Abstract
Background
The incidence of ischemic stroke has increased among adults aged 18 to 64 years, yet little is known about relationships between specific risk factors and outcomes. This study investigates in‐hospital and long‐term outcomes in patients with stroke aged <65 years with preexisting diabetes mellitus.
Methods and Results
Consecutive patients aged <65 years admitted to comprehensive stroke centers for acute ischemic stroke between 2003 and 2013 were identified from the Ontario Stroke Registry. Multinomial logistic regression was used to estimate adjusted odds ratio (OR [95% CI]) of in‐hospital mortality or direct discharge to long‐term or continuing care. Cox proportional hazards regression was used to estimate the adjusted hazards ratio (aHR [95% CI]) of long‐term mortality, readmission for stroke/transient ischemic attack, admission to long‐term care, and incident dementia. Predefined sensitivity analyses examined stroke outcomes among young (aged 18–49 years) and midlife (aged 50–65 years) subgroups. Among 8293 stroke survivors (mean age, 53.6±8.9 years), preexisting diabetes mellitus was associated with a higher likelihood of in‐hospital death (adjusted OR, 1.46 [95% CI, 1.14–1.87]) or direct discharge to long‐term care (adjusted OR, 1.65 [95% CI, 1.07–2.54]). Among stroke survivors discharged (N=7847) and followed up over a median of 6.3 years, preexisting diabetes mellitus was associated with increased hazards of death (aHR, 1.68 [95% CI, 1.50–1.88]), admission to long‐term care (aHR, 1.57 [95% CI, 1.35–1.82]), readmission for stroke/transient ischemic attack (aHR, 1.37 [95% CI, 0.21–1.54]), and incident dementia (aHR, 1.44 [95% CI, 1.17–1.77]). Only incident dementia was not increased for young stroke survivors.
Conclusions
Focused secondary prevention and risk factor management may be needed to address poor long‐term outcomes for patients with stroke aged <65 years with preexisting diabetes mellitus.
Keywords: dementia, diabetes mellitus, ischemic, longitudinal, midlife, stroke, young
Subject Categories: Ischemic Stroke, Cognitive Impairment, Secondary Prevention, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- aHR
adjusted hazard ratio
- LTC
long‐term care
Clinical Perspective
What Is New?
There was a higher likelihood of mortality in hospital and after discharge, recurrent stroke, incident dementia, and the need for long‐term care among adults with stroke and pre‐existing diabetes compared to stroke with no diabetes.
These risks were notable for stroke in midlife, as well for young stroke survivors, with the exception of dementia, which was uncommon for those aged <50 years.
What Are the Clinical Implications?
Optimizing diabetes mellitus care for vascular protection in stroke prevention and recovery may be needed to alleviate morbidity and mortality associated with young and middle‐age stroke.
The incidence of stroke shows a decreasing trend in recent decades, according to a US study.1 And yet there are exceptions,1, 2 including stroke among young adults,3 such as acute ischemic stroke having increased 36% from 2003 to 2012 among adults aged 35 to 44 years.4 Across multiple population‐based cohorts from Europe and the United States, pooled annualized median incidence of stroke in young adults increased 4.4% between the mid 1980s and the late 2000s.5 Although younger adults who sustain stroke tend to show better functional recovery compared with older adults,6 recovery can be highly variable, and few studies have identified risk factors associated with long‐term outcomes in younger stroke survivors. Questions remain surrounding at‐risk profiles in working‐aged7 and young stroke,8 which is highly pertinent given the increasing prevalence of premorbid cardiovascular risk factors (ie, diabetes mellitus, hypertension, obesity, dyslipidemia, and tobacco use) over the past decades in these stroke demographics.4
Diabetes mellitus is particularly relevant as it is associated with poor stroke outcomes among older adults.9 More recent young stroke studies report diabetes mellitus to be an independent risk factor for mortality and recurrent events10, 11; however, other long‐term outcomes that reflect independent living after stroke have received little to no research attention. This study follows adult patients with or without comorbid diabetes mellitus who were admitted for acute ischemic stroke at an age of ≤65 years. We investigate whether preexisting diabetes mellitus increases the likelihood and hazards of adverse in‐hospital and long‐term outcomes. Specifically, relative to patients with stroke without diabetes mellitus, we examined whether stroke with comorbid diabetes mellitus was associated with increased risks of in‐hospital and long‐term mortality and long‐term recurrent stroke and institutionalization. Recognizing the interplay between cerebrovascular disease, diabetes mellitus, and cognitive impairment,12, 13, 14, 15 we also examined associations between diabetes mellitus and incident dementia after stroke.
Methods
Study Design
In this retrospective study, we used data from the Ontario Stroke Registry to identify a cohort of consecutive patients presenting with an acute ischemic stroke to comprehensive stroke centers in Ontario, Canada, from 2003 to 2013. Registry data were linked via unique encoded identifiers with administrative databases at Institute for Clinical Evaluative Sciences. Although data sharing agreements prohibit making the data publicly available, access may be granted to those who meet prespecified criteria for confidential access via https://www.ices.on.ca/DAS. The study creation plan and underlying analytic code are available from the authors on request, understanding that the programs may rely on unique coding templates and/or macros.
