Abstract
Background
Data from the International Registry of Acute Aortic Dissection indicate that the guideline criterion of 5.5 cm for ascending aortic intervention misses many dissections occurring at smaller dimensions. Furthermore, studies of natural behavior have generally treated the aortic root and the ascending aorta as 1 unit despite embryological, anatomical, and functional differences. This study aims to disentangle the natural histories of the aforementioned aortic segments, allowing natural behavior to define specific intervention criteria for root and ascending segments of the aorta.
Methods and Results
Diameters of the aortic root and mid‐ascending segment were measured separately. Long‐term complications (dissection, rupture, and death) were analyzed retrospectively for 1162 patients with ascending thoracic aortic aneurysm. Cox regression analysis suggested that aortic root dilatation (P=0.017) is more significant in predicting adverse events than mid‐ascending aortic dilatation (P=0.087). Short stature posed as a serious risk factor. The dedicated risk curves for the aortic root and the mid‐ascending aorta revealed hinge points at 5.0 and 5.25 cm, respectively.
Conclusions
The natural histories of the aortic root and mid‐ascending aorta are uniquely different. Dilation of the aortic root imparts a significant higher risk of adverse events. A diameter shift for intervention to 5.0 cm for the aortic root and to 5.25 cm for the mid‐ascending aorta should be considered at expert centers.
Keywords: aneurysm, aortic root, dissection, mid‐ascending aorta, natural history, surgical threshold
Subject Categories: Clinical Studies
Nonstandard Abbreviations and Acronyms
- CE
composite end point
- DM
diabetes mellitus
- MAAE
major adverse aortic event
Clinical Perspective
What Is New?
The aortic root and the mid‐ascending aorta demonstrate increased risk of major adverse events at diameters <5.5 cm.
Aortic root dilatation is found to be more malignant than mid‐ascending aortic dilatation.
What Are the Clinical Implications?
Aortic root dilatation and mid‐ascending aortic dilatation deserve new and different management.
A surgical threshold of 5.0 cm should be considered for the aortic root.
A surgical threshold of 5.25 cm should be considered for the mid‐ascending aorta.
Aortic dissection is a devastating disease that threatens life without premonitory signs. The surgical guidelines of the American Heart Association,1 Society of Thoracic Surgeons, American Association for Thoracic Surgery, and European Society of Cardiology2 recommend preemptive repair of ascending aorta aneurysms at a diameter of 5.5 cm and 5.0 cm for patients with connective tissue aortopathies whose malevolent behavior dictates a more aggressive approach and earlier intervention. The cutoff value of 5.5 cm corresponds to a steep rise in the respective risk curve. The threshold for intervention for patients with bicuspid aortic valve has varied over the years, with the most recent dedicated bicuspid valve guidelines (American Heart Association, Society of Thoracic Surgeons, American Association for Thoracic Surgery, European Association for Cardio‐Thoracic Surgery) back to 5.5 cm.3 Beyond intervention criterion values, the major adverse aortic events (MAAEs), defined as dissection, rupture, and death, pose a considerable threat to patients, exceeding operative risk at experienced centers.
Despite improved access to healthcare services, increased awareness of clinicians, implemented screening policies, and improved surgical outcomes, the prevalence of thoracic aortic disease has been reported on the increase.4 The study by Evangelista et al from the International Registry of Acute Aortic Dissection demonstrated that 60% of acutely dissected ascending aortas present with a diameter <5.5 cm and 40% <5.0 cm.5 Our recent study showed that population dynamics at least partially explain this failure of standard criteria to prevent aortic dissection, specifically the exponential increase in the number of surgical candidates in the population, as the surgical cutoff value in the right “tail” of the bell curve is displaced leftward.6 Also, recent studies indicate that the aorta grows abruptly by about 0.8 cm at the moment of dissection itself,7 again suggesting earlier intervention. This mounting evidence suggests a closer look at criteria for surgical intervention on the ascending aorta.
The natural histories of the dilated aortic root (“root”) and mid‐ascending aorta (“mid”) have traditionally been left entangled in a single analysis. Even the guideline documents perceive these 2 segments as a single unit although they differ significantly in anatomy, embryology, and physiologic function. The diverse smooth muscle cell origin of the thoracic arteries and its implications to disease development have been long known.8 The root is populated by a smooth muscle subtype that originates from the lateral plate mesoderm,9 whereas the subtype of the ascending aorta is neural crest derived.9, 10
In this article, we explore the possibility that the natural history of the root and the mid differ, with each manifesting a different risk profile.
Methods
The data that support the findings of this study, as well as the analytic methods and study materials, are available from the corresponding author upon reasonable request. This investigation was approved by the Human Investigation Committee of the Yale University School of Medicine. The study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement.11
Data Origin and Inclusion and Exclusion Criteria
To test the hypothesis of differing natural histories of the root and the mid, the patients with aneurysms registered in the Aortic Institute's database at Yale–New Haven Hospital were studied retrospectively. This registry spans 3 decades and >3500 patients. The inclusion criteria were presence of root or mid at least 3.5 cm wide and retrievable data. Only computed tomography or magnetic resonance tomography scans were considered for the reassessment of diameters for the present study (Figure 1). Transesophageal and transthoracic ultrasounds were not included because the upper ascending aorta may evade visualization and the aortic root cannot be assessed with multiplanar reconstruction. Excluded were chronic aortic dissection and traumatic aortic injury as well as congenital aortic malformations.
