4) | 510 (70.1) | 365 (80.4) | <0.0001 |
| Hyperlipidemia | 1572 (51.3) | 1714 (57.1) | <0.0001 | 168 (52.7) | 322 (53.9) | 482 (53.3) | 432 (59.3) | 310 (68.3) | <0.0001 |
| Previous MI | 602 (19.6) | 286 (9.5) | 0.95 | 55 (17.2) | 86 (14.4) | 180 (19.9) | 160 (22) | 129 (28.4) | <0.0001 |
| CABG history | 394 (12.8) | 610 (20.3) | 0.51 | 21 (6.6) | 54 (9) | 99 (10.9) | 102 (14) | 79 (17.4) | <0.0001 |
| CHF history | 287* (9.4) | 355 (11.8) | 0.22 | 27 (8.5) | 38 (6.4) | 56 (6.2) | 66 (9.1) | 52 (11.5) | 0.005 |
| ESRD | 78 (2.5) | 297 (9.9) | 0.07 | 9 (2.8) | 9 (1.5) | 22 (2.4) | 24 (3.3) | 20 (4.4) | 0.06 |
| CVA/TIA | 291 (9.5) | 106 (3.5) | 0.77 | 31 (9.7) | 53 (8.9) | 68 (7.5) | 71 (9.8) | 63 (13.9) | 0.006 |
| COPD | 313 (10.2) | 239 (8) | 0.05 | 41 (12.9) | 48 (8.0) | 83 (9.2) | 57 (7.8) | 37 (8.2) | 0.09 |
| MI characteristics | |||||||||
| STEMI | |||||||||
| Anterior | 418 (31.5) | 484 (34.6) | 0.08 | 43 (31.4) | 111 (34.6) | 129 (30.6) | 127 (37.5) | 74 (41.3) | 0.08 |
| Inferior/posterior | 690 (51.9) | 704 (50.4) | 0.43 | 76 (55.5) | 172 (53.6) | 215 (51.1) | 169 (49.9) | 72 (40.2) | 0.04 |
| Thrombolytic therapy | 201 (15.1) | 172 (12.3) | 0.03 | 14 (10.2) | 25 (7.8) | 58 (13.8) | 50 (14.7) | 25 (14) | 0.04 |
| Primary PCI | 1131 (85.1) | 11 143 (81.8) | 0.02 | 111 (81) | 277 (86.3) | 357 (84.8) | 277 (81.7) | 121 (67.6) | <0.0001 |
| In‐hospital revascularization, nonprimary PCI and CABG | 193 (14.5) | 261 (18.7) | 0.004 | 32 (23.4) | 53 (16.5) | 71 (16.9) | 58 (17.1) | 47 (26.3) | 0.02 |
| Diagnostic angiography | 68 (5.1) | 62 (4.4) | 0.41 | 6 (4.4) | 8 (2.5) | 15 (3.6) | 16 (4.7) | 17 (9.5) | 0.006 |
| NSTEMI | |||||||||
| Thrombolytic therapy | 73 (4.2) | 26 (1.6) | <0.0001 | 3 (1.6) | 2 (0.7) | 12 (2.5) | 5 (1.3) | 4 (1.5) | 0.41 |
| Primary PCI | 769 (44.1) | 673 (41.9) | 0.20 | 73 (40.1) | 141 (51.1) | 227 (46.8) | 140 (36) | 92 (33.5) | <0.0001 |
| In‐hospital revascularization, nonprimary PCI and CABG | 573 (32.9) | 534 (33.2) | 0.82 | 56 (30.8) | 81 (29.3) | 165 (34) | 138 (35.5) | 94 (34.2) | 0.48 |
| Diagnostic angiography | 286 (16.4) | 233 (14.5) | 0.13 | 33 (18.1) | 36 (13) | 58 (12) | 63 (16.2) | 43 (15.6) | 0.19 |
| ICD | 105 (3.4) | 86 (2.9) | 0.22 | 8 (2.5) | 9 (1.5) | 20 (2.2) | 25 (3.4) | 24 (5.3) | 0.003 |
| Admission SBP, mm Hg | 141.2±29.9 | 140.7±29.8 | 0.48 | 138.6±31.6 | 136.6±27.8 | 141±28.5 | 142.3±30.7 | 144.4±31.7 | 0.0002 |
| Admission HR, beats/min | 82.2±20.6 | 82.1±21.9 | 0.81 | 80.0±22.2 | 79.9±19.9 | 81.3±21.3 | 82.3±21.8 | 87.6±24.2 | <0.0001 |
| Heart failure on admission | 262 (8.5) | 266 (8.9) | 0.07 | 32 (10) | 55 (9.2) | 83 (9.2) | 76 (10.4) | 51 (11.2) | 0.73 |
| LVEF | 47.1±13.1 | 47.9±12.3 | 0.02 | 49.8±12.4 | 47.6±12.4 | 47.9±12.2 | 47.8±11.8 | 46.7±12.7 | 0.03 |
| Troponin, ng/mL | 8.1 (2–31.6) | 6.2 (1.8–24.9) | 0.004 | 6.3 (1.9–29.4) | 11.7 (3–41.9) | 6.6 (1.9–25.4) | 5 (1.4–17.3) | 3.8 (1.1–16.2) | <0.0001 |
| LOS median | 5 (4–8) | 5 (3–7) | 0.20 | 4 (3–7) | 4 (3–7) | 4 (3–7) | 5 (4–8) | 5 (4–9) | <0.0001 |
| Beta‐blocker dose, % target | |||||||||
| Mode, % taking mode dose | 12.5 (90.5) | 25.0 (98.3) | 50 (94.2) | 100 (70.3) | |||||
| Metoprolol | 1841 (59.9) | 1979 (65.9) | <0.0001 | 155 (48.6) | 415 (69.5) | 657 (72.5) | 475 (65.2) | 277 (61) | <0.0001 |
| Carvedilol | 757 (24.6) | 584 (19.4) | <0.0001 | 40 (12.5) | 136 (22.8) | 161 (17.8) | 138 (19) | 109 (24) | 0.0002 |
| Discharge medications | |||||||||
| ASA | 2853 (92.8) | 2819 (93.8) | 0.12 | 297 (93.1) | 555 (93) | 857 (94.6) | 688 (94.5) | 422 (93) | 0.53 |
