Abstract
Background
It is known that dietary intake of polyunsaturated fatty acids may improve cardiac function. However, relatively high daily doses are required to achieve sufficient cardiac concentrations of beneficial omega‐3 fatty acids. The liver X receptor (LXR) is a nuclear hormone receptor and a crucial regulator of lipid homeostasis in mammals. LXR activation has been shown to endogenously reprogram cellular lipid profiles toward increased polyunsaturated fatty acids levels. Here we studied whether LXR lipid reprogramming occurs in cardiac tissue and exerts cardioprotective actions.
Methods and Results
Male 129SV mice were treated with the LXR agonist AZ876 (20 µmol/kg per day) for 11 days. From day 6, the mice were injected with the nonselective β‐agonist isoproterenol for 4 consecutive days to induce diastolic dysfunction and subendocardial fibrosis while maintaining systolic function. Treatment with isoproterenol led to a marked impairment of global longitudinal strain and the E/e' ratio of transmitral flow to mitral annular velocity, which were both significantly improved by the LXR agonist. Histological examination showed a significant reduction in isoproterenol‐induced subendocardial fibrosis by AZ876. Analysis of the cardiac lipid composition by liquid chromatography‐high resolution mass spectrometry revealed a significant increase in cardiac polyunsaturated fatty acids levels and a significant reduction in saturated fatty acids by AZ876.
Conclusions
The present study provides evidence that the LXR agonist AZ876 prevents subendocardial damage, improves global longitudinal strain and E/e' in a mouse model of isoproterenol‐induced cardiac damage, accompanied by an upregulation of cardiac polyunsaturated fatty acids levels. Cardiac LXR activation and beneficial endogenous cardiac lipid reprogramming may provide a new therapeutic strategy in cardiac disease with diastolic dysfunction.
Keywords: diastolic dysfunction, heart failure, lipids, liver X receptor, nuclear receptor
Subject Categories: Basic Science Research, Echocardiography, Fibrosis, Heart Failure, Lipids and Cholesterol
Nonstandard Abbreviations and Acronyms
- CTRL
control
- GLS
global longitudinal strain
- ISO
isoproterenol
- LXR
liver X receptor
Clinical Perspective
What Is New?
Pharmacological liver X receptor activation protects against catecholamine‐induced cardiac damage and diastolic dysfunction.
Liver X receptor activation endogenously reprograms the cardiac lipid profile toward higher levels of cardioprotective polyunsaturated fatty acids.
What Are the Clinical Implications?
New liver X receptor agonists may provide a future therapeutic approach for cardiac diseases with diastolic dysfunction such as heart failure with preserved ejection fraction.
Heart failure (HF) is a leading cause of morbidity and mortality worldwide.1 As many as 1 in 5 people are expected to develop HF during their lifetime. Two major forms of HF exist, HF with reduced ejection fraction and HF with preserved ejection fraction. Whereas multiple pharmacological interventions for the treatment of HF with reduced ejection fraction have been established, effective approaches for HF with preserved ejection fraction are still being sought.2
Numerous neurohormonal mechanisms are activated to preserve cardiac function during HF, such as the sympathetic nervous system.3 Two hormones that are essential for the sympathetic nervous system are the catecholamines epinephrine and norepinephrine.3 Under pathological conditions, as they occur during HF with reduced ejection fraction and HF with preserved ejection fraction, the chronic overstimulation by catecholamines becomes adverse, causing hypoxia, dysfunction in molecular signaling, and cardiac contractility, eventually leading to left‐ventricular remodeling, cardiac fibrosis, and cell death.3 Isoproterenol (ISO) is a synthetic sympathomimetic amine with structural similarities to epinephrine and binds almost exclusively to β‐adrenergic receptors. Prolonged and excessive β‐adrenoreceptor stimulation, for example by ISO, mimics the pathophysiological conditions of HF and induces serious myocardial damage.3 In the present model, ISO induces subendocardial cell death and fibrosis accompanied by a preserved ejection fraction but impaired global longitudinal strain (GLS) and a pathological E/e' ratio of transmitral flow to mitral annular velocity.4
The liver X receptors (LXR) α and β are nuclear hormone receptors that belong to the superfamily of ligand‐activated transcription factors.5 Owing to their involvement in lipid and glucose metabolism, as well as cholesterol homeostasis and inflammation, they became therapeutic drug targets of interest in pathologies such as atherosclerosis and metabolic disease.5
In the present study, we show that systemic application of the dual partial LXR agonist AZ876 significantly reduces catecholamine‐mediated cardiac damage and dysfunction in mice. In parallel, AZ876 endogenously reprograms the cardiac lipid profile toward higher levels of cardioprotective polyunsaturated fatty acids (PUFAs). Our findings suggest that LXR‐mediated cardiac lipid reprogramming may provide a promising approach to reduce catecholamine‐mediated cardiac damage and to improve diastolic dysfunction.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. All animal procedures were performed according to the guidelines of the Charité ‐ Universitaetsmedizin Berlin, Germany and were approved by the Landesamt für Gesundheit und Soziales (Berlin, Germany) for the use of laboratory animals and according to the current version of the German Law on the protection of animals.
