Abstract
Background
Recent trials comparing catheter ablation to medical therapy in patients with heart failure (HF) with symptomatic atrial fibrillation despite first‐line management have demonstrated a reduction in adverse outcomes. We performed an economic evaluation to estimate the cost‐utility of catheter ablation as second line therapy in patients with HF with reduced ejection fraction.
Methods and Results
A Markov model with health states of alive, dead, and alive with amiodarone toxicity was constructed, using the perspective of the Canadian healthcare payer. Patients in the alive states were at risk of HF and non‐HF hospitalizations. Parameters were obtained from randomized trials and Alberta health system data for costs and outcomes. A lifetime time horizon was adopted, with discounting at 3.0% annually. Probabilistic and 1‐way sensitivity analyses were performed. Costs are reported in 2018 Canadian dollars. A patient treated with catheter ablation experienced lifetime costs of $64 960 and 5.63 quality‐adjusted life‐years (QALY), compared with $49 865 and 5.18 QALYs for medical treatment. The incremental cost‐effectiveness ratio was $35 360/QALY (95% CI, $21 518–77 419), with a 90% chance of being cost‐effective at a willingness‐to‐pay threshold of $50 000/QALY. A minimum mortality reduction of 28%, or a minimum duration of benefit of >1 to 2 years was required for catheter ablation to be attractive at this threshold.
Conclusions
Catheter ablation is likely to be cost‐effective as a second line intervention for patients with HF with symptomatic atrial fibrillation, with incremental cost‐effectiveness ratio $35 360/QALY, as long as over half of the relative mortality benefit observed in extant trials is borne out in future studies.
Keywords: atrial fibrillation, catheter ablation, cost‐effectiveness, heart failure
Subject Categories: Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator, Cost-Effectiveness
Nonstandard Abbreviations and Acronyms
- CA
catheter ablation
- ICER
incremental cost‐effectiveness ratio
- MED
medical therapy
- MLHFQ
Minnesota Living with Heart Failure Questionnaire
- QALY
quality‐adjusted life‐year
Clinical Perspective
What Is New?
Catheter ablation for heart failure patients with atrial fibrillation refractory to first‐line medical management is cost effective, with incremental cost‐effectiveness ratio $35 360 Canadian dollars/quality‐adjusted life‐years.
A minimum relative mortality reduction of 28% over at least 2 years is required for catheter ablation to be cost effective at a threshold of $50 000 Canadian dollars/quality‐adjusted life‐years.
What Are the Clinical Implications?
Further trials are needed examining catheter ablation in older patients and at different stages in atrial fibrillation management.
Despite improvements in the prevention and care of heart disease, heart failure (HF) with reduced ejection fraction remains a common condition associated with significant mortality and morbidity.1, 2, 3 Atrial fibrillation (AF) frequently coexists in patients with HF.4, 5 The combination of HF and AF has been associated with increased hospitalizations, more severe symptoms, lower rates of AF control,6, 7, 8 and increased mortality.9
Rhythm control of AF with anti‐arrhythmic drugs has not been shown superior to medical rate control,10, 11 and may be harmful in patients with HF.12, 13 Catheter ablation (CA) is an alternative, non‐pharmacologic means of rhythm control in AF with recent evidence of benefit in patients with HF, including a 38% reduction in mortality or HF hospital admissions in the 2018 CASTLE‐AF (Catherter Ablation for Atrial Fibrillation with Heart Failure) randomized controlled trial (RCT).14 Putting aside, for now, the methodologic limitations of CASTLE‐AF, we can anticipate that increasing use of CA for AF in patients with HF will have significant economic implications. The upfront costs of catheter ablation have previously been characterized from $16 to $21 thousand Canadian dollars (CAD) ($12–$16 thousand US dollars [USD]) compared with annual medical therapy costs of $4 to $5 thousand CAD ($3–$4 USD) in AF.15 Economic evaluations can inform the attractiveness of catheter ablation from a cost and cost‐effectiveness perspective, and also determine the magnitude of comparative effectiveness that may inform future research. Two previous economic evaluations of catheter ablation in HF have been performed. Gao et al reported an incremental cost‐effectiveness ratio (ICER) of $55 942 Australian dollars (AUD)/quality‐adjusted life‐years (QALY) which was felt not to be cost‐effective at an ICER threshold of $50 000 AUD/QALY.16 Chew et al reported an ICER of $38 496 USD/QALY which was felt to be cost‐effective at a higher threshold of $100 000 USD/QALY, with a reasonable chance (75%) of being cost‐effective at the $50 000 USD/QALY threshold.17 Variations in the conclusions and modeling decisions of these 2 studies—for example, the use of baseline health utilities from AF, as opposed to HF, populations in Chew et al, and the assumption of a utility increment of 0.08 associated with maintenance of sinus rhythm; the use of background lifetime mortality rates extrapolated from CASTLE‐AF in both studies—necessitate additional studies to ensure convergence (or to explore divergence) of findings across different settings and conditions. We performed an economic evaluation of the expected cost‐utility of catheter ablation in patients with HF with AF in Alberta, Canada, using currently available estimates of CA effectiveness.
Methods
We performed a cost‐utility analysis of CA compared with usual medical management of AF in patients with HF, using data from available randomized controlled trials and meta‐analyses where available. A 30‐year time horizon was specified, equivalent to a lifetime time horizon given the high mortality rate of this population. The perspective was that of a public healthcare payer; costs were analyzed in 2018 Canadian dollars. All data and materials have been made publicly available at the Education & Research Archive of the University of Alberta and can be accessed at https://doi.org/10.7939/r3‐jbh9‐v007.