Figure 1. Distribution of the aortic root and mid‐ascending aorta diameters in size groups.
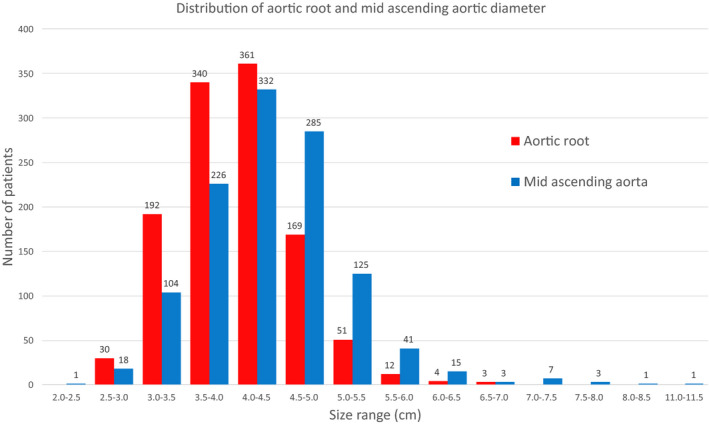
The diameter mean±SD for the root is 4.02±0.60 cm and for the mid is 4.33±0.77 cm.
End Points and Long‐Term Follow‐Up
Long‐term follow‐up was conducted according to the Yale Aortic Institute 4‐pronged policy.12 The outcomes assessed included acute type A aortic dissection, ascending aorta rupture, confirmed ascending aorta–related death, and death of unknown or other causes (Figure 2). Surgery interrupted the course of natural history, so our follow‐up stopped at the time of surgery. The remainder of patients outside of these outcomes are still alive and free of any aortic event. We also assessed 2 composite end points (CEs): (1) acute type A dissection, rupture, and ascending aorta–related death and (2) acute type A dissection, rupture, and all‐cause death. After reaching an outcome or surgery, the patients were censored. A positive family history was attributed to patients with at least 1 relative with a known aneurysm in any vascular distribution or dissection confirmed on imaging or autopsy.
Figure 2. Incidence of acute type A aortic dissection, ascending aorta rupture, ascending aorta death, and unknown or other cause of death and surgery.
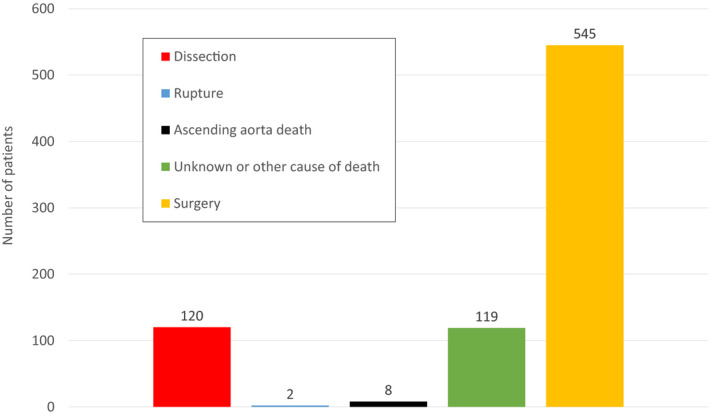
The remaining 368 patients are alive and free of any aorta‐related event.
Radiographic Definitions and Techniques
The clinical data of the 1162 included patients (Table 1) were accrued retrospectively from electronic medical records and hospital charts. The mid was measured at its maximum diameter, usually near the level of the pulmonary artery bifurcation and perpendicular to the vessel's centerline. Regarding root measurement, there is no consensus, but there are established methods.13, 14 In this study, root diameter was the average of 3 distances, from each commissure to the opposite cusp. Two distances were averaged for the bicuspid valves, approximately parallel and perpendicular to the long axis of the valve opening. One author (P.K.) performed the measurements, and 2 authors (M.Z., B.Z.) confirmed them. Reports from the Diagnostic Imaging at Yale were consulted, and any discrepancies were resolved by the senior author (J.A.E.). Aortic valves were characterized as bicuspid either echocardiographically or by direct intraoperative visualization. Marfan syndrome diagnosis was based on genetic testing or the constellation of signs, labeled clinically by the senior author (J.A.E.).
Table 1.
Patient Characteristics
| Patient Characteristics | All Patients | Nonsurgical Patients |
|---|---|---|
| Total number of patients | 1162 | 617 |
| Male | 791 (68.1) | 405 (65.6) |
| Female | 371 (31.9) | 212 (34.6) |
| Age, y | 70.2±13.5 | 71.9±13 |
| Height, cm | 173.2±11.5 | 172.7±11.9 |
| Body surface area, m2 | 1.99±0.28 | 1.99±0.3 |
| Bicuspid | 257 (22.1) | 42 (6.8) |
| Marfan, connective tissue disorders | 36 (3.1) | 16 (5.8) |
| Family history | ||
| Proven | 248 (21.3) | 114 (18.5) |
| Likely | 75 (6.5) | 35 (5.7) |
| Possible | 51 (4.4) | 33 (5.4) |
| Unknown | 136 (11.7) | 80 (13) |
| None | 652 (56.1) | 355 (57.5) |
| Previous cardiac surgeries | ||
| CABG | 37 (3.2) | 33 (5.3) |
| AVR | 64 (5.5) | 21 (3.4) |
| MVR | 13 (1.1) | 7 (1.1) |
| CABG+AVR | 17 (1.4) | 8 (1.3) |
| AVR+MVR | 2 (0.02) | 1 (0.2) |
| CABG+AVR+MVR | 2 (0.02) | 2 (0.3) |
| Ascending aorta–related death | 8 (0.69) | 8 (1.3) |
| Other cause of death | 119 (10.2) | 119 (19.3) |
| Hypertension | 716 (61.6) | 374 (60.6) |
| DM | 178 (15.3) | 75 (12.2) |
| Aortic root diameter, cm | 4.02±0.60 | 3.94±0.57 |
| Mid‐ascending aorta diameter, cm | 4.33±0.77 | 4.16±0.76 |
Data are provided as mean±SD or number (percentage). The nonsurgical group is a subsection of the all‐patients group. It was studied because the weeding out of surgical patients blunts the effect of censoring patients at the time of surgery, that is, before further aortic dilatation and adverse events. As patients with bicuspid aortic valve undergo surgery early, the nonsurgical group contains significantly fewer patients (P<0.001). AVR indicates aortic valve replacement; CABG, coronary artery bypass grafting; DM, diabetes mellitus; and MVR, mitral valve replacement.