Patients entered the cohort on the date of admission for stroke and classified as having preexisting diabetes mellitus if there was a record of diabetes mellitus from the Ontario Diabetes Database on a date that preceded the stroke. To ascertain relationships between preexisting diabetes mellitus and in‐hospital outcomes, patients with stroke were followed up to the time of mortality or discharge, whichever occurred first. To ascertain relationships between diabetes mellitus and long‐term outcomes, patients with stroke who were discharged were subsequently followed up until one of the following end points: all‐cause death, occurrence of a secondary outcome (readmission for stroke/transient ischemic attack [TIA], admission to long‐term care [LTC], or dementia), or the cohort end date of December 31, 2016, as shown in the flow chart (Figure 1).
Figure 1.
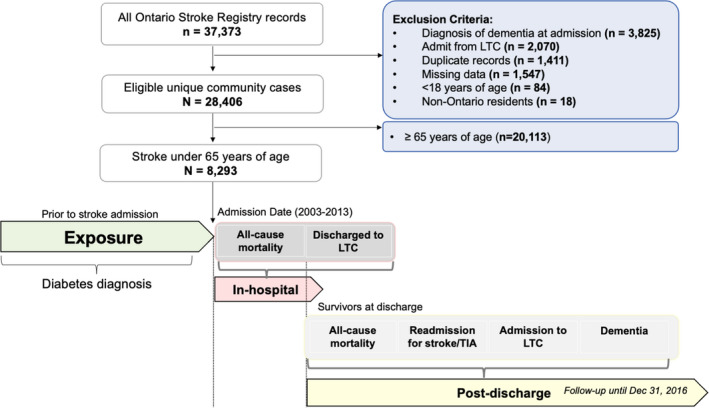
Flow chart showing the participants who were included in this study after accounting for exclusions by previous medical conditions, missing and duplicate data, study criteria, and ages <18 and >65 years.
Diabetes mellitus was ascertained by all available Ontario Diabetes Database records that preceded the stroke admission. A total of 8293 participants were available to test the in‐hospital outcomes. There were 4 outcomes tested among the stroke survivors who were discharged alive. LTC indicates long‐term care; and TIA, transient ischemic attack.
This study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre. Because the Institute for Clinical Evaluative Sciences is a prescribed entity under Ontario's Personal Health Information Protection Act, data collection for the Ontario Stroke Registry is permitted without patient consent.
Cohort Selection
We used the Ontario Stroke Registry to identify cases of ischemic stroke. Individuals from Ontario were included in this study if they sustained an ischemic stroke between the ages of 18 and 65 years at the time of hospital admission, over a time period between 2003 and 2013. Exclusion criteria were premorbid dementia, diagnosis of dementia at the time of admission, admission to hospital from an LTC facility, admission for hemorrhagic stroke, and non‐Ontario resident status (Figure 1).
Ascertainment of Diabetes Mellitus and Covariates
A diabetes mellitus diagnosis (type 1 or 2) was identified before the date of cohort entry via the Ontario Diabetes Database. Sociodemographic variables were part of the Ontario Stroke Registry and based on information abstracted from patient charts, including age, sex, race/ethnicity, income quintile, marital status, and urban/rural location. These variables were used as covariates in each of the models. The following clinical covariates were captured from the Ontario Stroke Registry or using Institute for Clinical Evaluative Sciences–derived validated algorithms from physician encounters in the ontario health insurance plan billing database or admissions from the Canadian Institute for Health Information Discharge Abstract Database: glucose on admission, atrial fibrillation, cancer, coronary artery disease, heart failure, hyperlipidemia, hypertension, renal disease, current smoking, and preadmission dependency. Current use of antihypertensive, antithrombotic, and lipid‐lowering medications was also included as confounders. Premorbid depression was a binary covariate that was accessed from the Ontario Mental Health Reporting System and the Canadian Institute for Health Information Discharge Abstract Database and defined conservatively as ≥1 hospitalization related to depressive disorders within 5 years before cohort entry. To account for stroke severity and functional status, we used the Canadian Neurological Scale score on admission and modified Rankin Scale score, respectively. Last, length of stay was an added covariate for the long‐term outcomes using groupings of 3 to 7, 8 to 14, and ≥15 days. Table S1 shows whole cohort associations between covariates and outcome measures.
Outcome Ascertainment
All‐cause mortality was the first in‐hospital outcome, compared with discharge to the community as the reference group. Discharge from hospital directly to LTC was the second in‐hospital outcome, which was also compared with discharge to the community as the reference group. Long‐term outcomes occurring after discharge included all‐cause mortality, admission for recurrent stroke/TIA, admission to LTC, and incident dementia. Admissions for recurrent stroke/TIA were captured using the Canadian Institute for Health Information Discharge Abstract Database, and incident dementia was ascertained using a previously established algorithm with a sensitivity of 79% and a specificity of 99%.16 Admission to LTC was captured via the continuous care reporting system and defined as cases with an admit date at any point during the follow‐up period, and a length of stay ≥10 days.