| ACE‐I/ARB | 2007 (65.3) | 2097 (69.8) | <0.001 | 192 (60.2) | 390 (65.3) | 634 (70) | 537 (73.8) | 344 (75.8) | <0.0001 |
| Statin | 2554 (83.2) | 2773 (92.3) | <0.001 | 276 (86.5) | 554 (92.8) | 846 (93.4) | 677 (93) | 420 (92.7) | 0.002 |
| Clopidogrel | 2169 (70.6) | 2157 (71.8) | <0.001 | 209 (65.5) | 466 (78.1) | 694 (76.6) | 565 (77.6) | 317 (69.8) | <0.0001 |
| Dual antiplatelet | 2077 (67.6) | 2251 (74.9) | <0.001 | 201 (63.0) | 439 (73.5) | 670 (73.9) | 546 (75.0) | 301 (66.3) | <0.0001 |
| Mortality, indexed to time of MI | |||||||||
| 2 y, Kaplan‐Meier % | 140 (4.6) | 121 (4.0) | 0.31 | 15 (4.7) | 25 (4.2) | 23 (2.5) | 35 (4.8) | 23 (5.1) | 0.09 |
| 3 y, Kaplan‐Meier % | 206 (6.7) | 152 (5.1) | 0.007 | 19 (6.0) | 31 (5.2) | 30 (3.3) | 46 (6.3) | 26 (5.7) | 0.06 |
Values are mean±SD, n (%), or median (interquartile range). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, aspirin; BMI, body mass index; CABG, coronary artery bypass graft; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ESRD, end‐stage renal disease; HR, heart rate; ICD, implantable cardioverter‐defibrillator; LOS, length of stay; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST‐segment–elevation myocardial infarction; and TIA, transient ischemic attack.
Includes patients with preadmission ICD and those discharged with an ICD.
Beta‐blocker therapy was not dictated by this study, but was managed individually by the patient's physician. As performed in the original report, beta‐blocker doses were indexed to the target doses (dose administered/target dose) used in prior clinical trials: metoprolol 200 mg/d,10, 11 carvedilol 50 mg/d12 (Coreg CR equivalent dose 80 mg/d), propranolol 180 mg/d,13 bisoprolol 10 mg/d,14 and atenolol 100 mg/d.15 Beta‐blocker doses were divided into 5 prespecified groups: no beta‐blocker, >0% to 12.5%, >12.5% to 25%, >25% to 50%, and >50% of the target dose.
Statistical Analysis
Patient characteristics were summarized as mean±SD or count (percent). Differences among groups were compared using χ2 tests for categorical variables and analysis of variance for continuous variables. A distribution‐free rank sum test was used for variables that deviated from normality. Median (interquartile range) was used to summarize these variables. The Kaplan‐Meier method was used to calculate 2‐ and 3‐year post‐MI survival in each study group.
Cox proportional hazards regression was used to test for the independent effects of beta‐blocker dosing on survival. Multivariable analysis included the covariates listed in Table 1. Random effects (shared frailty model) were also included for each of the recruiting hospitals to better model differences in mortality among them. Quadratic and cubic polynomial terms for continuous predictors were included to account for potential nonlinearity.
Propensity score analysis was also performed as an alternative adjustment for patient differences in the 5 beta‐blocker dose groups, as previously reported.9 To calculate the propensity score, we used mixed‐effects linear regression with random effects of the recruiting centers, continuous beta‐blocker dose (% of target dose) as a dependent variable, and the expanded control variable set reported in Table 1, including quadratic and cubic polynomial terms for continuous predictors. In that way, the propensity scores represent the predicted beta‐blocker dose given the extended set of patient characteristics. The propensity score was used as a control variable in the proportional hazards frailty regression model.