Dissection Diameter “Correction”
According to conventional knowledge, aortic size remains unchanged at dissection. However, recent understanding suggests that the aorta enlarges considerably at the moment of dissection itself. As different research groups agree on that fact,7, 15, 16, 17 we studied patients with dissections using the corrected predissectional mid size. The diameter just before dissection was estimated according to a formula published by our group,7 ≈0.8 cm less than measured at the postdissectional computed tomography scan. Rylski et al15 suggested that spontaneous and retrograde dissections result in similar geometrical changes regardless of patients' age, height, and weight. The mid enlarges at acute type A dissection, whereas the root remains unaffected and its diameter does not change.
Statistical Analysis
Cox regression was employed to investigate the role of root diameter, mid diameter, height, body surface area,18 female sex, age, hypertension, and diabetes mellitus (DM; Table 2). A total of 545 patients had undergone preemptive surgery that prevented both further aortic enlargement and the ensuing MAAEs. This confounding relationship requires careful statistical accommodation.
Table 2.
Cox Regression Analysis of Various Factors Associated With the First and Second CEs
| All Patients | Nonsurgical Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Hazard Ratio | 95% CI | P Value | Covariate | Hazard Ratio | 95% CI | P Value | |
| First CE | Mid | 0.97 | 0.95–0.99* | 0.040* | Mid | 1.00 | 0.98–1.03 | 0.885 |
| Root | 1.05 | 1.01–1.08* | 0.006* | Root | 1.06 | 1.02–1.09* | 0.001* | |
| Height | 0.97 | 0.95–0.99* | 0.004* | Height | 0.98 | 0.96–0.99* | 0.035* | |
| BSA | 1.15 | 0.48–2.75 | 0.757 | BSA | 0.67 | 0.28–1.62 | 0.375 | |
| Female | 1.16 | 0.72–1.87 | 0.533 | Female | 1.19 | 0.74–1.90 | 0.481 | |
| Age | 0.99 | 0.98–1.01 | 0.197 | Age | 0.98 | 0.97–0.99* | 0.003* | |
| Hypertension | 1.52 | 1.04–2.23* | 0.031* | Hypertension | 1.49 | 1.01–2.18* | 0.043* | |
| DM | 0.97 | 0.60–1.57 | 0.904 | DM | 1.21 | 0.74–1.95 | 0.450 | |
| Second CE | Mid | 0.98 | 0.97–1.00 | 0.087 | Mid | 1.00 | 0.99–1.02 | 0.704 |
| Root | 1.03 | 1.01–1.06* | 0.017* | Root | 1.04 | 1.01–1.07* | 0.003* | |
| Height | 0.97 | 0.95–0.98* | 0.000* | Height | 0.97 | 0.96–0.99* | 0.000* | |
| BSA | 1.31 | 0.70–2.45 | 0.401 | BSA | 0.95 | 0.51–1.77 | 0.871 | |
| Female | 1.05 | 0.75–1.47 | 0.791 | Female | 1.12 | 0.80–1.57 | 0.509 | |
| Age | 1.00 | 0.99–1.01 | 0.683 | Age | 0.99 | 0.98–1.00 | 0.182 | |
| Hypertension | 0.94 | 0.72–1.22 | 0.637 | Hypertension | 0.91 | 0.70–1.18 | 0.462 | |
| DM | 0.81 | 0.55–1.19 | 0.275 | DM | 0.95 | 0.65–1.40 | 0.797 | |
Interpretations should be cautious as elective surgery confounds the results. Root dilatation predisposes (hazard ratio >1) and height protects (hazard ratio <1) significantly (P<0.05; 95% CI not including 1) to the first and second CEs. The counterintuitive protective effect of mid dilatation in the all‐patients group, but not in the nonsurgical group, can be interpreted in clinical context. The milder disease virulence of mid dilatation offers a broader time window for preemptive repair, thus causing the epiphenomenon of protection in contrast with the more malevolent root dilatation that leads earlier to major adverse aortic events. BSA appears unimportant. Advanced age protects and hypertension predisposes to the first CE. Female sex and DM pose as bystanders. BSA indicates body surface area; CE, composite end point; DM, diabetes mellitus; mid, mid‐ascending aorta and root, aortic root.
connotes statistical significance.
