Abstract
Background
As transcatheter aortic valve replacement (TAVR) technology expands to healthy and lower‐risk populations, the burden and predictors of procedure‐related complications including the need for permanent pacemaker (PPM) implantation needs to be identified.
Methods and Results
Digital databases were systematically searched to identify studies reporting the incidence of PPM implantation after TAVR. A random‐ and fixed‐effects model was used to calculate unadjusted odds ratios (OR) for all predictors. A total of 78 studies, recruiting 31 261 patients were included in the final analysis. Overall, 6212 patients required a PPM, with a mean of 18.9% PPM per study and net rate ranging from 0.16% to 51%. The pooled estimates on a random‐effects model indicated significantly higher odds of post‐TAVR PPM implantation for men (OR, 1.16; 95% CI, 1.04–1.28); for patients with baseline mobitz type‐1 second‐degree atrioventricular block (OR, 3.13; 95% CI, 1.64–5.93), left anterior hemiblock (OR, 1.43; 95% CI, 1.09–1.86), bifascicular block (OR, 2.59; 95% CI, 1.52–4.42), right bundle‐branch block (OR, 2.48; 95% CI, 2.17–2.83), and for periprocedural atriorventricular block (OR, 4.17; 95% CI, 2.69–6.46). The mechanically expandable valves had 1.44 (95% CI, 1.18–1.76), while self‐expandable valves had 1.93 (95% CI, 1.42–2.63) fold higher odds of PPM requirement compared with self‐expandable and balloon‐expandable valves, respectively.
Conclusions
Male sex, baseline atrioventricular conduction delays, intraprocedural atrioventricular block, and use of mechanically expandable and self‐expanding prosthesis served as positive predictors of PPM implantation in patients undergoing TAVR.
Keywords: aortic disease, aortic valve, aortic valve implantation, aortic valve stenosis, atrioventricular block, pacemaker, transcathether aortic valve replacement
Subject Categories: Pacemaker, Aortic Valve Replacement/Transcather Aortic Valve Implantation
Nonstandard Abbreviations and Acronyms
- AR
aortic regurgitation
- CVA
cerebrovascular accident
- FU
follow up
- HB
heart block
- MACCE
major adverse cardiac or cerebrovascular event
- MCRS
Medtronic Corevalve Revealing System
- MR
mitral regurgitation
- NOP LBBB
new onset persistent left bundle‐branch block
- OCS
Observational Cohort Study
- PPM
permanent pacemaker
- RCT
randomized controlled trial
- SAVR
surgical atrial valve replacement
- SEV
self‐expandable valve
- TAVR
transcatheter aortic valve replacement
- VIV
valve‐in‐valve
Clinical Perspective
What Is New?
This meta‐analysis comprising 78 studies (31261 patients) provides a comprehensive analysis of the predictors of pacemaker implantation in patients undergoing transcathether aortic valve replacement.
Male sex, baseline atrioventricular conduction delays, and the use of mechanically expandable and self‐expanding prosthesis are associated with a higher need for permanent pacemakers after transcatheter aortic valve replacement.
What Are the Clinical Implications?
Timely identification of these high‐risk patients can alleviate the risk of periprocedural atrioventricular block and associated complications such as syncope and sudden cardiac death.
As the rheumatic etiology of aortic stenosis (AS) has significantly waned over time, age‐related AS remains the most common valvular disease in the developed world.1 Valve replacement is the only definite and effective treatment to improve survival in these patients, however, a multitude of coexisting comorbidities, including but not limited to chronic cardiac or pulmonary diseases, operative risks, extremes of age and poor physical health serve as barriers to surgical aortic valve replacement (SAVR). Transcatheter aortic valve replacement (TAVR) has recently emerged as a reasonable alternative to rescue these high‐risk patients.2 The first TAVR was performed in 2002, in France, on a 57‐year‐old man in whom SAVR was contraindicated due to multiple comorbidities.3 Almost 20 years later, the data indicates that not only is it superior to medical therapy in patients with severe AS, but is also non‐inferior to SAVR, even in low‐risk patients.4, 5, 6
However, like any other therapeutic intervention, the advent of TAVR has presented its own set of challenges urging the need for a favorable risk‐benefit estimation. With the widespread availability and expanded indication of TAVR to a lower‐risk healthy population, there are concerns about the rising trend of procedural complications associated with TAVR. A frequent issue encountered with this procedure is conduction defects requiring permanent pacemaker (PPM) implantation.7, 8 The bundle of His and the bundle branches run in the vicinity of where the prosthesis is being placed. These conduction abnormalities arise primarily due to the proximity of the aortic annulus to the atrioventricular conduction system that gets manipulated during the procedure.7 Data suggests that the prevalence of conduction defects post‐procedure also depends upon the type of valve implanted during the TAVR procedure.8 The 2 most common prostheses used are balloon‐expandable Edwards Sapien Valve (ESV) and self‐expanding Medtronic Corevalve Revealing System (MCRS) with a 5%–12% incidence of PPM implantation post‐procedure in the former and 24%–33% in the latter.9 Due to the manipulation of the old valve, aortic annulus dilatation and subsequent implantation of a prosthetic valve, conduction defects are common. In our study, we intend to identify various cardiac and non‐cardiac predictors that lead to PPM implantation following TAVR. We also aim to gauge the risk of conduction abnormalities based on the type of prosthesis and access site used in TAVR.
Methods
Data was obtained from published articles on the topic. All data can be obtained from the references mentioned in the supplementary file. The consolidated extracted data is available on demand.
Search Strategy
PubMed, Embase, Ovid, and Cochrane databases were queried with various combinations of keywords and medical subject headings (MeSH) to identify studies of interest. There were no time filters or language restrictions placed. Backward snowballing by screening the references of relevant articles were also performed to retrieve unidentified articles that were missed on the primary search. The MeSH used included 2 subsets: one for TAVR using the keywords “percutaneous prosthetic valve,” “transcatheter aortic valve replacement,” “TAVR,” “transcatheter aortic valve implantation,” “TAVI,” “percutaneous approach,” “minimal invasive aortic valve replacement,” “transapical aortic valve replacement,” and the other for PPM and heart block including “LAFB,” “LPFB,” “LBBB,” “pacemaker implantation,” “heart block,” “conduction abnormalities,” and “conduction delays.” The 2 subsets of MeSH were systematically combined using Boolean operators. The final results from all possible combinations were downloaded into an EndNote library. All randomized control trials (RCT) and observational cohort studies (OCS) until April 2021, were screened for relevance. Any OCS or RCT that assessed the post‐TAVR rate of atrioventricular conduction or cardiac rhythm abnormalities and subsequent PPM implantation during the same hospitalization or within 30‐days of TAVR procedure were included. To avoid the inclusion of duplicate data, we only selected the most contemporary data when overlapping study populations (according to the period of recruitment and participating institutions) were reported; however, we cautiously included all patients reporting different predictors from studies of overlapping populations. To measure the impact of the procedure on PPM implantation, all patients with prophylactic implantation of PPM before the TAVR procedure were excluded from the analysis.
Data Extraction
Raw data about the events of PPM implantation in different predictor comparison groups were extracted for analysis by the first 9 authors independently. Detailed study‐ and patient‐level baseline characteristics including the type of study design; recruitment period, region, and follow‐up duration; sample size, number of post‐TAVR PPM implantations, sex, age, procedural risk assessment (by logistic EuroSCORE [European System for Cardiac Operative Risk Evaluation] or STS‐PROM [Society of Thoracic Surgeons Predicted Risk of Mortality] score), and baseline comorbidities were abstracted. Additionally, data related to the access site (transfemoral versus trans subclavian, transapical versus transvascular), type of prosthesis (MCRS versus ESV versus LOTUS), inclusion criteria, and definition of outcomes were obtained from individual studies (Table S1). Finally, the post‐TAVR indications for PPM implantation in each article were also extracted. Based on previous reviews, the following proposed potential predictors were selected: age, sex, baseline conduction abnormalities, anatomical features, access route, and valve types. Case reports, review articles, conference papers, and articles with insufficient data or no control arms were excluded. Patients with prior PPM implantation unrelated to TAVR were also excluded from our analysis. All data was validated by the corresponding author; in case of missing data authors of the original article were contacted. The detailed search map is given in Data S1.
Statistical Analysis
The statistical analysis was performed using the DerSimonian and Laird (DL) and Mantel Haenszel (MH) methods on random‐ and fixed‐effects models, respectively. The unadjusted odds ratio (OR) for dichotomous outcomes of RCTs and OCS were calculated. The “test for overall effect” was reported as a z value corroborating the inference from the 95% confidence interval. To avoid the influence of study design on pooled estimates, a stratified analysis based on the type of study (OCS versus RCT) was performed. A subgroup analysis based on the type of implanted valve (mechanically expandable versus self‐expanding versus balloon‐expandable), access route (transfemoral versus trans subclavian), and procedure type (transapical versus transvascular) was also performed. Sensitivity analysis after exclusion of small studies with fewer than 200 patients was done to determine the impact of sample size on pooled estimates. Descriptive characteristics for continuous data were reported as mean and SD, whereas categorical variables were presented as frequencies and percentages. Higgins I‐squared (I2) statistical model was used to determine heterogeneity in outcomes of the included studies. The observed heterogeneity was regarded statistically significant if the I2 statistics P value was <0.05. Publication bias was illustrated graphically using a funnel plot. The methodological quality assessment of the included RCTs was performed using the risk of bias‐2 (RoB‐2) tool and the Oxford quality scoring system (Jadad score). The Newcastle‐Ottawa Scale was used for assessing non‐randomized studies. The probability value of two‐sided P<0.05 was considered statistically significant. All statistical analysis was performed using the Cochrane Review Manager (RevMan) version 5.3 and STATA software (version 16.0, STATA Corp., College Station, Texas).
