Abstract
Background
We assessed the associations between patient‐clinician relationships (communication and involvement in shared decision‐making [SDM]) and adherence to antihypertensive medications.
Methods and Results
The 2010 to 2017 Medical Expenditure Panel Survey (MEPS) data were analyzed. A retrospective cohort study design was used to create a cohort of prevalent and new users of antihypertensive medications. We defined constructs of patient‐clinician communication and involvement in SDM from patient responses to the standard questionnaires about satisfaction and access to care during the first year of surveys. Verified self‐reported medication refill information collected during the second year of surveys was used to calculate medication refill adherence; adherence was defined as medication refill adherence ≥80%. Survey‐weighted multivariable‐adjusted logistic regression models were used to measure the odds ratio (OR) and 95% CI for the association between both patient‐clinician constructs and adherence. Our analysis involved 2571 Black adult patients with hypertension (mean age of 58 years; SD, 14 years) who were either persistent (n=1788) or new users (n=783) of antihypertensive medications. Forty‐five percent (n=1145) and 43% (n=1016) of the sample reported having high levels of communication and involvement in SDM, respectively. High, versus low, patient‐clinician communication (OR, 1.38; 95% CI, 1.14–1.67) and involvement in SDM (OR, 1.32; 95% CI, 1.08–1.61) were both associated with adherence to antihypertensives after adjusting for multiple covariates. These associations persisted among a subgroup of new users of antihypertensive medications.
Conclusions
Patient‐clinician communication and involvement in SDM are important predictors of optimal adherence to antihypertensive medication and should be targeted for improving adherence among Black adults with hypertension.
Keywords: adherence, antihypertensive medication, black adults, communication, hypertension, patient‐clinician relationships, shared decision‐making
Subject Categories: Health Services, Hypertension, Quality and Outcomes, Treatment
Nonstandard Abbreviations and Acronyms
- CAHPS
consumer assessment of healthcare providers and systems
- HCAHPS
hospital consumer assessment of healthcare providers and systems
- MEPS
medical expenditure panel survey
- MEPS‐HC
household component of the medical expenditure panel survey
- MRA
medication refill adherence
- SAQ
self‐administered questionnaire
- SDM
shared decision‐making
Clinical Perspective
What Is New?
The effects of patient‐provider relationships on adherence to antihypertensive medication therapy were observed among Black adults but not among other racial/ethnic groups.
Our data elucidated the associations between patient‐provider relationships (communication and shared decision‐making) and adherence to antihypertensive medications using a large national longitudinal data set.
What Are the Clinical Implications?
Black patients may be more likely to benefit from effective communication and higher involvement in shared decision‐making with respect to antihypertensive medication adherence than are other ethnic groups.
It is possible that providers who communicate effectively and involve Black patients in shared decision‐making may be less likely to be biased against Black patients.
Black patients are likely to be more trusting of providers who communicate effectively and involve them in shared decision‐making and, consequently, are more likely to adhere to treatment recommendation.
Only 27% to 53% of Black adults are fully adherent to their antihypertensive medications,1, 2, 3, 4, 5, 6 which is associated with increased hypertension morbidity, mortality, and avoidable healthcare costs.7, 8, 9, 10 Black patients experience worse hypertension outcomes, potentially because of poorer adherence to antihypertensive medications, compared with all racial groups in the United States.3, 11, 12, 13, 14, 15 Interventions to increase adherence can improve clinical outcomes,16, 17, 18 but only a few have been well studied among Black patients.19, 20, 21
Medication adherence functions as a shared agreement between patients and clinicians.22 Patients are more receptive to clinical recommendations when there is an engaged partnership.23, 24 Existing conceptual frameworks have often placed adherence to medications as an intermediary outcome between patient‐clinician relationships and health outcomes.25, 26 Communication and shared decision‐making (SDM) have shown direct and indirect effects on medication adherence.25, 26 The indirect pathways between these tenets of the patient‐clinician relationships and medication adherence are mediated by proximal affective‐cognitive outcomes (eg, trust in clinicians, satisfaction with health care, and understanding) of the patient‐clinician relationship.26
Patients are more likely to be adherent to medications, in general, when they have good communication with their clinician.27 A few studies among small samples of Black patients (n=92–723) found a positive relationship between patient‐clinician communication and adherence to antihypertensive medications.28, 29, 30, 31 Three separate systematic reviews reported that there is insufficient data to determine the effect of SDM on medication adherence in the general population and among socioeconomically disadvantaged groups.26, 32, 33
Methodological limitations regarding the use of cross‐sectional study designs, small sample sizes of Black patients (n=92–723), lack of geographically representative populations, and self‐reported measures of adherence limit the scientific rigor of the extant evidence base for the association between patient‐clinician relationships and adherence. Disentangling these relationships with cross‐sectional and cohort studies, where exposures and outcomes are measured at single fixed time points, is difficult. For instance, patients with controlled blood pressure (BP) tend to be more adherent, yet adherence to antihypertensive medications is also shown to improve BP control.34, 35, 36 Patients with poor health outcomes, such as uncontrolled BP, also tend to have negative perceptions of interactions with clinicians.37, 38
We therefore sought to leverage a nationally representative healthcare utilization data set to assess the associations between patient‐clinician relationships (communication and involvement in SDM) and adherence to antihypertensive medications among Black adults with hypertension. A better understanding of the associations between patients' perspectives about their communication and SDM with clinicians on adherence could help guide the formulation of effective interventions to improve antihypertensive medication adherence among Black patients.
Methods
We will make our analytic methods available to other researchers upon request. Medical Expenditure Panel Survey (MEPS) is publically available. The data that support the findings of this study are available from the MEPS website: https://www.meps.ahrq.gov/mepsweb/. The use of this publically available data set did not require institutional review board approval.
Data Source and Setting
We analyzed data from Household Component of the Medical Expenditure Panel Survey (MEPS‐HC) from 2010 to 2017.39 MEPS‐HC data are collected from a nationally representative sample of households through an overlapping panel design (Figure 1). These data are collected in 5 rounds of interviews during a 2‐year period. Survey questionnaires are used to gather data on self‐reported health status, medical conditions, health insurance status, healthcare access, prescription medication use, and access to and satisfaction with the clinician. To verify and obtain detailed prescription medication use, respondents are asked to provide names of prescription medications obtained and to identify the pharmacy where medications were filled. MEPS seeks the consent of respondents to contact pharmacies to collect the following information about drugs obtained: payments, payers, date each prescription was filled, quantity dispensed, and the National Drug Code.40 The pharmacies also provide information on the number of times medications are filled within a given calendar year.40
Figure 1. Study design.
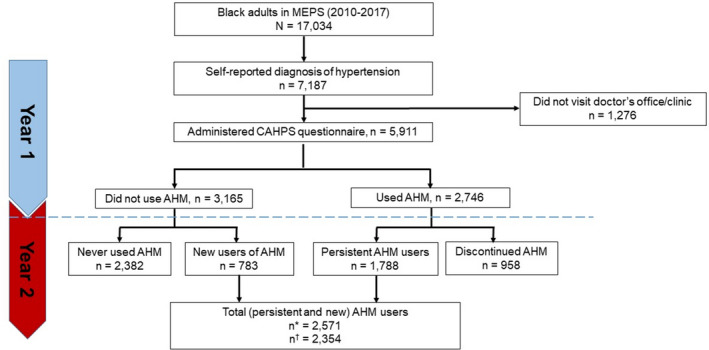
In this illustration, 2 years of data from 5 rounds of surveys are combined to create a cohort of participants who filled at least 1 prescription of antihypertensive medication. Data collected from rounds 1 through 3 in 2015 are used to define patient‐provider engagement factors (shared decision‐making, communication, and trust) and all covariates (individual characteristics and provider characteristics). On the other hand, the 2016 data are used to define medication refill adherence based on medication refill and the days' supply of filled drugs. MEPS indicates Medical Expenditure Panel Survey.