Statistical Analysis
Data analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC). Multinomial logistic regression was used to estimate the adjusted odds ratio (OR) of in‐hospital outcomes (ie, mortality or discharge from hospital directly to LTC) for patients with stroke with preexisting diabetes mellitus compared with those without. Among those discharged, we used Cox proportional hazards regression to estimate the adjusted hazard ratio (aHR) of all‐cause mortality, readmission for stroke/TIA, admission to LTC, and incident dementia. We opted to use a Fine and Gray competing risks framework to estimate cumulative incidence for each of the nonmortality outcomes, in which case all‐cause mortality was considered as a competing risk. This approach is suited to estimate absolute risk of an event over time, which others have noted relates more to prognosis than cause.17 To further characterize the influence of diabetes mellitus on stroke outcomes in young versus midlife adults, we conducted sensitivity analyses examining these associations in 2 prespecified age subgroups: (1) young stroke, between 18 and 49 years, and (2) midlife stroke, between 50 and 65 years.
Results
Cohort Description
A total of 8293 adults aged <65 years with acute ischemic stroke were identified for entry into the cohort and evaluated for in‐hospital outcomes (aged 53.6±8.9 years; 63.7% men, 36.3% women; Table 1). There were 2411 patients with stroke who had preexisting diabetes mellitus. To provide context, on querying the Ontario Diabetes Database, 63.3% of people diagnosed with diabetes mellitus were aged <65 years. The proportion of the cohort that was midlife was 6101 individuals (73.6%), whereas the remainder were young individuals. Marital status was available for a proportion of participants, whereas race/ethnicity details were sparse. A total of 446 (5.4%) died in hospital, leaving 7847 stroke survivors discharged from acute care and subsequently evaluated for long‐term outcomes over a median follow‐up time after discharge of 6.3 years, up to a maximum of 12 years.
Table 1.
Participant Demographics, Comorbidities, Stroke Characteristics, and Concomitant Medications for Adults With Stroke Aged <65 Years
| Variable | Overall Cohort | Diabetes Mellitus Absent | Diabetes Mellitus Present | Standardized Difference |
|---|---|---|---|---|
| (n=8293) | (n=5882) | (n=2411) | ||
| Demographics | ||||
| Age, mean±SD, y | 53.64±8.94 | 52.52±9.53 | 56.35±6.55 | 0.469 |
| Sex, % women | 36.3 | 36.5 | 36 | 0.009 |
| Ethnicity, % | ||||
| Chinese | 2.1 | 1.9 | 2.5 | 0.037 |
| South Asian | 1.8 | 1.2 | 3.2 | 0.132 |
| Other* | 96.1 | 96.8 | 94.4 | 0.121 |
| Income quintile, % | ||||
| Lowest | 25.6 | 23.9 | 30.0 | 0.138 |
| Second | 21.7 | 21.1 | 23.1 | 0.048 |
| Third | 19.2 | 19.7 | 18 | 0.042 |
| Fourth | 17.6 | 18.1 | 16.3 | 0.047 |
| Highest | 15.9 | 17.3 | 12.6 | 0.132 |
| Marital status, % | ||||
| Married | 24 | 24.4 | 23.0 | 0.032 |
| Common‐law | 2.8 | 3.1 | 2.0 | 0.037 |
| Single | 6.4 | 6.8 | 5.4 | 0.056 |
| Divorced | 2.8 | 2.8 | 2.9 | 0.008 |
| Widowed | 1.4 | 1.3 | 1.7 | 0.032 |
| Undetermined | 61.1 | 60.0 | 63.6 | 0.425 |
| Geographic location, % | ||||
| Urban | 85.6 | 85.5 | 85.9 | 0.009 |
| Rural | 14.4 | 14.5 | 14.1 | 0.009 |
| Comorbidities, % | ||||
| Hypertension | 55.0 | 45.2 | 78.8 | 0.738 |
| Dyslipidemia | 35.5 | 26.6 | 57.4 | 0.656 |
| Atrial fibrillation | 6.6 | 6.2 | 7.6 | 0.057 |
| Heart failure | 9.6 | 6.3 | 17.6 | 0.353 |
| Coronary artery disease | 15.9 | 10.8 | 28.4 | 0.453 |
| MI | 10.3 | 7.2 | 18.0 | 0.329 |
| Angina | 4.0 | 2.6 | 7.3 | 0.217 |
| PCI | 2.2 | 1.4 | 4.3 | 0.177 |
| CABG | 2.6 | 1.1 | 6.1 | 0.272 |
| Cancer | 5.2 | 5.6 | 4.1 | 0.068 |
| Renal dialysis | 2.8 | 1.3 | 6.5 | 0.269 |
| Depression | 9.9 | 9.2 | 11.6 | 0.001 |
