Abstract
Background
Guidelines recommend mineralocorticoid receptor antagonist (MRA) use in patients with left ventricular ejection fraction ≤40% following a myocardial infarction plus heart failure or diabetes mellitus, based on mortality benefit in the EPHESUS (Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) trial. The objective of this study was to evaluate the real‐world utilization of MRAs for patients with ST‐segment–elevation myocardial infarction (STEMI) with left ventricular dysfunction.
Methods and Results
The prospective, population‐based, Vancouver Coastal Health Authority STEMI database was linked with local outpatient cardiology records from 2007 to 2018. EPHESUS criteria were used to define post‐STEMI MRA eligibility (left ventricular ejection fraction ≤40% plus clinical heart failure or diabetes mellitus, and no dialysis‐dependent renal dysfunction). The primary outcome was MRA prescription among eligible patients at discharge and the secondary outcome was MRA prescription within 3 months postdischarge. Of 2691 patients with STEMI, 317 (12%) were MRA eligible, and 70 (22%) eligible patients were prescribed an MRA at discharge. Among eligible patients with no MRA at discharge, 12/126 (9.5%) with documented postdischarge follow‐up were prescribed an MRA within 3 months. In multivariable analysis, left ventricular ejection fraction (odds ratio [OR], 1.55 per 5% left ventricular ejection fraction decrease; 95% CI, 1.26–1.90) and calendar year (OR, 1.23 per year, 95% CI, 1.11–1.37) were associated with MRA prescription at discharge. Other prespecified variables were not associated with MRA prescription.
Conclusions
In this contemporary STEMI cohort, only 1 in 4 MRA‐eligible patients were prescribed an MRA within 3 months following hospitalization despite high‐quality evidence for use. Novel decision‐support tools are required to optimize pharmacotherapy decisions during hospitalization and follow‐up to target this gap in post‐STEMI care.
Keywords: acute coronary syndrome, aldosterone antagonist, heart failure
Nonstandard Abbreviations and Acronyms
- ACTION‐GWTG
Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With the Guidelines
- EPHESUS
Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study
- MRA
mineralocorticoid receptor antagonist
Clinical Perspective
What Is New?
Only 1 in 4 patients with left ventricular dysfunction following ST‐segment–elevation myocardial infarction who meet EPHESUS (Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) trial criteria receive a mineralocorticoid receptor antagonist within 3 months of ST‐segment–elevation myocardial infarction discharge.
What Are the Clinical Implications?
These findings support the need for novel decision‐support tools to facilitate uptake and optimization of mineralocorticoid receptor antagonists and other evidence‐based pharmacotherapy options during ST‐segment–elevation myocardial infarction and at follow‐up appointments.
Left ventricular (LV) dysfunction, with or without overt clinical heart failure (HF), occurs in ≈1 in 4 patients hospitalized for myocardial infarction (MI), and is associated with worse clinical outcomes.1, 2 Specifically, LV dysfunction is associated with higher mortality, including early all‐cause mortality and sudden cardiac death.3 Since 2004, guidelines have recommended the use of a mineralocorticoid receptor antagonist (MRA) in eligible patients who are post‐MI, based on the inclusion criteria of the EPHESUS (Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) trial.4, 5, 6 The EPHESUS trial demonstrated that, compared with placebo, an MRA started 3 to 14 days after hospital admission for acute MI reduced mortality in patients with LV dysfunction (LV ejection fraction [LVEF] ≤40%) and either clinical HF or diabetes mellitus.7 Importantly, this benefit occurred rapidly, with a significant reduction in 30‐day mortality,8 and was greatest when started early, with a 31% relative risk reduction in mortality when eplerenone was initiated 3 to 6 days post‐MI compared with a nonsignificant 6% reduction when initiated 7 to 14 days post‐MI.9 These findings were supported by mechanistic research demonstrating that MRAs reduce early myocardial remodeling and fibrosis after MI.10, 11 Therefore, the benefits of an MRA post‐MI may be time sensitive, and delayed initiation beyond 1 week may represent a missed opportunity to improve patient outcomes.
Despite the benefits of MRAs in patients with post‐MI LV dysfunction, use of these agents in clinical practice remains highly variable.12 In the US‐based National Cardiovascular Data Registry ACTION‐GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With the Guidelines) study from 2007 to 2011, only 14.5% of MRA‐eligible patients received an MRA at discharge,1 whereas use among eligible patients was 54.8% in a Spanish study from 2006 to 2008.13 Neither study showed a trend for increased adoption of MRA use over time, although MRA uptake may have been low because of the short duration of both studies and the short interval from publication of EPHESUS to each study. Furthermore, no study has specifically evaluated the initiation of MRA after discharge among patients with MI with LV dysfunction. Although the benefit of MRAs occurs early after MI, clinicians may choose not to prescribe an MRA during admission with the aim of titrating other MI therapies and initiating an MRA on follow‐up, which would not be captured by evaluating discharge prescriptions only.