Study Design
Figure 1 is an illustration of how the MEPS panel design data were leveraged to create a cohort of antihypertensive medication users. In this illustration, 2 years of data from 5 rounds of surveys are combined to create a cohort of participants who filled at least 1 prescription of antihypertensive medication. Data collected from rounds 1 through 3 in 2015 (year 1) were used to define patient‐clinician constructs (communication and SDM) and all potential confounders. The 2016 data (year 2) were used to define adherence measures based on medication refills and the days' supply of filled antihypertensive medications. Among the primary analysis cohort, we identified a subgroup of new users as patients who only began using antihypertensive medications in year 2 but had no prior records of antihypertensive medication use in year 1. The year 1 and 2 periods are referred to as the baseline and follow‐up periods, respectively.
Participant Selection
Figure 2 is a flowchart of participant selection. Our analysis included participants who reported a hypertension diagnosis and antihypertensive medication use. For the patient‐clinician communication and adherence analysis, we further restricted the primary analysis to only participants who reported visiting a clinic or a doctor's office in the past 12 months; the Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey questionnaire was administered to only participants who met this criterion. Additionally, participants were required to have had a usual source of care in the previous year to be included in the analysis for measuring the associations between SDM and adherence. This restriction was necessary because SDM is defined from both the CAHPS and access to healthcare survey questionnaires.
Figure 2. Participant selection.
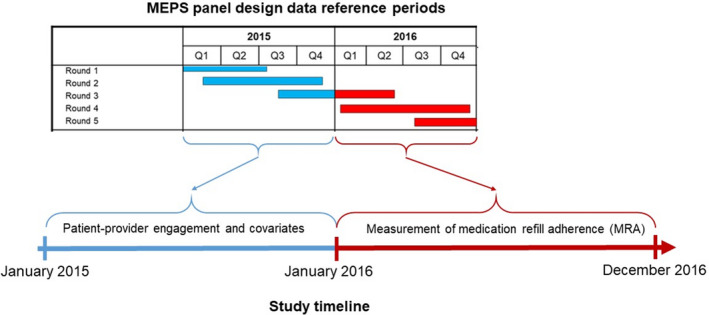
Black adults were identified from the 2010 to 2017 MEPS (Medical Expenditure Panel Survey) data. The year 1 data were used for identifying Black patients with a hypertension diagnosis and antihypertensive medication (AHM) use. Persistent use, discontinuation, and new use of AHMs were assessed from the year 2 data. *Sample for measuring associations between patient‐clinician communication and adherence to AHMs. †Sample for measuring associations between patient involvement in shared decision‐making and adherence to AHM. AHM users (n=217) were excluded if they lacked access to a usual source of care provider. CAHPS indicates Consumer Assessment of Healthcare Providers and Systems.
Measurement of Patient‐Clinician Constructs
Our analysis is based solely on patients' perspectives about their engagement with clinicians. MEPS participants' perspectives about access to care and the quality of health care they received were assessed with computer‐assisted personal interview and self‐administered supplemental paper questionnaires (Self‐Administered Questionnaire [SAQ]), respectively (https://www.meps.ahrq.gov/mepsweb/survey_comp/survey.jsp#Questionnaires). The healthcare quality measures in SAQ were adapted from the health plan version of CAHPS (Consumer Assessment of Healthcare Providers and Systems). CAHPS is a reliable and valid tool for capturing information about health plans' performances from racially/ethnically diverse consumers.41, 42, 43, 44 CAHPS' survey items have also been used to accurately measure patient‐clinician communication and SDM among all racial/ethnic groups.44, 45 Previous studies have examined the associations between medication adherence and patient‐clinician constructs defined from CAHPS survey items.46, 47, 48 We referred to human providers as clinicians in this study.
The patient‐clinician communication construct was created from participant responses (never, sometimes, usually, always) to the questions: how often does the care provider (1) listen carefully to the patient; (2) explain to the patient; (3) show respect to the patient; and (4) spend enough time with the patient? The Hospital CAHPS (HCAHPS) “top‐box” scoring approach was used to dichotomize responses to each item as 1 for “always” and 0 for any other response.49 These binary scores were then summed to generate a composite score of patient‐clinician communication ranging from 0 to 4 (“always” to all 4 items).29 Composite scores of 4 (“always”) were considered high and those <4 were considered low levels of patient‐clinician communication.49, 50
The SDM construct was defined from the 4 CAHPS items described above plus 3 additional questions about patients' satisfaction with their usual source of care provider: does the usual source of care provider (1) usually ask about and show respect for medical, traditional, and alternative treatments that the person is happy with (never/sometimes/usually/always)?; (2) ask the person to help make decisions between a choice of treatments (never/sometimes/usually/always)?; and (3) usually ask about prescription medications and treatments other doctors may give them (yes/no)?.51 The top‐box approach was used to dichotomize responses into 1 (always) and 0 (never, sometimes, usually) scores for the questions with Likert scale responses. “Yes” responses were coded as 1, whereas “no” responses were coded as 0. The sum of these scores ranged from 0 to 7, with SDM scores ≥6 considered as high and scores <6 considered as low based on the top‐box approach.49, 50
Outcomes
We considered medication refill adherence (MRA) as a measure of adherence to antihypertensive medications. The MEPS Prescribed Medicines Files provide detailed information on the type, dosage, and payment for each filled prescription for MEPS participants in each year. For each individual selected for our analysis, MRA was calculated as the percent of total days' supply of antihypertensives divided by number of days of study participation (365 days).52, 53 MRA has been previously used to measure adherence from the MEPS data set.47, 54 An overall MRA was obtained as the average of MRAs calculated for separate therapeutic classes of antihypertensive medications if a participant was using >1 antihypertensive agent from multiple therapeutic classes. Patients were considered to be adherent to antihypertensive medications if their overall MRA was ≥80%.55, 56, 57
Potential Confounders
The World Health Organization suggests multidimensional frameworks of adherence through which several predictors affect medication adherence at the individual patient, healthcare provider and healthcare system levels.58 We applied a directed acyclic graph to identify and illustrate the interrelationships between predictors of medication adherence as potential confounders of the associations between patient‐clinician relationships and adherence to antihypertensive medications (Figure 3). Through this directed acyclic graph, we identified social/economic, patient‐related, condition‐related, and antihypertensive therapy–related factors as an individual patient‐level potential confounder; a detailed list of these variables is provided in Table 1. These individual‐level factors can influence both the patient‐clinician relationships and adherence to antihypertensive medications directly or indirectly via provider characteristics (provider specialty, race, and sex) and healthcare system factors (health insurance status, source of payment of healthcare services, out‐of‐packet payment, and number of office visits).
Figure 3. Directed acyclic graph (DAG).