Patients
The target population was composed of patients similar to those enrolled in previous RCTs, of which CASTLE‐AF provided the most patients. Accordingly, our population was composed of patients with HF with reduced left ventricular ejection fraction and symptomatic paroxysmal or persistent AF, age 65 years, who did not have a response to antiarrhythmic drugs, had unacceptable side effects, or were unwilling to take these drugs.14 We did not model a sex‐ or ejection fraction‐specific effect, but patients in CASTLE‐AF were predominantly men (84%–87%) with persistent AF (65%–70%), median New York Heart Association functional class II with median left ventricular ejection fraction 32%. The majority had previously trialed amiodarone (57%–61%). Other trials of catheter ablation in AF enrolled a demographically and clinically similar group of patients, though some, like AATAC (Ablation vs Amiodarone for Treatment of AFib in Patients with CHF and an ICD), which also showed a mortality benefit of CA, differ in not requiring previous failure of anti‐arrhythmic therapy or not specifying whether a degree of AF‐attributable symptoms were required for study enrollment.12
Intervention and Comparator
In the 7 extant randomized trials of CA in patients with HF with AF, CA was performed at specialized cardiac centers using radiofrequency energy to achieve pulmonary vein isolation, with variations in the specific technique both within and between studies. Procedures were followed by a “blanking” period, usually of 3 months, during which patients could be cardioverted or receive repeat catheter ablation. The trials featured 526 CA procedures among 428 patients, for an inverse‐variance weighted mean of 1.32 (95% CI, 1.21–1.44) procedures per patient. Adverse events from each trial were summed to calculate the frequency of CA complications per person (Table 1). The adverse event frequencies were consistent with those seen in previous literature.18, 19, 20 No peri‐procedure deaths were observed in the HF trials, so a death rate of 0.1% was assumed based on a retrospective cohort of 32 569 patients undergoing CA without HF.21 The procedure was presumed to take 2 to 3 hours, with patients discharged on the same day,22 therefore a single day of disutility was assumed for each patient.
Table 1.
Procedure‐Related Costs and Complications
| Parameters | Risk/Mean (SD) ($CAD) | Probability Distribution ($CAD) | Source and Notes |
|---|---|---|---|
| Procedure costs and duration | |||
| Procedures per patient | 1.32 (0.06) | Gamma, a=506.13 b=383.43 | * |
| Total costs per procedure | $9498 CAD ($1211) | Gamma, a=61.466, b=0.006472 | †, ‡ |
| Duration of actual procedure | Negligible (2–4 h) | … | Assumed |
| Health status day of procedure | |||
| Health status | 0.25 | … | Assumed. |
| Duration | 1.0 day | … | Assumed. |
| Complications | |||
| Death | |||
| Risk | 0.10% | … | 21 |
| Tamponade | |||
| Risk | 0.70% | Beta, a=3, b=425 | * |
| Cost | $33 196 ($4234) | Gamma, a=61.466, b=0.001852 | * |
| Duration | 10.9 d | … | † |
| Pulmonary vein stenosis | |||
| Risk | 0.70% | Beta, a=3, b=425 | * |
| Cost | $16 524 ($2108) | Gamma, a=61.466, b=0.003720 | † |
| Duration | 11.9 d | … | † |
| Stroke | |||
| Risk | 0.47% | Beta, a=2, b=426 | * |
| Cost | $22 050 ($2813) | Gamma, a=61.466, b=0.002788 | † |
| Duration | 15.1 d | … | † |
| Effusion | |||
| Risk | 1.40% | Beta, a=6, b=422 | * |
| Cost | $14 549 ($1856) | Gamma, a=61.466, b=0.004225 | † |
| Duration | 7.3 d | … | † |
| HF admission | |||
| Risk | 1.17% | Beta, a=5, b=423 | |
| Cost | $15 651 ($1996) | Gamma, a=61.466, b=0.003927 | † |
| Duration | 11.4 d | … | † |
| Pneumonia | |||
| Risk | 0.47% | Beta, a=2, b=426 | |
| Cost | $12 973 ($1655) | Gamma, a=61.466, b=0.004738 | † |
| Duration | 7.6 d | … | † |
| Bleeding | |||
| Risk | 2.34% | Beta, a=9, b=418 | |
| Cost | $6531 ($829) | Gamma, a=61.466, b=0.003128 | ǁ |
| Duration | 2.5 d | … | Assumed |
Analysis performed in 2018 Canadian dollars using costs from publicly available Alberta data. Probability distributions in column 3 are generated solely from parameters for risk and mean and SD of costs provided in column 2. CAD indicates Canadian dollars.
Estimated from events per patients cumulatively in extant trials of catheter ablation in patients with atrial fibrillation and heart failure. Number of procedures per patient is an inverse‐variance weighted average.
Estimated from provider billing codes (Alberta Schedule of Medical Benefits) and case‐mix based ambulatory (Comprehensive Ambulatory Care Classification System) and inpatient (Canada Institute for Health Information Case Mix Group Plus) average encounter costs in 2017 to 2018 (http://www.ahw.gov.ab.ca/IHDA_Retrieval, accessed March 18, 2020) and validated by clinical experts. Distribution of costs including SDs were unavailable. Standard errors were assumed to be 25% of the mean cost. Gamma distributions for costs were imputed from means and standard deviations. No probabilistic distributions were specified for duration of hospitalizations since duration was not expected to affect results materially apart from its effects on the average costs of hospitalization. Specific Comprehensive Ambulatory Care Classification System and Canada Institute for Health Information Case Mix Group Plus codes available in Data S1.
Assumed 35% of cases involved a routine inpatient stay of 1 day, apart from acute complications.
Assumed analogous to an admission for heart failure with an angiogram.
Weighted average of costs related to a hospital admission for vascular surgery miscellaneous procedure and an uncomplicated overnight stay for observation with transfusion of packed red blood cells. Vascular repair was assumed to be required in 15% of peri‐procedural bleeds.18
The comparator was ongoing medical management of AF and HF (medical therapy [MED]). The frequencies of medication use were taken from supplementary data in CASTLE‐AF. As expected, patients in the MED group had a higher rate of anti‐arrhythmic drug use and digoxin. Otherwise, the medical management of both groups was similar and included standard HF medications, diuretics, and oral anticoagulation for stroke prophylaxis.