Because patients are censored at the point of surgery, results as tabulated are accurate as natural history. However, the weeding out of surgical patients prevents us from "seeing" what would have happened beyond that point in aortic diameter. We can blunt that effect by adding a separate analysis without the surgical patients, supplementing the combined analysis. This adjunct nonsurgical group was also analyzed with Cox regression for insight into the significance of the aforementioned factors in predicting dissection.
Initially, the risk curve of the ascending aorta as a single unit (Figure 3) was plotted (greatest diameter at either site, mid or root) without diameter correction to reproduce the traditional risk curves that were built several years before the notion of size correction. The term risk is used interchangeably with the term probability, which is defined as the ratio of the total number of events to the total number of aneurysms for each size group. Then the risk curves of the mid and the root were plotted separately for purposes of comparison (Figures 4 and 5). The curves presented are high‐order polynomial trend lines that best fit the data. The R 2 represents the proportion of the variance for MAAE that can be explained by diameter in our regression model.
Figure 3. Lifetime risk of the first composite end point (red line) and the second composite end point (black line) against the ascending aorta diameter of the 1162 patients.
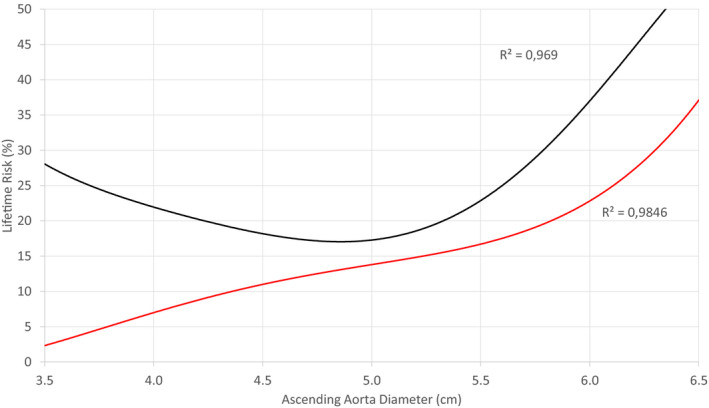
In view of the ascending aorta as a single unit, the slope of both curves increases considerably at the diameter of about 5.5 cm, thus reproducing the surgical cutoff value recommended by current guidelines. There was no correction for the mid‐ascending aorta size as this notion was proposed recently. Taking into consideration the success rates of repair at expert centers and the 15% risk of the first composite end point between 5.0 and 5.5 cm, the surgical threshold of 5.5 cm is arguably high. The aortas with low diameters demonstrate pseudo‐high risk because of a selection bias with the underrepresentation of healthy individuals. The R 2 are very close to 1, suggesting that the trend lines almost perfectly fit the data.
Figure 4. Lifetime risk of the first composite end point (red line) and the second composite end point (black line) against the mid‐ascending aorta diameter of the 1162 patients.
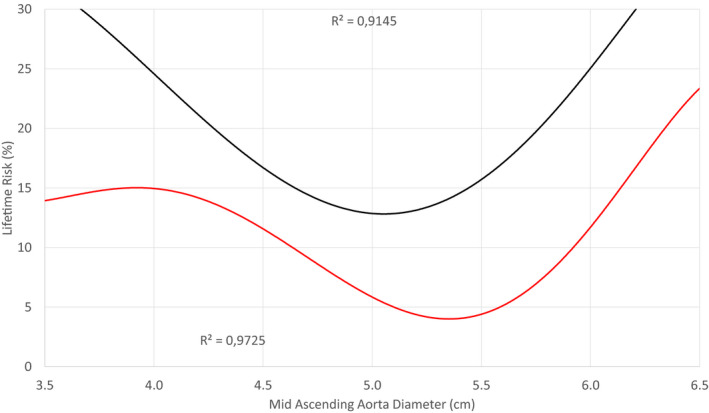
Mid‐ascending aorta diameter >5.25 cm poses a considerable increase of risk for the first and second composite end points. The aortas with low diameters demonstrate different degrees of pseudo‐high risk because of a selection bias with the underrepresentation of healthy individuals. At diameters >4.5 cm, the sample becomes representative. Note that the risk of the first composite end point, attributed to a mid 5.0 cm wide, is almost 6%. The R 2 are very close to 1, suggesting that the trend lines almost perfectly fit the data.
Figure 5. Lifetime risk of the first composite end point (red line) and the second composite end point (black line) against the aortic root diameter of the 1162 patients.
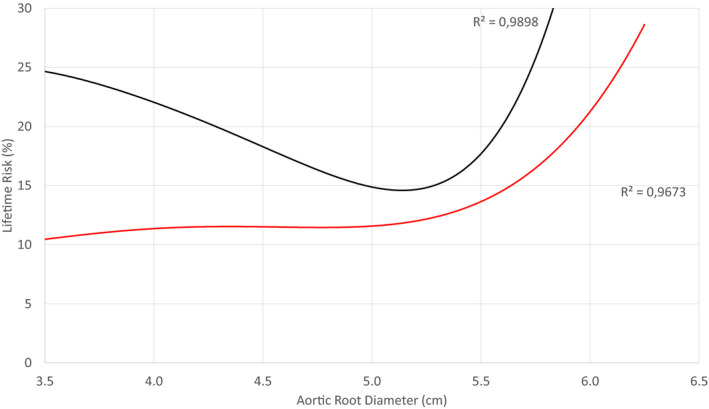
The risk of the first composite end point increases considerably at a diameter >5.0 cm. The risk of near normal‐sized aortas is overestimated because of a selection bias with the underrepresentation of healthy individuals. At diameters >4.5 cm, the sample becomes representative. Note that the risk of the first composite end point, attributed to an aortic root 5.0 cm wide, is almost 12%, which is double compared with the respective risk of a mid‐ascending aorta (Figure 4). The risk of the second composite end point increases at diameters approximately >5.0 cm. The R 2 are very close to 1, suggesting that the trend lines almost perfectly fit the data.