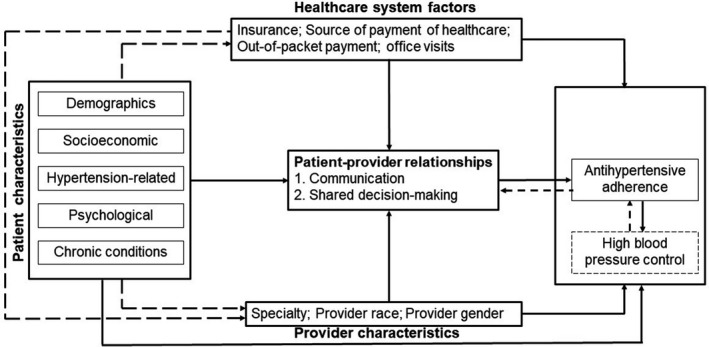
This DAG was used for identifying potential confounders of the associations between patient‐clinician relationships and adherence to antihypertensive medications. The direct paths from each set of confounders (patient characteristics, provider characteristics, and healthcare system factors) to the primary exposure (patient‐clinician relationships) and outcome (adherence) were modeled. Solid lines represent direct paths; dashed lines represent indirect paths or feedback loops.
Table 1.
Distribution of Baseline Covariates by Levels of Communication and SDM Among Black Patients With Hypertension
| Baseline Characteristics | Communication | Involvement in SDM | ||||
|---|---|---|---|---|---|---|
| Frequency, %* | SDT† | Frequency, %* | SDT† | |||
| Low (n=1426) | High (n=1145) | Low (n=1338) | High (n=1016) | |||
| Demographics | ||||||
| Age, mean (SD), y | 57 (13) | 59 (13) | 0.10 | 58 (13) | 59 (13) | 0.05 |
| Age categories, y | ||||||
| 18–44 | 232 (17) | 159 (15) | 0.06 | 201 (16) | 145 (16) | 0.01 |
| 45–64 | 721 (52) | 585 (52) | 0.00 | 684 (53) | 508 (51) | 0.04 |
| ≥65 | 473 (31) | 401 (33) | 0.04 | 453 (31) | 363 (33) | 0.06 |
| Sex | ||||||
| Women | 903 (64) | 751 (63) | 0.01 | 863 (63) | 665 (64) | 0.01 |
| Men | 523 (36) | 394 (37) | 0.01 | 475 (37) | 351 (36) | 0.01 |
| Geographic region of residence | ||||||
| Midwest | 248 (19) | 203 (18) | 0.03 | 238 (21) | 171 (16) | 0.11 |
| Northeast | 181 (11) | 161 (13) | 0.06 | 177 (12) | 138 (12) | 0.01 |
| South | 908 (63) | 736 (65) | 0.05 | 839 (61) | 662 (68) | 0.15 |
| West | 89 (7) | 45 (4) | 0.15 | 84 (7) | 45 (4) | 0.12 |
| Education | ||||||
| Up to high school | 520 (58) | 448 (57) | 0.02 | 494 (58) | 400 (56) | 0.03 |
| College and beyond | 906 (42) | 697 (43) | 0.02 | 844 (42) | 616 (44) | 0.03 |
| Speaking English at home | ||||||
| English | 1416 (99) | 1137 (99) | 0.03 | 1331 (99) | 1008 (99) | 0.01 |
| Non‐English | 10 (1) | 8 (1) | 0.03 | 7 (1) | 8 (1) | 0.01 |
| Socioeconomic factors | ||||||
| Marital status | ||||||
| Married | 910 (62) | 716 (58) | 0.09 | 867 (62) | 627 (58) | 0.08 |
| Not married | 516 (38) | 429 (42) | 0.09 | 471 (38) | 389 (42) | 0.08 |
| Employment status | ||||||
| Employed | 525 (42) | 449 (42) | 0.01 | 482 (42) | 392 (43) | 0.02 |
| Unemployed | 901 (58) | 696 (58) | 0.01 | 856 (58) | 624 (57) | 0.02 |
| Poverty status | ||||||
| Above poverty level | 998 (78) | 821 (78) | 0.00 | 936 (77) | 737 (79) | 0.05 |
| Below poverty level | 428 (22) | 324 (22) | 0.00 | 402 (23) | 279 (21) | 0.05 |
| Cost‐related barriers | ||||||
| Ever delay, forego, or make change in prescription medicine because of cost | 101 (11) | 38 (4) | 0.25 | 83 (10) | 40 (4) | 0.24 |
| Ever delay, forego, or make change in treatment because of cost | 131 (8) | 54 (3) | 0.21 | 122 (7) | 52 (4) | 0.14 |
| Hypertension‐related | ||||||
| Have blood checked in the past y | 1378 (98) | 1116 (98) | 0.01 | 1301 (98) | 992 (98) | 0.03 |
| Duration of hypertension, mean (SD), y | 13 (11) | 14 (11) | 0.04 | 13 (11) | 14 (11) | 0.04 |
| Antihypertensive therapy–related factors | ||||||
| Prior use of AHMs | 988 (68) | 800 (69) | 0.02 | 948 (70) | 714 (69) | 0.01 |
| Adherent (MRA in y 1 ≥80%) | 398 (40) | 339 (42) | 0.04 | 396 (41) | 301 (42) | 0.02 |
| Chronic comorbidities | ||||||
| Poor physical health | 254 (17) | 142 (11) | 0.18 | 227 (15) | 142 (13) | 0.07 |
| Poor mental health | 111 (8) | 53 (5) | 0.13 | 96 (7) | 56 (6) | 0.04 |
| Cognitive limitations | 194 (12) | 126 (10) | 0.08 | 185 (12) | 120 (10) | 0.06 |
| Coronary heart disease | 158 (11) | 130 (10) | 0.02 | 150 (11) | 121 (10) | 0.02 |
| Angina | 80 (6) | 46 (3) | 0.12 | 77 (6) | 41 (4) | 0.11 |
| Myocardial infarction | 122 (7) | 93 (8) | 0.00 | 121 (8) | 80 (6) | 0.07 |
| Other heart diseases | 266 (19) | 190 (17) | 0.07 | 244 (19) | 184 (18) | 0.03 |
| Stroke | 177 (12) | 134 (10) | 0.06 | 163 (11) | 128 (10) | 0.03 |
| Diabetes mellitus | 430 (30) | 396 (33) | 0.07 | 417 (30) | 355 (33) | 0.07 |
| Arthritis | 766 (55) | 579 (49) | 0.12 | 730 (54) | 532 (50) | 0.08 |
| Asthma | 211 (15) | 184 (16) | 0.02 | 201 (15) | 176 (17) | 0.05 |
| Chronic bronchitis | 85 (5) | 62 (5) | 0.01 | 81 (5) | 56 (5) | 0.01 |
| Cancer | 151 (11) | 117 (10) | 0.05 | 133 (10) | 117 (10) | 0.00 |
| Provider characteristics | ||||||
| Provider specialty | ||||||
| General/family practice/internal medicine | 381 (31) | 363 (38) | 0.14 | 402 (31) | 342 (38) | 0.15 |
| Other medical doctor | 37 (3) | 21 (2) | 0.08 | 32 (3) | 26 (3) | 0.01 |
| Specialist (cardiologist/nephrologist) | 133 (12) | 94 (10) | 0.08 | 134 (12) | 93 (11) | 0.04 |
| Provider not human†† | 717 (55) | 581 (53) | 0.05 | 755 (57) | 543 (51) | 0.11 |
| Other provider | 13 (1) | 14 (1) | 0.02 | 15 (1) | 12 (1) | 0.01 |
| Provider and patient are of the same sex | 568 (45) | 466 (47) | 0.03 | 596 (46) | 438 (45) | 0.02 |
| Provider and patient are of the same race | 129 (11) | 111 (12) | 0.05 | 139 (110) | 101 (13) | 0.10 |
| Healthcare system factors | ||||||
| Uninsured | 132 (8) | 72 (5) | 0.13 | 113 (8) | 60 (6) | 0.09 |
| Do not have usual source of payment | 183 (11) | 104 (8) | 0.11 | 141 (10) | 93 (8) | 0.05 |
| Payment source is Medicaid | 323 (20) | 263 (20) | 0.00 | 321 (21) | 230 (20) | 0.03 |
| Payment source is Medicare | 347 (23) | 276 (23) | 0.00 | 326 (22) | 257 (24) | 0.05 |
| Payment source is private insurance | 548 (44) | 483 (49) | 0.10 | 514 (44) | 430 (49) | 0.10 |
| Out‐of‐pocket payments, mean (SD), $ | 307 (629) | 323 (704) | 0.02 | 325 (686) | 303 (641) | 0.03 |
| No. of office visits, mean (SD) | 10 (19) | 9 (18) | 0.06 | 10 (20) | 9 (17) | 0.04 |
AHM indicates antihypertensive medication; MRA, medication refill adherence; SDM, shared decision‐making; and SDT, standardized difference test.