Male 129SV mice (Janvier Labs, France) were kept in a temperature‐controlled facility in individually ventilated cages. The amount of light was controlled to imitate a 12‐hour day‐night rhythm. Eight‐week‐old animals were randomized and divided into 4 groups (control[CTRL]‐vehicle [VEH] n=10, CTRL‐ISO n=10, AZ876‐VEH n=12, and AZ876‐ISO n=12). Mice either received a regular control diet (V1127‐000, ssniff, Soest, Germany) (CTRL) or a diet supplemented with the LXR agonist AZ876 (AZ876) at 20 μmol/kg per day. After 6 days, mice either received a daily subcutaneous NaCl (VEH) or ISO injection over 4 consecutive days. Two days after the last injection, the cardiac phenotype of the mice was characterized by both conventional and speckle tracking echocardiography. Post mortem analyses were performed in a blinded fashion. Additionally, histological analyses, quantitative reverse transcription‐polymerase chain reaction‐based analyses, and analytical analyses of lipid composition by liquid chromatography‐high resolution mass spectrometry were performed on heart tissue.
The cell experiments were performed with the murine cardiomyocyte cell line HL‐1.6 The cell line was initially provided by Prof. W.C. Claycomb (Louisiana State University, USA) and cultivated in Claycomb medium supplemented with 10% fetal bovine serum, 1% P/S, norepinephrine (0.1 mmol/L), and L‐glutamine (2 mmol/L).
HL‐1 cardiomyocytes were seeded in 6 well plates coated with fibronectin and gelatin. Starved cells were stimulated with the LXR agonist AZ876 for indicated time intervals and with indicated concentration.
Detailed methods are described in Data S1.
Results
The LXR Agonist AZ876 Attenuates ISO‐Induced Impairment in Cardiac Function
To investigate the cardiac effects of LXR activation in a mouse model of sympathetic activation and diastolic dysfunction,4 the LXR agonist AZ876 was administered to male 129SV mice. Mice were treated with either a CTRL diet or a diet supplemented with the LXR agonist AZ876 for 6 days, followed by 4 consecutive subcutaneous injections of VEH (NaCl) or ISO to induce cardiac damage (Figure 1A).
Figure 1. LXR agonist AZ876 attenuates ISO‐induced impairment in cardiac function.
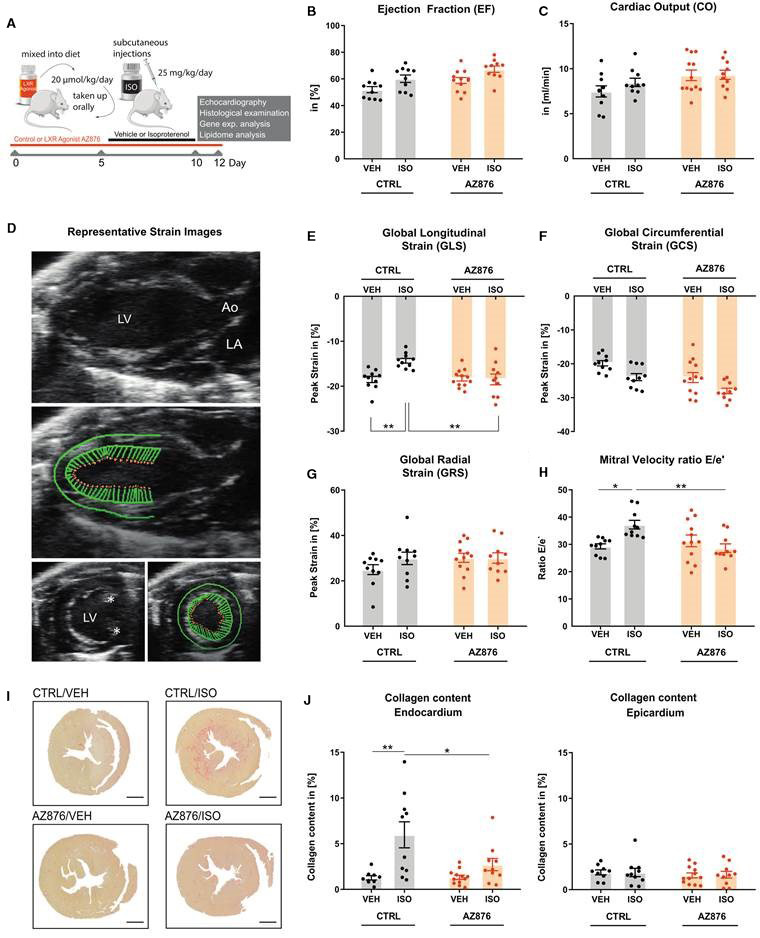
A, Study design. Male 129SV mice were treated with either a control diet (CTRL) or a diet supplemented with the LXR agonist AZ876) and received one subcutaneous injection on 4 consecutive days, either with NaCl (VEH) or with isoproterenol (ISO). B, Left ventricular ejection fraction and (C) left ventricular cardiac output were assessed by echocardiography. D, Representative strain images in long‐axis view (left) and short‐axis view (right) asterisks identify papillary muscles. E, Global longitudinal strain (GLS), (F) Global circumferential strain (GCS), and (G) Global radial strain (GRS). H, E/e' ratio of transmitral flow to mitral annular velocity. I, Representative images of collagen content in cardiac cross‐sections of the 4 treatment groups. Heart samples were fixated in paraffin and stained with Picrosirus red to visualize collagen content in red. J, Quantitative analysis of collagen content in the (sub‐) endocardium (left) and epicardium (right). Data presented as mean ± SEM, n=10–12 per group, *=P<0.05, **=P<0.01 2‐way ANOVA with Bonferroni post hoc test. Ao indicates aorta; LA, left atrium; and LV, left ventricle.
Two days after the last ISO injection, heart function was assessed by conventional echocardiography and speckle tracking echocardiography. Systolic ejection fraction and cardiac output were found to be unchanged and were neither affected by the LXR agonist nor by ISO administration (Figure 1B and 1C). Specific analysis of myocardial deformation was performed by speckle tracking echocardiography (Figure 1D). It is known that the subcutaneous application of ISO at 25 mg/kg per day over 4 consecutive days leads to an impairment in the global longitudinal elongation.5 However, the global radial strain, as well as the global circumferential strain, are not affected.5 In this study, the application of ISO also led to a significant impairment in GLS, while the global radial strain and global circumferential strain remained unchanged (Figure 1E through 1G). More important, application of the LXR agonist significantly improved the GLS compared with the group treated with ISO alone (Figure 1E through 1G). In addition, significant differences were found in an essential parameter for diastolic function, the E/e' ratio of transmitral flow to mitral annular velocity (Figure 1H). The administration of ISO alone led to a significant increase of E/e', which was prevented by additional treatment with the LXR agonist (Figure 1H). Because the ratio between early mitral inflow velocity and mitral annular early diastolic velocity (E/e'), has become central in the guidelines for diastolic assessment, these data suggest that LXR activation may exert beneficial actions on diastolic function.
As previously shown, ISO‐mediated impairment of GLS is associated with subendocardial fibrosis in the present model.4 Accordingly, a significant increase of subendocardial fibrosis (red) was found in the CTRL/ISO‐treated group as assessed by a Picrosirus red staining (Figure 1I and 1J). More important, the LXR agonist significantly reduced subendocardial fibrosis (Figure 1I and 1J). No fibrosis regulation was observed in the epicardium (Figure 1I and 1J).
Together these data show that pharmacological LXR activation reduces cardiac fibrosis and exerts beneficial actions on parameters of diastolic dysfunction.