CA Effectiveness, Duration of Benefit, and Ongoing Care
Based on the systematic review of Turagam et al,23 we assumed CA reduces mortality (base case relative risk [RR], 0.52; 95% CI, 0.33–0.81) and HF admissions (base case RR, 0.60; 95% CI, 0.39–0.93) (Table 2). We assumed no CA‐specific effect on health utility for our base case, apart from decrements attributable to outcomes or complications (eg, HF admission or peri‐procedural stroke), as the meta‐analytic estimate of CA effect on Minnesota Living With Heart Failure Questionnaire (MLHFQ) scores was not statistically significant in Turagam et al (corrected in erratum to the original publication).23, 24 This assumption is conservative, as numerous studies in AF patients with and without HF have demonstrated improvements in health‐related quality of life on disease‐specific instruments. However, the impact on generic health utilities is typically much lower than disease‐specific instruments imply, perhaps best shown in patients without HF in the recent CABANA trial.25 This assumption was explored in sensitivity analysis using a range of CA‐associated utility increments up to 0.20, with 0.05 felt to be a reasonable alternative assumption based on mapping from MLHFQ data (see below). We modeled differences in ongoing medical care for CA versus MED patients based on medication use at the end of CASTLE‐AF, and cardioversion and subsequent catheter ablation rates from various randomized trials; these differences in ongoing medical care were presumed to persist indefinitely. Based on a recent well‐conducted observational study of catheter ablation in patients with AF and HF, the duration of effectiveness for CA on mortality and HF requiring hospitalization was assumed to be 3 years.26 This is similar to the median follow‐up duration of CASTLE‐AF, which was 38 months.14
Table 2.
Model Assumptions—Treatment Effectiveness and Ongoing Medical Care
| Parameters | Estimate Assumed | Probability Distribution | Source |
|---|---|---|---|
| Effectiveness | |||
| Hospitalizations for HF | RR/annual risk | ||
| CA | RR, 0.60; 95% CI, 0.39‒0.93 | Lognormal, u=−0.511 s=0.222 | SR23 |
| MED | 11.7% | Beta, a=56.65, b=429.38 | CASTLE‐AF14 * |
| Hospitalizations, non‐HF | RR/risk | ||
| CA | RR, 0.99; 95% CI, 0.77‒1.28 | Lognormal, u=−0.010, s=0.130 | CASTLE‐AF |
| MED | 16.7% | Beta, a=38.40, b=191.04 | CASTLE‐AF* |
| Mortality | RR/Risk | ||
| CA | RR, 0.52; 95% CI, 0.33‒0.81 | Lognormal, u=−0.654 s=0.229 | SR23 |
| MED | |||
| Baseline mortality | 4.8% to 21.1% depending on age | … | CCDSS2, * |
| AF‐associated excess mortality risk (OR) | OR, 1.14; 95% CI, 1.03‒1.26 | Lognormal u=0.131, s=0.051 | SR,9 observational studies, adjusted estimates |
| Quality of Life | Utility difference/health utility | ||
| CA | +0.00 | … | SR23, * |
| MED | 0.66 (0.069) | Beta, a=30.56, b=15.74 | SR27, * |
| Ongoing medical care | |||
| Drug therapy | Frequency | ||
| Amiodarone | |||
| CA | 27% | Beta, a=48, b=129 | CASTLE‐AF |
| MED | 35% | Beta, a=64, b=118 | |
| ACEi/ARB | |||
| CA | 88% | … | |
| MED | 85% | … | |
| Beta‐blocker | |||
| CA | 91% | … | |
| MED | 90% | … | |
| CCB | |||
| CA | 5% | … | |
| MED | 3% | … | |
| Digoxin | |||
| CA | 14% | … | |
| MED | 34% | … | |
| Diuretics | |||
| CA | 85% | … | |
| MED | 90% | … | |
| OAC | |||
| CA | 90% | … | |
| MED | 90% | … | |
| Additional procedures | Annual risk | ||
| Cardioversions | |||
| CA | 4.3% | Beta, a=36.91, b=825.91 | RCTs of CAe.g.: 12 |
| MED | 7.9% | Beta, a=3.32, b=38.42 | AF‐CHF13, || |
| Catheter ablation | |||
| CA | … | … | |
| MED | 1.1% | Beta, a=47.06, b=4293.75 | AF‐CHF¶ |
ACEi indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; CA, catheter ablation; CCB, calcium channel blocker; CCDSS, Canadian Chronic Disease Surveillance System; HF, heart failure; MED, medical therapy; OAC, oral anticoagulant; RCT, randomized controlled trial; RR, relative risk; and SR, systematic review.
Interpolated from Kaplan‒Meier cumulative survival rates reported in CASTLE‐AF (Catherter Ablation for Atrial Fibrillation with Heart Failure).14 The resulting figures turned out to be similar to the yearly rates seen in AF‐CHF (Atrial Fibrillation and Congestive Heart Failure) trial.13
Mortality in patients with heart failure by sex and 10‐y age bands. Average mortality between men and women chosen for base case.
Systematic review showed a non‐significant improvement in Minnesota Living With Heart Failure Questionnaire score, −9.02, 95% CI, −19.75 to +1.71. Because of lack of health utility data, we have assumed, in our base case analysis, no impact on health utility/health‐related quality of life.
Based on mean EQ‐5D values from studies with 0% to 67% New York Heart Association III/IV patients reported in the systematic review of Dyer et al, 2010.27 A CI was considered the range of mean EQ‐5D for these studies, ie: 0.58 to 0.78.
In AF‐CHF, the medical rate control arm appeared to have a 2.9% yearly risk of cardioversion, compared with 19.1% in the anti‐arrhythmic drug arm. Given 31% of patients on amiodarone in the medical therapy group, this leads to a blended average risk of 7.9%, with 2.9% and 19.1% set as 95% confidence limits for the purposes of estimating beta‐distribution parameters.