Subgroup analysis was performed to test the hypothesis that the effect of 1‐year beta‐blocker status on subsequent mortality was equivalent in patients with (1) a history of congestive heart failure, (2) left ventricular ejection fraction ≤40%, (3) ST‐elevation MI, and (4) in‐hospital revascularization. Two versions of beta‐blocker status were used. First, we analyzed this variable in binary format, that is, patients who were on a beta‐blocker at 1 year versus those who were not. Subsequently, we used 5‐group analysis with all beta‐blocker dose categories including patients not on a beta‐blocker.
All tests were 2‐tailed, and conventional 5% significance level was used. Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Table 1 displays patient characteristics for the various treatment groups. Mean age was consistently 63 to 64 years across groups, and approximately two thirds were men, with a higher ratio in the higher‐dose groups. Other intergroup differences are shown in Table 1.
In the 3073 patients for which no follow‐up beta‐blocker information was obtained, there were no significant differences in age and sex compared with those in whom 1‐year follow‐up beta‐blocker information was obtained, whereas several significant differences were observed in other baseline characteristics, MI characteristics, and notably in the discharge medications (Table 1). The 2‐year mortality in this 1‐year landmark analysis did not differ (4.6% versus 4.0% in those in whom follow‐up beta‐blocker information was available), whereas there was a higher 3‐year mortality (6.7% versus 5.1%, P=0.007).
Among the 3004 one‐year post‐MI survivors in whom beta‐blocker dosing at 1 year was available, 319 (10.6%) were not treated with beta‐blockers, 597 (19.9%) were treated with >0% to 12.5% of the target dose, 906 (30.2%) were treated with >12.5% to 25% of the target dose, 728 (24.2%) were treated with >25% to 50% of the target dose, and 454 (15.1%) were treated with >50% of the target dose. Approximately 60% of the patients remained on the same dose at 1 year as at discharge. Figure 1 shows the distribution of changing dose categories from the discharge dose.
Figure 1. Histogram of change in beta‐blocker (BB) dosing from discharge to 1 year.
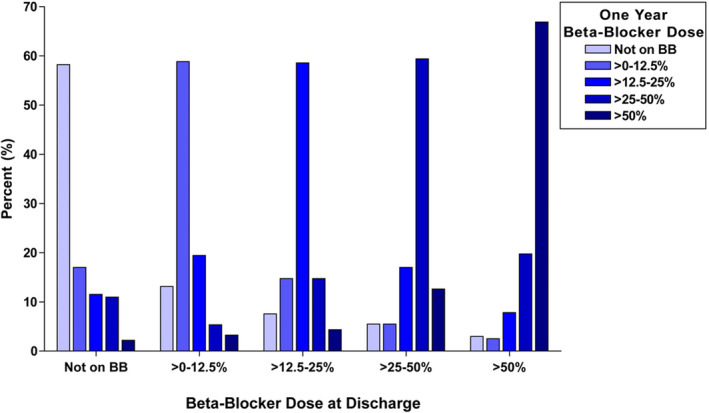
Of the 2685 patients treated with beta‐blockers, 1748 (65.1%) were treated with metoprolol, 653 (24.3%) were treated with carvedilol, 148 (5.5%) were treated with bisoprolol, 133 (5.0%) were treated with atenolol, and 3 (0.1%) were treated with propranolol.
The median follow‐up duration after 1 year in this cohort of 1‐year post‐MI survivors was 1.05 years (interquartile range, 0.98–1.22). Overall, when analyzed as a dichotomous variable, beta‐blocker therapy was not associated with improved survival (unadjusted hazard ratio [HR], 0.892; 95% CI, 0.551–1.447; P=0.53; adjusted HR, 0.806; 95% CI, 0.485–1.340; P=0.40) compared with no beta‐blocker therapy. Figure 2 shows the Kaplan‐Meier curves for the 5 beta‐blocker dose groups. The overall unadjusted effect of 1‐year beta‐blocker dose on subsequent survival was significant (P=0.03 by Cox proportional hazard analysis). As in the report of overall post‐MI survival in the OBTAIN study, the lowest observed mortality was in the >12.5% to 25% dose group. Multivariable analysis identified that many of the tested parameters were independently related to survival (Table 2). The multivariable adjusted HRs relative to the >12.5% to 25% target dose are shown in Figure 3. After multivariable adjustment, only the >25% to 50% dose showed a statistically significant increased mortality (HR, 1.755; 95% CI, 1.095–2.813; P<0.02) compared with the >12.5% to 25% dose group, with borderline increases in most of the other doses. Propensity score analysis showed significantly increased mortality in the no beta‐blocker group (HR, 1.997; 95% CI, 1.118–3.568; P<0.02), the >0% to 12.5% group (HR, 1.817; 95% CI, 1.094–3.016; P<0.02), and the >25% to 50% group (HR, 1.764; 95% CI, 1.105–2.815; P<0.02), compared with the >12.5% to 25% dose group. The mortality in the full‐dose group was not significantly higher (HR, 1.196; 95% CI, 0.687–2.083). There were no significant differences in survival among any of the other dose groups, not including the >12.5% to 25% dose group.
Figure 2. Kaplan‐Meier survival curves with landmark analysis from 1 year after myocardial infarction (MI) based on beta‐blocker (BB) dose at 1 year.