The LXR Agonist AZ876 Mediates Cardiac Anti‐Inflammatory Actions and Induces Enzymes Involved in the Synthesis of Omega‐3 Fatty Acids
Ligand‐activated LXR has been shown to exert potent anti‐inflammatory actions.5 To determine whether cardiac inflammation is altered by AZ876 in our model, we performed a quantitative polymerase chain reaction‐based expression array of inflammatory response and autoimmunity genes in cardiac tissue samples from our mice (Figure 2A and 2B). ISO‐treatment induced multiple proinflammatory genes including interleukins 1α, 6, and 18, chemokine (C‐C motif) receptors 1 and 2, and multiple chemokine (C‐C motif) ligands, which were reduced by the LXR agonist (Figure 2B). In addition, immunohistological staining of cardiac cross sections for the macrophage marker Mac‐3 revealed cardiac macrophage accumulation under ISO, which was attenuated with AZ876 (Figure 2C). Together, these data indicate an anti‐inflammatory action of AZ876 in the hearts of our model.
Figure 2. The LXR agonist AZ876 induces anti‐inflammatory cardiac actions and LXR‐target genes.
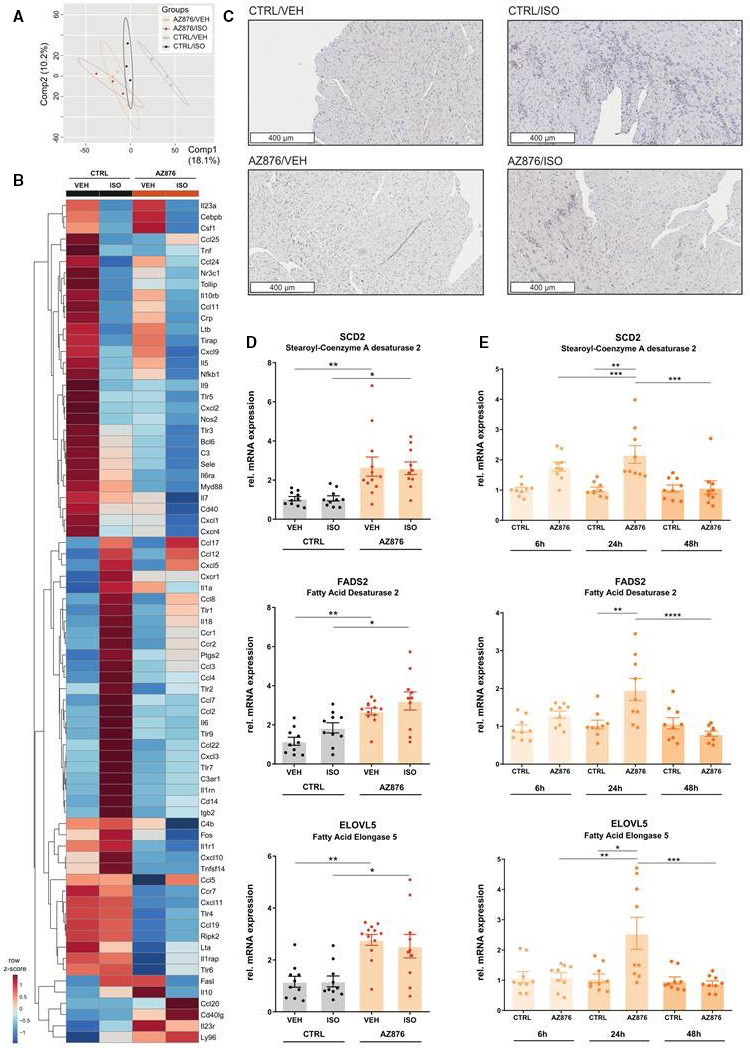
A and B, Reverse transcription quantitative polymerase chain reaction (PCR)‐based cardiac gene expression analysis of inflammatory response and autoimmunity genes (locked nucleic acid‐enhanced, SYBR® Green‐based PCR array) (n=3/ group) (A) Partial least squares discriminant analysis (PLS‐DA) of cardiac gene expression between the 4 groups, x‐axis, principal component 1 (Comp1); y‐axis, principal component 2 (Comp2), ellipses show 95% CI. B, Expression profiles of cardiac inflammatory and autoimmunity genes represented in a heatmap. Hierarchical clustering of normalized gene expression levels, shown as row z‐scores. Clustering and heatmap were generated using MetaboAnalyst (C) Representative images of macrophage infiltration into cardiac tissue visualized by immunohistological staining for Mac‐3, a macrophage surface glycoprotein. D, Relative gene expression of liver X receptor (LXR) target genes measured in apices of murine hearts. E, Relative gene expression of LXR target genes after 6h, 24h, and 48h of 10nM LXR agonist AZ876 stimulation, measured in HL‐1 cells. Data presented as mean ± SEM, n=10–12 per group, *= P<0.05, **=P<0.01 2‐way ANOVA with Bonferroni post hoc test (D), or n=3; N=3, mean ± SEM, *=P<0.05, **=P<0.01, ***=P<0.001, 1‐way ANOVA with Bonferroni post hoc test (E). AZ876 indicates LXR agonist AZ876; CTRL, control (dimethyl sulfoxide); ELOVL5, fatty acid elongase 5; FADS2, fatty acid desaturase 2; ISO, isoproterenol; SCD2, stearoyl‐CoA desaturase 2; and VEH, vehicle (NaCl).