Quality of the Included Studies
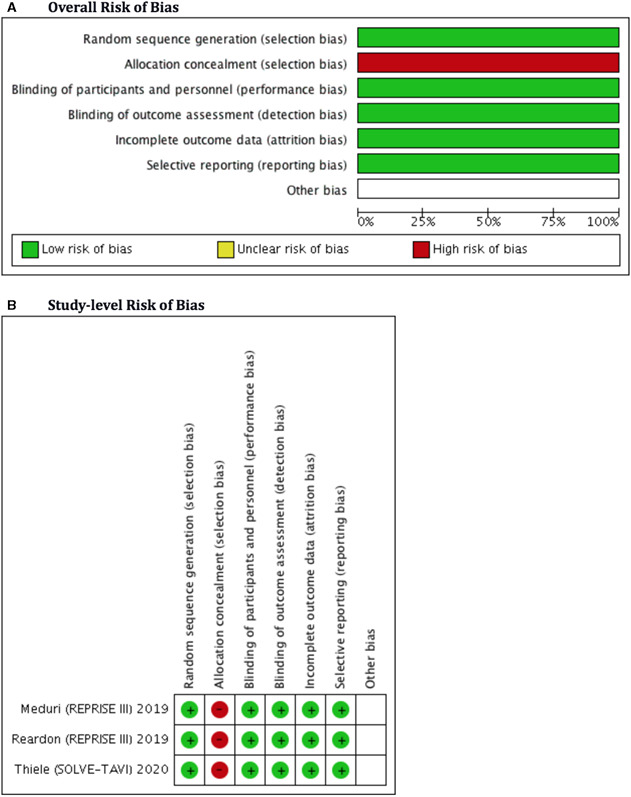
The overall quality of the included studies was high. The risk of bias‐2 (RoB‐2) tool used 5 different bias assessments: selection, detection, performance, attrition, and reporting. All 3 of the included RCTs in our meta‐analysis were open‐label, posing some theoretical risk to “allocation concealment,” however, the overall risk of selection bias was reduced due to adequate randomization. Because most RCTs used an “intention to treat model” or had a lower loss at follow‐up, the risk of attrition bias was minimal. Similarly, the risk of reporting, detection and performance bias was lower due to appropriate reporting and adequate blinding of outcome assessors, respectively. The RoB‐2 plots are given in Figure 1. 10, 11, 12 The methodological quality of included RCTs was also high on the Jadad scale with a score >3 (Table S2). Observational studies were mostly matched in terms of clinical profile and demographics to curtail selection bias. The Newcastle‐Ottawa Scale for assessing nonrandomized studies indicated the inclusion of high‐quality observational studies (score >7) (Table S3).
Figure 1. Overall (A) and study‐level (B) methodological bias assessment of the included randomized clinical trials with the Cochrane risk of bias tool‐2.
Results
Search Results
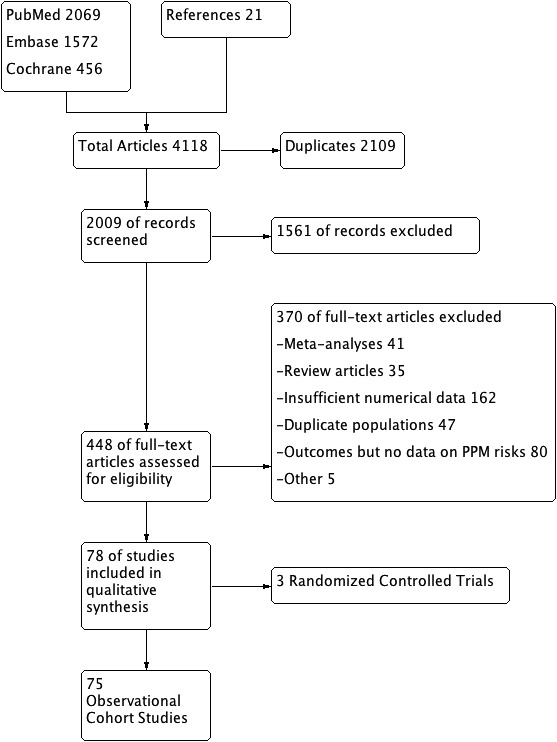
The initial search revealed 4118 articles. After the removal of irrelevant (1561) and duplicate (2109) items, 448 studies were selected for full‐text review. Of these, 370 articles were excluded based on different reasons including: review articles (35), meta‐analyses (41), insufficient data for analysis (162), duplicate population studies (47), no risk factors data (80), and other reasons (5). A total of 78 articles (3 RCTs, 75 observational studies) qualified for quantitative analysis. The Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) flow diagram is shown in Figure 2 and the PRISMA checklist is given in Data S2.
Figure 2. PRISMA flow diagram showing the included studies.
Study Characteristics
A total of 31 261 patients undergoing TAVR from 78 studies were included, of these 6212 (19.8%) received PPM, while 25 049 (80.2%) did not require a PPM.7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 Most of the studies were from the United States and Europe. Two of the RCTs were multi‐continental, recruiting patients from the US, Australia, Germany, and Brazil. All included studies were published between 2009 and 2020 with an average recruitment period of approximately 4 years. The mean age of the included population was 81±8 years, comprising on average 46% male patients. The proportion of PPM implantation across different baseline comorbidities was comparable between the 2 groups. The detailed baseline characteristics are given in Tables S4 and S5, while the procedure characteristics of TAVR are given in Table S6. The summary is illustrated in Figure 1. The overall study‐level rate of post‐TAVR PPM ranged from 0.16% to 51.1%. The need for PPM implantation across different baseline comorbidities was variable as shown in Table S7 and Figure 3. The etiology for PPM implantation was only mentioned in 19.9% of patients (n=1238/6212). Post‐TAVR complete atrioventricular block was the most commonly observed indication for PPM implantation; other causes included bradycardia, new‐onset left bundle‐branch block (LBBB), and trifascicular block (Table). Patients with a prior history of PPM before the index TAVR procedure were mostly excluded from the analysis of their respective study. Two studies (De‐Carlo and Hamandi et al) had prophylactic PPM implantation before the TAVR procedure in 158 patients; these patients were excluded from the analysis. Most PPM implantations were performed during the same hospitalization or within 30‐days of the TAVR procedure. Most studies employed a transfemoral approach for TAVR, while 31 studies used transapical access in about 32% of its population. Mechanical (LOTUS) self‐expanding (MCRS and Evolut R) and balloon‐expandable (ESV) aortic prosthesis were the major valves used in the included studies. MCRS was used in 55, while ESV and Lotus were used in 46 and 12 studies, respectively. The mean log EuroSCORE for patients among the included studies was around 18.9±10 and the mean Society of Thoracic Surgeons score was found to be 5.85. The overall follow‐up duration ranged from 2 to 36 months, with a mean follow‐up of 8.02 months (Tables S4 through S6).
Figure 3. Percentages of patients with (A) different comorbidities and (B) those with and without permanent pacemaker (PPM) implantation across different baseline comorbidities.
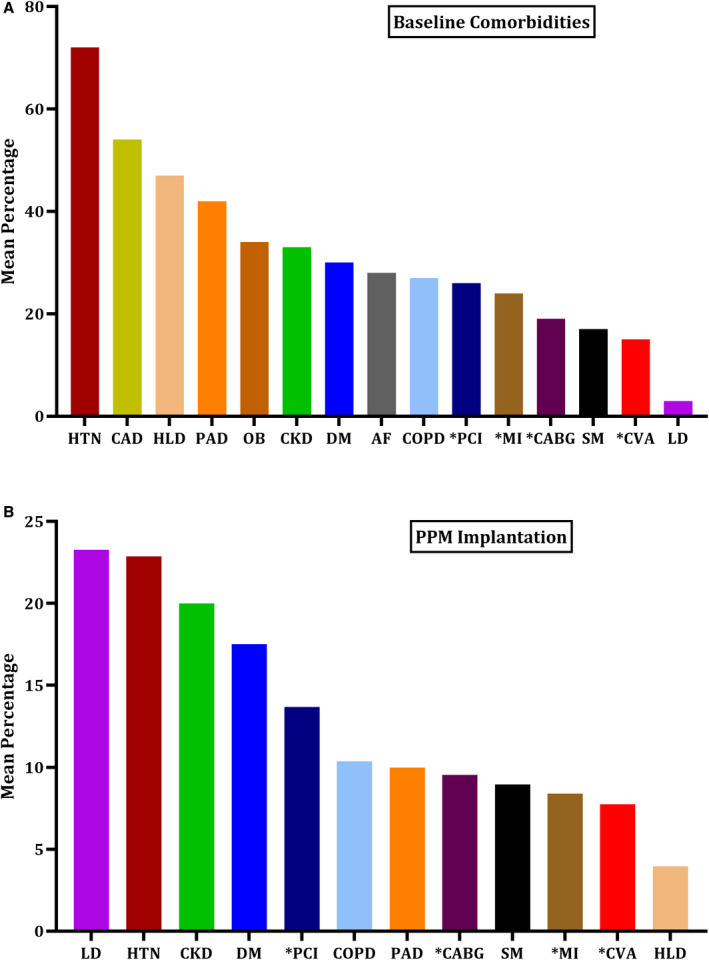
A, Mean percentage of comorbidities. B, Proportion of comorbidities in PPM vs no‐PPM groups. ACS indicates acute coronary syndrome; AF; atrial fibrillation; OB, obesity; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accidents; DM, diabetes; HLD, hyperlipidemia; HTN, hypertension; LD, Liver Disease; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; SM, smoking. Asterisk denotes “prior history of”.
Table 1.