Finally, the risk of the first CE was expressed as a function of both root and mid size in a risk matrix (Figure 6). Each cell represents a combination of root and mid diameters color coded according to the associated risk. The oblique black line delineates 2 areas: cells above the line represent the patients whose mids are larger, and cells below the line represent the patients whose roots are larger. A few cells have been left blank as they contain no patients. Green denotes low risk (<5%); yellow, intermediate risk (5%–10%); orange, high risk (10%–20%); and red, very high risk (>20%). The color clustering of these cells should be evocative of the elusive interplay between the root and mid diameters with regard to dissection, rupture, and ascending aorta–related death.
Figure 6. Risk matrix for the 1162 patients.
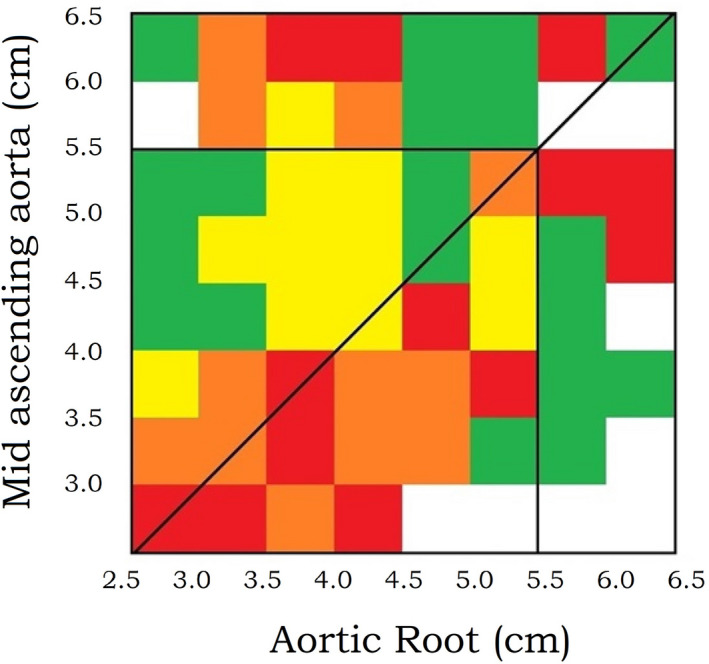
The risk of the first composite end point for each combination of aortic root and mid‐ascending aorta size is expressed on a color scale with green as low risk (<5%), yellow as intermediate risk (5%–10%), orange as high risk (10%–20%) and red as very high risk (>20%). Above the oblique line, the mid is more dilated than the root. Below the oblique line, the opposite is true. The matrix portrays considerable risk for both root and mid dilatation. However, its coloring conveys at first glance that root dilatation is more malignant than mid dilatation. The vertical and horizontal black lines inside the matrix outline the surgical threshold of 5.5 cm, therefore the top 2 rows and the far right 2 columns are biased because of surgery. The great number of cells (64) leads to the extended stratification of patients in an equal number of size groups. This increased data resolution entails high disturbance, that is, local deviation of the reported risk from the unobservable true risk. In this context, no attempts were made to explain local color clustering. Although the inclusion criterion was an ascending aorta at least 3.5 cm wide, after applying the correction formula for estimating the predissectional size, it was revealed that dissections have occurred in aortas with predissectional diameters <3.0 cm. Thus, the matrix's lower margins were set at 2.5 cm.
Results
Patient Characteristics and Aortic Size Distribution
A total of 1162 patients were included. Their baseline characteristics, including sex, age, height, body surface area, bicuspid aortic valve, Marfan syndrome, family history, previous cardiac surgery, hypertension, and DM, are reported in Table 1. The diameters were clustered into size groups (Figure 1). The mean±SD of the root was 4.02±0.60 cm and of the mid was 4.33±0.77 cm.
End Points and Cox Regression Models
The primary end point scores for the 1162 patients were 120 dissections, 2 ruptures, 8 ascending aorta–related deaths, and 119 deaths of other or unknown cause (Figure 2). A total of 545 patients underwent elective surgical repair. The remaining 368 are alive and free of aorta‐related events. Of the patients, 91 have ascending aortas >4.5 cm, and their last computed tomography scan was >1 year ago. The first CE includes 130 patients, and the second CE includes 249 patients. A subset of patients with dissections was under surveillance before their events, whereas other patients presented for the first time with dissections. Regarding the former group, the average time from the first and the last scans to dissection was 45.2 and 25.9 months, respectively. The 1042 patients with no dissections had, on average, their first and last scans, respectively, 47.7 and 26.1 months before reaching an end point or censoring. The average number of scans for each patient was 1.8.
Root dilatation and height emerge as significant factors. Paradoxically, mid dilatation appeared pseudo‐protective in the all‐patient group. Arguably, patients with mid aneurysms are more likely to undergo preemptive surgery than suffer a MAAE. The nonsurgical group was not protected, as surgery was not performed and dissection was not prevented. It was unveiled that the risk of a dilated mid is dwarfed by the risk of root dilatation. Height was negatively associated with adverse events, in contrast to body surface area, which appeared unimportant. Female sex and DM failed to demonstrate significance. Age as well as hypertension provided conflicting hazard ratios among the first and second CEs.