Frequencies are absolute counts, whereas percentages are weighted by the Medical Expenditure Panel Survey's sampling weights.
Standardized difference >0.10 indicates that the covariate is unbalanced between groups being compared.
Provider not human refers to healthcare institutions where patients received care, a particular human provider (e.g. physician, nurse, etc) is not assigned in this case.
Statistical Analysis
Descriptive analysis was conducted to compare the frequency and mean distributions of baseline covariates between participants grouped into high versus low levels of communication and SDM. Standardized difference tests were used to assess the balance of baseline covariates between the comparison groups. Next, we used logistic regression models weighted by MEPS survey weights59 to measure the odds ratios (ORs) and 95% CIs for the associations between each construct of the patient‐clinician relationships and adherence to antihypertensive medications. Because the literature shows a worsening trend of hypertension awareness and control in recent years, we fixed a linear time trend in calculating all adjusted ORs.60 We calculated all estimates (prevalences, averages, and ORs) and their standard errors based on standardized approaches for obtaining weighted estimates among subpopulations in MEPS.59 The goal of our primary analyses was to measure the following associations between each patient‐clinician construct and adherence, modeled as a binary outcome (MRA ≥80% versus <80%). Communication and SDM were modeled both as binary and ordinal‐scale independent variables.
Three sets of sensitivity analysis were performed to:
Test for the potential presence of reverse associations. The potential presence of reverse association between adherence and patient‐clinician relationships is illustrated in the directed acyclic graph (Figure 1) by a feedback loop from high BP to adherence and back to patient‐clinician relationships. For example, patients who have their BP under control are most likely to adhere and to rate their communication and involvement in SDM as high than when their high BP is not under control with antihypertensive treatment and vice versa. To test for the presence of a reverse association, we first repeated the analysis among new users of antihypertensive medications. We assumed that the reported baseline communication and SDM scores are not influenced by patients' adherence behaviors in year 1 since new users did not report using antihypertensives in year 1. Therefore, any observed associations between patient‐clinician relationships and adherence among new users are not influenced by prior adherence behaviors (assumption 1). Second, we explored whether prior adherence (MRA in year 1) was associated with the patients' perceptions of their communication and involvement in SDM; a lack of association would suggest that the reported patient‐clinician scores are not influenced by prior adherence (assumption 2). If both assumptions 1 and 2 hold true, then it is unlikely that the observed associations in the primary analysis are under the influence of potential reverse associations between patient‐clinician relationships and adherence.
Identify individual items of patient‐clinician constructs associated with adherence. We modeled individual items of both communication and SDM constructs as the main predictors of adherence by repeating the models described in the primary analysis.
Assess whether the associations between patient‐clinician relationships and adherence is modified by race/ethnicity. We tested for interaction between race/ethnicity (Black versus non‐Black individuals) and generated race/ethnicity‐specific data on the associations between patient‐clinician relationships and adherence.
Between 8% and 12% were missing a response to questions about patient‐clinician communication and SDM; therefore, we imputed values via random selection methods.61, 62 The distributions of single items and overall communication and SDM scores did not change after random selection imputation (Table S1 and S2).
Results
Participants
The primary analysis results reported are based on a total of 2571 (weighted n=2 039 511) Black adult patients with hypertension who had office/clinic visits in the past 12 months; 70% (weighted n=1 399 259) of these patients were persistent users and 30% (weighted n=640 252) started using antihypertensive medications during the second year of the MEPS surveys. Forty‐five percent of the sample had high levels of communication with their clinicians. Among the 2354 patients (weighted n=1 865 852) who had a usual source of care provider and had an office/clinic visit in the past 12 months, 43% reported having high levels of involvement in SDM. The distribution of responses to individual items are reported in Table S2.
Distribution of Participant Characteristics
Table 1 describes the distribution of baseline characteristics by binary levels of patient‐clinician communication (unweighted n=2571; weighted n=2 039 511) and patient involvement in the SDM process (unweighted n=2354; weighted n=1 865 852) among those who responded to CAHPS and the access to care questionnaire, respectively. The standardized difference tests (>0.10) showed that the majority of the baseline covariates were balanced between levels of communication except for geographic region of residence (West), healthcare cost–related barriers, healthcare system factors (uninsured, lack of usual source of payment), provider specialty (general medical doctor), and chronic comorbidities (poor physical and mental health, angina, arthritis). Similarly, geographic region of residence (Midwest, South, West), healthcare cost–related barriers, healthcare system factors (uninsured, lack of usual source of payment), provider specialty (general medical doctor, nonhuman), and chronic comorbidities (poor physical and mental health, angina, arthritis). The distribution of responses to patient‐clinician interactions and baseline characteristics were similar before and after missing data imputation (Table S3).
Patterns of Prevalence of Adherence by Levels of Patient‐Clinician Communication and Involvement in SDM
The overall prevalence of adherence to antihypertensive medications (MRA ≥80%) was 40% with a median MRA of 66% (interquartile range [IQR], 41%–98%). The median MRAs were significantly higher among patients with high (74.0; IQR, 41.1–98.6) compared with those with low (61.6; IQR, 32.9–95.9) levels of patient‐clinician communication (P<0.01). Similarly, patients with higher levels of involvement in SDM had higher median MRAs (74.0; IQR, 41.1–98.6) compared with those who did not feel highly involved (63.7; IQR, 32.9–98.6) (P=0.05).
Associations between Patient‐Clinician Relationships and Adherence to Antihypertensive Medications
The associations between patient‐clinician relationships and adherence to antihypertensive medications among Black adults and other racial/ethnic groups are reported in Tables 2 and 3. In multivariable logistic regression analysis, the odds of adherence to antihypertensive medications was 38% (OR, 1.38; 95% CI, 1.14–1.67) higher among Black patients who reported having high levels of communication with their clinicians, versus low levels of communication, after adjusting for social and economic, patient‐related, condition‐related, and healthcare system/healthcare team factors (Table 2). Black patients were also more likely to adhere to antihypertensive medications if they felt more involved with the decision‐making process of their health care than if they did not (OR, 1.32; 95% CI, 1.08–1.61) (Table 3). Both ordinal‐level communication and SDM scores were significantly associated with adherence to antihypertensive medication (Table S4). The odds of adherence increased by 14% and 8% for every unit increase in communication (OR, 1.14; 95% CI, 1.07–1.22) and SDM scores (OR, 1.08; 95% CI, 1.04–1.15), respectively (Table S4). These associations persisted among subgroups of new users of antihypertensive medications (Table S4).