Rate of catheter ablation in both medical rate control and anti‐arrhythmic drug groups was similar in the AF‐CHF trial—rate given here is for all patients in the trial.
Amiodarone use was less frequent in the patients with CA14; all patients on amiodarone were subject to extra laboratory and chest X‐ray monitoring according to guidelines.28 Six percent of amiodarone users were expected to develop hypothyroidism, leading to increased medication and monitoring costs.28 Amiodarone‐induced lung toxicity was also modeled, as below. Other amiodarone toxicities (eg, hepatotoxicity) were not included because of rarity and lack of evidence of impacts on cost and quality of life.
Model Structure and Baseline Risk Estimates
The initial costs and complications of CA were modeled as a decision tree capturing the first 3 months of therapy. Patients undergoing CA could experience a peri‐procedural death, stroke, other complication, or no complications. Those alive after their procedures entered a Markov model with a cycle length of 1 year, to track ongoing outcomes and QALYs. Patients in the MED arm proceeded directly to the Markov model (Figure 1 and Figure S1).
Figure 1. Markov Model of catheter ablation versus medical therapy.
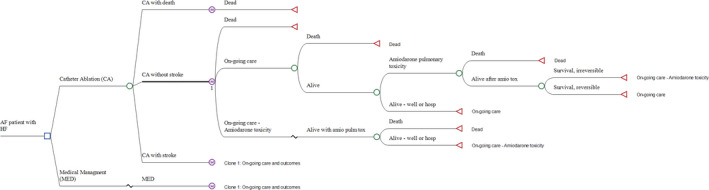
.
Markov model shown as a tree diagram. A simplified form shown as a state transition diagram is found in Figure S1. AF indicates atrial fibrillation; amio tox or amio pulm tox, amiodarone pulmonary toxicity; CA, catheter ablation; HF, heart failure; and MED, medical therapy. This figure was rendered in TreeAge Pro 2019 (TreeAge Software, LLC, Williamstown, MA).
The Markov model consisted of 3 states—Alive (labeled “ongoing care” in Figure 1), alive following irreversible amiodarone‐induced lung toxicity, and dead. Patients who had experienced a peri‐procedural stroke experienced the same health states, with a stroke‐related utility decrement. Patients who were alive could experience HF hospitalizations and non‐HF hospitalizations, with attendant costs and health utility decrements. They could also experience amiodarone‐induced lung toxicity, the risk of which was modeled as the frequency of amiodarone use multiplied by the risk of lung toxicity per amiodarone user.29 Amiodarone‐induced lung toxicity was modeled as an acute event with a separate transition subtree, featuring a specific risk of death, recovery to the alive state, and recovery to the alive with irreversible lung toxicity state, based on data from multiple sources, as synthesized in the previous cost‐utility study of Blackhouse et al., 2013 (Table S1).30
The baseline yearly risk of HF and non‐HF admissions were interpolated from supplementary cumulative survival data from CASTLE‐AF (Table 2, details in Data S1).14 The yearly risk of mortality was age dependent, from mortality estimates by 10 year age bands for patients with HF provided in the Canada Chronic Disease Surveillance System.2 Surveillance System estimates were inflated to reflect the additional burden of mortality imposed by concomitant AF from a systematic review of observational studies (OR, 1.14; 95% CI, 1.03 to 1.26).9
Costs
Costs were obtained in 2018 Canadian dollars (CAD) from publicly available Alberta sources. These are no charges to patients or private health insurers, but, rather, costs borne by the public healthcare payer under universal health care. Physician and procedure fees, and fees for certain outpatient laboratory tests, were obtained from Alberta’s Schedule of Medical Benefits. Briefly, the Schedule of Medical Benefits governs physician fees, and is negotiated between the Alberta Medical Association, Alberta Health and Wellness (the government ministry), and Alberta Health Services (the provincial corporate health authority), with the relative valuation of fee items between and within specialties determined by physician working groups.31 Ambulatory care costs, including emergency department costs, were obtained from the Comprehensive Ambulatory Care Classification System estimates. Inpatient costs were obtained from the Canada Institute for Health Information Case Mix Group Plus data set. Both sources provide costs for average patients in diagnosis‐based aggregates (http://www.ahw.gov.ab.ca/IHDA_Retrieval/selectCategory.do under “Health Costing”). Medication costs were obtained from the Alberta Blue Cross Interactive Drug Benefits List, and reflect prices negotiated between pharmaceutical companies and Alberta’s public drug funder. Estimated costs for study outcomes and ongoing medical care are provided in Table S1, with further details provided in Data S1. The additional ongoing costs of irreversible amiodarone‐induced lung toxicity were obtained from a literature source.32 Costs were discounted at a rate of 3.0% per year.
Utility
The primary model outcome was quality adjusted life years (QALYs), based on the health utility estimates provided in Table S2. While the weighted mean baseline Minnesota Living with Heart Failure Questionnaire score in the CA RCTs was ≈50, the 2 published algorithms translating MLHFQ to health utilities are based on small studies and led to divergent values.33, 34 Based on a 2010 review of EQ‐5D ratings from various HF trials, we assumed a base case utility of 0.66.27 Patients undergoing procedures or hospital admissions were assumed to experience a utility of 0.25 for the duration of the event.
Statistical Analysis
The primary, base case analysis was performed as probabilistic sensitivity analyses, with parameters assigned to distributions as follows. Effectiveness estimates were assigned a lognormal distribution based on published CIs. Event risks and the frequency of amiodarone use were assigned beta distributions, with parameters based on frequency data from their published literature sources. Beta‐distributions were assigned to health utilities, similarly parameterized. Costs were only available as the sum of mean or median values, therefore CIs of ±25% were presumed and used to extrapolate a symmetric gamma distribution. Probability distribution parameters are provided in Tables 1, 2, 3. The model was run with Monte Carlo sampling for 10 000 replicates to develop CIs and a cost‐effectiveness acceptability curve, presuming a threshold willingness‐to‐pay of $50 000 CAD/QALY (equivalent to $38 664 USD/QALY denominated in 2018 USD). Means and 95% CIs are reported, with lower and upper limits taken from the 2.5‐ and 97.5‐percentile values of the Monte Carlo outcome distributions.