To further prove cardiac LXR activation by AZ876 in our model, we next studied LXR target gene expression in left ventricular (LV) samples. Administration of the LXR agonist resulted in a significant increase in the expression of LXR target genes involved in the synthesis of omega‐3 fatty acids (FAs) compared with control (Figure 2D). Administration of ISO did not cause any significant changes in the expression of these genes compared with the control groups (Figure 2D). LXR‐mediated cardiac regulation of genes involved in lipid metabolism was next studied in HL‐1 cells. HL‐1 cells were stimulated for different time intervals with AZ876 (10 nmol/L). Consistently with the data in LV samples, ligand‐activated LXR resulted in a significant induction of stearoyl‐CoA desaturase2, FA desaturase 2, and FA elongase 5 after 24 hours. (Figure 2E). Together these results demonstrate that AZ876 induces cardiac anti‐inflammatory actions and LXR target gene expression involved in the process of lipid desaturation.
The LXR Agonist AZ876 Significantly Alters the Cardiac Lipid Profile Toward a Higher Content of Unsaturated FAs
Previously, LXR‐mediated anti‐inflammatory actions have been linked to its effects on lipid metabolism involving the synthesis of long chain PUFAs.7 To clarify whether the anti‐inflammatory actions of AZ876 are accompanied by beneficial actions on cardiac lipid composition, we next performed liquid chromatography‐high resolution mass spectrometry‐based lipidomics in LV samples from our mouse model.
Clustering of the treatment groups in a partial least squares discriminant analysis, shows that the groups treated with the LXR agonist (CTRL and ISO) differ markedly from the VEH‐treated groups (CTRL and ISO) (Figure 3A). Looking more closely at how these differences occur, shows that 33 lipid species are significantly regulated (VEH versus LXR agonist) as presented in the heat map (Figure 3B).
Figure 3. LXR agonist AZ876 significantly alters cardiac lipid composition toward a higher content in polyunsaturated fatty acids.
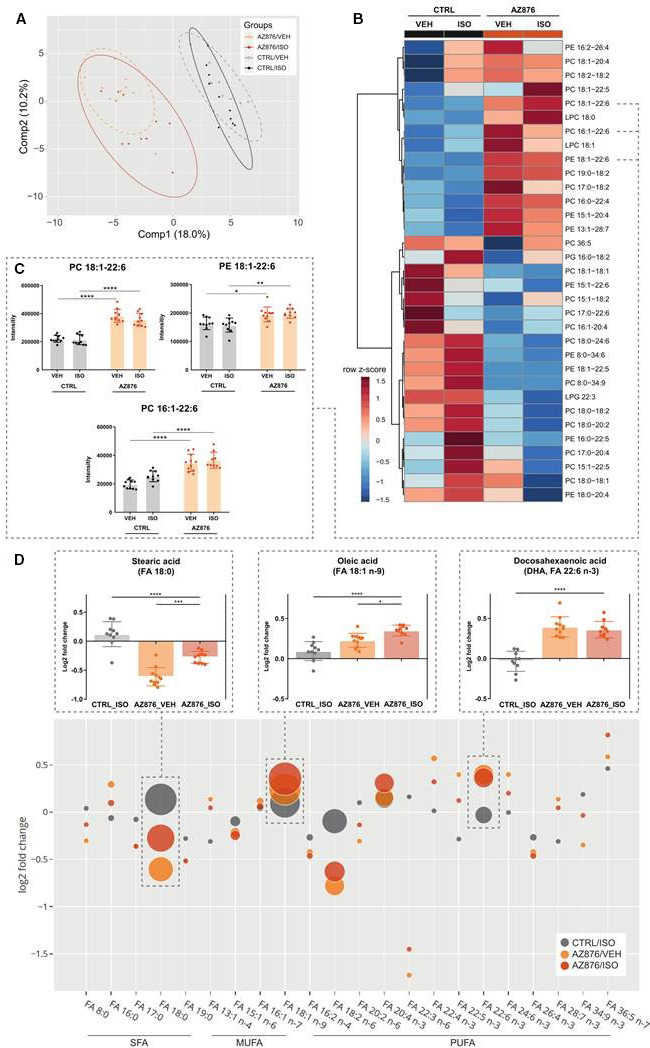
A, Partial least squares discriminant analysis (PLS‐DA) of the cardiac lipid profile between the 4 groups, x‐axis, principal component 1 (Comp1); y‐axis, principal component 2 (Comp2), ellipses show 95% CI. B, Heatmap of 33 significantly regulated cardiac lipid species (VEH vs AZ876). Mice were treated with either CTRL or AZ876 supplementation and received 4 consecutive subcutaneous injections of either VEH or ISO. Clustering and heat map were created with MetaboAnalyst. Clustering of normalized intensities represented as z‐scores, 2‐way ANOVA, followed by Benjamini‐Hochberg P value adjustment (P<0.05). C, Proposed beneficial lipid species containing monounsaturated FAs and the omega‐3 fatty acid docosahexaenoic acid (FA 22:6 n‐3, DHA). D, Log2‐fold change of significantly altered FA moieties compared with CTRL_VEH. Values less than zero on the y‐axis indicate lower fatty acid content, values greater than 1 indicate increase. The bubble size represents the measured intensity, which is analogous to the abundance of the FA. The intensities are normalized, and batch corrected. The FAs are sorted according to the degree of saturation. 