Table 2.
Associations Between Patient‐Provider Communication and Adherence to AHMs Among Prevalent and New Users
| Racial/Ethnic Groups | Prevalence of Refill Adherence by Levels of Communication, % (95% CI)* | OR (95% CI)† | ||
|---|---|---|---|---|
| Low | High | Unadjusted | Adjusted for Patient, Provider, and Healthcare System–Level Factors‡ | |
| Black patients | ||||
| All users, n=2571 | 35 (32–38) | 42 (39–45) | 1.42 (1.18–1.71) | 1.38 (1.14–1.67) |
| New users, n=783 | 29 (25–34) | 36 (31–41) | 1.47 (1.04–2.07) | 1.45 (1.01–2.07) |
| Non–Hispanic White patients | ||||
| All users, n=4771 | 50 (48–52) | 50 (48–52) | 0.98 (0.86–1.12) | 0.96 (0.86–1.12) |
| New users, n=1434 | 41 (37–45) | 46 (42–51) | 1.24 (0.96–1.58) | 1.20 (0.93–1.55) |
| Hispanic patients | ||||
| All users, n=1675 | 41 (37–44) | 43 (38–47) | 1.09 (0.86–1.39) | 1.02 (0.80–1.31) |
| New users, n=584 | 32 (26–38) | 33 (26–40) | 1.03 (0.67–1.57) | 0.91 (0.58–1.43) |
| Other race/ethnicity§ | ||||
| All users, n=689 | 43 (37–49) | 43 (36–51) | 1.02 (0.70–1.50) | 1.10 (0.73–1.65) |
| New users, n=257 | 38 (28–47) | 39 (26–52) | 1.04 (0.53–2.04) | 1.01 (0.47–2.16) |
Prevalence, odds ratios (ORs), and 95% CIs are weighted by Medical Expenditure Panel Survey's sampling weights.
Referent group is “low.”
Patient‐level factors: age; sex; geographic region of residence; education; speaks English at home; marital status; employment status; poverty status; ever delay, forego, or make change in prescription medicine because of cost; ever delay, forego, or make change in treatment because of cost; have blood checked in the past year; duration of hypertension; used antihypertension medications (AHMs) in the baseline year (adjusted for among only new users); adherent in year 1 (adjusted for among only new users); poor physical health; poor mental health; cognitive limitations; coronary heart disease; angina; myocardial infarction; other heart diseases; stroke; diabetes mellitus; arthritis; asthma; chronic bronchitis; cancer. Provider‐level factors: provider specialty; provider and patient are of the same sex; provider and patient are of the same race. Healthcare system–level factors: uninsured; usual source of payment; payment source; out‐of‐pocket payments; number of office visits.
Other race/ethnicity: Native Americans, Alaskans, Asians, Hawaiians, and Pacific Islanders.
Table 3.
Associations Between SDM and Adherence to AHMs Among Prevalent and New Users
| Racial/Ethnic Groups | Prevalence of Refill Adherence by Levels of SDM, % (95% CI)* | OR (95% CI)† | ||
|---|---|---|---|---|
| Low | High | Unadjusted | Adjusted for Patient‐, Provider‐, and Healthcare System–Level Factors‡ | |
| Black patients | ||||
| All users, n=2354 | 37 (34–39) | 40 (37–43) | 1.32 (1.09–1.60) | 1.32 (1.08–1.61 ) |
| New users, n=692 | 30 (26–35) | 25 (19–30) | 1.49 (1.03–2.14) | 1.59 (1.09–2.32 ) |
| White patients | ||||
| All users, n=4495 | 50 (48–52) | 51 (48–53) | 1.02 (0.89–1.17) | 1.01 (0.88–1.16) |
| New users, n=1344 | 42 (38–46) | 48 (43–53) | 1.25 (0.97–1.61) | 1.23 (0.95–1.60) |
| Hispanic patients | ||||
| All users, n=1494 | 42 (38–46) | 41 (37–46) | 0.97 (0.75–1.25) | 0.92 (0.71–1.20) |
| New users, n=510 | 34 (28–41) | 33 (25–40) | 0.93 (0.59–1.45) | 0.84 (0.51–1.37) |
| Other race/ethnicity§ | ||||
| All users, n=625 | 42 (36–47) | 47 (39–55) | 1.24 (0.82, 1.87) | 1.29 (0.84–1.99) |
| New users, n=232 | 37 (27–46) | 40 (26–55) | 1.16 (0.56–2.39) | 1.20 (0.51–2.83) |
SDM indicates shared decision‐making.
Prevalence, odds ratios (ORs), and 95% CIs are weighted by Medical Expenditure Panel Survey's sampling weights.
Referent group is “low.”
Patient‐level factors: age; sex; geographic region of residence; education; speaks English at home; marital status; employment status; poverty status; ever delay, forego, or make change in prescription medicine because of cost; ever delay, forego, or make change in treatment because of cost; have blood checked in the past year; duration of hypertension; used antihypertensive medications (AHMs) in the baseline year (adjusted for among only new users); adherent in year 1 (adjusted for among only new users); poor physical health; poor mental health; cognitive limitations; coronary heart disease; angina; myocardial infarction; other heart diseases; stroke; diabetes mellitus; arthritis; asthma; chronic bronchitis; cancer. Provider‐level factors: provider specialty; provider and patient are of the same sex; provider and patient are of the same race. Healthcare system–level factors: uninsured; usual source of payment; payment source; out‐of‐pocket payments; number of office visits.
Other race/ethnicity: Native Americans, Alaskans, Asians, Hawaiians, and Pacific Islanders.
Sensitivity Analysis
There were 783 (weighted n=640 252) and 692 (weighted n=571 084) new users of antihypertensive medications among participants with communication and SDM scores, respectively. Similar to the primary analysis, both high levels of communication (OR, 1.45; 95% CI, 1.01–2.07) (Table 2) and involvement in SDM (OR, 1.59; 95% CI, 1.09–2.32) (Table 3) were significantly associated with adherence to antihypertensive medications among new users, consistent with prevalent users. Prior adherence (measured in the baseline period) was neither associated with patients' perceptions of their communication (OR, 1.09; 95% CI, 0.87–1.35) nor their involvement in SDM process (OR, 1.03; 95% CI, 0.82–1.29). In the second set of sensitivity analysis, all 4 individual items that make up the communication construct were significantly associated with adherence to antihypertensive medications (Table S5). On the other hand, the additional 3 individual items that were combined with the 4 communication items were observed to not be significantly associated with adherence (Table S5). Black race/ethnicity significantly modified the associations between both constructs of the patient‐clinician relationships (communication [P interaction <0.001] and SDM [P interaction <0.01]) and adherence to antihypertensive medications. In contrast to the associations observed among Black patients, none of the associations between patient‐clinician constructs and adherence to antihypertensive medications were statistically significant among non–Hispanic White patients, Hispanic patients, and other (Native Americans, Alaskans, Asians, Hawaiians and Pacific Islanders) racial/ethnic groups (Tables 2 and 3).