Periprocedural Causes of PPM Implantation in Patients Undergoing TAVR for Severe Aortic Stenosis
| Periprocedural Events Leading to PPM in TAVR | No. of Patients | Percentage in the Known Causes |
|---|---|---|
| Third degree heart block | 941 | 76% |
| LBBB | 106 | 8.5% |
| Bradycardia | 60 | 4.84% |
| Second degree AV block | 45 | 3.63% |
| Second degree atrioventricular block associated with LBBB | 36 | 2.9% |
| First degree atrioventricular block | 35 | 2.82% |
| Tachy‐Brady syndrome | 34 | 2.58% |
| Symptomatic pause | 5 | 0.40% |
| Sick sinus syndrome | 9 | 0.72% |
| Alternating RBBB and LBBB | 4 | 0.32% |
| Afib with slow response | 4 | 0.32% |
| Afib with complete atrioventricular block | 4 | 0.32% |
| Total | 1238 | 100% |
All percentages are calculated among the known causes (1238). The reason for PPM implantation was not reported in 4924 cases. Afib indicates atrial fibrillation; LBBB, left bundle‐branch block; PPM, permanent pacemaker; RBBB, right bundle‐branch block; TAVR, transcatheter aortic valve replacement.
Pooled Analysis of Overall Studies
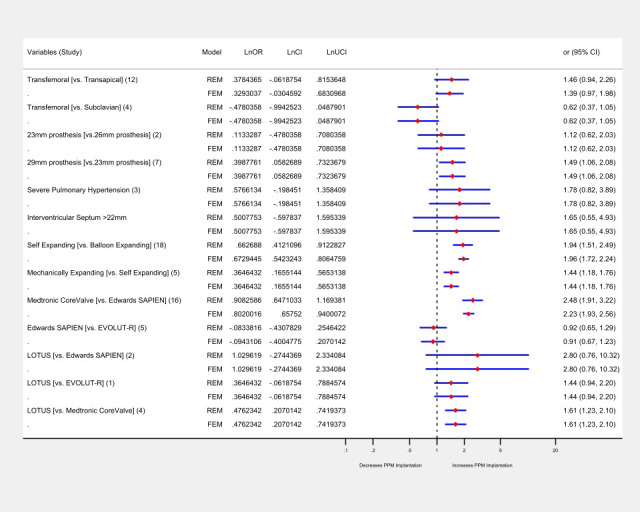
Twenty‐nine different potential predictors for the PPM implantation were evaluated. The number of patients having post‐TAVR PPM implantation (n=6212) from all studies contributed to the pooled OR calculation for each predictor. On a random effects model of binary data, the aggregate odds for post‐TAVR PPM implantation irrespective of the type of valve was higher in the male population compared with the female patients (OR, 1.16; 95% CI, 1.04–1.28). The baseline electrocardiographic conduction abnormalities, mobitz type‐1 second‐degree heart block (OR, 3.13; 95% CI, 1.64–5.93), mobitz type‐2 second‐degree heart block (OR, 3.89; 95% CI, 2.54–5.95), left anterior fascicular hemiblock (LAFB; OR, 1.43; 95% CI, 1.09–1.86), bifascicular block (OR, 2.59; 95% CI, 1.52–4.42), right bundle‐branch block (RBBB; OR, 2.48; 95% CI, 2.17–2.83), and intraprocedural atrioventricular block (OR, 4.17; 95% CI, 2.69–6.46) were associated with significantly higher odds of PPM implantation. The baseline predictor variables that were not statistically significantly associated with PPM implantation were age (OR, 1.19; 95% CI, 0.95–1.49), first‐degree heart block (OR, 1.09; 95% CI, 0.05–2.37), atrial fibrillation (AF; OR, 1.05; 95% CI, 0.93–1.20), left posterior fascicular hemiblock (LPFB; OR, 3.34; 95% CI, 1.1–11.13), left bundle branch block (LBBB; OR, 1.06; 95% CI, 0.87–1.29), severe pulmonary hypertension (OR, 1.78; 95% CI, 0.82–3.89), moderate/severe mitral regurgitation (MR; OR, 3.3; 95% CI, 0.59–18.32), unspecified heart failure; OR, 1.06; 95% CI, 0.72–1.55), and heart failure with preserved ejection fraction (OR, 1.01; 95% CI, 0.51–2.01). Of note, patients receiving 29 mm of prosthesis had significantly higher odds of PPM implantation compared with 23 mm prosthesis (OR, 1.49; 95% CI, 1.06–2.08). However, there appeared to be a statistically nonsignificant difference in the odds of PPM implantation between 23 mm versus 26 mm prosthesis (OR, 1.12; 95% CI, 0.62–2.03) and for patients with intraventricular septum size >11 mm (OR, 1.71; 95% CI, 0.17–17.41) and >22 mm (OR, 1.65; 95% CI, 0.55–4.93). The detailed valvular and anatomical variant estimates for PPM need are given Table S8.
Analysis of all predictors on a fixed‐effects model mirrored the findings of the random‐effects model with 2 exceptions; first‐degree heart block (OR, 0.35; 95% CI, 0.30–0.40) was found to be associated with a significantly lower risk, while LBBB (OR, 1.29; 95% CI, 1.14–1.46) had significantly higher odds of need for PPM. The detailed forest plots for both random and fixed effects are given in Figures S1 through S16. The heterogeneity in the outcomes of these studies was I2=0%, except for the studies comparing the RBBB and male populations, which showed significant heterogeneity (I2=52% and I2=74%, both P=<0.05), respectively (Figure 4). There was no significant difference in the odds of mortality in patients receiving PPM compared with those who did not receive PPM at 30 days and 1 year in 12 studies that included survival data (Figure 5).
Figure 4. Forest plot showing pooled estimates of demographic and electrocardiographic factors as potential predictors of permanent pacemaker (PPM) implantation in patients undergoing TAVR using random effects model (REM) and fixed effects model (FEM).
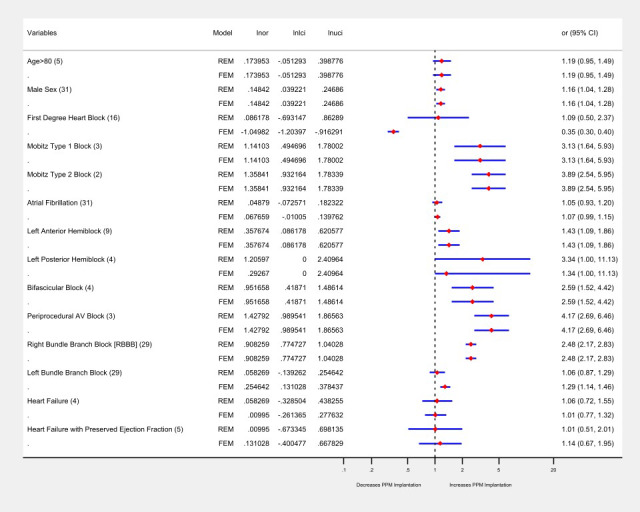
The dotted black line indicates null line (odds ratio of 1), to the right of null line indicates increased odds of PPM implantation. For each predictor the number of studies is in the parenthesis, the blue line indicates confidence interval, and the diamond red box signifies the point estimate. Lnor indicates natural log of odds ratio; lnlci, natural log of lower confidence interval; lnuci, natural log of uppper confidence interval.
Figure 5. Forest plot showing pooled estimates of procedural factors as potential predictors of permanent pacemaker (PPM) implantation in patients undergoing transcatheter aortic valve replacement (TAVR) using random effects model (REM) and fixed effects model (FEM).
On pooled analysis of continuous data, membranous septal length (MSL) was inversely, while the depth of prosthesis was directly, associated with the risk of PPM implantation. The mean MSL was 5.6 mm for patients requiring PPM implantation compared with 6.8 mm for those who did not require PPM, while the mean depth for prosthesis implantation for the former group was 6.86 mm compared with 5.34 mm in patients who did not require PPM (Figures S17 and S18).
Subgroup and Sensitivity Analyses
Overall, a head‐to‐head comparison based on the type of prosthesis favored the balloon‐expandable valves irrespective of the prevalence of different predictors. On a random‐effects model, the mechanically expandable valve (OR, 1.44; 95% CI, 1.18–1.76) and self‐expanding valves (OR, 1.93; 95% CI, 1.42–2.63) had higher PPM requirements compared with the self‐expanding and balloon‐expandable valves, respectively. Based on a breakdown data of 16 studies, MCRS implantation was associated with significantly higher odds of PPM implantation compared with ESV (OR, 2.48; 95% CI, 1.91–3.22). By contrast, the LOTUS valve implantation was associated with higher odds (OR, 1.61; 95% CI, 1.23–2.1) of PPM implantation compared with MCRS. Compared with EVOLUT‐R, the risk of PPM implantation was not significantly different in LOTUS and ESV (Table S9). There was no significant difference in the odds of PPM implantation in patients undergoing a transarterial versus transapical approach (OR 1.02; 95% CI, 0.1–10.1), transfemoral versus subclavian approach (OR 1.13; 95% CI, 0.6–2.1). These findings remained invariant on a fixed‐effects model. The heterogeneity among these studies ranged from I2=0% to I2=54% (Figure 6, Figures S14 through S16).
Figure 6. Forest plot showing the pooled estimate comparison of (A) self expanding vs balloon expandable and (B) mechanically expandable vs self‐expanding.
