Abstract
Background
Arterial hypertension affects cardiovascular outcome in patients with peripheral artery disease (PAD). We hypothesized that angioplasty of peripheral arterial stenoses decreases aortic (aBP) and brachial blood pressure (bBP).
Methods and Results
In an index cohort (n=30), we simultaneously measured aBP, bBP, augmentation index (AIx), and aortic pulse wave velocity (PWV) before and after angioplasty of the iliac and femoropopliteal arteries; diagnostic angiography served as a control. In an all‐comer registry cohort (n=381), we prospectively measured bBP in patients scheduled for angioplasty of the iliac, femoral, and crural arteries or diagnostic angiography. Systolic aBP decreased after iliac (Δ−25 mmHg; 95% CI, −30 to −20; P<0.0001) and femoropopliteal angioplasty (Δ−12 mmHg; 95% CI, −17 to −5; P<0.0001) as compared with diagnostic angiography. Diastolic aBP decreased after iliac (Δ−9 mmHg; 95% CI, −13 to −1; P=0.01) but not femoropopliteal angioplasty. In parallel, AIx significantly dropped, whereas PWV remained stable. In the registry cohort, systolic bBP decreased after angioplasty of the iliac (Δ−17 mmHg; 95% CI, −31 to −8; P=0.0005) and femoropopliteal arteries (Δ−10 mmHg; 95% CI, −23 to −1; P=0.04) but not the crural arteries, as compared with diagnostic angiography. Diastolic bBP decreased after iliac (Δ−10 mmHg; 95% CI, −17 to −2; P=0.01) and femoropopliteal angioplasty (Δ−9 mmHg; 95% CI, −15 to −1; P=0.04). Multivariate analysis identified baseline systolic bBP and site of lesion as determinants of systolic bBP drop after endovascular treatment.
Conclusions
Angioplasty of flow‐limiting stenoses in patients with peripheral artery disease lowers aortic and brachial blood pressure with more pronounced effects at more proximal lesion sites and elevated baseline systolic blood pressure. These data indicate a role of endovascular treatment to acutely optimize blood pressure in patients with peripheral artery disease.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02728479.
Keywords: arterial hypertension, augmentation index, blood pressure, peripheral artery disease, pulse wave velocity
Subject Categories: Peripheral Vascular Disease, Hypertension
Nonstandard Abbreviations and Acronyms
- aBP
aortic blood pressure
- AIx
augmentation index
- bBP
brachial blood pressure
- BTK
below the knee arteries
- CFA
common femoral artery
- HR
heart rate
- PWV
pulse wave velocity
Clinical Perspective
What Is New?
This is the first study that assessed invasively acute aortic and brachial blood pressure responses to peripheral angioplasties.
Endovascular treatment of flow‐limiting stenosis lowers aortic and brachial arterial blood pressure.
Beneficial effects are more pronounced with increased baseline systolic pressure and more proximal lesion site.
What Are the Clinical Implications?
Blood pressure control in patients with atherosclerotic cardiovascular disease reduces cardiovascular mortality.
Our data demonstrate that angioplasty of iliac and femoropopliteal artery lesions acutely improves blood pressure in patients with symptomatic peripheral artery disease.
The long‐term effects on blood pressure have yet to be determined.
Current prevention guidelines recommend a medical treatment for arterial hypertension in particular in patients with manifest cardiovascular disease.1, 2, 3, 4, 5, 6 Although blood pressure (BP) control in patients with peripheral artery disease (PAD) reduces cardiovascular mortality, the management of risk factors, including BP, is often suboptimal in these patients.7 Only 30% to 50% of treated patients with PAD achieve adequate BP control.7, 8
Isolated systolic hypertension occurs in up to 90% of patients with PAD.9 It is now appreciated that wave reflection and arterial stiffness are important determinants of age‐related isolated systolic hypertension.10, 11 In the context of PAD, arterial stenoses and occlusions may cause local reflection of the pulse wave increasing aortic augmentation and contribute to the development of isolated systolic hypertension. We have previously shown that peripheral arterial angioplasty acutely improves endothelium‐dependent flow‐mediated dilation and decreases local arterial stiffness associated with superficial femoral artery stenoses.12 Elimination of a proximal stenosis might affect wave reflection or shift it toward more distal sites in the lower limbs such as to the level of resistance arteries. However, the direct effect of peripheral arterial angioplasty on aortic blood pressure (aBP) and brachial blood pressure (bBP) has not been investigated to date. Therefore, we hypothesized that endovascular treatment of peripheral flow‐limiting arterial stenoses acutely reduces aBP and bBP.
Using serial and segmental invasive measurement of hemodynamics in the proximal to distal aorta in combination with vascular ultrasound, we demonstrate here for the first time that endovascular treatment of peripheral arterial stenoses through restitution of blood flow into the target leg significantly reduces aBP and bBP, improves augmentation index (AIx), whereas pulse wave velocity (PWV) remained unaffected. In a large prospective all‐comer registry of patients with PAD scheduled for endovascular treatment, these findings were corroborated with beneficial effects becoming more evident with higher systolic bBP and treatment of the more proximal lesion site.
Methods
The data that support the findings of this study are available from the first author upon reasonable request (hanslucas.busch@med.uni-duesseldorf.de).
We prospectively analyzed in an index cohort systolic bBP, diastolic bBP, heart rate (HR), ankle‐brachial index (ABI), and common femoral artery (CFA) flow at baseline 1 day before and 1 day after angioplasty of the iliac (n=15) and femoropopliteal arteries (n=10), and after mere diagnostic angiography (n=5). Moreover, we analyzed in these 30 patients intraprocedural systolic and diastolic aBP, AIx, aortic PWV, HR, and bBP before and after angioplasty, and diagnostic angiography in the catheterization laboratory.
As a second study group, we prospectively enrolled 381 patients scheduled for elective percutaneous transluminal angioplasty on an all‐comer basis within the Duesseldorf PTA Registry (PTA Registry–An Observational Study of Percutaneous Transluminal Angioplasty; Clinical Trial Registration–URL: http://www.clinicaltrials.gov. Unique identifier:: NCT02728479). Clinical baseline characteristics and bBP responses to angioplasty or angiography were analyzed (see Consolidated Standards of Reporting Trials diagram, Figure 1). The study was conducted after approval of the local ethics committee and in accordance with the Declaration of Helsinki. All patients gave written informed consent before the procedure.
Figure 1. CONSORT diagram of the prospective Duesseldorf percutaneous transluminal angioplasty study.