We analyzed a population‐based cohort of consecutive patients with ST‐segment–elevation MI (STEMI) with LV dysfunction from the Vancouver Coastal Health Authority STEMI database to determine the proportion of eligible patients with STEMI prescribed an MRA at discharge and within 3 months after hospitalization. Among MRA‐eligible patients, we sought to identify predictors and evaluate the contemporary frequency and temporal trends in MRA utilization.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results.
Experimental Design and Data Sources
We analyzed data from the Vancouver Coastal Health STEMI database, a prospective, population‐based study of all patients with STEMI treated at 1 of the 13 hospitals within the Vancouver Coastal Health region, which includes 2 percutaneous coronary intervention–capable quaternary‐care centers.14, 15 We linked the Vancouver Coastal Health STEMI database with local postdischarge outpatient cardiology encounters within the iClinic electronic medical record. We included all patients ≥18 years of age who were admitted for STEMI, managed with revascularization by primary percutaneous coronary intervention or fibrinolysis, and discharged from the hospital alive between June 26, 2007 and March 31, 2018. We excluded patients who had presented with out‐of‐hospital cardiac arrest, or had data missing on LVEF, diabetes mellitus status, or medications prescribed at discharge.
Outcomes
The primary outcome was the proportion of eligible patients with STEMI who were prescribed an MRA at discharge. The secondary outcome was the proportion of eligible patients with STEMI not prescribed an MRA at discharge who were subsequently prescribed an MRA within 3 months postdischarge. We defined MRA eligibility based on the EPHESUS trial inclusion criteria: LVEF ≤40% (based on the last evaluation by contrast ventriculography, echocardiography, or cardiac magnetic resonance imaging performed during the admission), plus either clinical HF (including HF on presentation or during hospitalization) or prior history of diabetes mellitus, and without dialysis‐dependent renal dysfunction.
Statistical Analysis
We reported continuous variables as means with SDs or medians with interquartile range for descriptive statistics, and categorical values as proportions using percentages (%). We performed univariate comparisons of characteristics between patients prescribed an MRA at discharge to those without MRA prescription at discharge using the Student's t test or Wilcoxon rank sum test for continuous variables and the χ2 test or Fisher exact test for categorical variables as appropriate. Additionally, we evaluated time trends in MRA prescription by comparing the proportion of MRA‐eligible patients with STEMI with MRA prescription at discharge by calendar year, and evaluated this trend using the Cochran‐Armitage test.
For the primary analysis evaluating variables associated with MRA prescription at discharge, we used multivariable logistic regression to calculate odds ratios (OR) and 95% CIs for a prespecified list of candidate variables available during the index admission. These candidate variables included calendar year, age, sex, anterior MI, hospital length‐of‐stay (as a continuous variable), LVEF (as a continuous variable), presentation to a percutaneous coronary intervention–capable hospital, calendar year, and angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker use within 24 hours of presentation.
For the secondary analysis evaluating variables associated with postdischarge MRA prescription among patients not prescribed an MRA at discharge, we used univariate analysis (χ2, Fisher exact, or Wilcoxon rank sum tests, as appropriate) for a list of candidate variables available during the follow‐up visit, including a modified congestion score16 (range 0–3 based on presence of jugular venous pressure elevation, crackles, and peripheral edema), serum creatinine, serum potassium, and LVEF on postdischarge imaging.
All analyses were performed using Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, NC). The threshold for statistical significance for all analyses was set at a 2‐sided P<0.05. One author (T.L.) had full access to all the data in the study and takes responsibility for its integrity and the data analysis. This study was approved by the Clinical Research Ethics Board of the University of British Columbia and the requirement for patient consent was waived.
Results
Cohort Characteristics
From June 26, 2007 to March 31, 2018, a total of 2691 patients with STEMI met our inclusion criteria (Figure 1). Of these, 317 (11.8%) were eligible for an MRA based on the EPHESUS trial criteria. The mean age of MRA‐eligible patients was 67 years, 22% were female, and 78% presented with an anterior MI. Mean LVEF was 33% (±7%), 48% had diabetes mellitus, and 69% had clinical HF (first documented on presentation in 19% and subsequently in the hospital in 50%) (Table 1).
Figure 1. Cohort derivation.