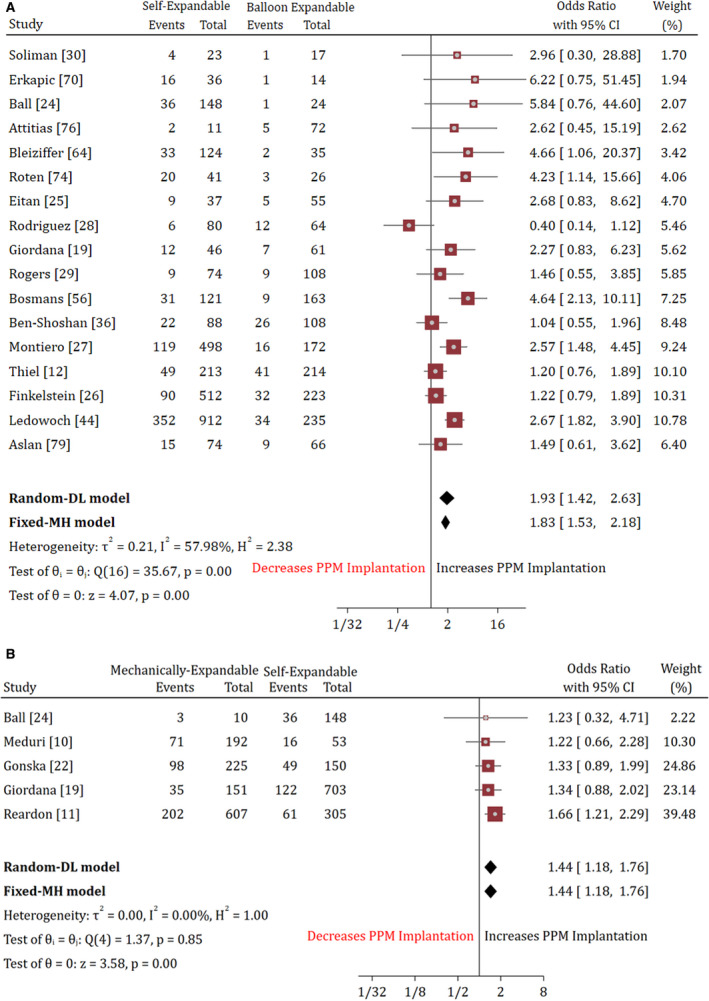
DL indicates DerSimonian and Laird; MH, Mantel‐Haenszel; PPM, permanent pacemaker.
Overall, a subgroup analysis based on the type of valve used, study design and access site mirrored the overall findings with few exceptions. In contrast to the pooled analysis, the summary estimates suggested higher odds of PPM implantation in patients with first‐degree heart block in MCRS (OR 1.95; 95% CI, 1.18–3.24). In concordance to the pooled analysis. Male sex (OR 1.33; 95% CI, 1.02–1.73), LAFB (OR 1.94, 95% CI, 1.11–3.38), intraprocedural atrioventricular block (OR 8.04; 95% CI, 3.53–18.29), and RBBB (OR 4.03; 95% CI, 2.47–6.56) remained the positive predictors of PPM implantation in a subset of patient undergoing MCRS‐only. For ESV and Evolut‐R valves, none of the previously mentioned predictors (except the intraprocedural atrioventricular block) appeared to have a significant influence on the need for PPM implantation. For individual valve types, we were able to assess only 5 to 10 predictors of PPM implantation (Table S9, Figures S19 through S21). More large scale studies are needed to determine the impact of other risk factors for PPM implantation across different valve types.
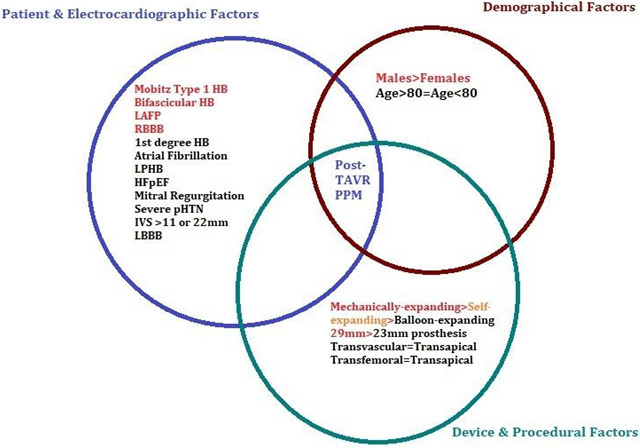
A sensitivity analysis on the “leave‐one‐out” strategy showed that the significantly lower odds of PPM implantation in patients with first‐degree heart block on a fixed‐effects model was driven by one study (Doshi et al) (Figure S22). There was no significant influence of any individual study on the pooled odds of PPM implantation across all other predictors (Figure S23 and S24). On a sensitivity analysis restricted to large studies of 200 patients or more, results remained consistent with the pooled results of the random‐effects model. Moreover, the summary estimates of OCS‐only (after exclusion of RCTs) and a subgroup analysis based on study design (OCS versus RCTs) also mirrored the results of the pooled analysis that included both OCS and RCT data (Table S10). The central illustration of all predictors is given in Figure 7 and the detailed study level PPM implantation rates for each predictor are given in Table S11.
Figure 7. Factors increasing the risk of PPM implantation post‐TAVR (red text indicates a higher risk).
Publication Bias
On the visual assessment of the funnel plots, no significant publication bias was detected for most of the predictors across all studies. Using the standard error, the vertical axis of the plot estimated the sample size of the study. Studies with a larger sample size were plotted on top and those with smaller populations appeared at the bottom of the plot. The horizontal spread indicated the individual effect size reflecting the overall power of the included studies. Our funnel plots were symmetrical, and most studies with low precision were spread evenly on both sides of the average line (Figure S25).
Discussion
The present meta‐analysis represents the most contemporary and largest evidence on the predictors of PPM implantation in patients with severe AS undergoing TAVR. Our findings revealed that male sex, pre‐TAVR baseline atrioventricular conduction abnormalities (including mobitz type‐1 second‐degree heart block, LAFB, RBBB), and intraprocedural atrioventricular block were associated with higher odds of PPM implantation, irrespective of the type of prosthesis or choice of the access site. A stratified analysis based on the prosthesis design showed a 2.4‐fold increased risk of PPM implantation with MCRS (self‐expanding) compared with ESV (balloon‐expandable), and 1.61 times higher odds of PPM‐need in LOTUS (mechanically expandable) compared with MCRS. The overall odds of PPM implantation remained identical in patients aged >80 years versus the younger population and those having first‐degree heart block, AF, prolonged PR‐interval, LPFB and LBBB, when compared with their corresponding control groups who had an absence of these rhythm abnormalities. The type of approach (transapical versus transvascular) or choice of access site (transfemoral versus trans‐subclavian) also had no impact on the risk of PPM implantation. Among the anatomical and valvular variants, the membranous septal length (MSL) was inversely, while the depth of prosthesis implantation was directly associated with the risk of PPM implantation. Larger devices (29 mm) had a higher risk of PPM implantation, while there was no impact of interventricular septum thickness, mitral regurgitation, or pulmonary hypertension on the need for PPM during TAVR. On subgroup analysis, only the MCRS data followed the results of the pooled analysis, indicating that the overall findings were mostly driven by the data obtained from patients receiving self‐expanding valves. The major post‐procedural etiology for PPM implantation was a periprocedural occurrence of high degree heart block, new‐onset LBBB, or persistent bradycardia.
It is imperative to identify patients at an increased risk of PPM implantation before a TAVR procedure, as timely detection of high‐risk patients can potentially prevent the occurrence of atrioventricular block and its associated complications (including syncope and sudden cardiac death). Also, patients with post‐TAVR atrioventricular nodal abnormalities are prone to prolonged hospitalization, putting a high financial burden on the healthcare budget.86 PPM predictors in this context can help in the effective allocation of limited resources. With all its benefits, PPM placement comes at the cost of loss of atrioventricular synchrony, lack of physiological heart rate control, and increased risk of bleeding and pocket infection.87, 88 Early detection of patients at high risk of PPM implantation and identification of pre‐specified predictors, therefore provides an opportunity to mitigate these risks and to favorably lower the harm‐benefit ratio.
Among the measured predictors for PPM implantation, the demographic risk factors including age and sex are of paramount importance. Current evidence on sex‐related differences in post‐TAVR complications and the need for PPM is conflicting in recently published studies.89, 90Our large‐scale analysis shows a 16% higher rate of PPM implantation in men. This can partly be explained by the relatively larger‐sized bioprosthesis (>25 mm) they receive, but mostly because of the higher prevalence of baseline comorbidities, putting men at a greater risk of procedural complications.63, 90 Additionally, our results also revealed a numerically higher rate of PPM use (by 19%) in a population age >80 years, however, the difference did not reach statistical significance. These findings contrast the results of Ramkumar et al. and Ledwoch et al. studies, which denoted a significantly higher risk of post‐TAVR PPM placement in octogenarians by 30% and 35%, respectively.37, 44 Amongst the cardiac predictors, the presence of a LAFB, bi‐fascicular block and second degree atrioventricular block are known to be associated with higher chances of receiving a PPM after TAVR.7, 8, 9 Our study echoes the same trend and expands these findings by demonstrating a 1.3‐, 2.1‐, and 3.1‐fold increase in the odds of the need for PPM implantation in LAFB, bi‐fascicular block and second degree atrioventricular block, respectively.9 Regarding the baseline first‐degree atrioventricular block, Dolci et al and Naveh et al showed an increased incidence of PPM placement at 1 year of TAVR.46, 91 By contrast, we believe that a first‐degree atrioventricular block is a mere delay of atrioventricular conduction rather than a true block and that is why our study demonstrated no impact of first‐degree heart block on the need for PPM implantation.