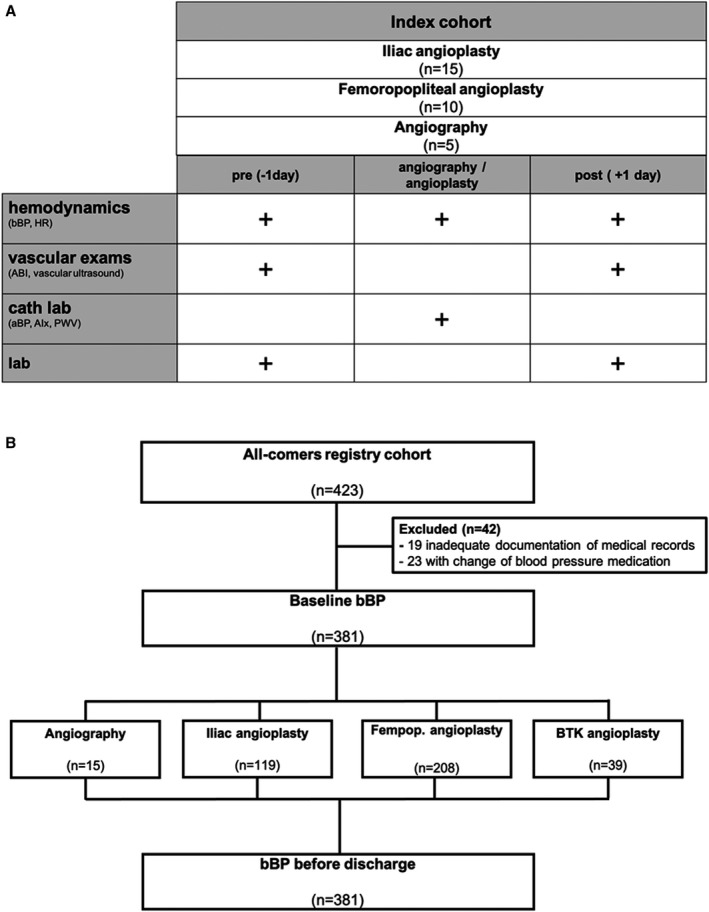
A, Index cohort with 30 patients. Clinical evaluations including office measurement of brachial blood pressure (bBP), ankle‐brachial index (ABI), heart rate (HR), and vascular assessment at baseline 1 day before and 1 day after intervention. Intraprocedural invasive serial hemodynamic measurement of segmental aortic blood pressure (aBP), augmentation index (AIx), pulse wave velocity (PWV), and bBP before and after angioplasty or diagnostic angiography. B, Registry cohort with office bBP recordings 1 day before and 1 day after angioplasty and angiography. Cath lab indicates catheterization laboratory; and Fempop., femoropopliteal.
Standard Angioplasty Strategy
Iliac lesions were treated with angioplasty (Passeo 35; Biotronik) followed by implantation of balloon‐expandable stents (Dynamic; Biotronik). Femoropopliteal arteries were treated with angioplasty, followed by drug‐coated balloon treatment (Passeo‐Lux 18; Biotronik), and if necessary, with self‐expandable nitinol stents (Innova; Boston Scientific). Below the knee (BTK) crural vessels were primarily treated with angioplasty, followed if necessary by implantation of balloon‐expandable drug‐eluting stents (Xience; Abbott Vascular).
Because we commonly treat a high‐risk collective of patients with a remarkable proportion of cardiovascular risk factors at our department, no anesthesia is applied during the procedure and thereafter for patient safety. Standard local anesthesia is applied at the arterial access site with lidocaine.
Office Measurement of bBP, HR, and CFA Flow in the Index Cohort
Office measurements were performed at baseline 1 day before and 1 day after intervention and included measurement of ABI, HR, and a standardize vascular ultrasound assessment (10 MHz transducer, Vivid I; GE Healthcare). HR and bBP were measured using an automated clinical digital sphygmomanometer (Dynamap Vital Signs Monitor, Dinamap; GE Healthcare). CFA blood flow was calculated as volume flow [ml/min] = π*r2[cm]*Vmean[cm/s]*60; see Consolidated Standards of Reporting Trials diagram Figure 1A). Local CFA resistance was calculated as
Mean arterial pressure was calculated with the mathematical equation .
Invasive Measurement of Aortic Blood Pressure, AIx, and Aortic PWV in the Index Cohort
aBP was obtained invasively before and after peripheral angioplasty and diagnostic angiography. In all cases (n=30), catheterization was performed via the CFA with a standard 6F sheath (Merit Medical). Recording of the aBP was performed with a pigtail catheter pullback in the aorta before and after angioplasty. We divided the aorta into 4 segments: ascending aorta, descending thoracic aorta, suprarenal aorta, and infrarenal aorta. In each segment, at least 4 cardiac cycles were recorded, and mean pressure was calculated. The length of the aorta was approximated with a calibrated measurement of the pigtail catheter length using the automated Philipps Xper IM catheterization laboratory software. The PWV was determined with a pigtail pull back from the aortic root to the aortic bifurcation under x‐ray film control, and the transit time of the pulse wave was measured using ECG gating.13 In case of femoropopliteal interventions, a standard crossover maneuver is performed to reach the culprit lesion. We used the HKWin software (HKWin v1.148.2; Medizin Technik Komponenten) to print out the original pressure contour waveforms invasively obtained. The AIx values were calculated by visual measurements based on the following formula Two blinded investigators calculated the PWV independently, and the mean PWV of both measurements was used. The time of the first systolic shoulder was also calculated by visual measurements. We assessed the reproducibility of our method for measuring PWV. Measurements were repeated in 15 out of 30 subjects. The coefficient of variation for PWV measurements was 6%, and the reproducibility analysis of PWV measurements showed that the mean difference was 0.13 m/s, with an SD of 0.86 m/s. Most of the values ranged within a mean±2 SD. Also, there was no significant interobserver difference (0.1 m/s with an SD of 0.8 m/s). Therefore, reliability of PWV measurements were satisfactory. bBP was measured using an automated clinical digital sphygmomanometer (Dynamap Vital Signs Monitor, Dinamap; GE Healthcare) right before and right after intervention and was correlated with aBP (Figure 1A).
Analyses of bBP in the Registry Cohort
Prospective analyses were based on the Duesseldorf PTA Registry and included data from patients who were scheduled for elective diagnostic angiographies or angioplasties of lower leg peripheral arteries. Clinical evaluations were performed in the outpatient clinic or on the ward at baseline 1 day before and 1 day after intervention. The clinical evaluations included staging to Rutherford classification, measurement of ABI, treadmill testing if possible, and a standardize ultrasound assessment (10‐MHz transducer; Vivid I; GE Healthcare) to identify target lesions (see Figure 1B for detailed information). bBP was measured using an automated clinical digital sphygmomanometer (Dynamap Vital Signs Monitor, Dinamap; GE Healthcare) at the upper arm. Patients receiving vasoactive medication during the procedure or any peri‐interventional change in BP‐lowering or pain medication were excluded. Patients were grouped according to the most proximal flow‐limiting stenosis.
Statistical Analysis
Categorical variables are presented as absolute numbers and percentages; statistical comparisons for these were made by the χ2 test. Continuous variables are expressed as mean values and SD and compared by the ANOVA F test. Changes in parameters (Δ) were calculated as postangioplasty values minus baseline (pre angioplasty) values and are expressed as means with 95% CIs. Within‐subject changes with single comparisons in hemodynamics were analyzed using a paired Student t test; when changes in parameters were compared among groups, we used 1‐way ANOVA with a post hoc Tukey correction for multiple comparisons. Linear relationships between continuous variables were expressed as Pearson r. Statistical significance was assumed at P≤0.05. To determine predictors of change in systolic bBP in both cohorts, a multiple linear regression analysis was performed with comorbidities, sex, hemodynamic characteristics (AIx, PWV, bBP), and localization of the proximal stenosis (iliac versus femoropopliteal versus BTK versus diagnostic angiography) as covariates. Data were analyzed using GraphPad Software’s Prism version 6.00 and IBM’s SPSS software version 22.0.