Table 3.
Base Case Analysis—Catheter Ablation Versus Medical Therapy
| Outcome | Mean | 95% CI |
|---|---|---|
| Cost (discounted) | ||
| CA | $64 960 | $55 715‒$75 647 |
| MED | $49 865 | $42 116‒$58 968 |
| Effectiveness (QALY, discounted) | ||
| CA | 5.63 | 4.38‒6.77 |
| MED | 5.18 | 4.06‒6.20 |
| Incremental | ||
| Cost ($CAD) | $15 095 | $10 631‒$19 806 |
| Effectiveness (QALY) | 0.45 | 0.15‒0.70 |
| Incremental cost‐effectiveness | ||
| ICER ($CAD/QALY) | $35 360 | $21 518‒$77 419 |
CIs taken from the 2.5 and 97.5 percentiles of the output distributions from probabilistic sensitivity analysis under base case assumptions. $CAD indicates Canadian dollars; CA, catheter ablation; ICER, incremental cost‐effectiveness ratio; MED, medical therapy; QALY, quality‐adjusted life‐years
One‐way sensitivity analyses were performed on the following variables, considered a priori to have the most potential influence on results: (1) effectiveness parameters including: relative risk of mortality, HF admissions, non‐HF admissions, effect of CA on health utility, and duration of benefit from CA; (2) CA characteristics including cost of procedure, number of procedures required per patient, and the frequency of peri‐procedural adverse events; (3) health utility in the alive state; (4) baseline risk of outcomes including HF admission, non‐HF admission, and mortality; (5) cost of HF admission; and (6) start age of the cohort.
Additional sensitivity analyses were performed on CA effectiveness on mortality. First, we assumed that CA would have no effect on mortality. Second, we modeled a fixed annual rate of background mortality, instead of using age‐banded HF‐specific rates. Finally, we ran the model with a discount rate of 3.0% per year. Analyses were performed in TreeAge Pro 2019 (TreeAge Software, LLC, Williamstown, MA). Institutional ethics review was not required for this study, which involved publicly available data and did not involve actual patients or patient information.
Results
Base Case Analysis
Expenditures in catheter ablation were higher than in MED, driven mainly by the increased upfront cost of the catheter ablation procedure(s), which amounted to, on average, $12 563 CAD with another $1312 for complications (Table S3). Most of the healthcare costs in both CA and MED were associated with the hospital admissions component, with patients with CA having slightly fewer HF admissions, but more non‐HF admissions, over time; the latter was because of longer overall survival. Patients with CA also had somewhat higher costs associated with ongoing medical care of HF and AF, also because of longer survival. While patients with CA were less likely to be amiodarone users, the marginal decrease in amiodarone‐associated costs, including those related to amiodarone‐induced pulmonary toxicity, was minimal ($1080 versus MED $1279).
In probabilistic analysis, CA was associated with average costs of $64 960 compared with $49 865 for MED, for average incremental costs of $15 095 (95% CI, $10 631–19 806) (Table 3). CA was associated with an additional 0.45 QALYs (95% CI, 0.15–0.70). The incremental cost effectiveness ratio (ICER) was $35 360/QALY (95% CI $21 518–77 419) (Table 3; see Figure S2 for ICER scatter plot). At a willingness‐to‐pay of $50 000 CAD/QALY, the probabilistic analysis with base case assumptions identifies a 90% chance of CA being cost‐effective; at willingness to pay thresholds of $25 000 CAD and $100 000 CAD, the probability that CA is the preferred strategy is 11% and 98%, respectively (see Figure S3 for cost‐effectiveness acceptability curve).
One‐Way Sensitivity Analysis
The tornado diagram (Figure S3) showed that the ICER was most sensitive to duration of benefit and the effectiveness of CA on all‐cause mortality. Benefits had to extend, at minimum, nearly 2 years to achieve an ICER <$50 000/QALY. If we assume a duration of benefit of 5 years, the longest follow‐up length of CASTLE‐AF, the ICER becomes $23 199/QALY.
Similarly, CA had to reduce mortality by at least 28% (RR≤0.72, ie: 70% of the HF benefit in relative risk reduction terms in the base case scenario), all other variables held equal, to be cost effective at this threshold. The model was otherwise robust to variations in other variables, including the estimated per‐procedure costs of CA, other CA effectiveness parameters, and peri‐procedural adverse event rates. Even with all peri‐procedural adverse event rates doubled and tripled, the resulting ICERs were $37 419/QALY and $41 678/QALY, respectively (shown in Figure 2 as “inflation factor for adverse events).
Figure 2. Catheter ablation vs medical therapy—tornado diagram with results of 1‐way sensitivity analyses.