Studies have shown a higher incidence of post‐TAVR atrioventricular blocks in patients with baseline conduction blocks, due to the manipulation of an already diseased conduction system.37, 44, 46, 61, 73, 92, 93 Pre‐procedure LBBB and RBBB resulted in up to 1.5 times greater risk of PPM implantation after TAVR.92, 93 In our study, RBBB conferred a 2.48 times greater risk of PPM implantation, much higher than the expected rise seen in previous studies. Intriguingly, baseline LBBB on our analysis did not increase the peri‐procedural odds of atrioventricular block or the need for PPM implantation on a random‐effects model. These effects were consistent across the different types of prosthesis and access sites used for the TAVR procedure. When comparing the risk of atrial arrhythmias induced conduction abnormalities, we found that AF had no impact on the need for PPM implantation after TAVR. These findings were in line with the previous literature that also demonstrated an identical rate of need for PPM.94 While a subset of the PARTNER registry showed that patients with sinus rhythm before TAVR and AF at discharge were twice more likely to get a PPM, patients with chronic AF had <6% risk of PPM, not significantly different from patients having no‐AF at baseline.94
On review, we found 40 previous meta‐analyses discussing the risk factors of PPM implantation, however in light of the current evidence the applicability of those studies is limited.92, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132 Most of these meta‐analyses included a smaller number of previously published studies ranging from 4 to 41 articles, missing a large amount of contemporary data. The selection criteria and measured predictors were limited with respect to conduction abnormalities evaluated, indications for TAVR, and in some incidences inclusive of SAVR patients. More importantly, these studies had conflicting results. By contrast, our meta‐analysis is the largest study (78 studies), including all patients who underwent TAVR for symptomatic AS (irrespective of the etiology), a wider range of demographics predictors, conduction abnormalities and procedural characteristics (29 predictors). Our study also provides a subgroup analysis on the type of valve and sensitivity analysis based on the sample size and study design. The detailed study‐level characteristics and differences of our study from previous meta‐analyses are given in Table S12.
Previous small‐scale studies have also shown that atrioventricular conduction disturbances and a subsequent requirement for PPM were more common after the implantation of non‐balloon expandable valves.111, 133 Our results validated these findings by demonstrating a 1.93 and 2.8 times higher rate of PPM implantation in the self‐expanding and mechanically expandable prosthesis compared with the balloon‐expandable valves. MCRS and LOTUS, being a self‐expanding and mechanically expandable valve increases the risk of complete heart block due to deeper implantation into the aortic annulus, tissue edema, and sustained pressure on the conduction pathway (atrioventricular node and left bundle branches).73 These effects might be delayed in the balloon‐expandable valves (ESV) due to the intermittent nature of expansion and lower risk of tissue impingement. Although relatively lower, the newer generation balloon‐expandable prosthesis is not devoid of the risk of PPM implantation. A study by Bisson and colleagues noted that in an effort to decrease a paravalvular leak, the newer ESV comes with an outer skirt, increasing the odds of PPM implantation.134 In contrast to the studies by Puls et al and Rouge et al that showed a higher prevalence of PPM implantation in transfemoral approach compared with trans subclavian access, we found no impact of the choice of the TAVR access site (transapical versus transvascular) and (transfemoral versus trans subclavian) on the need for PPM implantation.38, 135 To summarize, men, patients with baseline conduction abnormalities and those receiving the self‐expanding or mechanically expandable prosthesis are at higher risk of PPM implantation after TAVR.
Limitations
Our study is constrained by the limitations of the included studies. A multivariate logistic regression model is required to control for potential confounders and to obtain an independent impact of the predictor. Patient‐level data were missing to determine the adjusted odds of PPM predictors. For the same reason, we could not assess the impact of the procedure technique and could not account for the differential use of medications or other causes of atrioventricular conduction abnormalities. The impact of unmeasured confounding factors and operators' skills could not be measured. Although we selected a wide range of potential, previously proven predictors, the available data for some comparisons were sparse. Due to the lack of extended follow‐up data the long‐term effectiveness of PPM could not be evaluated. It is also important to note that the reasons for PPM implantation were variable in included studies, hence PPM implantation in our analysis should not be interpreted as a surrogate marker of atrioventricular conduction disturbances. The need for PPM in post‐TAVR patients can be influenced by several economic and logistic factors out of the scope of the current study.
Conclusions
Patients with baseline conduction abnormalities, men, and those receiving mechanical‐ or self‐expanding larger‐sized prostheses for transcatheter aortic valve replacement are at an increased risk of pacemaker implantation. Given the clinical and economic impact of TAVR, interventionists should cautiously risk‐stratify and identify patients at a high risk of the need for PPM.
Sources of Funding
None.
Disclosures
None.
Supporting information
Data S1
Tables S1–S12
Figures S1–S25
References 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.020906
For Sources of Funding and Disclosures, see page 13.
References
- 1.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. DOI: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, et al. Transcatheter aortic‐valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. DOI: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 3.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;10624:3008–3008. DOI: 10.21542/gcsp.2016.32. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. DOI: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 5.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. DOI: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. DOI: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 7.Khawaja MZ, Rajani R, Cook A, Khavandi A, Moynagh A, Chowdhary S, Spence MS, Brown S, Khan SQ, Walker N, et al. Permanent pacemaker insertion after CoreValve transcatheter aortic valve implantation: incidence and contributing factors (the UK CoreValve Collaborative). Circulation. 2011;123:951–960. DOI: 10.1161/CIRCULATIONAHA.109.927152. [DOI] [PubMed] [Google Scholar]
- 8.Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PS, El‐Chami MF, Herrmann HC, Mack M, Makkar RR, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv. 2015;8:60–69. DOI: 10.1016/j.jcin.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 9.De Carlo M, Giannini C, Bedogni F, Klugmann S, Brambilla N, De Marco F, Zucchelli G, Testa L, Oreglia J, Petronio AS. Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation. Am Heart J. 2012;163:492–499. DOI: 10.1016/j.ahj.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Meduri CU, Kereiakes DJ, Rajagopal V, Makkar RR, O'Hair D, Linke A, Waksman R, Babliaros V, Stoler RC, Mishkel GJ, et al. Pacemaker implantation and dependency after transcatheter aortic valve replacement in the REPRISE III trial. J Am Heart Assoc. 2019;8:e012594. DOI: 10.1161/JAHA.119.012594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reardon MJ, Feldman TE, Meduri CU, Makkar RR, O'Hair D, Linke A, Kereiakes DJ, Waksman R, Babliaros V, Stoler RC, et al. Two‐year outcomes after transcatheter aortic valve replacement with mechanical vs self‐expanding valves: the REPRISE III randomized clinical trial. JAMA cardiology. 2019;4:223–229. DOI: 10.1001/jamacardio.2019.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiele H, Kurz T, Feistritzer HJ, Stachel G, Hartung P, Eitel I, Marquetand C, Nef H, Doerr O, Lauten A, et al. Comparison of newer generation self‐expandable vs. balloon‐expandable valves in transcatheter aortic valve implantation: the randomized SOLVE‐TAVI trial. Eur Heart J. 2020;41:1890–1899. DOI: 10.1093/eurheartj/ehaa036. [DOI] [PubMed] [Google Scholar]