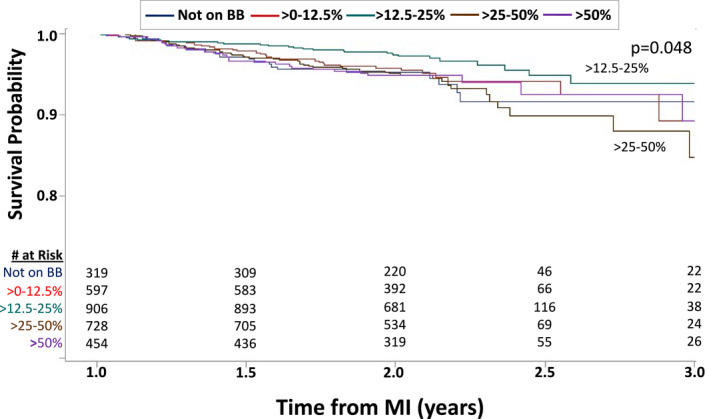
The top (>12.5%–25%) and bottom (>25–50%) survival curves are labeled. The legend provides the doses for the overlapping blue, red, and purple curves.
Table 2.
Hazard Ratios and 95% Confidence Intervals From Multivariable Analysis of Mortality
| Predictor | HR [95% CI] | P Value |
|---|---|---|
| Beta‐blocker dose | see Figure 3 | |
| Age | 1.045 [1.027–1.062] | <0.0001 |
| BMI* | −0.129±0.032 | 0.0001 |
| BMI2 * | 0.0017±0.0004 | <0.0001 |
| Hypertension | 2.204 [1.215–3.373] | 0.007 |
| ESRD | 3.098 [1.821–5.271] | <0.0001 |
| History of CHF | 1.826 [1.205–2.766] | 0.004 |
| Primary PCI | 0.518 [0.332–0.808] | 0.004 |
| CABG | 1.579 [1.055–2.363] | 0.03 |
| In‐hospital revascularization | 0.585 [0.380–0.902] | 0.02 |
HRs for continuous variables are associated with a 1‐unit increase in the measure. BMI indicates body mass index; CABG, coronary artery bypass graft; CHF, congestive heart failure; ESRD, end‐stage renal disease; HR, hazard ratio; and PCI, percutaneous coronary intervention.
Because HR is not constant across the range of BMI, model regression coefficient rather than HR is reported.
Figure 3. Hazard ratios for the multivariable and propensity score analyses for the various beta‐blocker (BB) groups relative to the >12.5% to 25% dose.
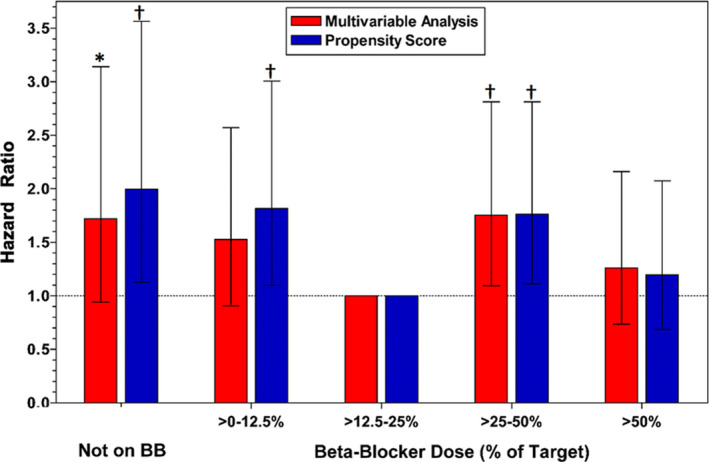
*P<0.08 versus >12.5% to 25% dose. † P<0.02 versus >12.5% to 25% dose.
Among the specified subgroup analyses, only history of congestive heart failure demonstrated significant interaction with beta‐blocker effects on survival. In the binary analysis, only the patients with history of congestive heart failure showed beta‐blocker benefit (HR, 0.442; 95% CI, 0.212–0.920; P=0.029), whereas no effect was observed for patients without history of congestive heart failure (HR, 1.232; 95% CI, 0.641–2.366; P=0.53; P=0.04 for the interaction). The effect remained after multivariable adjustment (P=0.014). In the beta‐blocker dose analysis, there was also a significant interaction with beta‐blocker effects on survival (P=0.011 for the interaction). In patients with a history of congestive heart failure, mortality was higher for those in all beta‐blocker dose categories except the >25% to 50% dose relative to the >12.5% to 25% dose (not taking beta‐blockers HR, 3.080; 95% CI, 1.141–8.319; P=0.027; >0%–12.5% dose HR, 2.727; 95% CI, 1.068–6.963; P=0.036; >50–100% dose HR, 1.919; 95% CI, 1.041–3.536; P=0.037). In patients without a history of congestive heart failure, only the >25% to 50% dose had inferior outcomes compared with the >12.5% to 25% dose (HR, 2.223; 95% CI, 1.313–3.764; P=0.0029). The difference among beta‐blocker effects in this subgroup analysis persisted after multivariable adjustment (P=0.030). Beta‐blocker effect was also different in patients with ST‐segment–elevation myocardial infarction versus non–ST‐segment–elevation myocardial infarction (P<0.0001); however, this difference was no longer significant after multivariable adjustment (P=0.65). None of the other subgroups (patients with left ventricular ejection fraction above or below 40%, patients with in‐hospital revascularization versus without) demonstrated a significant interaction with a beta‐blocker.