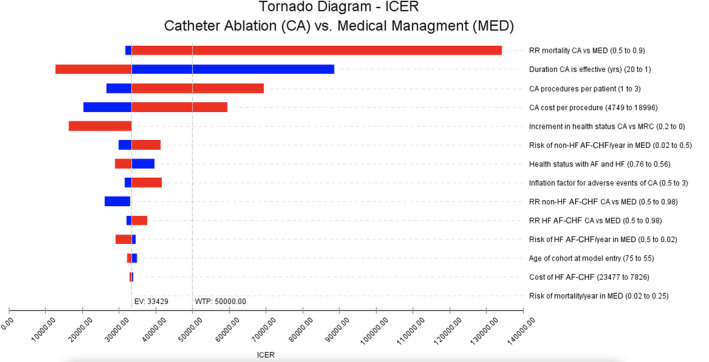
Vertical line intersecting the incremental cost‐effectiveness ratio axis at $33 429 Canadian dollars/quality‐adjusted life‐years (QALY) represents incremental cost‐effectiveness with all parameters set to base case assumptions (note that this varies slightly from the probabilistic sensitivity results reported as base case in this study, since the Tornado diagram is based on a deterministic analysis). As each parameter is varied across the range provided in parentheticals in the right‐hand column, the bars represent the range of incremental cost‐effectiveness ratio values obtained. Blue bars represent the effect of decreasing the parameter relative to its base case value, red bars represent the effect of increasing the parameter relative to its base case value. For example, relative risk mortality catheter ablation vs medical therapy, when set to 0.5 (down from base case estimate of 0.52) led to incremental cost‐effectiveness ratio=$31 730 Canadian dollars/QALY, and when set to 0.9 (up from base case estimate of 0.52), led to incremental cost‐effectiveness ratio=$134 176/QALY; the horizontal bar for this parameter captures the interval $31 730/QALY‒$134 176/QALY. Larger bars indicate that the result is more sensitive to the parameter when varied across the given range. The most influential parameters, ie, those with the largest bars, are found at the top of the graph. AF indicates atrial fibrillation; AF‐CHF, Atrial Fibrillation and Congestive Heart Failure trial; CA, catheter ablation; EV, expected value; HF, heart failure; ICER, incremental cost‐effectiveness ratio; MED, medical therapy; RR, relative risk; and WTP, willingness to pay.
If CA were to improve health utility, then, based on mapping35 from MLHFQ change‐scores,23 it would likely produce an increase of 0.05. At this level, the ICER would be $26 536/QALY. If there were no mortality benefit of CA, a health utility improvement of 0.10 lasting 3 years would produce an ICER value <$50 000/QALY in combination with the base case HF hospitalization relative reduction.
Additional sensitivity analyses were performed on the CA effectiveness on mortality. First, we assumed no mortality difference. Patients with CA ended up with slightly fewer QALYs and increased costs despite the reduction in HF admission associated with CA, leading CA to be dominated (more costly and less effective) by MED. Under the assumption of no mortality benefit, a health utility increment of 0.10 or more (see above) was the only alternative parameterization of any single variable that was able to generate an ICER of <$50 000/QALY CA. Second, using fixed annual background mortality rate instead of age‐banded background mortality rates from Canadian Chronic Disease Surveillance reports did not materially change ICER results, even on one‐way sensitivity analysis across a range from 2% to 25% mortality per year (results not shown).
Finally, with a discount rate of 1.5%, results were materially unchanged (see Table S4).
Discussion
Our economic evaluation suggests that catheter ablation is associated with both increased costs and increased quality‐adjusted life years compared with ongoing medical therapy of patients with HF with symptomatic AF despite previous trials of medical therapy, and may be cost‐effective considering the commonly cited willingness‐to‐pay threshold of $50 000 CAD/QALY in healthcare settings.36, 37, 38 These conclusions were robust to one‐way variations in most input variables, with the exception of CA mortality effectiveness and duration of benefit.
Cost‐effectiveness analyses of CA in the regular AF population have had heterogenous conclusions, with ICERs ranging from dominated, to €3434 EUR/QALY.39 While many have indicated that CA in the regular AF population may be attractive, some of these cost‐effectiveness analyses presumed a benefit on stroke risk from the restoration of sinus rhythm, which was not borne out by the recent CABANA trial.10 In patients with HF and comorbid AF, only 2 previous cost‐effectiveness analyses have examined the cost‐utility of CA.16, 17 Gao et al, 2019, produced a model similar to our own, but concluded that their base case ICER was unfavorable, at $55 942 AUD/QALY. One difference appears to have been different means of estimating mortality in our respective models. Gao et al. reconstructed trial patient data from CASTLE‐AF and fitted separate parametric survival curves to CA and MED patients. After 5 years, patients with CA experienced ongoing mortality at the same rate as patients receiving MED, with transition probabilities extrapolated from the parametric curve for MED long‐term. Chew et al. used a similar parametric extrapolation yet came to conclusion of cost‐effectiveness in the US context, using more favorable base‐case assumptions about the utility benefit of CA than ours. We modeled baseline mortality using annual mortality rates varying by 10‐year age bands according to HF surveillance data from the Public Health Agency of Canada,2 with patients with CA experiencing reduced mortality for 3 years according to the meta‐analyzed pooled relative risk from RCTs of CA.23 This approach generated average yearly mortality rates similar to those seen in CASTLE‐AF during the trial period (Data S1). Particularly where an intervention‐driven difference in mortality does not also need to be extrapolated past the duration of trial, our approach situates the intervention in conditions of “real world” background mortality.
The 2 major limitations to our analysis are methodologic limitations, and generalizability concerns about the current evidence for CA in HF. The mortality benefit in CASTLE‐AF was extraordinary (hazard ratio [HR], 0.54; 95% CI, 0.34–0.84), exceeding the observed HF hospitalization benefit.14 The CASTLE‐AF results are close to those of a preceding trial, AATAC (HR, 0.44; 95% CI, 0.20–0.97),12 and the mortality benefit appears to be driven by cardiovascular death, suggesting that the mechanism of benefit may be other than HF mitigation. However, multiple methodologic concerns with CASTLE‐AF have been pointed out, including early stopping, excessive loss‐to‐follow‐up, open‐label design, and a small number of primary end‐point events relative to most HF trials.40 The overall body of literature is heavily dependent on CASTLE‐AF.41 Thus, whether catheter ablation is truly effective in reducing HF admissions and mortality in patients with HF with AF remains an open question. Economic evaluation, uniquely, provides insight into the minimum degree of benefit future studies need to demonstrate for CA to be cost‐effective in the Canadian context; if CA results in a mortality benefit as low as 28%, representing 58% of the observed relative risk reduction in the present RCTs, it will remain attractive from a cost‐effectiveness perspective in many healthcare systems. The health utility benefit associated with CA is an important parameter for future studies to clarify, since an increment in health utility would offset the degree of mortality benefit required for CA to be cost‐effective, and may, on its own, be cost‐effective at higher willingness‐to‐pay thresholds.