Results
Index Cohort
aBP Decreases After Iliac and Femoropopliteal Angioplasty
Segmental measurement of systolic aBP and diastolic aBP was obtained in 30 patients before and after peripheral angioplasty or mere diagnostic angiography. Details on patient characteristics are presented in Table 1. Of these patients, 15 underwent iliac angioplasty (100% stent), 10 patients underwent femoropopliteal angioplasty (90% stent), and 5 patients underwent diagnostic angiography only. No major complications occurred in the index cohort (Table S1).
Table 1.
Baseline Clinical and Demographic Characteristics of 30 Patients With Peripheral Artery Disease (Index Cohort) Assessed by Invasive Hemodynamic Measurements Before and After Angioplasty
| Baseline Characteristics | Total | Iliac | Fempop | Diagnostic | P Value |
|---|---|---|---|---|---|
| No. | 30 (100) | 15 (50) | 10 (33) | 5 (17) | |
| Age, y | 71±10 | 69±10 | 74±8 | 68±11 | 0.38 |
| Men | 22 (73) | 11 (73) | 7 (70) | 4 (80) | 0.92 |
| Smoker | 20 (67) | 11 (73) | 6 (60) | 3 (60) | 0.74 |
| Hypertension | 30 (100) | 15 (100) | 10 (100) | 5 (100) | NA |
| Hyperlipidemia | 23 (77) | 12 (80) | 7 (70) | 4 (80) | 0.83 |
| CAD | 25 (83) | 12 (80) | 8 (80) | 5 (100) | 0.55 |
| Diabetes mellitus | 11 (37) | 6 (40) | 4 (40) | 1 (20) | 0.7 |
| Renal failure | 13 (43) | 6 (40) | 5 (50) | 2 (40) | 0.87 |
| Aspirin | 28 (93) | 15 (100) | 9 (90) | 4 (80) | 0.26 |
| Clopidogrel | 25 (83) | 14 (93) | 9 (90) | 2 (40) | 0.017* |
| Statin | 26 (87) | 13 (87) | 9 (90) | 4 (80) | 0.87 |
| Antihypertensive treatment | 30 (100) | 15 (100) | 10 (100) | 5 (100) | NA |
| ACE | 25 (83) | 12 (80) | 9 (90) | 4 (80) | 0.78 |
| ARB | 4 (13) | 3 (20) | 1 (10) | 0 (0) | 0.49 |
| CBB | 10 (33) | 6 (40) | 3 (30) | 1 (20) | 0.69 |
| β‐blocker | 15 (50) | 7 (47) | 5 (50) | 3 (60) | 0.88 |
| Clinical stage | |||||
| Rutherford 2–3 | 24 (80) | 13 (87) | 6 (60) | 5 (100) | 0.12 |
| Rutherford 4 | (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Rutherford 5–6 | 6 (20) | 2 (13) | 4 (40) | 0 (0) | 0.12 |
| Baseline ABI | 0.53±0.09 | 0.52±0.1 | 0.53±0.12 | 0.53±0.03 | 0.97 |
| Procedural characteristics | |||||
| No. of stents | |||||
| 0 | 6 (20) | 0 (0) | 1 (10) | 5 (100) | <0.0001* |
| 1 | 11 (37) | 4 (27) | 7 (70) | 0 (0) | 0.02* |
| 2 | 10 (33) | 9 (60) | 1 (10) | 0 (0) | 0.008* |
| ≥3 | 3 (10) | 2 (13) | 1 (10) | 0 (0) | 0.7 |
| Length of target lesion, mm | … | 44.0±7 | 165.0±67 | 180±80 | <0.0001* |
| Length of stented segment, mm | … | 46.0±9 | 172.0±71 | 0 (0) | <0.0001* |
| Target vessel diameter, mm | … | 8.4±0.7 | 6.0±0.9 | 6.6±1 | <0.0001* |
| Occlusion | 16 (53) | 6 (40) | 7 (70) | 3 (60) | 0.3 |
| ABI before discharge | 0.85±0.16 | 0.92±0.07 | 0.92±0.07 | 0.54±0.03 | <0.0001* |
Categorical variables are presented as absolute numbers (percentages); statistical comparisons for these were made by the χ2 test. Continuous variables are expressed as mean±SD and compared by the ANOVA F test. P values represent the overall difference between the 3 groups. ABI indicates ankle‐brachial index of the target leg; ACE, angiotensin‐converting‐enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CBB, calcium channel blocker; Fempop, femoropopliteal.
Significant difference between groups (P<0.05).
As depicted in Figure 2, iliac and femoropopliteal angioplasty and stenting led to a significant decrease in systolic aBP in all segments of the aorta. Mean systolic difference after iliac interventions was −24 mmHg and −11 mmHg after femoropopliteal intervention. Systolic aBP remained stable after diagnostic angiography (Table S2).
Figure 2. Segmental, serial, invasive aortic blood pressure (aBP) measurements before and after endovascular treatment measured from proximal (A) to distal (D) sites of the aorta.
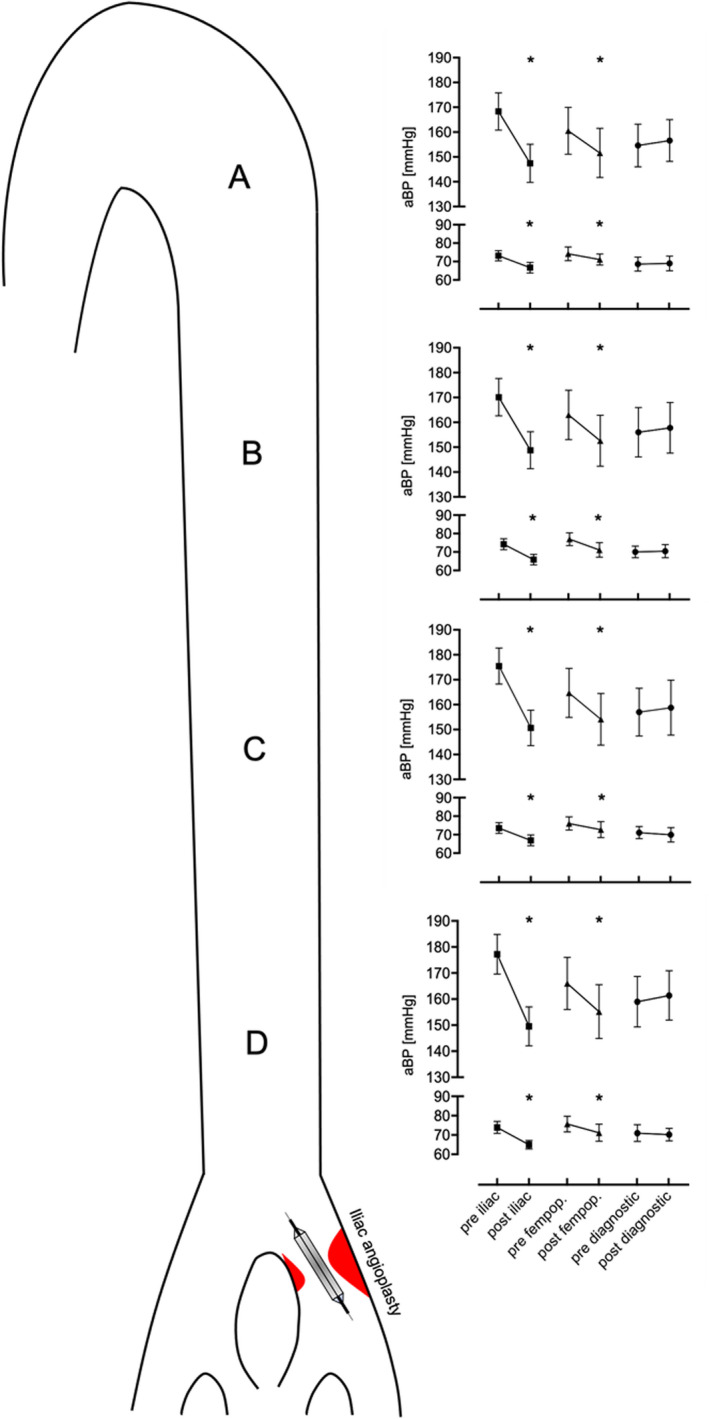
Bars indicate standard error of the mean. *P<0.05 vs baseline (paired t test). ■ indicates iliac angioplasty (n=15); ▲, femoropopliteal (fempop.) angioplasty (n=10); •, diagnostic angiography (n=5).