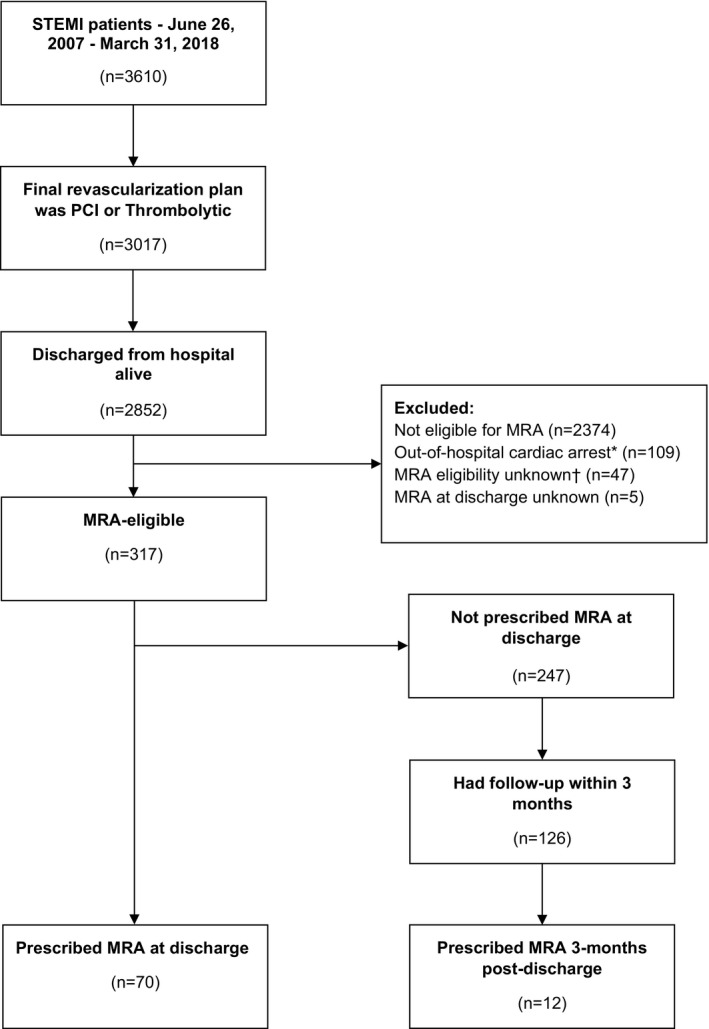
*Status systemically collected from 2012 onward. Participants with unknown status were included in the analysis. †Missing data for left ventricular ejection fraction, clinical heart failure, diabetes mellitus, and/or dialysis. MRA indicates mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Table 1.
Characteristics of MRA‐Eligible Patients With STEMI
| Characteristic |
Total MRA‐Eligible (n=317) |
No MRA at Discharge (n=247) |
MRA at Discharge (n=70) |
P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 67.4 (12.9) | 67.9 (12.9) | 65.7 (12.5) | 0.214 |
| Female patients, n (%) | 68 (21.5) | 55 (22.3) | 13 (18.6) | 0.506 |
| BMI, mean (SD) | 27.0 (5.2) | 26.8 (5.2) | 27.8 (5.0) | 0.165 |
| STEMI admission details | ||||
| Anterior infarct, n (%) | 248 (78.2) | 193 (78.1) | 55 (78.6) | 0.938 |
| HF on presentation, n (%) | 59 (18.6) | 49 (19.8) | 10 (14.3) | 0.292 |
| Admitted to PCI‐capable hospital, n (%) | 216 (68.1) | 172 (69.6) | 44 (62.9) | 0.283 |
| PCI, n (%) | 310 (97.8) | 241 (97.6) | 69 (98.6) | 0.615 |
| Fibrinolysis only, n (%) | 7 (2.2) | 6 (2.4) | 1 (1.4) | 0.615 |
| In‐hospital HF, n (%) | 207 (65.3) | 151 (61.1) | 56 (80.0) | 0.003 |
| In‐hospital cardiac arrest, n (%) | 23 (12.9) | 12 (9.3) | 11 (22.4) | 0.020 |
| Unknown, n | 139 | 118 | 21 | |
| Hospital length‐of‐stay, median (IQR), d | 4.7 (3.2–8.3) | 4.5 (3.2–8.2) | 5.4 (3.3–9.8) | 0.593 |
| ≤3 d, n (%) | 64 (20.2) | 51 (20.6) | 13 (18.6) | 0.702 |
| Cardiovascular disease and risk factors, n (%) | ||||
| Diabetes mellitus | 151 (47.6) | 127 (51.4) | 24 (34.3) | 0.011 |
| Prior HF | 18 (5.7) | 12 (4.9) | 6 (8.6) | 0.236 |
| Prior MI | 72 (22.7) | 54 (21.9) | 18 (25.7) | 0.497 |
| Prior PCI | 48 (15.1) | 32 (13.0) | 16 (22.9) | 0.041 |
| Current/recent smoker | 69 (21.8) | 54 (21.9) | 15 (21.4) | 0.938 |
| Hypertension | 211 (66.6) | 164 (66.4) | 47 (67.1) | 0.907 |
| Dyslipidemia | 157 (49.5) | 122 (49.4) | 35 (50.0) | 0.929 |
| Clinical and laboratory values | ||||
| LVEF | ||||
| Mean (SD) | 32.6 (6.6) | 33.4 (6.4) | 29.5 (6.5) | <0.001 |
| Median (IQR) | 35 (30–38) | 35 (30–40) | 30 (25–35) | |
| ≤20%, n (%) | 38 (12.0) | 24 (9.7) | 14 (20.0) | 0.019 |
| Admission heart rate, mean (SD), bpm | 87.7 (26.2) | 88.0 (26.1) | 86.7 (27.0) | 0.711 |
| Admission SBP, mean (SD), mm Hg | 137.6 (31.8) | 138.1 (30.8) | 135.8 (35.4) | 0.599 |
| Admission hemoglobin, mean (SD), g/L | 140.7 (18.6) | 140.3 (18.5) | 142.1 (18.9) | 0.475 |
| Admission creatinine, median (IQR), µmol/L | 97 (81–113) | 97 (79–114) | 97 (86–110) | 0.454 |