2‐way ANOVA followed by a Benjamini‐Hochberg P value adjustment (P<0.05). Stearic acid, oleic acid, and docosahexaenoic acid are shown in magnification. Data presented as mean ± SEM, n=10–12 per group, *=P<0.05, ***=P<0.01, ****=P<0.0001, 1‐way (D) or 2‐way ANOVA (C) with Bonferroni post hoc test. AZ876 indicates LXR agonist AZ876; CTRL, control (diet); ELOVL5, fatty acid elongase 5; FA, fatty acid; FADS2, fatty acid desaturase 2; ISO, isoproterenol; LPC, lysophosphatidyl‐choline; MUFA, monounsaturated fatty acids; LPG, lysophosphatidylglycerol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PUFA, polyunsaturated fatty acids; SCD2, stearoyl‐CoA desaturase 2; SFA, saturated fatty acids; and VEH, vehicle (NaCl).
Individual phosphatidylcholine and phosphatidylethanolamine lipid species consisting of monounsaturated FAs and PUFAs, especially the cardioprotective FA docosahexaenoic acid (FA 22:6 n‐3), are found in a higher abundance in the groups treated with the LXR agonist (Figure 3C). To gain a deeper understanding of the degree of saturation of lipids species in the heart tissue, the abundances of the individual FAs were calculated (Figure 3D). The abundance of stearic acid (FA 18:0), the most abundant saturated FA, is reduced in the groups treated with the LXR agonist (Figure 3D). Among the monounsaturated FAs oleic acid (FA 18:1 n‐9) had the highest abundance, and was reduced in heart tissue of mice treated with the LXR agonist (Figure 3D). Consistently with the induction of LXR‐target genes involved in lipid desaturation, the highly abundant PUFA, docosahexaenoic acid, was significantly induced by the LXR agonist AZ876 (Figure 3D).
Taken together, these data show that pharmacological LXR activation induces overall cardiac lipid abundance by a predominant induction of lipid species containing cardioprotective PUFAs, which may be linked to the anti‐inflammatory and cardioprotective actions of AZ876 in our model.
Discussion
The present study demonstrates cardioprotective effects of the partial dual LXR agonist AZ876 in a murine model of ISO‐induced cardiac damage. The assessment of cardiac function revealed a significant improvement in GLS and E/e' by AZ876 compared with ISO alone. The improvement in cardiac function was accompanied by reduced formation of subendocardial fibrosis. In parallel, AZ876 induced anti‐inflammatory actions in the heart that may be linked to an LXR‐mediated increase in the endogenous synthesis of cardiac PUFAs. This was supported by AZ876‐mediated induction of cardiac FA elongases and desaturases involved in the synthesis of PUFAs and by liquid chromatography‐high resolution mass spectrometry‐based cardiac lipid analysis, ultimately showing a significant decrease in saturated FAs and a significant increase of renowned PUFAs, such as docosahexaenoic acid.