Risk Curves and Risk Matrixes
The risk curves of the first and second CEs were plotted against the greater diameter of the ascending aorta as a single unit (Figure 3). To reflect the common practice throughout the past decades, size correction was omitted. The resultant curve reproduced the threshold of 5.5 cm, showing that the sample is representative.
Separately, the risk curves of the first and second CEs were plotted against the diameter of the mid (Figure 4) and the root (Figure 5). The increased risk cutoff values were 5.25 and 5.0 cm, respectively. These hinge points are shifted leftward compared with the aforementioned 5.5 cm surgical threshold recommended by the current guidelines. The selection bias in near normal aortas causes overestimation of risk in this size group because of the underrepresentation of healthy individuals. Of note, a root with 5.0 cm diameter is twice as likely to dissect, rupture, or kill (first CE) as an equally sized mid.
The risk of the first CE was depicted as a function of both root and mid diameters in a risk matrix (Figure 6). Risk is considerable on both sides of the oblique line, yet unevenly distributed. Risk is greater underneath this line, where the root is larger. Hence root dilatation is more perilous than mid dilatation. Beyond the repair threshold of 5.5 cm, the apparent risk is confounded because of surgery.
Discussion
Aortic dissection is a devastating, preventable disease. Although the risk of ascending aorta–related MAAEs is argued to be low,19 the prevalence of thoracic aortic disease has been reported on the rise,4 and the International Registry of Acute Aortic Dissection highlights that 60% of acute type A dissections occur in ascending aortas <5.5 cm wide. The recent understanding that aortas enlarge abruptly at dissection by ≈0.8 cm,7 or more,15 implies that this percentage was underestimated, as dissected aortas were routinely registered with their postdissectional diameter. The firmly held misconception that the aortic diameter remains unchanged at dissection was a silent assumption in shaping the recommendations for surgical repair. Therefore, the current practice is called into question and the need for earlier intervention becomes pressing, especially in the setting of improved surgical outcomes.
Limitations
The limitations of this study mainly relate to its retrospective nature. Biases were addressed early and well thought out during statistical interpretation. The sample of this study is large and representative, as shown by the risk curve of the ascending aorta as a single unit, with its hinge point at 5.5 cm. There is a sampling bias in aortas around 4.0 cm wide, with the upshot being an overestimation of risk in normal sizes. This bias can be filtered out as it is inconsequential, and the sample becomes representative at >4.5 cm. An attrition bias is inevitable in such a large study that spans decades; however, it was kept very low. A total of 794 patients (68%) reached an end point or surgery. Of the remaining patients, only 91 (7.8%) have ascending aortas >4.5 cm and had their last computed tomography scan >1 year ago. Nevertheless, most of these 91 patients undergo routine echocardiography; therefore, they are not considered dropouts. Old imaging modalities were less likely retrievable; nevertheless, this type of recall bias had a minimal impact as it was random and the risk curve of the ascending aorta as a single unit reproduced the surgical threshold of 5.5 cm, thus attesting for the representativeness of the data. Confounding is evident, as surgery prevents both aortic enlargement and the ensuing MAAEs, therefore distorting their causative relationship. Regression analysis is limited by this confounding association; however, it is not devoid of clinical implications.
Unique Nature of Root Versus Mid
The distribution of the root and mid diameters in size groups, per se, brings into question their homogenized surgical management, as the root is characterized by lesser mean and SD (Figure 1). This discrepancy implies diverging natural histories with embryology likely playing a key role as diverse embryonic cell lines converge to form the ascending aorta. The neural crest populates the ascending segment while the lateral plate mesoderm supplies the root with smooth muscle of a specific kind.8, 9, 10 The adjacent structures and the diverse anatomic‐physiologic standpoint may be of paramount importance for root and mid remodeling during adulthood, contributing further to their diverse risk profile. The safe conclusion is that the natural histories of the root and the mid are unique.
Unethical Randomized Study
As surgical repair of ascending aorta aneurysms is well established, and withholding of this treatment may be considered inappropriate, randomized trials may not be ethical.20 The best surrogate for studying the natural history of dilatation is retrospective observation. Yale's database is now robust enough to allow the detailed analysis of 1162 patients with aneurysms. The data were accurately recollected, and the aortas were carefully remeasured.
Root and Mid Deserve Individual Criteria and Management
This unique analysis offers a new perspective of root and mid dilatation with strong clinical implications. These 2 structures deserve new and different management.
Root Dilatation More Dangerous Than Mid Dilatation
The regression analyses offer a valuable insight into the natural history of aortic dilatation. The derived associations, when carefully interpreted, can serve as a stepping stone for further studies. Root dilatation and short stature were significant risk factors in all groups. At second reading, the <1 hazard ratio (Table 2) of the mid diameter in the all‐patients group is not suggesting a protective effect. Taking into consideration the malevolent behavior of root aneurysms, this hazard ratio reflects the milder disease virulence of mid aneurysms that offered a broader time window for repair that does offer a protective effect. After partially controlling for the confounder of surgery by eliminating the surgical patients, it was reconfirmed in the nonsurgical group that root dilatation is more dangerous than mid dilatation.