| Peak creatinine, median (IQR), µmol/L | 109 (92–137) | 110 (90–140) | 107.5 (96–133) | 0.948 |
| Medications within 24 h of admission, n (%) | ||||
| Aspirin | 314 (99.1) | 244 (98.8) | 70 (100.0) | 1.000 |
| P2Y12 inhibitor | 309 (97.5) | 239 (96.8) | 70 (100.0) | 0.127 |
| Enoxaparin | 105 (33.1) | 75 (30.4) | 30 (42.9) | 0.050 |
| Statin | 306 (96.5) | 238 (96.4) | 68 (97.1) | 0.751 |
| ACEI/ARB | 258 (81.4) | 205 (83.0) | 53 (75.7) | 0.167 |
| β‐blocker | 266 (83.9) | 212 (85.8) | 54 (77.1) | 0.081 |
| MRA | 8 (2.5) | 0 (0.0) | 8 (11.4) | <0.001 |
| Medications at discharge, n (%) | ||||
| Aspirin | 311 (98.1) | 243 (98.4) | 68 (97.1) | 0.617 |
| P2Y12 inhibitor | 302 (95.3) | 234 (94.7) | 68 (97.1) | 0.403 |
| Warfarin | 135 (42.6) | 115 (46.6) | 20 (28.6) | 0.007 |
| Statin | 316 (99.7) | 246 (99.6) | 70 (100.0) | 1.000 |
| ACEI/ARB | 280 (88.3) | 211 (85.4) | 69 (98.6) | 0.002 |
| β‐blocker | 311 (98.1) | 241 (97.6) | 70 (100.0) | 0.345 |
| Optimal medical therapy† | 263 (83.0) | 197 (79.8) | 66 (94.3) | 0.004 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; bpm, beats per minute; HF, heart failure; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; and STEMI, ST‐segment–elevation myocardial infarction.
Defined as use of the combination of aspirin plus P2Y12 inhibitor plus ACEI/ARB plus β‐blocker.
Prescription of MRA at Discharge
In total, 70 (22.0%) of MRA‐eligible patients received an MRA prescription at discharge. In univariate analysis, lower LVEF, lower prevalence of diabetes mellitus, HF in the hospital, prior percutaneous coronary intervention, and in‐hospital cardiac arrest were significantly associated with MRA discharge prescription (Table 1). Additionally, use of an MRA or enoxaparin within 24 hours of presentation and discharge prescription for warfarin, angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker, and optimal medical therapy were each associated with MRA prescription (Table 1). When considering MRA use based on calendar year, there was a significant trend for increased MRA prescription at discharge among MRA‐eligible patients over time (P<0.001) (Figure 2).
Figure 2. Proportion of MRA‐eligible patients with STEMI* with MRA prescription at discharge by calendar year*†.
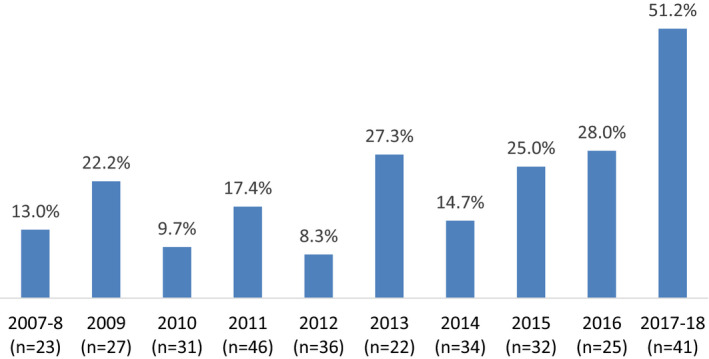
*MRA‐eligible patients with STEMI: Left ventricular ejection fraction ≤40% plus either clinical heart failure or prior history of diabetes mellitus, and without dialysis‐dependent renal dysfunction. †n denotes the number of MRA‐eligible patients with STEMI in that year. MRA indicates mineralocorticoid receptor antagonist; and STEMI, ST‐segment–elevation myocardial infarction.