Cardioprotective actions of pharmacological LXR activation have been described previously. Kuipers and colleagues demonstrated that the full dual LXR agonist T0901317 prevented pressure‐induced cardiac hypertrophy; however, this was associated with increased liver weight and hyperlipidemia in the T0901317‐treated group.8 In addition, Cannon and colleagues showed that AZ876 reduced LV hypertrophy improved systolic function and protected against cardiac fibrosis in a transverse aortic constriction model.9 Importantly, AZ876 did not increase liver weight or plasma triglycerides.9 These studies clearly demonstrated that pharmacological LXR activation is able to exert beneficial cardiac actions. However, the underlying biochemical and molecular mechanisms are still incompletely understood.10
Here we propose that ligand‐activated LXR may exert cardiac antifibrotic actions through inhibition of an inflammatory response that might be linked to an increased LXR‐mediated synthesis of cardiac anti‐inflammatory PUFAs. It is well known that LXR mediates anti‐inflammatory actions.5 In parallel, LXR induces the endogenous synthesis of PUFAs.7 Several studies have suggested a link between the cellular abundance of PUFAs and an inhibition of proinflammatory responses.7, 11, 12 Rong and colleagues recently demonstrated that ligand‐activated LXR drives the incorporation of PUFAs into cellular membrane phospholipids by the induction of LPCAT3 (lysophosphatidylcholine acyltransferase), an enzyme involved in phospholipid remodeling.12 This process suppressed inflammation by inhibiting activation of the proinflammatory kinases c‐Src (proto‐oncogene tyrosine‐protein kinase) and JNK (c‐Jun N‐terminal kinase) and by reducing the availability of proinflammatory mediators including arachidonic acid.12 To finally prove that the elevated cardiac PUFA levels induced by AZ876 are indeed responsible for its anti‐inflammatory and antifibrotic actions, additional experiments are required. In addition to our assumption, it is well known that LXR can directly affect antifibrotic and anti‐inflammatory signaling by transcriptional mechanisms.10 In particular, the repression of cardiac NF‐kappaB (nuclear factor kappa‐light‐chain‐enhancer of activated B cells) signaling by LXR plays an important role in this context.13
Antifibrotic actions and an improvement of diastolic dysfunction by PUFA administration have been recently also investigated in clinical studies. In a recent trial including 31 patients with ischemic HF, the administration of PUFAs (2 g/day for 8 weeks, 46% eicosapentaenoic acid and 38% docosahexaenoic acid) led to an improvement in GLS, a reduction in E/e' ratio, and reduced myocardial fibrosis, data consistent with our observations.14 Antifibrotic actions of PUFAs have also been described in LV remodeling after acute myocardial infarction.15
In summary, this study demonstrates that pharmacological LXR activation results in a beneficial endogenous cardiac lipid reprogramming, involving the induction of cardiac PUFA levels. These LXR‐mediated cardiac lipid changes are associated with anti‐inflammatory and pronounced antifibrotic actions resulting in an improvement of diastolic function. Whether these data point toward a clinical beneficial action of LXR‐ligands in heart diseases accompanied by fibrosis and diastolic dysfunction, such as HF with preserved ejection fraction, requires further investigation.
Sources of Funding
This work was supported by the DZHK (German Centre for Cardiovascular Research). DR is supported by a research grant of the Sonnenfeld Stiftung. NB is a participant in the BIH (Berlin Institute of Health) ‐ Charité Junior Clinician Scientist Program funded by the Charité ‐ Universitaetsmedizin Berlin and the Berlin Institute of Health. UK is supported by the DZHK; BER 5.4 PR, the Deutsche Forschungsgemeinschaft (DFG–KI 712/10‐1), the Bundesministerium für Bildung und Forschung/Bundesinstitut für Risikobewertung (BMBF/ BfR1328‐564m), and the Einstein Foundation/Foundation Charité (EVF‐BIH‐2018–440).
Disclosures
None.