Discussion
We show Black adults with hypertension who self‐report as having high levels of communication and a high degree of involvement with their clinicians in making decisions about treatment were more likely to take antihypertensive medications as prescribed compared with those with lower levels of communication and involvement in SDM. These associations were independent of all provider characteristics: the clinician's race/ethnicity, sex, and specialty. Individual‐ and healthcare system–level factors did not influence these associations nor did the observed associations appear to have been influenced by potential reverse associations between patient‐clinician relationships and adherence. In contrast, neither communication nor SDM were associated with adherence to antihypertensive medications among non–Hispanic White patients, Hispanic patients, and other racial/ethnic groups. This suggests that the observed associations between patient‐provider relationships and adherence are robust. Our analysis provides new data on the association between SDM and adherence to antihypertensive medications among Black adult patients with hypertension and also expands the growing evidence base on the potential benefits of patient‐clinician communication on adherence to antihypertensive medications among Black patients.28, 29, 30, 31 Most importantly, we have addressed methodological limitations that have been identified in previously published studies on this topic.
The observed positive associations between communication and adherence corroborates data from studies conducted among Black patients28, 29, 30, 31 and among the general population.27 However, our findings contradict those previously reported by Cooper et al (2011)63 among 279 underserved primary care patients with hypertension randomized to physicians who received communication skills training versus a control physician group. There are no published data on the associations between SDM and adherence to antihypertensive medications specifically among Black adults. Among a racially diverse population, overall medication adherence to either oral hypoglycemic, lipid‐lowering, or antihypertensive medication increased with higher scores of SDM, although no association was observed among only users of antihypertensive medications.48 Schoenthaler et al (2018)64 also reported that SDM was positively associated with adherence to antihypertensive medications among 43 Black patients and 32 White patients with hypertension. In a systematic review of intervention studies, SDM interventions did not improve adherence to medications among the general population.65
The effects of patient‐clinician communication and SDM on medication adherence has largely been postulated via indirect pathways involving affective‐cognitive outcomes including knowledge, attitudes, and satisfaction with care, which are all known determinants of adherence to medications.26, 58 It has also been theorized that effective communication increases patients' knowledge about their medications and medical conditions. The complex regimens required for patients with more severe hypertension and/or comorbidities,66 both more common among Black individuals, may particularly require effective communication to enhance long‐term adherence. Indeed, we observed that patients who reported that their clinician always, versus not, explained things in a way that was easy to understand were more likely to be adherent (OR, 1.40; 95% CI, 1.15–1.72). Also, by listening attentively, respecting the sociocultural views of Black patients, and making them partners in decisions about their medications, clinicians may be better positioned to intervene by providing accurate evidence‐based information and or switching medication in the instance where patients are experiencing drug adverse effects. Our data confirmed that patients who reported that their clinician always listened carefully, versus not, were more likely to be adherent (OR, 1.38; 95% CI, 1.13–1.69).
The lack of association between patient‐provider relationships and adherence among non–Hispanic White patients, Hispanic patients, and other racial/ethnic groups was unexpected. We suspect that Black patients with hypertension may have more complex drug regimens67 compared with other racial/ethnic groups because of the disproportionately higher severity of hypertension (uncontrolled BP) and burden of chronic comorbidities.68, 69 Therefore, it is plausible that higher levels of patient‐provider communication and involvement in SDM probably had higher effects on mitigating the adverse effects of complex antihypertensive regimens on adherence among Black patients but not among other racial/ethnic groups. Additional research is needed to test empirically the hypothesis that our observed racial/ethnic disparities in the associations between patient‐provider communication and involvement in SDM are moderated by hypertension symptom severity and the complexity of the antihypertensive regimen. It is also possible that providers who communicate effectively and involve Black patients in SDM may be less likely to be biased against Black patients. Black patients are likely to be more trusting of such providers and, consequently, more likely to adhere to treatment recommendations. Future research on whether patient‐provider relationships can close racial/ethnic disparities in adherence to antihypertensive medications is warranted.
Strengths and Limitations
Several limitations of this study need to be considered when interpreting our findings. First, because this is an observational study we cannot rule out the effects of potential unmeasured confounding although we adjusted for several patient‐, provider‐, and healthcare system–level confounders. Second, while MRA is a validated measure of refill adherence, it was measured based on self‐reported medication use and may therefore be liable to recall bias. Any such bias, however, would have had a differential effect on measured associations since we expect recall bias to be similar between the groups compared by levels of communication and SDM. MRA as a refill adherence measure does not account for switching between antihypertensive medications after initiation. Third, we measured communication and SDM from the patient's perspective without the clinician's perspective. However, it has been shown that patient‐perceived communication and SDM are more important in assessing patient outcomes.26 Fourth, we could not ascertain from the data how long patients and clinicians interacted with each other. However, given that responses reflect the patient's experience with a clinician in the past 12 months and that on average patients had 9 (SD, 19) office visits during the same period, it is highly likely that patients and clinicians had an established ongoing relationship. Fifth, because the MEPS data do not include unique provider identification numbers, we could not verify whether patients received antihypertensive therapy from the same provider for whom they had provided responses to regarding their communication and involvement in the SDM process. However, given that the patients included in our analysis had frequent office visits and that 81% reported that their clinician asked about other medications that were prescribed by other doctors, we believe that our findings reflect patients' experiences with clinicians in general, irrespective of whether the clinician was the prescriber of the antihypertensive medications.
Notwithstanding these limitations, our study also features strengths that enhance its relevance for practice and research. First, our sensitivity analysis shows that the observed associations are robust against the effects of potential reverse associations between patient‐clinician relationships and adherence. Second, by using a longitudinal cohort study design to leverage a national survey data set, we have contributed critical data on the associations between patient‐clinician relationships and adherence to antihypertensive medications among Black adults in the United States. Third, our findings are directly translatable to clinical practice because we defined patient‐clinician relationships from standard survey instruments that are currently in use by healthcare providers in the United States.
Conclusions
Patient‐clinician communication and SDM were identified as modifiable predictors of adherence to antihypertensive medications among Black patients but not among non–Hispanic White patients, Hispanic patients, or other racial/ethnic groups. Development of testable interventions to enhance these elements will support further assessment of the relationship between these patient‐clinician factors and adherence to antihypertensive medications among Black adults. Based on our findings, we recommend that clinicians and healthcare systems consider emphasizing communication and SDM processes within patient‐centered models as a strategy to improve adherence to antihypertensive medications among adult Black patients with hypertension.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1‐S5
(J Am Heart Assoc. 2021;10:e019943. DOI: 10.1161/JAHA.120.019943.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019943.
For Sources of Funding and Disclosures, see page 13.