Similarly, iliac and femoropopliteal angioplasty and stenting led to a significant decrease in diastolic aBP but not diagnostic angiography. Mean diastolic difference after iliac intervention was −8 mmHg and −4 mmHg after femoropopliteal intervention. There was no significant change in diastolic aBP after diagnostic angiography (Table S2). Iliac angioplasty had the most pronounced systolic and diastolic aBP lowering effect (Table S3).
Impact of Angioplasty on Mechanical Indices of the Aorta
Aortic PWV did not differ at baseline between the groups (Figure 3A, Table S4) and was not affected by diagnostic angiography or angioplasty of arteries. Angioplasty did not change timing of the reflected pressure wave to the aorta (Figure 3B, Table S4). In patients with iliac lesions, the baseline AIx was significantly greater compared with patients with dominant femoropopliteal lesions and patients with diagnostic angiography (Table S4). Iliac angioplasty achieved the most pronounced AIx‐lowering effect, whereas there was no change in AIx after diagnostic angiography (Figure 3C, Table S4).
Figure 3. Physicomechanical properties of the aorta.
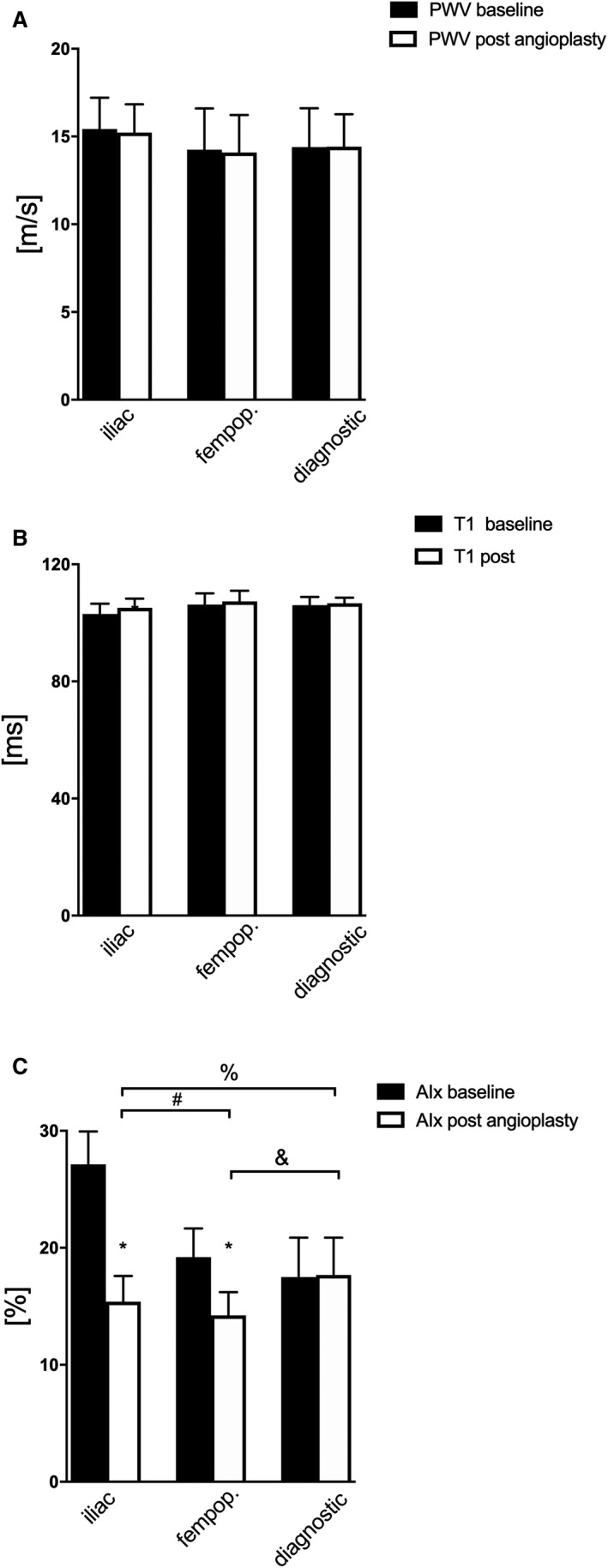
A, Aortic pulse wave velocity (PWV) before (black bars) and after (white bars) peripheral angioplasty of the iliac (n=15) femoropopliteal (fempop.) (n=10) arteries and during diagnostic angiography (n=5). B, Timing of the reflected pressure wave (T1) to the aorta before (black bars) and after (white bars) angioplasty or diagnostic angiography. C, Augmentation index (AIx) before (black bars) and after (white bars) elective angioplasty and diagnostic angiography. Bars indicate mean and standard error of the mean. *P<0.05 vs baseline (paired t test). %, &, and #, P<0.05 (1‐way ANOVA).
Intraprocedural Relation of aBP to bBP
The degree of intraprocedural bBP lowering after angioplasty was comparable to intraprocedural aBP lowering (Figure S1, Table S2, Table S3). The intraprocedural change in aBP correlated with the change in bBP (Figure 4). HR was not affected by angioplasty or angiography (Table S2).
Figure 4. Relation of intraprocedural changes in aortic blood pressure (aBP) and brachial blood pressure (bBP) before and after angioplasty.