In the prespecified multivariable analysis, LVEF (OR, 1.55 per 5% LVEF decrease; 95% CI, 1.26–1.90; P<0.001) and calendar year (OR, 1.23 per year increase, 95% CI, 1.11–1.37, P<0.001) were independently associated with MRA prescription (Table 2). Patients with a LVEF 31% to 40% comprised the majority of MRA‐eligible patients not receiving an MRA at discharge (Figure 3). No other candidate variable was associated with MRA prescription at discharge. In post hoc multivariable analysis adding history of diabetes mellitus and peak creatinine to the model, LVEF and calendar year remained independently associated with MRA prescription at discharge (Table S1). Similar patterns emerged in subgroup analysis of patients with diabetes mellitus (Table S2).
Table 2.
Association Between Candidate Variables and MRA Prescription at Discharge Among MRA‐Eligible Patients With STEMI
| Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | P Value | |
|---|---|---|---|
| LVEF (per 5% decrease) | 1.53 (1.26–1.87) | 1.55 (1.26–1.90) | <0.001 |
| Calendar year (per year increase) | 1.22 (1.10–1.35) | 1.23 (1.11–1.37) | <0.001 |
| Age, per 10‐y decrease | 1.14 (0.93–1.40) | 1.14 (0.90–1.44) | 0.278 |
| Male patients | 1.23 (0.63–2.39) | 1.21 (0.57–2.56) | 0.614 |
| Nonanterior location | 0.99 (0.52–1.88) | 1.06 (0.53–2.12) | 0.863 |
| Non‐PCI‐capable hospital | 1.36 (0.78–2.37) | 1.41 (0.78–2.57) | 0.256 |
| Hospital length‐of‐stay, per day increase | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) | 0.976 |
| No ACEI/ARB use within 24 h of presentation | 1.58 (0.84–2.99) | 1.38 (0.69–2.76) | 0.367 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; OR, odds ratio; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Multivariable adjustment for other covariates listed in this table.
Figure 3. Distribution of MRA‐eligible patients with STEMI by left ventricular ejection fraction and MRA prescription at discharge.
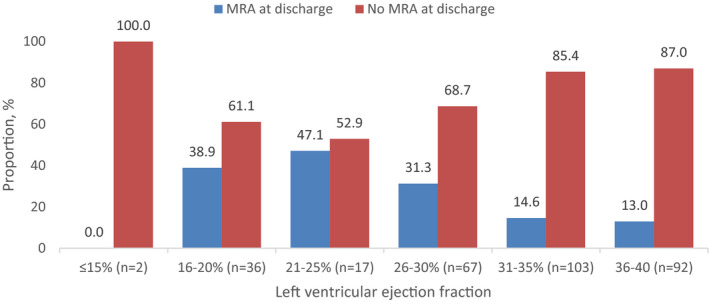
MRA indicates mineralocorticoid receptor antagonist; and STEMI, ST‐segment–elevation myocardial infarction.
Prescription of MRA Within 3 Months After Discharge
Of the remaining 247 MRA‐eligible patients with STEMI who were not prescribed an MRA at discharge, 126 (51%) had a documented cardiology follow‐up within 3 months, of whom 12 (9.5%) were prescribed an MRA. Among patients who had local cardiology follow‐up within 3 months, the median time from discharge to first cardiology follow‐up was 53 days (interquartile range, 36–70). In univariate analysis, patients who were prescribed an MRA at postdischarge follow‐up had lower LVEF on postdischarge cardiac imaging (median LVEF 32.5%) than those not prescribed an MRA postdischarge (median LVEF 40%, P=0.042; Table 3). Other candidate variables were not associated with MRA prescription postdischarge. Overall, a cumulative 82/317 (25.9%) MRA‐eligible patients were prescribed an MRA within 3 months after discharge from STEMI hospitalization.
Table 3.
Association Between MRA Prescription Within 3 Months and Selected Variables Among 126 MRA‐Eligible Patients With STEMI Not Prescribed an MRA at Discharge
| No MRA (n=114) | MRA (n=12) | P Value | |
|---|---|---|---|
| LVEF, median (IQR), % |
40.0 (35.0–49.0) n=43 |
32.5 (30.0–33.0) n=5 |
0.042 |
| Modified congestion score†, n (%) | 0.289 | ||
| 0 | 92 (80.7) | 10 (83.3) | |
| 1 | 16 (14.0) | 1 (8.3) | |
| 2 | 5 (4.4) | 0 | |
| 3 | 1 (0.9) | 1 (8.3) | |
| Serum potassium | |||
| Median (IQR), mmol/L |
4.4 (4.1–4.7) n=35 |
3.5 (–) n=1 |
0.091 |
| >5.5 mmol/L | n=0 | n=0 | … |
| Serum creatinine, median (IQR), µmol/L |
94.0 (82.0–118.0) n=39 |
99.5 (87.0–112.0) n=2 |
1.000 |
IQR indicates interquartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; and STEMI, ST‐segment–elevation myocardial infarction.