Body surface area was proven less important than height, recapping that height normalization is more useful for risk estimation.21 The results for age and hypertension were inconclusive. Younger age and hypertension might predispose to MAAEs. Female sex and DM posed as bystanders, heating up the debate about their repercussions. Female sex has been firmly linked to worse outcomes,22 and DM or antidiabetic medications23, 24 have been conjectured to be protective.
Sampling Bias in Small Size Ranges
The risk curves play a pivotal role in clinical decision making. The large sample of this study was representative of enlarged aortas, and the risk curve of the ascending aorta as a single unit reproduced the anticipated threshold value of the current guidelines at 5.5 cm. On the other end of the spectrum, both CEs appear unexpectedly frequent for aortas with small diameters. This overestimation is attributed to a sampling bias. Although the majority of the human population (97.18%25) has a normal aorta of <4.0 cm and rarely suffers a dissection, the small fraction of people with normal‐sized aortas who ultimately undergo thoracic computed tomography scans is more likely to be comorbid in some way (eg, family history) and ultimately present with MAAEs. Distinguishing these patients is a challenge unless there is a known associated risk factor (“guilt by association”) as discussed in a recent article by our group,26 especially positive family history and gene variants.27
Threshold Modification
As the surgical threshold for the ascending aorta comes to the forefront, the disentanglement of root and mid natural histories acquires new interest. Their separate risk curves are introduced in this study, unveiling cutoff values that blended with each other in the classical ascending‐aorta risk curve.28 This landmark curve was reproduced by large retrospective29 and prospective30 studies that fueled the current guidelines and established the threshold of 5.5 cm. These studies established the regression analysis of risk against aortic size and its hinge point as the surgical threshold. In the same context, from the present study, the mid risk curve presents a hinge point at ≈5.25 cm, and the root risk curves even earlier at 5.0 cm. These findings advocate for earlier intervention, especially in root aneurysms. Both of these thresholds are accommodated by our recent advocacy for a “left shift” to a simple 5.0 cm criterion for established centers of excellence that can deliver ascending surgery at relatively low risk.7
Color Risk Matrix
Τhe ascending aorta risk curve is not the sum of the root and mid curves because each of the 1162 patients has a unique combination of root and mid diameters. To investigate the interplay of these 2 factors regarding the risk of dissection, rupture, and ascending aorta–related death, the risk matrix was generated (Figure 6). It is a visual representation of risk that conveys at first glance the correlation of root size, mid size, and probability of the first CE.
The risk matrix portrays considerable risk on both sides of the oblique line, that is, for both root and mid dilatation. Moreover, the coloring conveys at first glance that root dilatation is more malignant than mid dilatation. Further analysis is uncertain because >5.5 cm the indication for surgery confounds the observations and sudden aortic death may be unheralded.
The great number of cells (64) leads to the extended stratification of patients in an equal number of size groups. This increased data resolution entails high disturbance, that is, local deviation of the reported risk from the unobservable true risk. In this context, no attempts were made to explain local color clustering. Although the inclusion criterion was an ascending aorta at least 3.5 cm wide, after applying the correction formula for estimating the predissectional size, it was revealed that dissections have occurred in aortas with predissectional diameters of <3.0 cm. Thus, the matrix's lower margins were set at 2.5 cm.
A leftward shift at lower surgical cutoff values would reduce the MAAEs, especially for the dilated roots. Apropos, the risk matrix reinforces that there are patients with near normal or mildly enlarged aortas who do suffer a dissection. With the available tools, it is difficult to detect these patients among their countless peers and triage them out of harm's way.
Conclusions
The persistence of MAAEs suggests a new approach for patients with ascending aorta aneurysms. Root dilatation is associated with a greater risk of MAAEs compared with mid dilatation. Shorter stature predisposed to dissection, rupture, and ascending aorta–related death. The root and mid have a diverse natural history of complications. The former exceeds the latter in malevolence, whereas both demonstrate a steep increase of risk at diameters below the standard surgical cutoff value of 5.5 cm. The hinge point at the risk curve of the root is at 5.0 cm and of the mid is at 5.25 cm. Both can be accommodated by our previously recommended surgical criterion of 5.0 cm at any root or ascending site.
Sources of Funding
None.
Disclosures
Dr Elefteriades reports commercial affiliations as a principal with CoolSpine, a member of the Data/Safety Monitoring Board of Terumo, and a consultant with CryoLife. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 10.