From a generalizability perspective, the source RCTs enrolled patients with reduced ejection fraction HF and symptomatic AF despite previous trials of medical therapy. In CASTLE‐AF, there was the additional criteria of having been intolerant of, failed, or unwilling to trial amiodarone therapy. Our cost‐effectiveness analyses therefore supports the use of CA as second‐line therapy of AF in patients with HF, and is unable to determine to whether CA should be considered earlier in the management of patients with HF and AF. It is worth noting that all patients in CASTLE‐AF had implanted devices. Our analysis may apply to as few as 7.8% of patients with HF and AF meeting the CASTLE‐AF inclusion and exclusion criteria, though CA still appeared effective in patients not meeting the inclusion criteria in this observational study.42 It also remains uncertain whether CA should be targeted to select groups of patients with HF, since the subgroup analyses of CASTLE‐AF appeared to show benefit in men, age <65 years, with left ventricular ejection fraction ≥25%.14 Further trials examining the use of CA in patients with HF with new onset AF and specific other subgroups will be needed to avoid inappropriate indication creep that might lead to diminished returns and potential harms.
There are additional limitations of the present economic evaluation. For example, cost‐effectiveness was not directly measured in CASTLE‐AF and other key trials. Key utility inputs were extrapolated from other studies. Given the results of our 1‐way sensitivity analysis, these limitations are probably minor relative to the uncertainty imposed by the evidence base.
In summary, current guidelines recommend catheter ablation in patients with HF with symptomatic AF. The American Heart Association gives a IIb recommendation that select patients may benefit from lower mortality and reduced HF hospitalization,43 while the Canadian Cardiovascular Society recommends it in symptomatic patients after an adequate trial of antiarrhythmic therapy.44 Our results support both guidelines by showing that CA is likely cost‐effective. Readers should avoid extrapolating these findings to patient groups that were not included in the randomized effectiveness trials, and more data on the effectiveness of CA compared with continued medical management, particularly on mortality and quality of life benefit, and in alternative patient populations, is needed. Until then, CA should be seen as a reasonably cost‐effective second‐line intervention for symptomatic AF in patients with reduced ejection fraction HF, based on the current available evidence.
Sources of Funding
None.
Disclosures
J.G.A. reports research grants and personal fees from Medtronic, research grants from Baylis, and personal fees from Biosense‐Webster. S.K. is supported by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta. Financial support from the above sources was not used to fund this study. The above‐mentioned companies, to our knowledge, are unaware of this study or its results, and had no influence in the conceptualization, planning, analysis, or reporting of this work. The remaining authors have no disclosures to report.
Supporting information
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019599.
For Sources of Funding and Disclosures, see page 10.
References
- 1.Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ. 2012;184:E765–E773. DOI: 10.1503/cmaj.111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada . Report From the Canadian Chronic Disease Surveillance System: Heart Disease in Canada, 2018. Ottawa, ON: Public Health Agency of Canada; 2018. [Google Scholar]
- 3.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. DOI: 10.1016/S0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 4.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2d–8d. DOI: 10.1016/S0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. DOI: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 6.Aleong RG, Sauer WH, Davis G, Bristow MR. New‐onset atrial fibrillation predicts heart failure progression. Am J Med. 2014;127:963–971. DOI: 10.1016/j.amjmed.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure‐Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. DOI: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 8.Silva‐Cardoso J, Zharinov OJ, Ponikowski P, Naditch‐Brule L, Lewalter T, Brette S, Steg PG. Heart failure in patients with atrial fibrillation is associated with a high symptom and hospitalization burden: the RealiseAF survey. Clin Cardiol. 2013;36:766–774. DOI: 10.1002/clc.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath‐Ordoubadi F, Neyses L. A meta‐analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676–683. DOI: 10.1093/eurjhf/hfp085. [DOI] [PubMed] [Google Scholar]
- 10.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. DOI: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJM, Tijssen JGP, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. DOI: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 12.Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. DOI: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 13.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JMO, Buxton AE, Camm AJ, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. DOI: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 14.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. DOI: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 15.Khaykin Y, Morillo CA, Skanes AC, McCracken A, Humphries K, Kerr CR. Cost comparison of catheter ablation and medical therapy in atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:907–913. DOI: 10.1111/j.1540-8167.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Moodie M. Modelling the lifetime cost‐effectiveness of catheter ablation for atrial fibrillation with heart failure. BMJ Open. 2019;9.e031033 DOI: 10.1136/bmjopen-2019-031033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chew DS, Loring Z, Anand J, Fudim M, Lowenstern A, Rymer JA, Weimer KED, Atwater BD, DeVore AD, Exner DV, et al. Economic evaluation of catheter ablation of atrial fibrillation in patients with heart failure with reduced ejection fraction. Circ Cardiovasc Qual Outcomes. 2020;13.e007094 DOI: 10.1161/CIRCOUTCOMES.120.007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardioll. 2012;59:143–149. DOI: 10.1016/j.jacc.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts‐Thomson KC, Brooks AG, Sanders P. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol. 2013;6:1082–1088. DOI: 10.1161/CIRCEP.113.000768. [DOI] [PubMed] [Google Scholar]