Range 0 to 3 based on presence of jugular venous pressure elevation, crackles, and peripheral edema.
Discussion
In this contemporary cohort of patients with STEMI, ≈12% had MRA‐eligible LV dysfunction according to the EPHESUS trial criteria, yet only 1 in 4 MRA‐eligible patients were prescribed an MRA within 3 months after hospitalization, despite high‐quality evidence of early mortality benefit.7 MRA use increased significantly over the 11‐year timeframe of our study, suggesting increased adherence to guideline recommendations over time. Lower LVEF was the only independent clinical predictor of MRA prescription at discharge and follow‐up among MRA‐eligible patients; no other patient‐level variables were independently associated with MRA prescription. The relatively infrequent prescription of MRA to eligible patients highlights an important gap between evidence‐based guidelines and current treatment patterns for patients with acute MI, which has lessened over time.
We extend the results of the ACTION‐GWTG data set from the United States, in which only 14.5% of MRA‐eligible patients with STEMI were prescribed an MRA at discharge, by assessing for delayed MRA initiation postdischarge.1 Our results demonstrate that MRAs are infrequently prescribed among eligible patients after STEMI discharge. This is consistent with the under‐use of MRAs among outpatients with chronic HF with reduced ejection fraction, in which only ≈1 in 3 eligible patients with HF with reduced ejection fraction receive an MRA.17, 18 Initiation of MRAs among eligible patients during their STEMI admission therefore represents an opportunity to maximize early benefits and increase the likelihood of outpatient use. Unlike ACTION‐GWTG, we found a significant improvement in MRA prescription at discharge over time, which may reflect the longer available timeframe and more recent data available within our database. Our study further confirms the observation from ACTION‐GWTG that patients meeting the EPHESUS criterion on the basis of having diabetes mellitus without overt HF are less likely to be prescribed an MRA.4, 6 In our study, patients with LV dysfunction and diabetes mellitus without clinical HF accounted for nearly one third of MRA‐eligible patients. Therefore, this subgroup of patients with diabetes mellitus and American College of Cardiology/American Heart Association stage B HF represents a target subgroup for quality improvement initiatives targeting the MRA treatment gap. Furthermore, in the present study, patients with lower LVEF were associated with a higher likelihood of being prescribed an MRA, likely reflecting prescribers' impression of the benefit–harm assessment based on patient prognosis. However, there was a mortality benefit across the LVEF range studied in the EPHESUS trial.7, 19 This highlights that clinicians caring for patients post‐MI with LV dysfunction should consider therapy even among patients with only “mildly” reduced LVEF.
Several patient factors may be perceived as barriers to early initiation of MRAs during the initial STEMI hospitalization, such as hypotension, renal dysfunction, or hyperkalemia.1, 7, 12 A recent analysis of the RALES (Randomized Aldactone Evaluation Study) and EMPHASIS‐HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) trials demonstrated a minimal effect of MRAs on blood pressure in patients with HF with reduced ejection fraction with systolic blood pressure ≤105 mm Hg at baseline, indicating that the presence of asymptomatic hypotension alone should not lead to the decision to withhold MRA therapy.20 Furthermore, while renal dysfunction and hyperkalemia are certainly important considerations before starting an MRA, they can be mitigated by ensuring early postdischarge follow‐up with repeat bloodwork. In practice, clinicians may elect to delay MRA initiation until renin‐angiotensin system blockers and β‐blockers are optimized,18 thereby missing an opportunity to add a therapy that reduces mortality during the period of highest risk.