| Preadmission disability, % | ||||
| Dependent | 10.2 | 8.2 | 15.2 | 0.219 |
| Independent | 89.8 | 91.8 | 84.8 | 0.219 |
| Glucose on admission, % | 2.4 | 0.5 | 7.1 | 0.353 |
| Smoking, % | 39.1 | 40.9 | 34.6 | 0.129 |
| Stroke characteristics | ||||
| CNS score on admission, % | ||||
| Mild | 72.0 | 71.2 | 74.0 | 0.063 |
| Moderate | 18.3 | 19.1 | 16.3 | 0.073 |
| Severe | 9.7 | 9.7 | 9.7 | 0.001 |
| Score, mean±SD | 8.54±2.79 | 8.54±2.81 | 8.55±2.73 | 0.003 |
| Length of stay, mean±SD, d | 11.08±18.89 | 10.64±19.10 | 12.16±18.31 | 0.081 |
| Modified Rankin Scale score at discharge | ||||
| Score, mean±SD | 2.51±1.54 | 2.44±1.53 | 2.68±1.56 | 0.1558 |
| Concomitant medications, % | ||||
| Lipid‐lowering agent | 76.7 | 76.6 | 77.8 | 0.028 |
| Antihypertensive | 67.9 | 60.6 | 86.4 | 0.611 |
| Antithrombotic/coagulant | 24.2 | 25.7 | 20.6 | 0.121 |
Information is tabulated separately for those with and without diabetes mellitus. We used the standardized difference for means or proportions to compare patients with and without diabetes mellitus, with clinically meaningful differences defined as an absolute value >0.20 (at least a small effect size). CABG indicates coronary artery bypass grafting; CNS, Canadian Neurological Scale; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
An algorithm was used to classify some ethnic groups (i.e., Chinese and South Asian) but not all ethnic groups (i.e., Other).
Diabetes Mellitus and In‐Hospital Outcomes
Patients with stroke and diabetes mellitus had a 46% higher likelihood of death during their hospital stay, versus being discharged to the community, compared with those without diabetes mellitus (adjusted OR, 1.46 [95% CI, 1.14–1.87]). Patients with stroke and diabetes mellitus had a 65% higher likelihood of being discharged directly to LTC after stroke, versus discharge to the community, compared with those without diabetes mellitus (adjusted OR, 1.65 [95% CI, 1.07–2.54]). Subgroup analyses indicated that these associations were driven primarily by the midlife subgroup (Table 2). The frequency of adverse in‐hospital outcomes was less common in the young stroke subgroup (ie, 3.9% of 2192 young patients with stroke died in hospital, whereas 0.5% were discharged directly to LTC).
Table 2.
Adjusted Associations Between Diabetes Mellitus and In‐Hospital and Long‐Term Stroke Outcomes
| In‐Hospital Outcomes | Total Cohort | Young Adults | Midlife Adults |
|---|---|---|---|
| Sample, N event/total | 446/8293 | 85/2192 | 361/6101 |
| All‐cause mortality | 1.46 (1.135–1.872) | 1.05 (0.486–2.274) | 1.49 (1.136–1.948) |
| Sample, N event/total | 113/7847 | 10/2107 | 103/5740 |
| Discharged to LTC | 1.65 (1.065–2.544) | 1.11 (0.126–9.812) | 1.61 (1.029–2.508) |
| Long‐term outcomes from discharge | |||
| Sample, N event/total | 1606/7847 | 228/2107 | 1378/5740 |
| Mortality from discharge | 1.68 (1.497–1.882) | 1.78 (1.250–2.546) | 1.61 (1.426–1.814) |
| Sample, N event/total | 961/7847 | 133/2107 | 828/5740 |
| Admitted to LTC | 1.57 (1.350–1.817) | 1.73 (1.085–2.760) | 1.50 (1.279–1.748) |
| Sample, N event/total | 1512/7847 | 351/2107 | 1161/5740 |
| Recurrent stroke/TIA | 1.37 (1.212–1.540) | 1.60 (1.190–2.146) | 1.32 (1.156–1.501) |
| Sample, N event/total | 485/7847 | 44/2107 | 441/5740 |
| Incident dementia | 1.44 (1.165–1.767) | 0.98 (0.415–2.295) | 1.41 (1.136–1.740) |
The in‐hospital outcomes were based on multinomial logistic regression, for which adjusted odds ratios (95% CIs) are reported. The long‐term, postdischarge outcomes were based on Cox proportional hazard regression models, for which adjusted hazard ratios (95% CIs) are reported. The corresponding events and sample sizes are provided for each outcome. LTC indicates long‐term care; and TIA, transient ischemic attack.