- 20.Freeman JV, Tabada GH, Reynolds K, Sung SH, Liu TI, Gupta N, Go AS. Contemporary procedural complications, hospitalizations and emergency visits after catheter ablation for atrial fibrillation. Am J Cardiol. 2018;121:602–608. DOI: 10.1016/j.amjcard.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cappato R, Calkins H, Chen S‐A, Davies W, Iesaka Y, Kalman J, Kim Y‐H, Klein G, Natale A, Packer D, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798–1803. DOI: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Deyell MW, Leather RA, Macle L, Forman J, Khairy P, Zhang R, Ding L, Chakrabarti S, Yeung‐Lai‐Wah JA, Lane C, et al. Efficacy and safety of same‐day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:609–619. DOI: 10.1016/j.jacep.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Turagam MK, Garg J, Whang W, Sartori S, Koruth JS, Miller MA, Langan N, Sofi A, Gomes A, Choudry S, et al. Catheter ablation of atrial fibrillation in patients with heart failure: a meta‐analysis of randomized controlled trials. Ann Intern Med. 2019;170:41–50. DOI: 10.7326/M18-0992. [DOI] [PubMed] [Google Scholar]
- 24.[No authors listed]. Correction: catheter ablation of atrial fibrillation in patients with heart failure. Ann Intern Med. 2019;170:668–669. [DOI] [PubMed] [Google Scholar]
- 25.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. DOI: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuel M, Abrahamowicz M, Joza J, Beauchamp ME, Essebag V, Pilote L. Long‐term effectiveness of catheter ablation in patients with atrial fibrillation and heart failure. Europace. 2020;22:739–747. DOI: 10.1093/europace/euaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ‐5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13. DOI: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassallo P, Trohman RG. Prescribing amiodarone: an evidence‐based review of clinical indications. JAMA. 2007;298:1312–1322. DOI: 10.1001/jama.298.11.1312. [DOI] [PubMed] [Google Scholar]
- 29.Vorperian VR, Havighurst TC, Miller S, January CT. Adverse effects of low dose amiodarone: a meta‐analysis. J Am Coll Cardiol. 1997;30:791–798. DOI: 10.1016/S0735-1097(97)00220-9. [DOI] [PubMed] [Google Scholar]
- 30.Blackhouse G, Assasi N, Xie F, Gaebel K, Campbell K, Healey JS, O'Reilly D, Goeree R. Cost‐effectiveness of catheter ablation for rhythm control of atrial fibrillation. Int J Vasc Med. 2013;2013.262809 DOI: 10.1155/2013/262809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberta Health and Wellness . Alberta Health Care Insurance Plan: Schedule of Medical Benefits as of 01 October 2019. Edmonton, AB: Government of Alberta; 2019. Available at: https://open.alberta.ca/publications/somb‐2019‐10‐01. Accessed April 16, 2021 [Google Scholar]
- 32.Tarride JE, Hopkins RB, Burke N, Guertin JR, O'Reilly D, Fell CD, Dion G, Kolb M. Clinical and economic burden of idiopathic pulmonary fibrosis in Quebec. Canada. Clinicoecon Outcomes Res. 2018;10:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havranek EP, McGovern KM, Weinberger J, Brocato A, Lowes BD, Abraham WT. Patient preferences for heart failure treatment: utilities are valid measures of health‐related quality of life in heart failure. J Card Fail. 1999;5:85–91. DOI: 10.1016/S1071-9164(99)90030-1. [DOI] [PubMed] [Google Scholar]
- 34.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health‐related quality of life data acquired in the baseline phase of the CARE‐HF study. Eur J Heart Fail. 2005;7:243–251. DOI: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Calvert MJ, Freemantle N, Yao G, Cleland JG, Billingham L, Daubert JC, Bryan S. Cost‐effectiveness of cardiac resynchronization therapy: results from the CARE‐HF trial. Eur Heart J. 2005;26:2681–2688. DOI: 10.1093/eurheartj/ehi662. [DOI] [PubMed] [Google Scholar]
- 36.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]
- 37.Clement FM, Harris A, Li JJ, Yong K, Lee KM, Manns BJ. Using effectiveness and cost‐effectiveness to make drug coverage decisions. JAMA. 2009;302:1437–1443. DOI: 10.1001/jama.2009.1409. [DOI] [PubMed] [Google Scholar]
- 38.Neumann PJ, Cohen JT, Weinstein MC. Updating cost‐effectiveness–the curious resilience of the $50,000‐per‐QALY threshold. N Engl J Med. 2014;371:796–797. DOI: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 39.Chang AY, Kaiser D, Ullal A, Perino AC, Heidenreich PA, Turakhia MP. Evaluating the cost‐effectiveness of catheter ablation of atrial fibrillation. Arrhythm Electrophysiol Rev. 2014;3:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Packer M, Kowey PR. Building castles in the sky: catheter ablation in patients with atrial fibrillation and chronic heart failure. Circulation. 2018;138:751–753. DOI: 10.1161/CIRCULATIONAHA.118.034583. [DOI] [PubMed] [Google Scholar]
- 41.Packer M. Methodological and clinical heterogeneity and extraction errors in meta‐analyses of catheter ablation for atrial fibrillation in heart failure. J Am Heart Assoc. 2019;8:e013779. DOI: 10.1161/JAHA.119.013779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noseworthy PA, Van Houten HK, Gersh BJ, Packer DL, Friedman PA, Shah ND, Dunlay SM, Siontis KC, Piccini JP, Yao X. Generalizability of the CASTLE‐AF trial: Catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm. 2020;17:1057–1065. DOI: 10.1016/j.hrthm.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. DOI: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 44.Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. DOI: 10.1016/j.cjca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Dusman RE, Stanton MS, Miles WM, Klein LS, Zipes DP, Fineberg NS, Heger JJ. Clinical features of amiodarone‐induced pulmonary toxicity. Circulation. 1990;82:51–59. DOI: 10.1161/01.CIR.82.1.51. [DOI] [PubMed] [Google Scholar]
- 46.Owens DK, Sanders GD, Harris RA, McDonald KM, Heidenreich PA, Dembitzer AD, Hlatky MA. Cost‐effectiveness of implantable cardioverter defibrillators relative to amiodarone for prevention of sudden cardiac death. Ann Intern Med. 1997;126:1–12. DOI: 10.7326/0003-4819-126-1-199701010-00001. [DOI] [PubMed] [Google Scholar]
- 47.Tengs TO, Lin TH. A meta‐analysis of quality‐of‐life estimates for stroke. Pharmacoeconomics. 2003;21:191–200. DOI: 10.2165/00019053-200321030-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