Discussion
This landmark analysis of the OBTAIN study was designed to evaluate whether there is benefit to beta‐blocker therapy beyond 1 year after MI. When analyzed dichotomously, beta‐blocker therapy beyond 1 year after an MI was not associated with improved survival. However, on the basis of the primary OBTAIN study9 results, we hypothesized there would be a dose‐dependent effect of beta‐blocker dosing in 1‐year survivors after an acute MI. Both multivariate and propensity score analyses of survival by dose suggested that patients treated with >12.5% to 25% of the target dose have enhanced survival compared with no beta‐blocker therapy and other beta‐blocker doses. The present study provides important data on the role of beta‐blocker dose and therapy in 1‐year survivors after MI.
CAPRICORN (Carvedilol Post‐Infarct Survival Control in LV Dysfunction)12 is the only reperfusion‐era randomized clinical trial to demonstrate a survival benefit of beta‐blocker therapy; in this trial of carvedilol versus placebo in patients after MI with left ventricular ejection fraction ≤40%, the survival curves continued to diverge for 2.5 years, suggesting long‐term benefit for this low ejection fraction group. Our findings in the subgroup of patients with a history of congestive heart failure support the continued benefit of beta‐blocker therapy in patients after MI with left ventricular dysfunction and/or heart failure. Two recent landmark analyses at 1 year after MI have been reported. In these observational studies of 138316 and 194617 patients, there was no benefit to beta‐blocker therapy when analyzed as a dichotomous variable. In a report from the French national health insurance database including 73 450 patients after MI who had been treated with beta‐blockers for at least 1 year, there was no significant increase in mortality associated with beta‐blocker discontinuation (HR, 1.13; 95% CI, 0.94–1.36).18 The dichotomous analysis in this OBTAIN report is consistent with these findings. However, beta‐blocker dose may be an important modulator of beta‐blocker benefit.9, 19 It is notable that the same dose (25% of the target dose used in randomized clinical trials) was found to be the optimal dose in this report as in the original OBTAIN report.9 Furthermore, in this subsample of the OBTAIN study, ≈40% of patients had a change in dose from the discharge dose used in the analysis of the original OBTAIN cohort.9 The only inconsistent finding in the current analysis is the lack of benefit of the >12.5% to 25% dose relative to the >50% dose. This may relate to this latter group being the smallest of the beta‐blocker treatment groups. In a report from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) registry,20 a landmark analysis was performed at 3 years after MI in patients aged ≥65 years old (mean age, 75 years), stratified by no beta‐blocker, <50% of target dose, and ≥50% of target dose. No difference in outcomes was noted. However, there was no delineation between the 12.5% and 25% dose. Furthermore, the authors note that the findings were consistent in patients with and without heart failure or systolic dysfunction, questioning the generalizability of these findings. Therefore, the present report provides further support that the medical discussion around the use of beta‐blockers after MI, even long‐term use, must center on dose and not simply use. This is a major paradigm change, because the vast majority of studies in this field do not address dose. It is important to note that there has not been a single randomized clinical trial of beta‐blockers after acute MI that tested multiple doses.
Although the present findings are intriguing, they should be considered exploratory in nature. Beta‐blocker dosing was at the physician's discretion. Although there are no data on the reasons for down‐titration, maintenance of the same dose, or up‐titration Figure 1, clearly shows the inertial effects of discharge dosing across the spectrum of doses. In real‐world cardiac practice, target doses from clinical trials are often not achieved.21
A variety of mechanisms have been suggested to underlie the improved survival associated with beta‐blocker therapy after MI. These include anti‐ischemic effects, prevention of reinfarction, and reduction in arrhythmic sudden death. In the modern era of reperfusion therapies and aspirin and statin use, the role of beta‐blockers for the first two mechanisms may have substantially diminished. Its role in the prevention of arrhythmic sudden death, however, may still be vital. VALIANT (Valsartan in Acute Myocardial Infarction Trial)22 reported the monthly rate of sudden death after MI. This peaked in the first month after MI and rapidly declined over 6 months. By 12 months, this appeared to plateau. Although the mechanism of sudden death in the early post‐MI period is likely multifactorial,23 an autopsy study suggested that half of the sudden deaths occurring beyond 1 year after acute MI are presumed arrhythmic. Olsson et al.24 demonstrated that metoprolol reduced sudden death substantially following acute MI, with an increasing effect over 3 years. There are no data evaluating the dose response for the protective effect of beta‐blockers against sudden death. In light of the potential detrimental effects of higher doses of beta‐blockers, other approaches for primary prevention may need to be considered. It is interesting to note that primary prevention implantable cardioverter defibrillators following acute MI have not provided any survival benefit over a period of several years.25, 26
There are potential detrimental effects of higher doses of beta‐blockers. In a compilation of data from randomized clinical trials of beta‐blockers in heart failure,27 significant adverse effects included hypotension and bradycardia. These are both dose dependent. Unappreciated downstream effects can be considered, such as diminished physical activity. It is well known that outcomes post‐MI are improved with increased physical activity.28 Clearly, further research is needed in this area to better identify the individual balance of beneficial and adverse effects that occur across the clinically used dose spectrum.