References
- 1.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College οf Cardiology Foundation/American Heart Association task force on practice guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. DOI: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 2.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. DOI: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 3.Borger MA, Fedak PWM, Stephens EH, Gleason TG, Girdauskas E, Ikonomidis JS, Khoynezhad A, Siu SC, Verma S, Hope MD, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve‐related aortopathy: full online‐only version. J Thorac Cardiovasc Surg. 2018;156:e41–e74. DOI: 10.1016/j.jtcvs.2018.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population‐based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. DOI: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 5.Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, Trimarchi S, Braverman AC, Myrmel T, et al. Insights from the International Registry of Acute Aortic Dissection: a 20‐year experience of collaborative clinical research. Circulation. 2018;137:1846–1860. DOI: 10.1161/CIRCULATIONAHA.117.031264. [DOI] [PubMed] [Google Scholar]
- 6.Paruchuri V, Salhab KF, Kuzmik G, Gubernikoff G, Fang H, Rizzo JA, Ziganshin BA, Elefteriades JA. Aortic size distribution in the general population: explaining the size paradox in aortic dissection. Cardiology. 2015;131:265–272. DOI: 10.1159/000381281. [DOI] [PubMed] [Google Scholar]
- 7.Mansour AM, Peterss S, Zafar MA, Rizzo JA, Fang H, Charilaou P, Ziganshin BA, Darr UM, Elefteriades JA. Prevention of aortic dissection suggests a diameter shift to a lower aortic size threshold for intervention. Cardiology. 2018;139:139–146. DOI: 10.1159/000481930. [DOI] [PubMed] [Google Scholar]
- 8.Gittenberger‐de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–1594. DOI: 10.1161/01.ATV.19.7.1589. [DOI] [PubMed] [Google Scholar]
- 9.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin‐dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. DOI: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. DOI: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E , Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. DOI: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Peterss S, Charilaou P, Ziganshin BA, Elefteriades JA. Assessment of survival in retrospective studies: the Social Security Death Index is not adequate for estimation. J Thorac Cardiovasc Surg. 2017;153:899–901. DOI: 10.1016/j.jtcvs.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, Bolen MA, Connolly HM, Cuéllar‐Calàbria H, Czerny M, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28:119–182. DOI: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Elefteriades JA, Mukherjee SK, Mojibian H. Discrepancies in measurement of the thoracic aorta: JACC review topic of the week. J Am Coll Cardiol. 2020;76:201–217. DOI: 10.1016/j.jacc.2020.03.084. [DOI] [PubMed] [Google Scholar]
- 15.Rylski B, Blanke P, Beyersdorf F, Desai ND, Milewski RK, Siepe M, Kari FA, Czerny M, Carrel T, Schlensak C, et al. How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol. 2014;63:1311–1319. DOI: 10.1016/j.jacc.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Rylski B, Branchetti E, Bavaria JE, Vallabhajosyula P, Szeto WY, Milewski RK, Desai ND. Modeling of predissection aortic size in acute type a dissection: more than 90% fail to meet the guidelines for elective ascending replacement. J Thorac Cardiovasc Surg. 2014;148:944–948.e941. DOI: 10.1016/j.jtcvs.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Koechlin L, Macius E, Kaufmann J, Gahl B, Reuthebuch O, Eckstein F, Berdajs DA. Aortic root and ascending aorta dimensions in acute aortic dissection. Perfusion. 2020;35:131–137. DOI: 10.1177/0267659119858848. [DOI] [PubMed] [Google Scholar]
- 18.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Medicine. 1916;17:863–871. DOI: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 19.Guo MH, Appoo JJ, Saczkowski R, Smith HN, Ouzounian M, Gregory AJ, Herget EJ, Boodhwani M. Association of mortality and acute aortic events with ascending aortic aneurysm: a systematic review and meta‐analysis. JAMA Netw Open. 2018;1:e181281. DOI: 10.1001/jamanetworkopen.2018.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elefteriades JA, Ziganshin BA. Familial legacy of thoracic aortic aneurysm. Circulation. 2020;142:929–931. DOI: 10.1161/CIRCULATIONAHA.120.049124. [DOI] [PubMed] [Google Scholar]
- 21.Zafar MA, Li Y, Rizzo JA, Charilaou P, Saeyeldin A, Velasquez CA, Mansour AM, Bin Mahmood SU, Ma W‐G, Brownstein AJ, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155:1938–1950. DOI: 10.1016/j.jtcvs.2017.10.140. [DOI] [PubMed] [Google Scholar]
- 22.Boczar KE, Coutinho T. Sex considerations in aneurysm formation, progression, and outcomes. Can J Cardiol. 2018;34:362–370. DOI: 10.1016/j.cjca.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 23.He X, Liu X, Liu W, Wang B, Liu Y, Li Z, Wang T, Tan R, Gao B, Zeng H. Association between diabetes and risk of aortic dissection: a case‐control study in a Chinese population. PLoS One. 2015;10:e0142697. DOI: 10.1371/journal.pone.0142697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel K, Zafar MA, Ziganshin BA, Elefteriades JA. Diabetes mellitus: Is it protective against aneurysm? A narrative review. Cardiology. 2018;141:107–122. DOI: 10.1159/000490373. [DOI] [PubMed] [Google Scholar]
- 25.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55:841–857. DOI: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 26.Ziganshin BA, Elefteriades JA. Guilt by association: a paradigm for detection of silent aortic disease. Ann Cardiothorac Surg. 2016;5:174–187. DOI: 10.21037/acs.2016.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faggion Vinholo T, Brownstein AJ, Ziganshin BA, Zafar MA, Kuivaniemi H, Body SC, Bale AE, Elefteriades JA. Genes associated with thoracic aortic aneurysm and dissection: 2019 update and clinical implications. Aorta (Stamford). 2019;7:99–107. DOI: 10.1055/s-0039-3400233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coady MA, Rizzo JA, Elefteriades JA. Developing surgical intervention criteria for thoracic aortic aneurysms. Cardiol Clin. 1999;17:827–839. DOI: 10.1016/S0733-8651(05)70118-1. [DOI] [PubMed] [Google Scholar]
- 29.Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, Elefteriades JA. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–491; discussion 489–491. DOI: 10.1016/S0022-5223(97)70360-X. [DOI] [PubMed] [Google Scholar]
- 30.Elefteriades JA. Natural history of thoracic aortic aneurysms: Indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–S1880; discussion S1892‐S1878. DOI: 10.1016/S0003-4975(02)04147-4. [DOI] [PubMed] [Google Scholar]