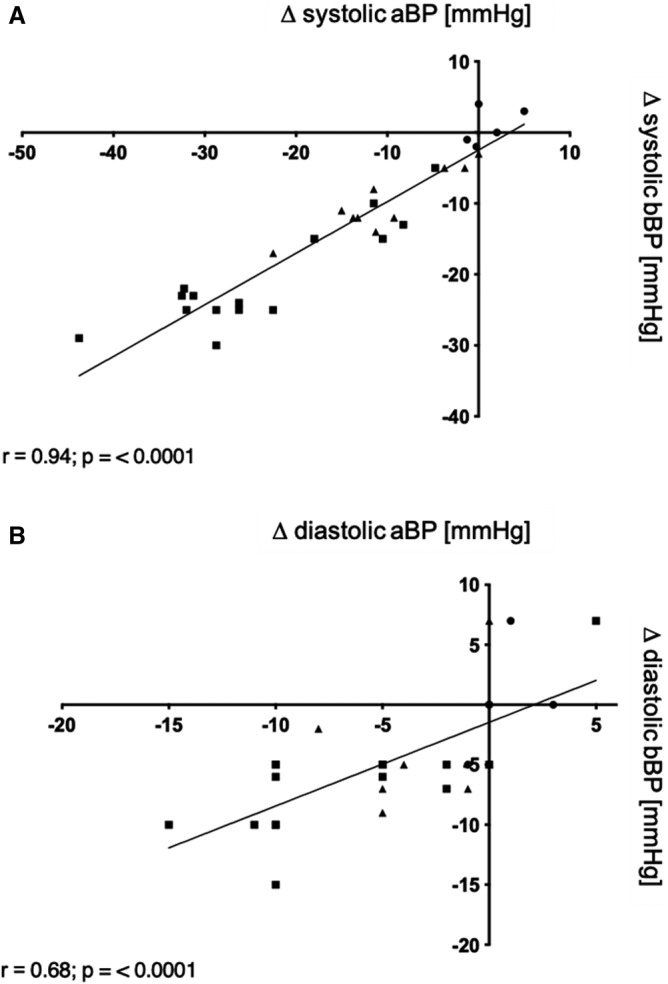
A, Correlation of angioplasty‐associated changes in invasively measured systolic aBP (Δ systolic aBP) and systolic bBP (Δ systolic bBP) determined via sphygmometry. B, Correlation of changes in diastolic aBP (Δ diastolic aBP) and diastolic bBP (Δ diastolic bBP). ■ indicates iliac angioplasty (n=15); ▲, femoropopliteal angioplasty (n=10); and •, diagnostic angiography (n=5).
Reduced bBP and Increased CFA Flow in Follow‐Up Control 1 Day After Angioplasty
Office measurements of HR, bBP, and CFA flow at baseline 1 day before and 1 day after angioplasty or angiography are shown in Figure 5. HR was not affected by diagnostic angiography and angioplasty of arteries (Figure 5A, Table S5). Peripheral angioplasty led to a significant lowering of bBP, with the most pronounced effect after iliac angioplasty (Figure 5B; Table S5). The degree of bBP lowering 1 day after angioplasty was comparable to intraprocedural bBP lowering. Baseline CFA blood flow into the target leg was significantly lower in dominant iliac lesions as compared with femoropopliteal lesions and diagnostic angiography (Figure 5D, Table S5). Although bBP decreased significantly after angioplasty of iliac and femoropopliteal lesions, CFA blood flow increased in the intervened leg with the most pronounced effect after iliac angioplasty, whereas there was a modest increase in flow after femoropopliteal angioplasty (Figure 5D, Table S5). There was no change in blood flow in the untreated leg (Figure 5C, Table S5). Likewise, ABI increased in the treated leg (Table 1). Baseline CFA resistance of the target leg was significantly higher in dominant iliac lesions as compared with femoropopliteal lesions and diagnostic angiography (Table S5). Although flow into the treated leg increased, CFA resistance decreased, with the most pronounced effect after iliac angioplasty (Table S5). Because bBP decreased and flow in the untreated leg remained unchanged, CFA resistance decreased in the untreated leg (Table S5), with the most pronounced effect after contralateral iliac angioplasty.
Figure 5. Endovascular treatment of iliacal and femoral flow‐limiting stenosis is associated with selective improvement/restitution of peripheral blood flow into the treated leg and reduction in brachial blood pressure (bBP) in the index cohort.
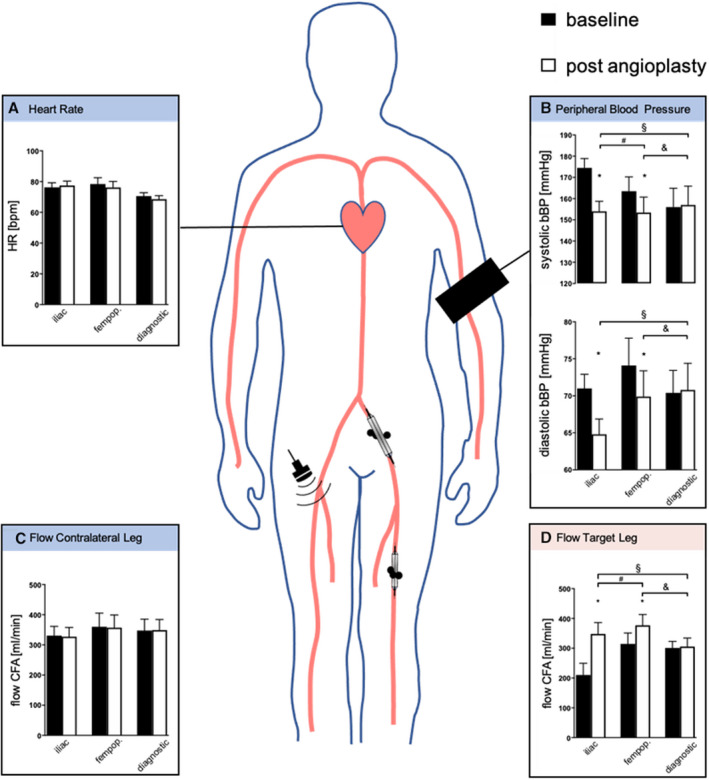
A, Heart rate (HR) measured at baseline 1 day before (black bars) and 1 day after (white bars) angioplasty of the iliac (n=15) and femoropopliteal (fempop.) (n=10) arteries, and after diagnostic angiography (n=5). B, Systolic bBP and diastolic bBP 1 day before (black bars) and 1 day after angioplasty (white bars) and diagnostic angiography. C, Common femoral artery (CFA) blood flow in the untreated leg 1 day before (black bars) and 1 day after (white bars) angioplasty of the contralateral leg. D, Target leg CFA blood flow 1 day before (black bars) and 1 day after (white bars) angioplasty and diagnostic angiography. Bars are mean and error bars are standard error of the mean. *P<0.05 vs baseline (paired t test). §, #, and &, P<0.05 (1‐way ANOVA).