- 13.Hamandi M, Tabachnick D, Lanfear AT, Baxter R, Shin K, Zingler B, Mack MJ, DiMaio JM, Kindsvater S. Effect of new and persistent left bundle branch block after transcatheter aortic valve replacement on long‐term need for pacemaker implantation. Bayl Univ Med Cen Proc. 2020;33:157–162. DOI: 10.1080/08998280.2020.1717906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma E, McCauley B, Ghosalkar DS, Atalay M, Collins S, Parulkar A, Sheikh W, Ahmed MB, Chu A. Aortic valve calcification as a predictor of post‐transcatheter aortic valve replacement pacemaker dependence. Cardiol Res. 2020;11:155. DOI: 10.14740/cr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochman J, Zbroński K, Kołtowski Ł, Parma R, Ochała A, Huczek Z, Rymuza B, Wilimski R, Dąbrowski M, Witkowski A, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis utilizing the next‐generation fully retrievable and repositionable valve system: mid‐term results from a prospective multicentre registry. Clin Res Cardiol. 2020;109:570–580. DOI: 10.1007/s00392-019-01541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karacop E, Enhos A. Predictive role of ventricular repolarization parameters for the occurrence of complete heart block in patients undergoing transcatheter aortic valve implantation. Ann Noninvasive Electrocardiol. 2020;25:e12734. DOI: 10.1111/anec.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ay NK. Impact of age on long term survival following transcatheter aortic valve implantation. J Geriatr Cardiol. 2019;16:265–271. DOI: 10.11909/j.issn.1671-5411.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko H, Hoelschermann F, Seifert M, Tambor G, Okamoto M, Moeller V, Neuss M, Butter C. Predictors of permanent pacemaker implantation after transcatheter aortic valve implantation for aortic stenosis using Medtronic new generation self‐expanding CoreValve Evolut R. Heart Vessels. 2019;34:360–367. DOI: 10.1007/s00380-018-1236-z. [DOI] [PubMed] [Google Scholar]
- 19.Giordano A, Corcione N, Ferraro P, Morello A, Conte S, Testa L, Bedogni F, Iadanza A, Berti S, Regazzoli D, et al. Comparative one‐month safety and effectiveness of five leading new‐generation devices for transcatheter aortic valve implantation. Sci Rep. 2019;19:1–2. DOI: 10.1038/s41598-019-53081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doshi R, Decter DH, Meraj P. Incidence of arrhythmias and impact of permanent pacemaker implantation in hospitalizations with transcatheter aortic valve replacement. Clin Cardiol. 2018;41:640–645. DOI: 10.1002/clc.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhardwaj A, Ramanan T, Sawant AC, Sinibaldi E, Pham M, Khan S, Qureshi R, Agrawal N, Khalil C, Hansen R, et al. Quality of life outcomes in transcatheter aortic valve replacement patients requiring pacemaker implantation. J Arrhythm. 2018;34:441–449. DOI: 10.1002/joa3.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonska B, Keßler M, Wöhrle J, Rottbauer W, Seeger J. Influence of permanent pacemaker implantation after transcatheter aortic valve implantation with new‐generation devices. Neth Heart J. 2018;26:620–627. DOI: 10.1007/s12471-018-1194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousif N, Obeid S, Binder R, Denegri A, Shahin M, Templin C, Lüscher TF. Impact of gender on outcomes after transcatheter aortic valve implantation. J Geriatr Cardiol. 2018;15:394–400. DOI: 10.11909/j.issn.1671-5411.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball TN, Vasudevan A, Mi Ko J, Assar MD, McCullough PA, Stoler RC. Analysis of electrocardiographic intervals before and after transcatheter aortic valve implantation to predict the need for permanent pacing. Proc (Bayl Univ Med Cent). 2018;31:407–413. DOI: 10.1080/08998280.2018.1471884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eitan A, Witt J, Stripling J, Haselbach T, Rieß FC, Schofer J. Performance of the Evolut‐R 34 mm versus Sapien‐3 29 mm in Transcatheter aortic valve replacement patients with larger annuli: early outcome results of Evolut‐R 34 mm as compared with Sapien‐3 29 mm in patients with Annuli ≥26 mm. Catheter Cardiovasc Interv. 2018;92:1374–1379. DOI: 10.1002/ccd.27588. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein A, Steinvil A, Rozenbaum Z, Halkin A, Banai S, Barbash I, Guetta V, Segev A, Danenberg H, Orvin K, et al. Efficacy and safety of new‐generation transcatheter aortic valves: insights from the Israeli transcatheter aortic valve replacement registry. Clin Res Cardiol. 2019;108:430–437. DOI: 10.1007/s00392-018-1372-6. [DOI] [PubMed] [Google Scholar]
- 27.Monteiro C, Ferrari ADL, Caramori PRA, Carvalho LAF, Siqueira DAA, Thiago LEKS, Perin M, Lima VC, Guérios E, Brito Junior FS. Permanent pacing after transcatheter aortic valve implantation: incidence, predictors and evolution of left ventricular function. Arq Bras Cardiol. 2017;109:550–559. DOI: 10.5935/abc.20170170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enríquez‐Rodríguez E, Amat‐Santos IJ, Jiménez‐Quevedo P, Martín‐Morquecho I, Tirado‐Conte G, Pérez‐Vizcayno MJ, Gómez de Diego JJ, Arnold R, Aldazábal A, Rojas P, et al. Comparison of the hemodynamic performance of the balloon‐expandable SAPIEN 3 versus self‐expandable Evolut R transcatheter valve: a case‐matched study. Rev Esp Cardiol (Engl Ed). 2018;71:735–742. DOI: 10.1016/j.rec.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Rogers T, Steinvil A, Buchanan K, Alraies MC, Koifman E, Gai J, Torguson R, Okubagzi P, Ben‐Dor I, Pichard A, et al. Contemporary transcatheter aortic valve replacement with third‐generation balloon‐expandable versus self‐expanding devices. J Interv Cardiol. 2017;30:356–361. DOI: 10.1111/joic.12389. [DOI] [PubMed] [Google Scholar]
- 30.Soliman H, Alrabaat K, Aboalaazm T, Mostafa S, Samy A. Outcome of transcatheter aortic valve implantation in high risk patients with severe aortic stenosis. Egypt Heart J. 2017;69:261–271. DOI: 10.1016/j.ehj.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Mourik MS , Geenen LM, Delewi R, Wiegerinck EM, Koch KT, Bouma BJ, Henriques JP, de Winter RJ , Baan J, Vis MM. Predicting hospitalisation duration after transcatheter aortic valve implantation. Open Heart. 2017;4:e000549. DOI: 10.1136/openhrt-2016-000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Kley F , van Rosendael PJ , Katsanos S, Kamperidis V, Marsan NA, Karalis I, de Weger A , Palmen M, Bax JJ, Schalij MJ, et al. Impact of age on transcatheter aortic valve implantation outcomes: a comparison of patients aged ≤80 years versus patients >80 years. J Geriatr Cardiol. 2016;13:31–36. DOI: 10.11909/j.issn.1671-5411.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaman S, McCormick L, Gooley R, Rashid H, Ramkumar S, Jackson D, Hui S, Meredith IT. Incidence and predictors of permanent pacemaker implantation following treatment with the repositionable Lotus™ transcatheter aortic valve. Catheter Cardiovasc Interv. 2017;90:147–154. DOI: 10.1002/ccd.26857. [DOI] [PubMed] [Google Scholar]
- 34.Kahraman S, Dogan A, Kalkan AK, Guler A, Pak M, Yilmaz E, Gurbak I, Panc C, Demir AR, Tosu AR, et al. Evaluation of Tp‐e interval, Tp‐e/QT and Tp‐e/QTc ratio in aortic valve stenosis before and after transcatheter aortic valve implantation. J Electrocardiol. 2018;51:949–954. DOI: 10.1016/j.jelectrocard.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Sawaya FJ, Spaziano M, Lefèvre T, Roy A, Garot P, Hovasse T, Neylon A, Benamer H, Romano M, Unterseeh T, et al. Comparison between the SAPIEN S3 and the SAPIEN XT transcatheter heart valves: a single‐center experience. World J Cardiol. 2016;8:735. DOI: 10.4330/wjc.v8.i12.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben‐Shoshan J, Konigstein M, Zahler D, Margolis G, Chorin E, Steinvil A, Arbel Y, Aviram G, Granot Y, Barkagan M, et al. Comparison of the Edwards SAPIEN S3 versus Medtronic Evolut‐R devices for transcatheter aortic valve implantation. Am J Cardiol. 2017;119:302–307. DOI: 10.1016/j.amjcard.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Ramkumar S, Rashid HN, Zaman S, McCormick L, Gooley R, Jackson D, Meredith IT. Feasibility and clinical outcomes in nonagenarians undergoing transcatheter aortic valve replacement with the LOTUS™ valve. J Geriatr Cardiol. 2016;13:636–638. DOI: 10.11909/j.issn.1671-5411.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rougé A, Huttin O, Aslam R, Vaugrenard T, Jouve T, Angioi M, Maureira P. Mid‐term results of 150 TAVI comparing apical versus femoral approaches. J Cardiothorac surg. 2015;10:147. DOI: 10.1186/s13019-015-0360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gauthier C, Astarci P, Baele P, Matta A, Kahn D, Kefer J, Momeni M. Mid‐term survival after transcatheter aortic valve implantation: results with respect to the anesthetic management and to the access route (transfemoral versus transapical). Ann Card Anaesth. 2015;18:343. DOI: 10.4103/0971-9784.159804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ki BV, Kooiman KM, Yong ZY, Wiegerinck EM, Damman P, Bouma BJ, Tijssen JG, Piek JJ, Knops RE, Baan J Jr. Predictors and permanency of cardiac conduction disorders and necessity of pacing after transcatheter aortic valve implantation. Pacing Clin Electrophysiol. 2014;37:1520–1529. DOI: 10.1111/pace.12460. [DOI] [PubMed] [Google Scholar]
- 41.Simms AD, Hogarth AJ, Hudson EA, Worsnop VL, Blackman DJ, O'Regan DJ, Tayebjee MH. Ongoing requirement for pacing post‐transcatheter aortic valve implantation and surgical aortic valve replacement. Interact Cardiovasc Thorac Surg. 2013;17:328–333. DOI: 10.1093/icvts/ivt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nuis RJ, Dager AE, van der Boon RM , Jaimes MC, Caicedo B, Fonseca J, Van Mieghem NM, Benitez LM, Umana JP, O’Neill WW, et al. Patients with aortic stenosis referred for TAVI: treatment decision, in‐hospital outcome and determinants of survival. Neth Heart J. 2012;20:16–23. DOI: 10.1007/s12471-011-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulse M, Viel T, Danner BC, Jacobshagen C, Teucher N, Hanekop G, Schöndube F, Hasenfuß G, Seipelt RG, Schillinger W. The risk‐to‐benefit ratio of transcatheter aortic valve implantation in specific patient cohorts: a single‐centre experience. Clin Res Cardiol. 2012;101:553–563. DOI: 10.1007/s00392-012-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ledwoch J, Franke J, Gerckens U, Kuck KH, Linke A, Nickenig G, Krülls‐Münch J, Vöhringer M, Hambrecht R, Erbel R, et al. Incidence and predictors of permanent pacemaker implantation following transcatheter aortic valve implantation: analysis from the German transcatheter aortic valve interventions registry. Catheter Cardiovasc Interv. 2013;82:E569–E577. DOI: 10.1002/ccd.24915. [DOI] [PubMed] [Google Scholar]