Diabetes Mellitus and Long‐Term Outcomes
The proportion of stroke survivors who died after discharge was 20.5%, 12.2% were admitted to LTC, and 19.3% were readmitted for stroke/TIA over a median of 6.3 years of follow‐up. In the case of readmission for stroke among those without diabetes mellitus, there were 18.0% and 14.9% for midlife and young subgroups, respectively, which is in contrast to the 24.8% and 25.2% of midlife and young stroke and diabetes mellitus. Patients with stroke and diabetes mellitus had a 68% increased hazard of all‐cause mortality (aHR, 1.68 [95% CI, 1.50–1.88]), a 37% increased hazard of recurrent stroke (aHR, 1.37 [95% CI, 0.21–1.54]), and a 57% increased hazard of admission to LTC (aHR, 1.57 [95% CI, 1.35–1.82]), compared with the discharged patients with stroke and no diabetes mellitus (Figure 2). These associations were significant in both the young and the midlife subgroups (Table 2).
Figure 2.
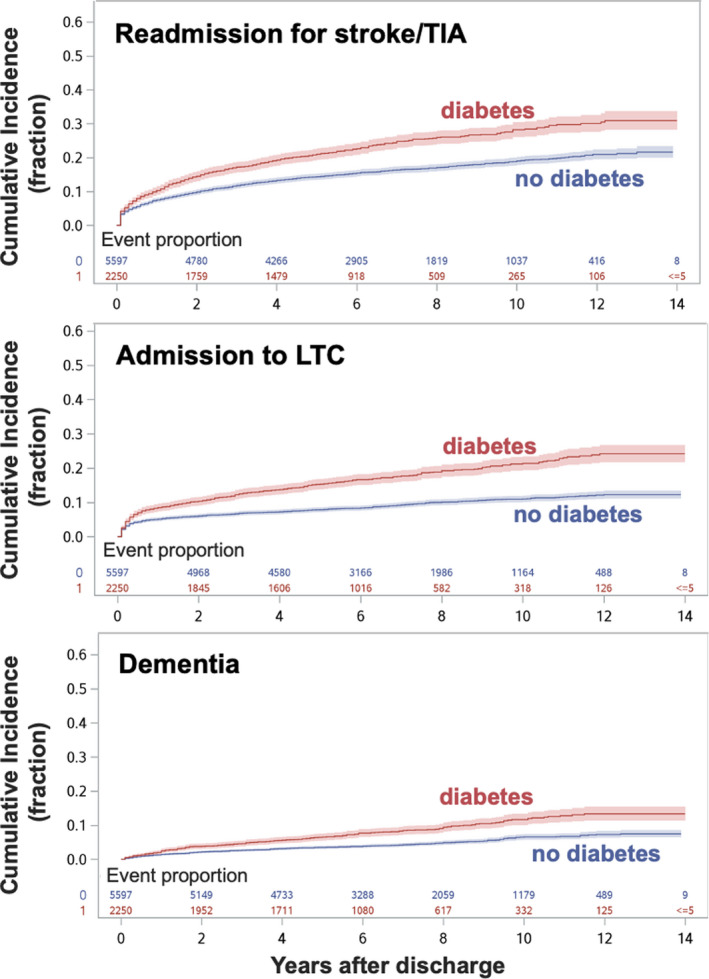
Cumulative incidence curves (fractional units) are shown as a function of time after discharge among all stroke survivors.
The panels (top to bottom) show 3 of the long‐term outcomes of interest: readmission for stroke/transient ischemic attack (TIA), admission to long‐term care (LTC), and incident dementia, respectively. Stroke and preexisting diabetes mellitus increased the risk of an event in all 3 of these outcomes compared with stroke and no diabetes mellitus. The x axis shows the longitudinal follow‐up period in years. Individuals with stroke and preexisting diabetes mellitus are represented by a red line, whereas individuals with stroke and no diabetes mellitus are represented by the blue lines. Corresponding CIs are shown as the shaded color area. Numeric data denote the proportion of people who do or not reach the event proportion at successive years after discharge.
A total of 485 (6.2%) patients were diagnosed with incident dementia after discharge and over the follow‐up period. Patients with stroke and diabetes mellitus had a 44% increased hazard of incident dementia after stroke (aHR, 1.44 [95% CI, 1.17–1.77]; Figure 2 and Table 2), compared with stroke and no diabetes mellitus. Notably, this heightened risk of dementia among those with diabetes mellitus was significant only for the midlife subgroup, of whom 441 of 5740, or 7%, were diagnosed with dementia over the follow‐up period. Additional results can be found in Data S1.
Discussion
In Ontario, patients with stroke who were aged <65 years, particularly those between 50 and 65 years, were more likely to die in hospital, and to be discharged directly to LTC from hospital, if they had preexisting diabetes mellitus. Among midlife and younger patients with stroke who were discharged alive, diabetes mellitus was associated with subsequent increased hazards of readmission for stroke/TIA, admission to LTC, incident dementia, and mortality, with the exception of dementia in the younger group. Although midlife stroke may be viewed optimistically from the perspective of survival and functional recovery, prognoses must be counterbalanced against the more complex burden of stroke in the context of comorbid disease.