Limitations
The major limitation of the current study is that it is an observational cohort in which there were differences in patient characteristics among the treatment groups. Although these differences were adjusted in multivariable and propensity score analyses and provided consistent results, there may be unrecognized confounders that were not accounted for. The dose‐dependent effects could differ among different beta‐blockers. Yet, this information is the best currently available and provides important impetus to change our paradigm of clinical care and investigation related to beta‐blocker use after MI. Finally, dose was determined at the 1‐year time point and not reassessed; the reasons for dose maintenance or change are also unknown.
Conclusions
Given the tremendous advances that have been made in the management of acute MI and post‐MI treatments, it is necessary to reconsider the role of beta‐blocker therapy. However, the relevant question is no longer whether all patients with acute MI should be treated with beta‐blockers. The OBTAIN study9 and this substudy support the need for a new paradigm that addresses which patients should be treated, for how long, and at what dose. An important role for personalized medicine in the management of this diverse patient population must be incorporated. Further randomized clinical trials are required to prospectively test dosing and treatment durations for beta‐blocker therapy following acute MI.
Appendix
OBTAIN (Outcomes of Beta‐Blocker Therapy After Myocardial Infarction) Investigators
The following investigators, coordinators, and centers participated in the study: P. Desai, M. Betzen, D. DeLuna, Amarillo Heart Clinical Research Institute, Amarillo; J. Whitehill, J. Hatch, L. Janak, R. Cherry, Austin Heart P.A., Austin; E. Gonzalez, I. Cruz, Baptist Cardiac & Vascular Institute, Miami; E. Johnson, C. Allbritton, V. Derrick, Baptist Memorial Hospital, Memphis; R. Fishman, V. Assalone, L. Mahon, Bridgeport Hospital, Bridgeport; D. Lustgarten, M. Rowen, M. Bessette, B. Alemy, Fletcher Allen Health Care, Burlington; D. Dan, K. Picardi, Fuqua Heart Center, Atlanta; C. Schuger, J. Dzidowski, M. McCarthy, P. Fields, S. Alexander, Henry Ford Hospital, Detroit; G. Nair, R. Kovacs, D. Beasley, T. Strickland, J. Marks, IU Health Methodist Research Institute, Indianapolis; S. Beau, J. Tableriou, B. Griffin, Little Rock Cardiology Clinic, Little Rock; J. Shani, M. Stokes‐McCarthy, G. Tan‐Augenstein, G. Pace, H. Brosnan, Maimonides Medical Center, New York; J. Hayes, K. Mancl, K. Maassen, Marshfield Clinic, Marshfield; D. Fintel, L. Karpf, T. Abraham, K. Campione, E. Martin, Northwestern Memorial Hospital, Chicago; D. Bello, I. Viera Fleetwood, M. Tinetti, R. Rock, Orlando Regional Medical Center, Orlando; J. Simonson, S. Barnes, J. Letexier, M. Strothman, J. Mattson, Park Nicollet Institute, Minneapolis; C. Albert Shoultz III, S. Ali, Providence Healthcare Network; D. Abbott, M. Medeiros, J. McKeon, Rhode Island Hospital/Brown Medical School, Providence; A. Nichols, T. Edwards, C. Watts, C. Alley, Riverside Methodist Hospital, Columbus; M. Romanelli, D. Steffen, R. Henschel, S. Teller, L. Froehlich, R. Bess, St. John Hospital & Medical Center, Detroit; W. Warnica, B. Smith, D. Eichman, D. Scarcelli, University of Calgary, Calgary; N. Badhwar, P. Malone, D. Green, S. Iyer, University of California at San Francisco, San Francisco; R. Germany, C. Murray, G. Straughn, K. Drennan, University of Oklahoma, Oklahoma City; O. Marroquin, L. Dennis, C. Farrow, L. Baxendell, S. Grate, M. Enlow, University of Pittsburgh Medical Center, Pittsburgh; W. Zareba, I. Chaudhary, P. Laduke, V. Conary‐Rocco, L. Caufield, C. Patterson, University of Rochester—Strong Memorial Hospital, Rochester; A. Warner, J. Johnson, West Los Angeles VA Medical Center, Los Angeles; J. Germano, W. Drewes, B. George, B. Yoo, D. Patel, Winthrop University Hospital, Mineola.
Steering Committee
R. Bonow*, Northwestern University, Chicago; M. Cuffe*, Hospital Corporation of America; A. Dyer, Northwestern University, Chicago; P. Greenland, Northwestern University, Chicago J. Goldberger*, Northwestern University, Chicago; R. O’Rourke, University of Texas Health Science Center at San Antonio, San Antonio; Y. Rosenberg*, National Heart, Lung, and Blood Institute, Bethesda; P. Shah*, Cedar‐Sinai Medical Center, Los Angeles; S. Smith*, University of North Carolina, Chapel Hill.