Quality improvement efforts are required to further optimize guideline‐directed post‐STEMI therapy decisions. For example, improved strategies in prescriber education regarding the indications for MRAs in post‐MI LV dysfunction beyond its use in chronic HF with reduced ejection fraction may improve its overall appropriate utilization. Current routine practices of clinician education through institutional cardiology rounds or academic detailing programs may, however, have limited long‐term efficacy in meaningfully overcoming prescribing inertia.21, 22 Therefore, other strategies should also be explored, such as an expanded role for clinical pharmacists to initiate post‐MI therapy based upon standardized clinical criteria.23, 24 Furthermore, decision‐support tools may be developed to improve use of evidence‐based therapies before discharge. For example, automated point‐of‐care alerts in the context of integrated electronic medical records can prompt a clinician to prescribe an MRA based upon a patient's date of index STEMI, LVEF on imaging, and current list of post‐MI therapies. Similar strategies of automated evidence‐based electronic medical record alerts among patients with HF have shown increased use of optimal medical therapy in that population.25
Strengths and Limitations
The major strength of this study is the inclusion of consecutive patients with STEMI managed within a regional network of hospitals over an 11‐year timeframe, using standardized data collection tools. Nevertheless, this study should also be interpreted in the context of some limitations. First, our data set did not include documentation of loop diuretic use, in‐hospital serum potassium, or all blood pressure or serum creatinine measurements, and reasons for not prescribing an MRA may not have been documented during clinical encounters. Therefore, a subset of MRA‐eligible patients may have had a valid contraindication to therapy that we could not ascertain, though only 8.6% patients in the ACTION‐GWTG had a contraindication to an MRA.1 Second, when abstracting data into the database, the lower end of a reported LVEF range was used. This may have led to some patients with “mid‐range” LVEF (eg, 40%–45%), outside the EPHESUS eligibility criteria, to be classified as MRA eligible. Despite this, MRA use was low among all LVEF categories, particularly within patients with LVEF 31% to 35%. Finally, among patients without an MRA prescribed at discharge, postdischarge MRA use could only be assessed in the 51% who had local cardiology follow‐up documented within the iClinic electronic medical record, and patients who received follow‐up in another health region were not captured. However, we anticipate that MRA use would be similar or lower than the group with close cardiology follow‐up.
Conclusions
In our contemporary regional STEMI cohort, only 1 in 4 MRA‐eligible patients were prescribed an MRA within 3 months following hospitalization, despite high‐quality evidence that early use reduces mortality. Aside from lower LVEF and calendar year, MRA utilization was not associated with any patient‐ or institutional‐level characteristics, suggesting unexplained variability in clinical practice. Novel decision‐support tools are required to optimize guideline‐directed therapy decisions during hospitalization and follow‐up to target this gap in post‐STEMI care.
Sources of Funding
This study was funded by the Vancouver Coastal Health Research Institute.
Disclosures
Dr Cairns has received research grant support from Medtronic, Sanofi Aventis, AstraZeneca, Bayer, Boston Scientific, and Edwards Laboratories; has received speaker honoraria from Pfizer/BMS, Bayer, and Servier; has received consulting fees from St Jude Medical, Bayer, BMS, and Servier; DSMBs: ARTESiA (apixaban; CIHR, BMS), COMPASS (rivaroxaban; Bayer); and ATLASICD (CIHR, Boston Scientific), OCEAN (rivaroxaban; CIHR, Bayer, Biotronik). Dr Fordyce serves on advisory boards for Bayer, Novo Nordisk, Boehringer Ingelheim, and Sanofi. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
(J Am Heart Assoc. 2021;10:e019167. DOI: 10.1161/JAHA.120.019167.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019167
For Sources of Funding and Disclosures, see page 9.
See Editorial by Schupp et al.
References
- 1.Rao KK, Enriquez JR, De Lemos JA, Alexander KP, Chen AY, McGuire DK, Fonarow GC, Das SR. Use of aldosterone antagonists at discharge after myocardial infarction: results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry‐Get with the Guidelines (GWTG). Am Heart J. 2013;166:709–715. DOI: 10.1016/j.ahj.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Sutton NR, Li S, Thomas L, Wang TY, de Lemos JA, Enriquez JR, Shah RU, Fonarow GC. The association of left ventricular ejection fraction with clinical outcomes after myocardial infarction: findings from the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry‐Get With the Guidelines (GWTG) Medicare‐linked database. Am Heart J. 2016;178:65–73. DOI: 10.1016/j.ahj.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Miller AL, Dib C, Li L, Chen AY, Amsterdam E, Funk M, Saucedo JF, Wang TY. Left ventricular ejection fraction assessment among patients with acute myocardial infarction and its association with hospital quality of care and evidence‐based therapy use. Circ Cardiovasc Qual Outcomes. 2012;662–671. DOI: 10.1161/CIRCOUTCOMES.112.965012. [DOI] [PubMed] [Google Scholar]