In contrast to the 46% increased hazard observed herein, the association between diabetes mellitus and in‐hospital mortality has not been entirely consistent previously.8, 9, 18, 19 The association between diabetes mellitus and admission to LTC, including those aged <50 years who were initially discharged, is a novel finding that calls for action, given that these stroke survivors are among the 6.6% of LTC residents aged <65 years. The result compliments reports of heightened risks of adverse functional outcomes6 and recurrent stroke10, 11 among young and midlife stroke survivors with diabetes mellitus. Among the cardiovascular risk factors investigated in the Netherlands young stroke longitudinal follow‐up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation study, diabetes mellitus yielded the highest, albeit nonsignificant, aHR for recurrent stroke and composite vascular events (1.44) among vascular risk factors.20 Herein, we demonstrate a significant risk of recurrent stroke associated with diabetes mellitus (aHR, 1.78 for the young subgroup), possibly attributable to a larger sample size and controlling for death as a competing risk. The observed long‐term risk of death associated with diabetes mellitus is consistent with studies of working‐aged South Asians in the United Kingdom21 and young adults in Finland.11
Poststroke cognitive impairment is a major burden for stroke survivors of all ages.15, 22, 23 The 44% increased hazard of dementia in this study was predominantly driven by the midlife subgroup who were on average aged 58 years at the time of stroke. Cognitive status in the years that precede a dementia diagnosis would be highly valuable, such as screening for mild cognitive impairment; however, these data were unavailable in the current study. A previous study found that processing speed, working memory, and attention were impaired among young adults with first‐time ischemic stroke, followed up for 11 years24; however, an effect of diabetes mellitus on cognitive outcomes was not assessed. Thus, to our knowledge, this is the first study to report diabetes mellitus as a risk for dementia in working‐aged stroke survivors. Incident dementia in the current <65 years of age sample should be viewed as relatively early age of onset; for comparison, the Ontario census data only start to report dementia prevalence for adults aged >65 years.
Patients would have received a high standard of care implemented at comprehensive stroke centers across Ontario. Given the high observed long‐term risks associated with diabetes mellitus in this study, one interpretation is that the standard of care may not be sufficient to mitigate the risk of the long‐term adverse outcomes associated with diabetes mellitus, or that adherence to recommended therapies was not optimal, both of which merit further exploration. Although outside of the scope of this study, repeated assessments, diabetes mellitus control data, and adjustment for potentially time‐varying concomitant medication use after discharge would have been useful to further address the potential for confounding in the assessment of long‐term risks. Glycemic targets and glucose‐lowering medications are important future directions to better understand these stroke outcomes.25 Best practice guidelines state that glycated hemoglobin ≤7.0% is desirable for diabetes mellitus management after stroke; however, one trial on intensive treatment of hyperglycemia showed no evidence of improved functional outcomes for adults with stroke and diabetes mellitus.26 Subcutaneous injections of dulaglutide, a glucagon‐like peptide 1 receptor agonist, reduced ischemic stroke in the Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial; and yet this drug did not appear to alleviate stroke severity among those participants who had a stroke over the course of the median follow‐up of 5.4 years.27 Educational resources for stroke clinicians as well as stroke survivors with diabetes mellitus may help to address potential gaps in treating younger adults with stroke and diabetes mellitus. Strategies that foster adherence and participation in structured risk factor management among adults with stroke and diabetes mellitus are warranted.28 The pathophysiology of stroke is more complex in the face of comorbid diabetes mellitus. Integrity of the blood‐brain barrier, neuroinflammation, propensity for lacunar infarcts, and impaired cerebral autoregulation should be further investigated as mediators of poorer outcomes among adults with diabetes mellitus.29
This study benefitted from a large prospectively collected sample of consecutive cases, but some limitations must be acknowledged. Our diabetes mellitus exposure was ascertained robustly on the basis of physician billing and admissions records; however, we are unable to distinguish between type 1 and type 2 diabetes mellitus,11 which may carry different mortality risks.30 Although we accounted for glucose on admission, glycemic control and concomitant medications were unavailable to be included as time‐varying covariates to long‐term outcomes. Stroke subtypes and small‐vessel disease could not be ascertained.19, 31 Participants without stroke were not included to estimate interactive effects of stroke and diabetes mellitus. South Asian and Chinese ethnicity data were available for only a small proportion of the cohort; future research is needed to characterize associations between diabetes mellitus and stroke among different racial/ethnic groups. Despite a large sample size, we observed relatively low frequency of some outcomes (eg, 2.1% of the young patients with stroke got dementia). The follow‐up period (mean, 6.3 years) may not have been long enough to fully ascertain hazards, warranting longer observational studies, particularly in young patients with stroke.