*Current members (all original members are listed).
Observational Study Monitoring Board
R. Byington, Wake Forest University Health Science, Winston‐Salem, N.C.; Z. Feng, Fred Hutchinson Cancer Research Center, Seattle; S. Goldstein, Henry Ford Hospital, Detroit; J. Kirkpatrick, Hospital of the University of Pennsylvania; C. Love, New York University Medical Center, New York; S. Singh, Veteran Affairs Medical Center, Washington, D.C.
National Heart, Lung, and Blood Institute
Y. Rosenberg, S. Goldberg, M. Kwak, A. Rao, P. Srinivas.
Northwestern University Clinical and Data Coordinating Center
J. Goldberger, C. Ball, J. Cahill, A. Schaechter, D. Alexander, K. Ma, T. Plant, A. Rosenfeld, J. Scofic, J. Simon, H. Subačius.
Sources of Funding
This research was supported by grant No. 5U01HL080416 from the National Heart, Lung, and Blood Institute of the National Institutes of Health and the Miami Heart Research Institute.
Disclosures
None.
Acknowledgments
The authors thank all of the site investigators and research coordinators for their support of this study.
(J Am Heart Assoc. 2021;10:e019017. DOI: 10.1161/JAHA.120.019017.)
For Sources of Funding and Disclosures, see page 10.
See Editorial by Schupp et al.
Contributor Information
Jeffrey J. Goldberger, Email: j-goldberger@miami.edu.
OBTAIN (Outcomes of Beta‐Blocker Therapy After Myocardial Infarction) Investigators:
P. Desai, M. Betzen, D. DeLuna, J. Whitehill, J. Hatch, L. Janak, R. Cherry, E. Gonzalez, I. Cruz, E. Johnson, C. Allbritton, V. Derrick, R. Fishman, V. Assalone, L. Mahon, D. Lustgarten, M. Rowen, M. Bessette, B. Alemy, D. Dan, K. Picardi, C. Schuger, J. Dzidowski, M. McCarthy, P. Fields, S. Alexander, G. Nair, R. Kovacs, D. Beasley, T. Strickland, J. Marks, S. Beau, J. Tableriou, B. Griffin, J. Shani, M. Stokes‐McCarthy, G. Tan‐Augenstein, G. Pace, H. Brosnan, J. Hayes, K. Mancl, K. Maassen, D. Fintel, L. Karpf, T. Abraham, K. Campione, E. Martin, D. Bello, I. Viera Fleetwood, M. Tinetti, R. Rock, J. Simonson, S. Barnes, J. Letexier, M. Strothman, J. Mattson, C. Albert Shoultz, III, S. Ali, D. Abbott, M. Medeiros, J. McKeon, A. Nichols, T. Edwards, C. Watts, C. Alley, M. Romanelli, D. Steffen, R. Henschel, S. Teller, L. Froehlich, R. Bess, W. Warnica, B. Smith, D. Eichman, D. Scarcelli, N. Badhwar, P. Malone, D. Green, S. Iyer, R. Germany, C. Murray, G. Straughn, K. Drennan, O. Marroquin, L. Dennis, C. Farrow, L. Baxendell, S. Grate, M. Enlow, W. Zareba, I. Chaudhary, P. Laduke, V. Conary‐Rocco, L. Caufield, C. Patterson, A. Warner, J. Johnson, J. Germano, W. Drewes, B. George, B. Yoo, D. Patel, R. Bonow, M. Cuffe, A. Dyer, P. Greenland, J. Goldberger, R. O’Rourke, Y. Rosenberg, P. Shah, R. Byington, Z. Feng, S. Goldstein, J. Kirkpatrick, C. Love, S. Singh, Y. Rosenberg, S. Goldberg, M. Kwak, A. Rao, P. Srinivas, J. Goldberger, C. Ball, J. Cahill, A. Schaechter, D. Alexander, K. Ma, T. Plant, A. Rosenfeld, J. Scofic, J. Simon, and H. Subačius
References
- 1.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. DOI: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 2.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. DOI: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 3.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. DOI: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 4.Roffi M, Patrono C, Collet J‐P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. DOI: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 5.Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. Circulation. 2011;124:2458–2473. DOI: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 6.Bangalore S, Makani H, Radford M, Thakur K, Toklu B, Katz SD, DiNicolantonio JJ, Devereaux PJ, Alexander KP, Wetterslev J, et al. Clinical outcomes with β‐blockers for myocardial infarction: a meta‐analysis of randomized trials. Am J Med. 2014;127:939–953. DOI: 10.1016/j.amjmed.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, Xie JX, Liu LS. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo‐controlled trial. Lancet. 2005;366:1622–1632. DOI: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen TR. Six‐year follow‐up of the Norwegian multicenter study on timolol after acute myocardial infarction. N Engl J Med. 1985;313:1055–1058. [DOI] [PubMed] [Google Scholar]
- 9.Goldberger JJ, Bonow RO, Cuffe M, Liu L, Rosenberg Y, Shah PK, Smith SC Jr, Subacius H; Investigators O . Effect of beta‐blocker dose on survival after acute myocardial infarction. J Am Coll Cardiol. 2015;66:1431–1441. DOI: 10.1016/j.jacc.2015.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The MIAMI Trial Research Group . Metroprolol in acute myocardial infarction (MIAMI): a randomized placebo‐controlled international trial. Eur Heart J. 1985;6:199–226. [PubMed] [Google Scholar]