- 45.Akin I, Kische S, Paranskaya L, Schneider H, Rehders TC, Trautwein U, Turan G, Bänsch D, Thiele O, Divchev D, et al. Predictive factors for pacemaker requirement after transcatheter aortic valve implantation. BMC Cardiovasc Disord. 2012;12:87. DOI: 10.1186/1471-2261-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagur R, Rodés‐Cabau J, Gurvitch R, Dumont É, Velianou JL, Manazzoni J, Toggweiler S, Cheung A, Ye J, Natarajan MK, et al. Need for permanent pacemaker as a complication of transcatheter aortic valve implantation and surgical aortic valve replacement in elderly patients with severe aortic stenosis and similar baseline electrocardiographic findings. JACC Cardiovasc Interv. 2012;5:540–551. DOI: 10.1016/j.jcin.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Gilard M, Eltchaninoff H, Iung B, Donzeau‐Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, et al. Registry of transcatheter aortic‐valve implantation in high‐risk patients. N Engl J Med. 2012;366:1705–1715. DOI: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 48.Muñoz‐García AJ, Hernández‐García JM, Jiménez‐Navarro MF, Alonso‐Briales JH, Domínguez‐Franco AJ, Fernández‐Pastor J, Hernández JP, Cordero AB, Rodríguez JA, de Teresa‐Galván E . Factors predicting and having an impact on the need for a permanent pacemaker after CoreValve prosthesis implantation using the new Accutrak delivery catheter system. JACC Cardiovasc Interv. 2012;5:533–539. DOI: 10.1016/j.jcin.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Saia F, Lemos PA, Bordoni B, Cervi E, Boriani G, Ciuca C, Taglieri N, Mariani J Jr, Filho RK, Marzocchi A. Transcatheter aortic valve implantation with a self‐expanding nitinol bioprosthesis: prediction of the need for permanent pacemaker using simple baseline and procedural characteristics. Catheter Cardiovasc Interv. 2012;79:712–719. DOI: 10.1002/ccd.23336. [DOI] [PubMed] [Google Scholar]
- 50.Salinas P, Moreno R, Calvo L, Jiménez‐Valero S, Galeote G, Sánchez‐Recalde A, López‐Fernández T, Garcia‐Blas S, Iglesias D, Riera L, et al. Clinical and prognostic implications of atrial fibrillation in patients undergoing transcatheter aortic valve implantation. World J Cardiol. 2012;4:8. DOI: 10.4330/wjc.v4.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeter T, Linke A, Haensig M, Merk DR, Borger MA, Mohr FW, Schuler G. Predictors of permanent pacemaker implantation after Medtronic CoreValve bioprosthesis implantation. Europace. 2012;14:1759–1763. DOI: 10.1093/europace/eus191. [DOI] [PubMed] [Google Scholar]
- 52.Van der Boon RM, Van Mieghem NM, Theuns DA, Nuis RJ, Nauta ST, Serruys PW, Jordaens L, van Domburg RT , de Jaegere PP . Pacemaker dependency after transcatheter aortic valve implantation with the self‐expanding Medtronic CoreValve System. Int J Cardiol. 2013;168:1269–1273. DOI: 10.1016/j.ijcard.2012.11.115. [DOI] [PubMed] [Google Scholar]
- 53.Mouillet G, Lellouche N, Lim P, Meguro K, Yamamoto M, Deux JF, Monin JL, Bergoënd E, Dubois‐Randé JL, Teiger E. Patients without prolonged QRS after TAVI with CoreValve device do not experience high‐degree atrio‐ventricular block. Catheter Cardiovasc Interv. 2013;81:882–887. DOI: 10.1002/ccd.24657. [DOI] [PubMed] [Google Scholar]
- 54.Liang M, Devlin G, Pasupati S. The incidence of transcatheter aortic valve implantation‐related heart block in self‐expandable Medtronic Core‐Valve and balloon‐expandable Edwards valves. J Invasive Cardiol. 2012;24:173–176. [PubMed] [Google Scholar]
- 55.Pilgrim T, Wenaweser P, Meuli F, Huber C, Stortecky S, Seiler C, Zbinden S, Meier B, Carrel T, Windecker S. Clinical outcome of high‐risk patients with severe aortic stenosis and reduced left ventricular ejection fraction undergoing medical treatment or TAVI. PLoS One. 2011;6:e27556. DOI: 10.1371/journal.pone.0027556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosmans JM, Kefer J, De Bruyne B, Herijgers P, Dubois C, Legrand V, Verheye S, Rodrigus I. Procedural, 30‐day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg. 2011;12:762–767. DOI: 10.1510/icvts.2010.253773. [DOI] [PubMed] [Google Scholar]
- 57.D'Ancona G, Pasic M, Unbehaun A, Hetzer R. Permanent pacemaker implantation after transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg. 2011;13:373–376. DOI: 10.1510/icvts.2011.274456. [DOI] [PubMed] [Google Scholar]
- 58.Ewe SH, Delgado V, Ng AC, Antoni ML, van der Kley F , Marsan NA, de Weger A , Tavilla G, Holman ER, Schalij MJ, et al. Outcomes after transcatheter aortic valve implantation: transfemoral versus transapical approach. Ann Thorac Surg. 2011;92:1244–1251. DOI: 10.1016/j.athoracsur.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 59.Fraccaro C, Buja G, Tarantini G, Gasparetto V, Leoni L, Razzolini R, Corrado D, Bonato R, Basso C, Thiene G, et al. Incidence, predictors, and outcome of conduction disorders after transcatheter self‐expandable aortic valve implantation. Am J Cardiol. 2011;107:747–754. DOI: 10.1016/j.amjcard.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 60.Guetta V, Goldenberg G, Segev A, Dvir D, Kornowski R, Finckelstein A, Hay I, Goldenberg I, Glikson M. Predictors and course of high‐degree atrioventricular block after transcatheter aortic valve implantation using the CoreValve Revalving System. Am J Cardiol. 2011;108:1600–1605. DOI: 10.1016/j.amjcard.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Calvi V, Conti S, Pruiti GP, Capodanno D, Puzzangara E, Tempio D, Di Grazia A, Ussia GP, Tamburino C. Incidence rate and predictors of permanent pacemaker implantation after transcatheter aortic valve implantation with self‐expanding CoreValve prosthesis. J Interv Card Electrophysiol. 2012;34:189–195. DOI: 10.1007/s10840-011-9634-5. [DOI] [PubMed] [Google Scholar]
- 62.Chorianopoulos E, Krumsdorf U, Pleger ST, Katus HA, Bekeredjian R. Incidence of late occurring bradyarrhythmias after TAVI with the self‐expanding CoreValve® aortic bioprosthesis. Clin Res Cardiol. 2012;101:349–355. DOI: 10.1007/s00392-011-0398-9. [DOI] [PubMed] [Google Scholar]
- 63.Hayashida K, Morice MC, Chevalier B, Hovasse T, Romano M, Garot P, Farge A, Donzeau‐Gouge P, Bouvier E, Cormier B, et al. Sex‐related differences in clinical presentation and outcome of transcatheter aortic valve implantation for severe aortic stenosis. J Am Coll Cardiol. 2012;59:566–571. DOI: 10.1016/j.jacc.2011.10.877. [DOI] [PubMed] [Google Scholar]
- 64.Bleiziffer S, Ruge H, Hörer J, Hutter A, Geisbüsch S, Brockmann G, Mazzitelli D, Bauernschmitt R, Lange R. Predictors for new‐onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:524–530. DOI: 10.1016/j.jcin.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 65.Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, Iung B, Donzeau‐Gouge P, Tribouilloy C, Debrux JL, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–197. DOI: 10.1093/eurheartj/ehq261. [DOI] [PubMed] [Google Scholar]
- 66.Baan J Jr, Yong ZY, Koch KT, Henriques JP, Bouma BJ, Vis MM, Cocchieri R, Piek JJ, de Mol BA . Factors associated with cardiac conduction disorders and permanent pacemaker implantation after percutaneous aortic valve implantation with the CoreValve prosthesis. Am Heart J. 2010;159:497–503. DOI: 10.1016/j.ahj.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Ewe SH, Ajmone Marsan N, Pepi M, Delgado V, Tamborini G, Muratori M, Ng AC, van der Kley F , de Weger A , Schalij MJ, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–1120. DOI: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Ferreira ND, Caeiro D, Adão L, Oliveira M, Gonçalves H, Ribeiro J, Teixeira M, Albuquerque A, Primo J, Braga P, et al. Incidence and predictors of permanent pacemaker requirement after transcatheter aortic valve implantation with a self‐expanding bioprosthesis. Pacing Clin Electrophysiol. 2010;33:1364–1372. DOI: 10.1111/j.1540-8159.2010.02870.x. [DOI] [PubMed] [Google Scholar]
- 69.Godino C, Maisano F, Montorfano M, Latib A, Chieffo A, Michev I, Al‐Lamee R, Bande M, Mussardo M, Arioli F, et al. Outcomes after transcatheter aortic valve implantation with both Edwards‐SAPIEN and CoreValve devices in a single center: the Milan experience. JACC Cardiovasc Interv. 2010;3:1110–1121. DOI: 10.1016/j.jcin.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Erkapic D, Kim WK, Weber M, Mollmann H, Berkowitsch A, Zaltsberg S, Pajitnev DJ, Rixe J, Neumann T, Kuniss M, et al. Electrocardiographic and further predictors for permanent pacemaker requirement after transcatheter aortic valve implantation. Europace. 2010;12:1188–1190. DOI: 10.1093/europace/euq094. [DOI] [PubMed] [Google Scholar]
- 71.Haworth P, Behan M, Khawaja M, Hutchinson N, de Belder A , Trivedi U, Laborde JC, Hildick‐Smith D. Predictors for permanent pacing after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;76:751–756. DOI: 10.1002/ccd.22457. [DOI] [PubMed] [Google Scholar]
- 72.Piazza N, Nuis RJ, Tzikas A, Otten A, Onuma Y, García‐García H, Schultz C, Van Domburg R, van Es GA , Van Geuns R, et al. Persistent conduction abnormalities and requirements for pacemaking six months after transcatheter aortic valve implantation. EuroIntervention. 2010;6:475–484. DOI: 10.4244/EIJ30V6I4A80. [DOI] [PubMed] [Google Scholar]