Conclusions
Given threats to survival and independence, aggressive prevention and risk factor management strategies may be needed to address poor subacute and long‐term outcomes for younger patients with stroke with preexisting diabetes mellitus. The findings reinforce the need to identify causal clinical and neurodegenerative factors that increase the risk of poststroke dementia. More research is needed to identify whether improving diabetes mellitus care can lead to greater time living independently after stroke for those aged <65 years.
Sources of Funding
This study was funded by the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery, by the Canadian Institutes of Health Research, by the Alzheimer’s Association (United States), by Brain Canada, by Sunnybrook Health Sciences Centre Department of Psychiatry, and by the Sunnybrook Research Institute LC Campbell Cognitive Neurology Unit. This study was supported by Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information. Drs Kapal and Swartz hold Mid‐Career Awards from the Heart and Stroke Foundation of Canada. Dr Kapal holds the Lillian Love Chair in Women’s Health from the University Health Network/University of Toronto. Dr MacIntosh received an Independent Investigator award from the Brain and Behavior Research Foundation. Dr Swardfager holds the Heart and Stroke/ Richard Lewar Centre of Excellence/ Canadian Heart Failure Society/ Boehringer Ingelheim‐Lilly Cardiometabolic Young Investigator Award.
Disclosures
J. Colby‐Milley (formerly of Sunnybrook Research Institute) is now a paid employee of F. Hoffmann‐La Roche Ltd (as of July 29, 2019), but their role is unrelated to the present work. The remaining authors have no disclosures to report.
Supporting information
Data S1
Table S1
(J Am Heart Assoc. 2021;10:e019991. DOI: 10.1161/JAHA.120.019991.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019991
For Sources of Funding and Disclosures, see page 9.
References
- 1.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, et al. Stroke incidence is decreasing in whites but not in blacks: a population‐based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky stroke study. Stroke. 2010;41:1326–1331. DOI: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madsen TE, Khoury J, Alwell K, Moomaw CJ, Rademacher E, Flaherty ML, Woo D, Mackey J, De Los Rios La Rosa F, Martini S, et al. Sex‐specific stroke incidence over time in the Greater Cincinnati/Northern Kentucky Stroke Study. Neurology. 2017;89:990–996. DOI: 10.1212/WNL.0000000000004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, de Leeuw FE. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology. 2019;92:e2444–e2454. DOI: 10.1212/WNL.0000000000007533. [DOI] [PubMed] [Google Scholar]
- 4.George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol. 2017;74:695. DOI: 10.1001/jamaneurol.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekker MS, Boot EM, Singhal AB, Sin Tan K, Debette S, Tuladhar AM, de Leeuw FE. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018;17:790–801. DOI: 10.1016/S1474-4422(18)30233-3. [DOI] [PubMed] [Google Scholar]
- 6.Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, et al. Functional recovery after ischemic stroke ‐ a matter of age: data from the Austrian Stroke Unit Registry. Neurology. 2012;78:279–285. DOI: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- 7.Daniel K, Wolfe CDA, Busch MA, McKevitt C. What are the social consequences of stroke for working‐aged adults? Stroke. 2009;40:e431‐40. DOI: 10.1161/STROKEAHA.108.534487. [DOI] [PubMed] [Google Scholar]
- 8.Maaijwee NAMM, Rutten‐Jacobs LCA, Schaapsmeerders P, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, et al. Ischaemic stroke in young adults: risk factors and long‐term consequences. Nat Rev Neurol. 2014;10:315–325. DOI: 10.1038/nrneurol.2014.72. [DOI] [PubMed] [Google Scholar]
- 9.Megherbi S‐E, Milan C, Minier D, Couvreur G, Osseby G‐V, Tilling K, Di Carlo A, Inzitari D, Wolfe CDA, Moreau T, et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: data from the European BIOMED stroke project. Stroke. 2003;34:688–694. DOI: 10.1161/01.STR.0000057975.15221.40. [DOI] [PubMed] [Google Scholar]
- 10.Nedeltchev K, Der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle HP, Schroth G, Remonda L, Sturzenegger M, Fischer U, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005;76:191–195. DOI: 10.1136/jnnp.2004.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putaala J, Liebkind R, Gordin D, Thorn LM, Haapaniemi E, Forsblom C, Groop PH, Kaste M, Tatlisumak T. Diabetes mellitus and ischemic stroke in the young: clinical features and long‐term prognosis. Neurology. 2011;76:1831–1837. DOI: 10.1212/WNL.0b013e31821cccc2. [DOI] [PubMed] [Google Scholar]