- 11.Hjalmarson Å, Herlitz J, Málek I, Rydén L, Vedin A, Waldenström A, Wedel H, Elmfeldt D, Holmberg S, Nyberg G, et al. Effect on mortality of metoprolol in acute myocardial infarction: a double‐blind randomized trial. Lancet. 1981;2:823–827. DOI: 10.1016/S0140-6736(81)91101-6. [DOI] [PubMed] [Google Scholar]
- 12.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. DOI: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 13.A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–1714. [DOI] [PubMed] [Google Scholar]
- 14.CIBIS‐II Investigators and Committees . The Cardiac Insufficiency Bisoprolol Study II (CIBIS II): a randomized trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 15.Viskin S, Kitzis I, Lev E, Zak Z, Heller K, Villa Y, Zajarias A, Laniado S, Belhassen B. Treatment with beta‐adrenergic blocking agents after myocardial infarction: from randomized trials to clinical practice. J Am Coll Cardiol. 1995;25:1327–1332. DOI: 10.1016/0735-1097(94)00552-2. [DOI] [PubMed] [Google Scholar]
- 16.Puymirat E, Riant E, Aissaoui N, Soria A, Ducrocq G, Coste P, Cottin Y, Aupetit JF, Bonnefoy E, Blanchard D, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016;354:i4801. DOI: 10.1136/bmj.i4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JJ, Kim SH, Kang SH, Yoon CH, Cho YS, Youn TJ, Chae IH, Choi DJ. Effect of beta‐blockers beyond 3 years after acute myocardial infarction. J Am Heart Assoc. 2018;7:e007567. DOI: 10.1161/JAHA.117.007567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann A, Maura G, Weill A, Alla F, Danchin N. Clinical events after discontinuation of β‐blockers in patients without heart failure optimally treated after acute myocardial infarction: a cohort study on the French healthcare databases. Circ Cardiovasc Qual Outcomes. 2018;11:e004356. DOI: 10.1161/CIRCOUTCOMES.117.004356. [DOI] [PubMed] [Google Scholar]
- 19.Allen JE, Knight S, McCubrey RO, Bair T, Muhlestein JB, Goldberger JJ, Anderson JL. β‐blocker dosage and outcomes after acute coronary syndrome. Am Heart J. 2017;184:26–36. DOI: 10.1016/j.ahj.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Shavadia JS, Holmes DN, Thomas L, Peterson ED, Granger CB, Roe MT, Wang TY. Comparative effectiveness of beta‐blocker use beyond 3 years after myocardial infarction and long‐term outcomes among elderly patients. Circ Cardiovasc Qual Outcomes. 2019;12:e005103. DOI: 10.1161/CIRCOUTCOMES.118.005103. [DOI] [PubMed] [Google Scholar]
- 21.Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, et al. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail. 2013;15:1173–1184. DOI: 10.1093/eurjhf/hft134. [DOI] [PubMed] [Google Scholar]
- 22.Solomon SD, Zelenkofske S, McMurray JJV, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. DOI: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 23.Pouleur A‐C, Barkoudah E, Uno H, Skali H, Finn PV, Zelenkofske SL, Belenkov YN, Mareev V, Velazquez EJ, Rouleau JL, et al. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597–602. DOI: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]
- 24.Olsson G, Rehnqvist N, Sjogren A, Erhardt L, Lundman T. Long‐term treatment with metoprolol after myocardial infarction: effect on 3 year mortality and morbidity. J Am Coll Cardiol. 1985;5:1428–1437. [DOI] [PubMed] [Google Scholar]
- 25.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of an implantable cardioverter‐defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. DOI: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 26.Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, Kornacewicz‐Jach Z, Sredniawa B, Lupkovics G, Hofgärtner F, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. DOI: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 27.Ko DT, Hebert PR, Coffey CS, Curtis JP, Foody JM, Sedrakyan A, Krumholz HM. Adverse effects of beta‐blocker therapy for patients with heart failure: a quantitative overview of randomized trials. Arch Intern Med. 2004;164:1389–1394. DOI: 10.1001/archinte.164.13.1389. [DOI] [PubMed] [Google Scholar]
- 28.Ekblom O, Ek A, Cider A, Hambraeus K, Borjesson M. Increased physical activity post‐myocardial infarction is related to reduced mortality: results from the SWEDEHEART Registry. J Am Heart Assoc. 2018;7:e010108. DOI: 10.1161/JAHA.118.010108. [DOI] [PMC free article] [PubMed] [Google Scholar]