References
- 1.Charles H, Good CB, Hanusa BH, Chang CC, Whittle J. Racial differences in adherence to cardiac medications. J Natl Med Assoc. 2003;95:17–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings DM, Wu JR, Cene C, Halladay J, Donahue KE, Hinderliter A, Miller C, Garcia B, Penn D, Tillman J, et al. Perceived social standing, medication nonadherence, and systolic blood pressure in the rural south. J Rural Health. 2016;32:156–163. DOI: 10.1111/jrh.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes HM, Luo R, Hanlon JT, Elting LS, Suarez‐Almazor M, Goodwin JS. Ethnic disparities in adherence to antihypertensive medications of medicare part D beneficiaries. J Am Geriatr Soc. 2012;60:1298–1303. DOI: 10.1111/j.1532-5415.2012.04037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11:54–65. DOI: 10.3121/cmr.2013.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiner JF, Ho PM, Beaty BL, Dickinson LM, Hanratty R, Zeng C, Tavel HM, Havranek EP, Davidson AJ, Magid DJ, et al. Sociodemographic and clinical characteristics are not clinically useful predictors of refill adherence in patients with hypertension. Circ Cardiovasc Qual Outcomes. 2009;2:451–457. DOI: 10.1161/CIRCOUTCOMES.108.841635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Baik SH. Race/Ethnicity, disability, and medication adherence among medicare beneficiaries with heart failure. J Gen Intern Med. 2014;29:602–607. DOI: 10.1007/s11606-013-2692-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosworth HB, Dudley T, Olsen MK, Voils CI, Powers B, Goldstein MK, Oddone EZ. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119:70.e9–70.e15. DOI: 10.1016/j.amjmed.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 8.Butler MJ, Tanner RM, Muntner P, Shimbo D, Bress AP, Shallcross AJ, Sims M, Ogedegbe G, Spruill TM. Adherence to antihypertensive medications and associations with blood pressure among African Americans with hypertension in the Jackson Heart Study. J Am Soc Hypertens. 2017;11:e5. DOI: 10.1016/j.jash.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173:46–51. DOI: 10.1001/2013.jamainternmed.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. DOI: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 11.Wall HK, Ritchey MD, Gillespie C, Omura JD, Jamal A, George MG. Vital signs: prevalence of key cardiovascular disease risk factors for million hearts 2022––United States, 2011–2016. MMWR Morb Mortal Wkly Rep. 2018;67:983–991. DOI: 10.15585/mmwr.mm6735a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics––2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. DOI: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 13.Xie Z, St Clair P, Goldman DP, Joyce G. Racial and ethnic disparities in medication adherence among privately insured patients in the United States. PLoS One. 2019;14:e0212117. DOI: 10.1371/journal.pone.0212117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y, Song S, Zhang L, Trisolini MG, Labresh KA, Smith SC Jr, Zheng ZJ. Disparities in premature cardiac death among US counties from 1999–2017: temporal trends and key drivers. J Am Heart Assoc. 2020;9:e016340. DOI: 10.1161/JAHA.120.016340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics (US) . Health, United States, 2018 [Internet]. Hyattsville (MD): National Center for Health Statistics (US); 2019. PMID: 31944640. [PubMed] [Google Scholar]
- 16.Gagne JJ, Choudhry NK, Kesselheim AS, Polinski JM, Hutchins D, Matlin OS, Brennan TA, Avorn J, Shrank WH. Comparative effectiveness of generic and brand‐name statins on patient outcomes: a cohort study. Ann Intern Med. 2014;161:400–407. DOI: 10.7326/M13-2942 [DOI] [PubMed] [Google Scholar]
- 17.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. DOI: 10.1056/NEJMsa1107913 [DOI] [PubMed] [Google Scholar]
- 18.Dragomir A, Cote R, Roy L, Blais L, Lalonde L, Berard A, Perreault S. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Med Care. 2010;48:418–425. DOI: 10.1097/MLR.0b013e3181d567bd [DOI] [PubMed] [Google Scholar]
- 19.Conn VS, Ruppar TM, Chase JA, Enriquez M, Cooper PS. Interventions to improve medication adherence in hypertensive patients: systematic review and meta‐analysis. Curr Hypertens Rep. 2015;17:94. DOI: 10.1007/s11906-015-0606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;2014:CD000011. DOI: 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruppar TM, Dunbar‐Jacob JM, Mehr DR, Lewis L, Conn VS. Medication adherence interventions among hypertensive black adults: a systematic review and meta‐analysis. J Hypertens. 2017;35:1145–1154. DOI: 10.1097/HJH.0000000000001260 [DOI] [PubMed] [Google Scholar]
- 22.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. DOI: 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 23.Bussell JK, Cha E, Grant YE, Schwartz DD, Young LA. Ways health care providers can promote better medication adherence. Clin Diabetes. 2017;35:171–177. DOI: 10.2337/cd016-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper LA, Beach MC, Johnson RL, Inui TS. Delving below the surface. Understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21:S21–S27. DOI: 10.1111/j.1525-1497.2006.00305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Street RL Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician‐patient communication to health outcomes. Patient Educ Couns. 2009;74:295–301. DOI: 10.1016/j.pec.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 26.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35:114–131. DOI: 10.1177/0272989X14551638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47:826–834. DOI: 10.1097/MLR.0b013e31819a5acc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogedegbe G, Harrison M, Robbins L, Mancuso CA, Allegrante JP. Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study. Ethn Dis. 2004;14:3–12. [PubMed] [Google Scholar]
- 29.Schoenthaler A, Allegrante JP, Chaplin W, Ogedegbe G. The effect of patient‐provider communication on medication adherence in hypertensive black patients: does race concordance matter? Ann Behav Med. 2012;43:372–382. DOI: 10.1007/s12160-011-9342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenthaler A, Chaplin WF, Allegrante JP, Fernandez S, Diaz‐Gloster M, Tobin JN, Ogedegbe G. Provider communication effects medication adherence in hypertensive African Americans. Patient Educ Couns. 2009;75:185–191. DOI: 10.1016/j.pec.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenthaler A, Knafl GJ, Fiscella K, Ogedegbe G. Addressing the social needs of hypertensive patients: the role of patient‐provider communication as a predictor of medication adherence. Circ Cardiovasc Qual Outcomes. 2017;10:e003659. DOI: 10.1161/CIRCOUTCOMES.117.003659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, Elwyn G. Do interventions designed to support shared decision‐making reduce health inequalities? A systematic review and meta‐analysis. PLoS One. 2014;9:e94670. DOI: 10.1371/journal.pone.0094670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuntz JL, Safford MM, Singh JA, Phansalkar S, Slight SP, Her QL, Lapointe NA, Mathews R, O'Brien E, Brinkman WB, et al. Patient‐centered interventions to improve medication management and adherence: a qualitative review of research findings. Patient Educ Couns. 2014;97:310–326. DOI: 10.1016/j.pec.2014.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breekveldt‐Postma NS, Penning‐van Beest FJ, Siiskonen SJ, Koerselman J, Klungel OH, Falvey H, Vincze G, Herings RM. Effect of persistent use of antihypertensives on blood pressure goal attainment. Curr Med Res Opin. 2008;24:1025–1031. DOI: 10.1185/030079908X280554 [DOI] [PubMed] [Google Scholar]