Registry Cohort
bBP Decreases After Iliac and Femoropopliteal Angioplasty
The baseline characteristics of 381 study subjects who had undergone diagnostic angiography or angioplasty of iliac, femoropopliteal, and BTK arteries are shown in Table 2. Patients were grouped according to the most proximal stenosis. In total, 119 patients underwent iliac intervention, 208 patients underwent femoropopliteal intervention, 39 patients were treated BTK only, and 15 patients underwent diagnostic angiography. At baseline, the ABI in 381 patients was 0.5±0.11 and increased significantly to 0.9±0.1 before discharge. Technical success was achieved in 376 cases (99%), with no major complications (Table S6). Patients with crural BTK interventions were significantly more often affected by critical limb ischemia as indicated by Rutherford classification 4–6 with a significantly lower baseline ABI. As depicted in Table S7, clinical baseline and procedural characteristics were similar between the registry cohort and the index cohort.
Table 2.
Baseline Clinical and Demographic Characteristics of the All‐Comer Registry Cohort
| Baseline Characteristics | Total | Iliac | Fempop | BTK | Diagnostic | P Value |
|---|---|---|---|---|---|---|
| No. | 381 (100) | 119 (31) | 208 (55) | 39 (10) | 15 (4) | |
| Age, y | 71±2 | 70±8 | 72±8 | 72±11 | 68±12 | 0.09 |
| Men | 289 (76) | 85 (71) | 160 (77) | 32 (82) | 12 (80) | 0.5 |
| Smoker | 221 (58) | 78 (66) | 114 (55) | 20 (50) | 9 (60) | 0.22 |
| Hypertension | 337 (88) | 106 (89) | 187 (90) | 32 (82) | 12 (80) | 0.38 |
| Hyperlipidemia | 290 (76) | 100 (84) | 149 (71) | 29 (75) | 12 (80) | 0.09 |
| CAD | 265 (70) | 81 (68) | 145 (70) | 28 (72) | 11 (73) | 0.96 |
| Diabetes mellitus | 170 (45) | 40 (34) | 98 (47) | 26 (68) | 6 (40) | 0.003* |
| Renal failure | 159 (42) | 40 (34) | 91 (44) | 22 (57) | 6 (40) | 0.07 |
| Aspirin | 351 (92) | 115 (97) | 190 (91) | 33 (85) | 13 (87) | 0.13 |
| Clopidogrel | 305 (80) | 102 (86) | 169 (81) | 28 (71) | 6 (40) | <0.0001* |
| Statin | 287 (75) | 96 (81) | 150 (72) | 31 (79) | 10 (67) | 0.27 |
| Antihypertensive treatment | 350 (92) | 108 (90) | 194 (93) | 33 (86) | 15 (100) | 0.19 |
| ACE | 210 (56) | 70 (59) | 110 (53) | 22 (57) | 8 (53) | 0.77 |
| ARB | 96 (25) | 30 (25) | 55 (26) | 8 (21) | 3 (20) | 0.84 |
| CBB | 133 (35) | 45 (38) | 71 (34) | 12 (31) | 5 (33) | 0.85 |
| β‐blocker | 207 (54) | 75 (63) | 132 (64) | 23 (59) | 7 (47) | 0.6 |
| Clinical stage | ||||||
| Rutherford 2–3 | 218 (57) | 96 (81) | 112 (54) | 7 (18) | 3 (20) | <0.0001* |
| Rutherford 4 | 34 (9) | 10 (8) | 18 (9) | 3 (7) | 3 (20) | 0.5 |
| Rutherford 5–6 | 129 (34) | 13 (11) | 78 (37) | 29 (75) | 9 (60) | <0.0001* |
| Baseline ABI | 0.50±0.11 | 0.53±0.11 | 0.50±0.1 | 0.48±0.15 | 0.51±0.07 | 0.036* |
| Procedural characteristics | ||||||
| No. of stents implanted | ||||||
| 0 | 100 (26) | 4 (3) | 50 (24) | 31 (80) | 15 (100) | <0.0001* |
| 1 | 128 (34) | 43 (37) | 80 (38) | 5 (13) | 0 (0) | 0.0005* |
| 2 | 119 (31) | 59 (49) | 57 (27) | 3 (7) | 0 (0) | <0.0001* |
| ≥3 | 34 (9) | 13 (11) | 21 (10) | 0 (0) | 0 (0) | 0.1 |
| Length of target lesion, mm | … | 42.0±6 | 158.0±51 | 33.0±12 | 210±78 | <0.0001* |
| Length of stented segment, mm | … | 44.0±5 | 164.0±60 | 12±3 | 0 (0) | <0.0001* |
| Target vessel diameter, mm | … | 8.1±0.6 | 6.2±0.6 | 2.9±0.7 | 6.9±1.2 | <0.0001* |
| Occlusion | 250 (66) | 62 (52) | 142 (68) | 33 (86) | 13 (87) | 0.0001* |
| ABI before discharge | 0.9±0.1 | 0.92±0.1 | 0.92±0.12 | 0.84±0.1 | 0.54±0.11 | <0.0001* |
Categorical variables are presented as absolute numbers (percentages); statistical comparisons for these were made by the χ2 test. Continuous variables are expressed as mean±SD and compared by the ANOVA F test. P values represent the overall difference between the 4 groups. ABI indicates ankle‐brachial index of the target leg; ACE, angiotensin‐converting‐enzyme; ARB, angiotensin receptor blocker; BTK, below the knee; CAD, coronary artery disease; CBB, calcium channel blocker; and Fempop, femoropopliteal.
Significant difference between groups (P<0.05).
Systolic and diastolic bBP as measured on the day before and on the day after angioplasty decreased significantly after iliac and femoropopliteal angioplasties, but not after BTK artery angioplasty and diagnostic angiography (Figure 6, Table S8 and Table S9).
Figure 6. Change in brachial blood pressure (bBP) following angioplasty in the registry cohort.
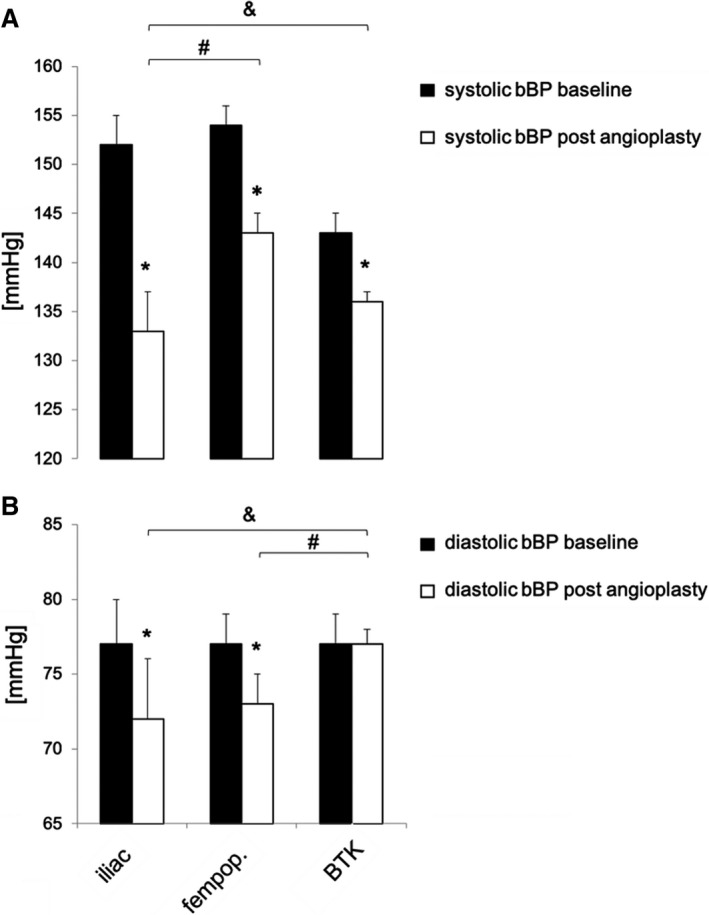
Office systolic bBP (A) and diastolic bBP (B) at baseline as measured on the day before (black bars) and on the day after (white bars) elective angioplasty of iliac (n=119), femoropopliteal (fempop.) (n=208), and below‐the‐knee (BTK) (n=39) arteries. Bars indicate mean standard error of the mean. *P<0.05 vs baseline (paired t test). & and #, P<0.05 (1‐way ANOVA).