- 12.Feinkohl I, Price JF, Strachan MWJ, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimer’s Res Ther. 2015;7:46. DOI: 10.1186/s13195-015-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardigan T, Ward R, Ergul A. Cerebrovascular complications of diabetes: focus on cognitive dysfunction. Clin Sci. 2016;130:1807–1822. DOI: 10.1042/CS20160397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biessels GJ, Strachan MWJ, Visseren FLJ, Jaap Kappelle L, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246–255. DOI: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 15.Swardfager W, MacIntosh BJ. Depression, type 2 diabetes, and poststroke cognitive impairment. Neurorehabil Neural Repair. 2017;31:48–55. DOI: 10.1177/1545968316656054. [DOI] [PubMed] [Google Scholar]
- 16.Jaakkimainen RL, Bronskill SE, Tierney MC, Herrmann N, Green D, Young J, Ivers N, Butt D, Widdifield J, Tu K. Identification of physician‐diagnosed Alzheimer’s disease and related dementias in population‐based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimer’s Dis. 2016;54:337–349. DOI: 10.3233/JAD-160105. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. DOI: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marini C, Totaro R, De Santis F, Ciancarelli I, Baldassarre M, Carolei A. Stroke in young adults in the community‐based L’Aquila registry: incidence and prognosis. Stroke. 2001;32:52–56. DOI: 10.1161/01.STR.32.1.52. [DOI] [PubMed] [Google Scholar]
- 19.Arboix A, Rivas A, García‐Eroles L, de Marcos L, Massons J, Oliveres M. Cerebral infarction in diabetes: clinical pattern, stroke subtypes, and predictors of in‐hospital mortality. BMC Neurol. 2005;5:9. DOI: 10.1186/1471-2377-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutten‐Jacobs LCA, Maaijwee NAM, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, van der Vlugt MJ, van Dijk EJ, de Leeuw FE. Long‐term risk of recurrent vascular events after young stroke: the FUTURE study. Ann Neurol. 2013;74:592–601. DOI: 10.1002/ana.23953. [DOI] [PubMed] [Google Scholar]
- 21.Gunarathne A, Patel JV, Potluri R, Gammon B, Jessani S, Hughes EA, Lip GYH. Increased 5‐year mortality in the migrant South Asian stroke patients with diabetes mellitus in the United Kingdom: the West Birmingham Stroke Project. Int J Clin Pract. 2008;62:197–201. DOI: 10.1111/j.1742-1241.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- 22.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre‐stroke and post‐stroke dementia: a systematic review and meta‐analysis. Lancet Neurol. 2009;8:1006–1018. DOI: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 23.Tham W, Auchus AP, Thong M, Goh ML, Chang HM, Wong MC, Chen CPLH. Progression of cognitive impairment after stroke: one year results from a longitudinal study of Singaporean stroke patients. J Neurol Sci. 2002;204:49–52. DOI: 10.1016/S0022-510X(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 24.Schaapsmeerders P, Maaijwee NA, van Dijk EJ, Rutten‐Jacobs LCA, Arntz RM, Schoonderwaldt HC, Dorresteijn LDA, Kessels RPC, de Leeuw FE. Long‐term cognitive impairment after first‐ever ischemic stroke in young adults. Stroke. 2013;44:e81. DOI: 10.1161/STROKEAHA.111.000792. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Yu X, Zheng Y, Li J, Wang Y, Lin Y, He Z, Zhao W, Chen C, Qiu K, et al. Association of glucose‐lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020;8:192–205. DOI: 10.1016/S2213-8587(19)30422-X. [DOI] [PubMed] [Google Scholar]
- 26.Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, Fansler A, Van de Bruinhorst K, Janis S, Durkalski‐Mauldin VL, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. J Am Med Assoc. 2019;322:326–335. DOI: 10.1001/jama.2019.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerstein HC, Hart R, Colhoun HM, Diaz R, Lakshmanan M, Botros FT, Probstfield J, Riddle MC, Rydén L, Atisso CM, et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. 2020;8:106–114. DOI: 10.1016/S2213-8587(19)30423-1. [DOI] [PubMed] [Google Scholar]
- 28.Marzolini S, Balitsky A, Jagroop D, Corbett D, Brooks D, Grace SL, Lawrence D, Oh PI. Factors affecting attendance at an adapted cardiac rehabilitation exercise program for individuals with mobility deficits poststroke. J Stroke Cerebrovasc Dis. 2016;25:87–94. DOI: 10.1016/j.jstrokecerebrovasdis.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Marzolini S, Robertson AD, Oh P, Goodman JM, Corbett D, Du X, MacIntosh BJ. Aerobic training and mobilization early post‐stroke: cautions and considerations. Front Neurol. 2019;10:1187. DOI: 10.3389/fneur.2019.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putaala J, Curtze S, Hiltunen S, Tolppanen H, Kaste M, Tatlisumak T. Causes of death and predictors of 5‐year mortality in young adults after first‐ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40:2698–2703. DOI: 10.1161/STROKEAHA.109.554998. [DOI] [PubMed] [Google Scholar]
- 31.Putaala J, Haapaniemi E, Kurkinen M, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts, leukoaraiosis, and long‐term prognosis in young ischemic stroke patients. Neurology. 2011;76:1742–1749. DOI: 10.1212/WNL.0b013e31821a44ad. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1