- 35.Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: a systematic review and meta‐analysis. Medicine (Baltimore). 2017;96:e5641. DOI: 10.1097/MD.0000000000005641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler MJ, Tanner RM, Muntner P, Shimbo D, Bress AP, Shallcross AJ, Sims M, Ogedegbe G, Spruill TM. Adherence to antihypertensive medications and associations with blood pressure among African Americans with hypertension in the Jackson Heart Study. J Am Soc Hypertens. 2017;11:581–588. DOI: 10.1016/j.jash.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg R, Shen C, Sambamoorthi N, Kelly K, Sambamoorthi U. Type of multimorbidity and patient‐doctor communication and trust among elderly medicare beneficiaries. Int J Family Med. 2016;2016:8747891. DOI: 10.1155/2016/8747891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong K, Rose A, Peters N, Long JA, McMurphy S, Shea JA. Distrust of the health care system and self‐reported health in the United States. J Gen Intern Med. 2006;21:292–297. DOI: 10.1111/j.1525-1497.2006.00396.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadeq R, Chowdhury SRM, Gwet KL. Sample designs of the medical expenditure panel survey household component, 1996‐2006 and 2007‐2016. https://www.meps.ahrq.gov/data_files/publications/mr33/mr33.shtml: Agency for Healthcare Research and Quality, 2019. [Google Scholar]
- 40.Hill SC, Roemer M, Stagnitti MN. Outpatient Prescription Drugs: Data Collection and Editing in the 2011 Medical Expenditure Panel Survey. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 41.Hargraves JL, Hays RD, Cleary PD. Psychometric properties of the consumer assessment of health plans study (CAHPS) 2.0 adult core survey. Health Serv Res. 2003;38:1509–1527. DOI: 10.1111/j.1475-6773.2003.00190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hays RD, Shaul JA, Williams VS, Lubalin JS, Harris‐Kojetin LD, Sweeny SF, Cleary PD. Psychometric properties of the CAHPS 1.0 survey measures. Consumer assessment of health plans study. Med Care. 1999;37:MS22–MS31. DOI: 10.1097/00005650-199903001-00003 [DOI] [PubMed] [Google Scholar]
- 43.Morales LS, Elliott MN, Weech‐Maldonado R, Spritzer KL, Hays RD. Differences in CAHPS adult survey reports and ratings by race and ethnicity: an analysis of the National CAHPS benchmarking data 1.0. Health Serv Res. 2001;36:595–617. [PMC free article] [PubMed] [Google Scholar]
- 44.Weech‐Maldonado R, Carle A, Weidmer B, Hurtado M, Ngo‐Metzger Q, Hays RD. The consumer assessment of healthcare providers and systems (CAHPS) cultural competence (CC) item set. Med Care. 2012;50:S22–S31. DOI: 10.1097/MLR.0b013e318263134b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carle AC, Weech‐Maldonado R, Ngo‐Metzger Q, Hays RD. Evaluating measurement equivalence across race and ethnicity on the CAHPS cultural competence survey. Med Care. 2012;50:S32–S36. DOI: 10.1097/MLR.0b013e3182631189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer AM, Parker MM, Schillinger D, Katon W, Adler N, Adams AS, Moffet HH, Karter AJ. Associations between antidepressant adherence and shared decision‐making, patient‐provider trust, and communication among adults with diabetes: diabetes study of Northern California (DISTANCE). J Gen Intern Med. 2014;29:1139–1147. DOI: 10.1007/s11606-014-2845-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milky G, Thomas J 3rd. Shared decision making, satisfaction with care and medication adherence among patients with diabetes. Patient Educ Couns. 2020;103:661–669. DOI: 10.1016/j.pec.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 48.Ratanawongsa N, Karter AJ, Parker MM, Lyles CR, Heisler M, Moffet HH, Adler N, Warton EM, Schillinger D. Communication and medication refill adherence: the diabetes study of Northern California. JAMA Intern Med. 2013;173:210–218. DOI: 10.1001/jamainternmed.2013.1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Medicare & Medicaid Services . 2020. [cited 2020 06/27/2020]. Available at: https://hcahpsonline.org/en/summary‐analyses/#Overview.
- 50.Centers for Medicare and Medicaid Services . In Calculation of HCAHPS scores: from raw data to publicly reported results. 2011. Available at: https://www.hcahpsonline.org/globalassets/hcahps/technical‐specifications/calculation‐of‐hcahps‐scores2.pdf.
- 51.Levine DM, Landon BE, Linder JA. Trends in patient‐perceived shared decision making among adults in the United States, 2002–2014. Ann Fam Med. 2017;15:552–556. DOI: 10.1370/afm.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. DOI: 10.1345/aph.1H018 [DOI] [PubMed] [Google Scholar]
- 53.Hamilton RA, Briceland LL. Use of prescription‐refill records to assess patient compliance. Am J Hosp Pharm. 1992;49:1691–1696. [PubMed] [Google Scholar]
- 54.Haas K, Ben Miled Z, Mahoui M. Medication adherence prediction through online social forums: a case study of fibromyalgia. JMIR Med Inform. 2019;7:e12561. DOI: 10.2196/12561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51:S11–S21. DOI: 10.1097/MLR.0b013e31829b1d2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baumgartner PC, Haynes RB, Hersberger KE, Arnet I. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front Pharmacol. 2018;9:1290. DOI: 10.3389/fphar.2018.01290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut‐point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25:2303–2310. DOI: 10.1185/03007990903126833 [DOI] [PubMed] [Google Scholar]
- 58.Sabaté E. Adherence to Long‐Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 59.Machlin S, Yu W, Zodet M. Medical Expenditure Panel Survey Computing Standard Errors for MEPS Estimates. Rockville, MD: AHRQ; 2005. [Google Scholar]
- 60.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. DOI: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shrive FM, Stuart H, Quan H, Ghali WA. Dealing with missing data in a multi‐question depression scale: a comparison of imputation methods. BMC Med Res Methodol. 2006;6:57. DOI: 10.1186/1471-2288-6-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quintero M, LeBoulluec A. Missing data imputation for ordinal data. Int J Comput Appl. 2018;181:10–16. DOI: 10.5120/ijca2018917522 [DOI] [Google Scholar]
- 63.Cooper LA, Roter DL, Carson KA, Bone LR, Larson SM, Miller ER 3rd, Barr MS, Levine DM. A randomized trial to improve patient‐centered care and hypertension control in underserved primary care patients. J Gen Intern Med. 2011;26:1297–1304. DOI: 10.1007/s11606-011-1794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoenthaler A, Rosenthal DM, Butler M, Jacobowitz L. Medication adherence improvement similar for shared decision‐making preference or longer patient‐provider relationship. J Am Board Fam Med. 2018;31:752–760. DOI: 10.3122/jabfm.2018.05.180009 [DOI] [PubMed] [Google Scholar]
- 65.Mathijssen EG, van den Bemt BJ, van den Hoogen FH, Popa CD, Vriezekolk JE. Interventions to support shared decision making for medication therapy in long term conditions: a systematic review. Patient Educ Couns. 2020;103:254–265. DOI: 10.1016/j.pec.2019.08.034 [DOI] [PubMed] [Google Scholar]
- 66.Pantuzza LL, Ceccato M, Silveira MR, Junqueira LM, Reis AM. Association between medication regimen complexity and pharmacotherapy adherence: a systematic review. Eur J Clin Pharmacol. 2017;73:1475–1489. DOI: 10.1007/s00228-017-2315-2 [DOI] [PubMed] [Google Scholar]
- 67.Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among US adults with hypertension. Circ Cardiovasc Qual Outcomes. 2017;10:e003166. DOI: 10.1161/CIRCOUTCOMES.116.003166 [DOI] [PubMed] [Google Scholar]
- 68.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Hall WD, Jones WE, Kountz DS, Lea JP, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. DOI: 10.1161/HYPERTENSIONAHA.110.152892 [DOI] [PubMed] [Google Scholar]
- 69.Ferdinand KC. Improving approaches to hypertension treatment in African Americans: lessons learned from the Jackson Heart Study. J Clin Hypertens (Greenwich). 2013;15:362–364. DOI: 10.1111/jch.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S5