Univariate and multivariate analysis identified baseline systolic bBP and site of arterial lesion (Table S10) as predictors of the change in systolic bBP.
Discussion
This study shows for the first time that (1) angioplasty of flow‐limiting stenosis in patients with PAD effectively decreased systolic and diastolic aBP and bBP, (2) this acute beneficial effect was dependent on baseline systolic bBP and was more pronounced at proximal sites of arterial lesion, and (3) was associated with favorable reduction of AIx, whereas PWV as an index of physicomechanical properties of the aorta itself remained stable.
Patient Population
This study was conducted in a representative large cohort of high‐risk patients with PAD affected by a remarkable proportion of cardiovascular risk factors. The prevalence of these comorbidities is even higher than in the recently published VOYAGER PAD (Vascular Outcomes Study of Aspirin Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization for Peripheral Artery Disease) trial and COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, and the previously reported German national average in hospitalized patients with PAD.14, 15, 16 The present study shows that endovascular interventions can be safely and efficiently performed in an all‐comer multimorbid patient cohort with excellent hemodynamic improvement as demonstrated by a high technical success rate, improved ABI, and low complication rate. The safety profile is remarkable, with no conversion to vascular surgery and no major adverse limb events.
Antihypertensive Treatment in Patients With PAD
Patients with symptomatic PAD have a markedly increased risk for death, cardiovascular events and, especially in those with critical limb ischemia, limb loss.16 This poor outcome might also be because PAD and critical limb ischemia are often underdiagnosed and undertreated.16 Patients with PAD are less intensively treated with antihypertensive drugs as compared with other cardiovascular diseases,9 and BP is often uncontrolled.17 Meta‐analyses and randomized controlled trials have shown that a 10 mmHg reduction in systolic bBP or a 5 mmHg reduction in diastolic bBP is associated with significant reductions in all major cardiovascular events by ≈20% and all‐cause mortality up to 15%.1, 18, 19 Notably, the effect size of lowering in aBP and bBP seen in our study after endovascular treatment was in the same range as seen with many antihypertensive drug regimens.1
aBP and bBP Lowering
Our study is the first to demonstrate that peripheral arterial angioplasty leads to acute BP lowering in patients with symptomatic PAD. More than 80% of the investigated patients were affected by arterial hypertension, most of them already under antihypertensive treatment. This study confirms a high prevalence of hypertension in patients affected by PAD.9 Of the hypertensive subjects, the majority were characterized by isolated systolic and not by diastolic hypertension. In the treated patients, peripheral angioplasty led to lowering of systolic and diastolic aBP, with the effect being greater on systolic pressure. Furthermore, our data indicate that the localization of the proximal stenosis plays a pivotal role. The more proximal the location, the more pronounced the BP‐lowering effect is. Angioplasty of proximal iliac lesions had the strongest BP‐lowering effect. This significant aortic effect correlated to the degree of bBP lowering. In nearly 400 patients in the all‐comer registry cohort, we observed a similar bBP response to angioplasty confirming the initial results. Therefore, our study indicates that angioplasty can achieve a remarkable acute aBP‐ and bBP‐lowering effect.
Murgo et al investigated the effect of bilateral manual femoral compression on the aortic pressure waveform in men.20 Bilateral femoral compression caused an increase of ≈10 to ≈20 mmHg in systolic aBP, accompanied by a rise in diastolic pressure of ≈4 mmHg. Baksi et al invasively investigated 20 subjects’ pressure and flow velocity with a sensor‐tipped intra‐arterial wire at multiple locations distal to the proximal aorta before, during, and following occlusion of the left femoral artery by thigh cuff inflation.21 Supra systolic cuff inflation led to a significant increase in aortic mean arterial pressure by ≈4 mmHg, whereas local femoral artery mean arterial pressure increased by ≈6 mmHg. Again, the increase in BP in these experimental studies with acute induction of flow‐limiting interventions in individuals without PAD was in the order of magnitude as the decrease of BP observed in our study after removal of flow‐limiting stenosis.
Angioplasty Improves Regional Blood Flow in the Target Leg
Conversely to aBP and bBP lowering, iliac artery angioplasty led to a significant increase in CFA blood flow of the target leg, whereas angioplasty in the more distal femoral arteries led to a modest increase in CFA blood flow. Factors governing blood flow to the affected extremity include the number, severity, and location of stenotic lesions, and the presence of collateral vessels. It is well understood that conduit vessel function is impaired in patients affected by PAD. Our previous results have indicated a local response to peripheral angioplasty by an improved local flow‐mediated vasodilation response to angioplasty.12 In this study, angioplasty of conduit vessels improved arterial flow and perfusion pressure, as indicated by an elevated ABI.
Impact of Angioplasty on Physicomechanical Indices of the Aorta
Overall, the patients were a high‐risk population as indicated by a high prevalence of comorbidities such as diabetes mellitus, chronic kidney failure, and coronary artery disease. This aggregation of vascular risk factors translates into advanced stages of aortic stiffness, as indicated by a high baseline aortic PWV. Aortic PWV remained stable after angioplasty or mere diagnostic angiography. This indicates that peripheral angioplasty does not acutely change physicomechanical properties of the aorta in patients with advanced PAD. However, we observed an acute lowering of central AIx through peripheral angioplasty, and significant predictors of systolic bBP‐lowering magnitude were baseline bBP and proximal lesion site. This suggests that in patients with proximal lesions, these may act as major pulse reflection sites increasing systolic BP by aortic augmentation. Thus, the change in AIx by angioplasty may be explained by a primary decrease of the contribution of pressure wave reflected at the proximal lesion and thereby a secondary decrease in BP, thus decreasing augmentation pressure.