- 4.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. DOI: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, et al. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). Circulation. 2004;110:588–636. DOI: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 6.Jeger R, Ben LH, Sujayeva V, Dizdarevic‐Hudic L, Kastrati A, Terkelsen PJ, Bertelli L, Simpson IA, Gilard M, Karamfiloff K, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2017;39:119–177. DOI: 10.1093/eurheartj/ehx393. [DOI] [Google Scholar]
- 7.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. DOI: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, van Veldhuisen DJ, Zannad F, Krum H, Mukherjee R, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46:425–431. DOI: 10.1016/j.jacc.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Adamopoulos C, Ahmed A, Fay R, Angioi M, Filippatos G, Vincent J, Pitt B, Zannad F; EPHESUS Investigators . Timing of eplerenone initiation and outcomes in patients with heart failure after acute myocardial infarction complicated by left ventricular systolic dysfunction: insights from the EPHESUS trial. Eur J Heart Fail. 2009;11:1099–1105. DOI: 10.1093/eurjhf/hfp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi M, Tsutamoto T, Wada A, Tsutsui T, Ishii C, Ohno K, Fujii M, Taniguchi A, Hamatani T, Nozato Y, et al. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post‐infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation. 2003;107:2559–2565. DOI: 10.1161/01.CIR.0000068340.96506.0F. [DOI] [PubMed] [Google Scholar]
- 11.Brandimarte F, Blair JEA, Manuchehry A, Fedele F, Gheorghiade M. Aldosterone receptor blockade in patients with left ventricular systolic dysfunction following acute myocardial infarction. Cardiol Clin. 2008;26:91–105. DOI: 10.1016/j.ccl.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Rassi AN, Cavender MA, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, Peacock WF, Laskey WK, Rosas SE, Zhao X, et al. Temporal trends and predictors in the use of aldosterone antagonists post‐acute myocardial infarction. J Am Coll Cardiol. 2013;61:35–40. DOI: 10.1016/j.jacc.2012.08.1019. [DOI] [PubMed] [Google Scholar]
- 13.López‐de‐Sá E, Martínez A, Anguita M, Dobarro D, Jiménez‐Navarro M. Aldosterone receptor antagonist use after myocardial infarction. data from the REICIAM registry. Article in Spanish. Rev Esp Cardiol. 2011;64:981–987. DOI: 10.1016/j.recesp.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Fordyce CB, Cairns JA, Singer J, Lee T, Park JE, Vandegriend RA, Perry M, Largy W, Gao M, Ramanathan K, et al. Evolution and impact of a regional reperfusion system for ST‐elevation myocardial infarction. Can J Cardiol. 2016;32:1222–1230. DOI: 10.1016/j.cjca.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Moghaddam N, Wong GC, Cairns JA, Goodman SG, Perry‐Arnesen M, Tocher W, Mackay M, Singer J, Lee T, Rao SV, et al. Association of anemia with outcomes among ST‐segment‐elevation myocardial infarction patients receiving primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2018;11:e007175. DOI: 10.1161/CIRCINTERVENTIONS.118.007175. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–843. DOI: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 17.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018;72:351–366. DOI: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 18.Savarese G, Carrero J‐J, Pitt B, Anker SD, Rosano GMC, Dahlström U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail. 2018;20:1326–1334. DOI: 10.1002/ejhf.1182. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira JP, Rossello X, Pitt B, Rossignol P, Zannad F. Eplerenone in patients with myocardial infarction and “mid‐range” ejection fraction: an analysis from the EPHESUS trial. Clin Cardiol. 2019;42:1106–1112. DOI: 10.1002/clc.23261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serenelli M, Jackson A, Dewan P, Jhund PS, Petrie MC, Rossignol P, Campo G, Pitt B, Zannad F, Ferreira JP, et al. Mineralocorticoid receptor antagonists, blood pressure, and outcomes in heart failure with reduced ejection fraction. JACC Heart Fail. 2020;8:188–198. DOI: 10.1016/j.jchf.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyrkorn R, Langaas HC, Giverhaug T, Espnes KA, Rowett D, Spigset O. Academic detailing as a method of continuing medical education. Adv Med Educ Pract. 2019;10:717–725. DOI: 10.2147/AMEP.S206073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agee N, Komenaka IK, Drachman D, Bouton ME, Caruso DM, Foster KN. The effectiveness of grand rounds lectures in a community‐based teaching hospital. J Surg Educ. 2009;66:361–366. DOI: 10.1016/j.jsurg.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Tsuyuki RT, Al Hamarneh YN, Jones CA, Hemmelgarn BR. The effectiveness of pharmacist interventions on cardiovascular risk. J Am Coll Cardiol. 2016;67:2846–2854. DOI: 10.1016/j.jacc.2016.03.528. [DOI] [PubMed] [Google Scholar]
- 24.Bhat S, Kansal M, Kondos GT, Groo V. Outcomes of a pharmacist‐managed heart failure medication titration assistance clinic. Ann Pharmacother. 2018;52:724–732. DOI: 10.1177/1060028018760568. [DOI] [PubMed] [Google Scholar]
- 25.Kao DP, Trinkley KE, Lin C‐T. Heart failure management innovation enabled by electronic health records. JACC Heart Fail. 2020;8:223–233. DOI: 10.1016/j.jchf.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