Supporting information
Acknowledgments
The authors thank Beata Hoeft for her excellent technical assistance. Parts of this work will be used in the PhD thesis of DR. Open access funding enabled and organized by Projekt DEAL.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019473
For Sources of Funding and Disclosures, see page 8.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. DOI: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al; and Authors/Task Force . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;2016(37):2129–2200. DOI: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. DOI: 10.1038/nrcardio.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyhoff N, Lohr D, Foryst‐Ludwig A, Klopfleisch R, Brix S, Grune J, Thiele A, Erfinanda L, Tabuchi A, Kuebler WM, et al. Characterization of myocardial microstructure and function in an experimental model of isolated subendocardial damage. Hypertension. 2019;74:295–304. DOI: 10.1161/HYPERTENSIONAHA.119.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. DOI: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ Jr. HL‐1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. DOI: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P, Spann N, Kaikkonen M, Lu M, Oh D, Fox J, Bandyopadhyay G, Talukdar S, Xu J, Lagakos W, et al. NCoR repression of LXRs restricts macrophage biosynthesis of insulin‐sensitizing omega 3 fatty acids. Cell. 2013;155:200–214. DOI: 10.1016/j.cell.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuipers I, Li J, Vreeswijk‐Baudoin I, Koster J, van der Harst P , Sillje HH, Kuipers F, van Veldhuisen DJ , van Gilst WH , de Boer RA . Activation of liver X receptors with T0901317 attenuates cardiac hypertrophy in vivo. Eur J Heart Fail. 2010;12:1042–1050. DOI: 10.1093/eurjhf/hfq109. [DOI] [PubMed] [Google Scholar]
- 9.Cannon MV, Yu H, Candido WM, Dokter MM, Lindstedt EL, Sillje HH, van Gilst WH , de Boer RA . The liver X receptor agonist AZ876 protects against pathological cardiac hypertrophy and fibrosis without lipogenic side effects. Eur J Heart Fail. 2015;17:273–282. DOI: 10.1002/ejhf.243. [DOI] [PubMed] [Google Scholar]
- 10.Cannon MV, van Gilst WH , de Boer RA . Emerging role of liver X receptors in cardiac pathophysiology and heart failure. Basic Res Cardiol. 2016;111:3. DOI: 10.1007/s00395-015-0520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oishi Y, Spann NJ, Link VM, Muse ED, Strid T, Edillor C, Kolar MJ, Matsuzaka T, Hayakawa S, Tao J, et al. SREBP1 contributes to resolution of pro‐inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 2017;25:412–427. DOI: 10.1016/j.cmet.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rong X, Albert C, Hong C, Duerr M, Chamberlain B, Tarling E, Ito A, Gao J, Wang BO, Edwards P, et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013;18:685–697. DOI: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Yin R, Ernest R, Li Y, Zhelyabovska O, Luo J, Yang Y, Yang Q. Liver X receptors are negative regulators of cardiac hypertrophy via suppressing NF‐kappaB signalling. Cardiovasc Res. 2009;84:119–126. DOI: 10.1093/cvr/cvp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oikonomou E, Vogiatzi G, Karlis D, Siasos G, Chrysohoou C, Zografos T, Lazaros G, Tsalamandris S, Mourouzis K, Georgiopoulos G, et al. Effects of omega‐3 polyunsaturated fatty acids on fibrosis, endothelial function and myocardial performance, in ischemic heart failure patients. Clin Nutr. 2019;38:1188–1197. DOI: 10.1016/j.clnu.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Heydari B, Abdullah S, Shah R, Francis SA, Feng JH, McConnell J, Harris W, Antman EM, Jerosch‐Herold M, Kwong RY. Omega‐3 fatty acids effect on post‐myocardial infarction ST2 levels for heart failure and myocardial fibrosis. J Am Coll Cardiol. 2018;72:953–955. DOI: 10.1016/j.jacc.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two‐dimensional strain‐a novel software for real‐time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. DOI: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–633. DOI: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, Kanazawa M, VanderGheynst J, Fiehn O, Arita M. MS‐DIAL: data‐independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods. 2015;12:523–526. DOI: 10.1038/nmeth.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaeger C, Lisec J. Statistical and multivariate analysis of MS‐based plant metabolomics data. Methods Mol Biol. 2018;1778:285–296. DOI: 10.1007/978-1-4939-7819-9_20. [DOI] [PubMed] [Google Scholar]
- 20.Ito K, Murphy D. Application of ggplot2 to Pharmacometric Graphics. CPT Pharmacometrics Syst Pharmacol. 2013;2.e79. DOI: 10.1038/psp.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–W494. DOI: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salatzki J, Foryst‐Ludwig A, Bentele K, Blumrich A, Smeir E, Ban Z, Brix S, Grune J, Beyhoff N, Klopfleisch R, et al. Adipose tissue ATGL modifies the cardiac lipidome in pressure‐overload‐induced left ventricular failure. PLoS Genet. 2018;14.e1007171. DOI: 10.1371/journal.pgen.1007171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