We conclude that treatment of such lesions increases blood flow into the treated leg with concomitant drop of systemic BP, which together results in a decrease of local vascular resistance that decreases wave reflection as measured by AIx.22 Arterioles account for one half of systemic vascular resistance, whereas the other half is contributed to by other elements of arterial circulation including conduit arteries.23 Tanaka et al demonstrated in healthy women that AIx was the primary predictor of systemic vascular resistance.24
One limitation of the present study is that we only measured changes in BP in a short time frame after angioplasty. Although it is known that chronic BP control is dependent on a complex network of pressure control systems, with the kidney being a main regulator,25 our results provide novel insight that underscore the potential of endovascular treatment to acutely optimize BP, in particular in those patients with high systolic bBP and more proximal lesion sites. Jacomella et al demonstrated that the effect of lower limb angioplasty on AIx lasted for 3 months after endovascular treatment.26 Further studies with longer follow‐up are required to investigate if lower limb angioplastyof proximal lesions, in particular, may play a role in long term BP management in these high‐risk patients.
Conclusions
Our data demonstrate that peripheral arterial angioplasty of iliac and femoropopliteal lesions acutely improve BP in patients with symptomatic PAD independent of comorbidities. Although physicomechanical properties of the aorta remain stable during angioplasty, increase of flow into the treated leg with concomitant drop of systemic BP together results in a decrease in peripheral vascular resistance of the target leg and concomitant reduced wave reflection that overall ameliorated AIx. Further studies are needed to evaluate if this acute effect persists and provides long‐term added benefits of angioplasties, and whether this translates into lower major adverse cardiac and cerebrovascular event rates in this high‐risk population.
Sources of Funding
None.
Disclosures
None.
Supporting information
Table S1–S10
Figure S1
Acknowledgments
The authors thank Dr Wagenhäuser and Dr Horn for discussion and advice. Open access funding was enabled and organized by ProjektDEAL.
(J Am Heart Assoc. 2021;10:e019724. DOI: 10.1161/JAHA.120.019724.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019724
For Sources of Funding and Disclosures, see page 12.
References
- 1.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, ESC Scientific Document Group , et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. DOI: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao‐Melacini P, Zhang X, Pais P, Agapay S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet. 2016;388:761–775. DOI: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 3.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959–968. DOI: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 4.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective SC, Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. DOI: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 5.Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:e71–e126. DOI: 10.1016/j.jacc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Aboyans V, Ricco J‐B, Bartelink M‐L, Björck M, Brodmann M, Cohnert T, Collet J‐P, Czerny M, De Carlo M, Debus S, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. DOI: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. DOI: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 8.Ya'qoub L, Peri‐Okonny P, Wang J, Patel KK, Stone N, Smolderen K. Blood pressure management in patients with symptomatic peripheral artery disease: insights from the portrait registry. Eur Heart J Qual Care Clin Outcomes. 2019;5:79–81. DOI: 10.1093/ehjqcco/qcy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safar ME, Priollet P, Luizy F, Mourad JJ, Cacoub P, Levesque H, Benelbaz J, Michon P, Herrmann MA, Blacher J. Peripheral arterial disease and isolated systolic hypertension: the ATTEST study. J Hum Hypertens. 2009;23:182–187. DOI: 10.1038/jhh.2008.121. [DOI] [PubMed] [Google Scholar]
- 10.Heiss C, Pitcher A, Belch JJF, De Carlo M, Reinecke H, Baumgartner I, Mazzolai L, Aboyans V. The year in cardiology: aorta and peripheral circulation. Eur Heart J. 2020;41:501–508b. DOI: 10.1093/eurheartj/ehz939. [DOI] [PubMed] [Google Scholar]
- 11.Chirinos JA, Segers P, Hughes T, Townsend R. Large‐artery stiffness in health and disease: jacc state‐of‐the‐art review. J Am Coll Cardiol. 2019;74:1237–1263. DOI: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinen Y, Stegemann E, Sansone R, Benedens K, Wagstaff R, Balzer J, Rassaf T, Lauer T, Kelm M, Heiss C. Local association between endothelial dysfunction and intimal hyperplasia: relevance in peripheral artery disease. J Am Heart Assoc. 2015;4:e001472. DOI: 10.1161/JAHA.114.001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath IG, Nemeth A, Lenkey Z, Alessandri N, Tufano F, Kis P, Gaszner B, Cziraki A. Invasive validation of a new oscillometric device (arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28:2068–2075. DOI: 10.1097/HJH.0b013e32833c8a1a. [DOI] [PubMed] [Google Scholar]
- 14.Anand SS, Caron F, Eikelboom JW, Bosch J, Dyal L, Aboyans V, Abola MT, Branch KRH, Keltai K, Bhatt DL, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the compass trial. J Am Coll Cardiol. 2018;71:2306–2315. DOI: 10.1016/j.jacc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Bonaca MP, Bauersachs RM, Anand SS, Debus ES, Nehler MR, Patel MR, Fanelli F, Capell WH, Diao L, Jaeger N, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382:1994–2004. DOI: 10.1056/NEJMoa2000052. [DOI] [PubMed] [Google Scholar]
- 16.Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Luders F, Gebauer K, Roeder N, Berger K, Malyar NM. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36:932–938. DOI: 10.1093/eurheartj/ehv006. [DOI] [PubMed] [Google Scholar]
- 17.Smolderen KG, Gosch K, Patel M, Jones WS, Hirsch AT, Beltrame J, Fitridge R, Shishehbor MH, Denollet J, Vriens P, et al. PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): overview of design and rationale of an international prospective peripheral arterial disease study. Circ Cardiovasc Qual Outcomes. 2018;11:e003860. DOI: 10.1161/CIRCOUTCOMES.117.003860. [DOI] [PubMed] [Google Scholar]
- 18.Brunstrom M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta‐analysis. JAMA Intern Med. 2018;178:28–36. DOI: 10.1001/jamainternmed.2017.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. DOI: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 20.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62:105–116. DOI: 10.1161/01.CIR.62.1.105. [DOI] [PubMed] [Google Scholar]
- 21.John Baksi A, Davies JE, Hadjiloizou N, Baruah R, Unsworth B, Foale RA, Korolkova O, Siggers JH, Francis DP, Mayet J, et al. Attenuation of reflected waves in man during retrograde propagation from femoral artery to proximal aorta. Int J Cardiol. 2016;202:441–445. DOI: 10.1016/j.ijcard.2015.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson IB, MacCallum H, Hupperetz PC, van Thoor CJ, Cockcroft JR, Webb DJ. Changes in the derived central pressure waveform and pulse pressure in response to angiotensin ii and noradrenaline in man. J Physiol. 2001;530:541–550. DOI: 10.1111/j.1469-7793.2001.0541k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyton AC. Systemic circulation. Textbook of Medical Physiology. 1986;218–229. [Google Scholar]
- 24.Tanaka H, Dinenno FA, Hunt BE, Jones PP, DeSouza CA, Seals DR. Hemodynamic sequelae of age‐related increases in arterial stiffness in healthy women. The American journal of cardiology. 1998;82:1152–1155. DOI: 10.1016/S0002-9149(98)00578-5. [DOI] [PubMed] [Google Scholar]
- 25.Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science. 1991;252:1813–1816. DOI: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 26.Jacomella V, Shenoy A, Mosimann K, Kohler MK, Amann‐Vesti B, Husmann M. The impact of endovascular lower‐limb revascularisation on the aortic augmentation index in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2013;45:497–501. DOI: 10.1016/j.ejvs.2013.01.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S10
Figure S1