- 73.Rodés‐Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. DOI: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 74.Roten L, Wenaweser P, Delacrétaz E, Hellige G, Stortecky S, Tanner H, Pilgrim T, Kadner A, Eberle B, Zwahlen M, et al. Incidence and predictors of atrioventricular conduction impairment after transcatheter aortic valve implantation. Am J Cardiol. 2010;106:1473–1480. DOI: 10.1016/j.amjcard.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Lefevre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schächinger V, De Bruyne B, Eltchaninoff H, Thielmann M, Himbert D, et al. One year follow‐up of the multi‐centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32(2):148–157. DOI: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Attias D, Himbert D, Ducrocq G, Détaint D, Al‐Attar N, Iung B, Francis F, Maury JM, Brochet E, Enguerrand D, et al. Immediate and mid‐term results of transfemoral aortic valve implantation using either the Edwards Sapien™ transcatheter heart valve or the Medtronic CoreValve® System in high‐risk patients with aortic stenosis. Arch Cardiovasc Dis. 2010;103:236–245. DOI: 10.1016/j.acvd.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Petronio AS, De Carlo M, Bedogni F, Marzocchi A, Klugmann S, Maisano F, Ramondo A, Ussia GP, Ettori F, Poli A, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv. 2010;3:359–366. [DOI] [PubMed] [Google Scholar]
- 78.Thielmann M, Wendt D, Eggebrecht H, Kahlert P, Massoudy P, Kamler M, Erbel R, Jakob H, Sack S. Transcatheter aortic valve implantation in patients with very high risk for conventional aortic valve replacement. Ann Thorac Surg. 2009;88:1468–1474. DOI: 10.1016/j.athoracsur.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 79.Aslan S, Demir AR, Çelik Ö, Kalkan AK, Uzun F, Güner A, Topel Ç, Ertürk M. Usefulness of membranous septum length in the prediction of major conduction disturbances in patients undergoing transcatheter aortic valve replacement with different devices. Kardiol Pol. 2020;78:1020–1028. DOI: 10.33963/KP.15538. [DOI] [PubMed] [Google Scholar]
- 80.Hamdan A, Guetta V, Klempfner R, Konen E, Raanani E, Glikson M, Goitein O, Segev A, Barbash I, Fefer P, et al. Inverse relationship between membranous septal length and the risk of atrioventricular block in patients undergoing transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2015;8:1218–1228. [DOI] [PubMed] [Google Scholar]
- 81.Jilaihawi H, Zhao Z, Du R, Staniloae C, Saric M, Neuburger PJ, Querijero M, Vainrib A, Hisamoto K, Ibrahim H, et al. Minimizing permanent pacemaker following repositionable self‐expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:1796–1807. DOI: 10.1016/j.jcin.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 82.Matsushita K, Kanso M, Ohana M, Marchandot B, Kibler M, Heger J, Peillex M, Trimaille A, Hess S, Grunebaum L, et al. Periprocedural predictors of new‐onset conduction abnormalities after transcatheter aortic valve replacement. Circ J. 2020;84:1875–1883. DOI: 10.1253/circj.CJ-20-0257. [DOI] [PubMed] [Google Scholar]
- 83.Tretter JT, Mori S, Anderson RH, Taylor MD, Ollberding N, Truong V, Choo J, Kereiakes D, Mazur W. Anatomical predictors of conduction damage after transcatheter implantation of the aortic valve. Open heart. 2019;6:e000972. DOI: 10.1136/openhrt-2018-000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaid S, Sengupta A, Tsoi M, Khan A, Ahmad H, Goldberg J, Dangas GD, Sharma SK, Kini AS, Tang G. Impact of membranous septal length on new permanent pacemaker implantation after sapien 3 tavr. J Am Coll Cardiol. 2020;75:1474. DOI: 10.1016/S0735-1097(20)32101-X. [DOI] [Google Scholar]
- 85.Ahmad M, Patel JN, Loc BL, Vipparthy SC, Divecha C, Barzallo PX, Kim M, Baman T, Barzallo M, Mungee S. Association between body mass index and permanent pacemaker implantation after Transcatheter Aortic Valve Replacement (TAVR) with Edwards SAPIEN™ 3 TAVR Valves: a single‐center experience. Cureus. 2019;11:209–219. DOI: 10.7759/cureus.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chevreul K, Brunn M, Cadier B, Haour G, Eltchaninoff H, Prat A, Leguerrier A, Blanchard D, Fournial G, Iung B, et al. Cost of transcatheter aortic valve implantation and factors associated with higher hospital stay cost in patients of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Arch Cardiovasc Dis. 2013;106:209–219. DOI: 10.1016/j.acvd.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 87.Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, Shinn T, St. John Sutton M. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. DOI: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 88.Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony: implications for therapy. J Am Coll Cardiol. 2009;54:764–776. DOI: 10.1016/j.jacc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 89.D’Ascenzo F, Gonella A, Moretti C, Omedè P, Salizzoni S, La Torre M, Giordana F, Barbanti M, Ussia GP, Brambilla N, et al. Gender differences in patients undergoing TAVI: a multicentre study. EuroIntervention. 2013;9:367–372. DOI: 10.4244/EIJV9I3A59. [DOI] [PubMed] [Google Scholar]
- 90.Buja P, Napodano M, Tamburino C, Petronio AS, Ettori F, Santoro G, Ussia GP, Klugmann S, Bedogni F, Ramondo A, et al. Comparison of variables in men versus women undergoing transcatheter aortic valve implantation for severe aortic stenosis (from Italian Multicenter CoreValve registry). Am J Cardiol. 2013;111:88–93. DOI: 10.1016/j.amjcard.2012.08.051. [DOI] [PubMed] [Google Scholar]
- 91.Dolci G, Vollema EM, van der Kley F , de Weger A , Marsan NA, Delgado V, Bax JJ. One‐year follow‐up of conduction abnormalities after transcatheter aortic valve implantation with the SAPIEN 3 valve. Am J Cardiol. 2019;124:1239–1245. DOI: 10.1016/j.amjcard.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 92.Siontis GC, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, Wenaweser P, Windecker S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta‐analysis. J Am Coll Cardiol. 2014;64:129–140. DOI: 10.1016/j.jacc.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 93.Fischer Q, Himbert D, Webb JG, Eltchaninoff H, Muñoz‐García AJ, Tamburino C, Nombela‐Franco L, Nietlispach F, Moris C, Ruel M, et al. Impact of preexisting left bundle branch block in transcatheter aortic valve replacement recipients. Circ Cardiovasc Interv. 2018;11:e006927. DOI: 10.1161/CIRCINTERVENTIONS.118.006927. [DOI] [PubMed] [Google Scholar]
- 94.Biviano AB, Nazif T, Dizon J, Garan H, Abrams M, Fleitman J, Hassan D, Kapadia S, Babaliaros V, Xu KE, et al. Atrial fibrillation is associated with increased pacemaker implantation rates in the placement of AoRTic transcatheter valve (PARTNER) trial. J Atr Fibrillation. 2017;10. DOI: 10.4022/jafib.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Erkapic D, De Rosa S, Kelava A, Lehmann R, Fichtlscherer S, Hohnloser SH. Risk for permanent pacemaker after transcatheter aortic valve implantation: a comprehensive analysis of the literature. J Cardiovasc Electrophysiol. 2012;23:391–397. DOI: 10.1111/j.1540-8167.2011.02211.x. [DOI] [PubMed] [Google Scholar]
- 96.Gozdek M, Ratajczak J, Arndt A, Zieliński K, Pasierski M, Matteucci M, Fina D, Jiritano F, Meani P, Raffa GM, et al. Transcatheter aortic valve replacement with Lotus and Sapien 3 prosthetic valves: a systematic review and meta‐analysis. J Thorac Dis. 2020;12:893. DOI: 10.21037/jtd.2019.12.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhan Y, Saadat S, Soin A, Kawabori M, Chen FY. A meta‐analysis comparing transaxillary and transfemoral transcatheter aortic valve replacement. J Thorac Dis. 2019;11:5140. DOI: 10.21037/jtd.2019.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zafar MR, Mustafa SF, Miller TW, Alkhawlani T, Sharma UC. Outcomes after transcatheter aortic valve replacement in cancer survivors with prior chest radiation therapy: a systematic review and meta‐analysis. Cardio‐Oncology. 2020;6:1. DOI: 10.1186/s40959-020-00062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xi Z, Liu T, Liang J, Zhou YJ, Liu W. Impact of postprocedural permanent pacemaker implantation on clinical outcomes after transcatheter aortic valve replacement: a systematic review and meta‐analysis. J Thorac Dis. 2019;11:5130. DOI: 10.21037/jtd.2019.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shoar S, Batra S, Gulraiz A, Ikram W, Javed M, Hosseini F, Naderan M, Shoar N, John J, Modukuru VR, et al. Effect of pre‐existing left bundle branch block on post‐procedural outcomes of transcatheter aortic valve replacement: a meta‐analysis of comparative studies. Am J Cardiovasc Dis. 2020;10:294. [PMC free article] [PubMed] [Google Scholar]
- 101.Biondi‐Zoccai G, Peruzzi M, Abbate A, Gertz ZM, Benedetto U, et al. Network meta‐analysis on the comparative effectiveness and safety of transcatheter aortic valve implantation with CoreValve or Sapien devices versus surgical replacement. Heart Lung Vessel. 2014;6:232–243. [PMC free article] [PubMed] [Google Scholar]
- 102.Alperi A, Muntané‐Carol G, Freitas‐Ferraz AB, Junquera L, Del Val D, Faroux L, Philippon F, Rodés‐Cabau J. Overcoming the transcatheter aortic valve replacement Achilles heel: conduction abnormalities—a systematic review. Ann Cardiothorac Surg. 2020;9:429–441. DOI: 10.21037/acs-2020-av-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee HA, Chou AH, Wu VC, Chen DY, Lee HF, Lee KT, Chu PH, Cheng YT, Chang SH, Chen SW. Balloon‐expandable versus self‐expanding transcatheter aortic valve replacement for bioprosthetic dysfunction: a systematic review and meta‐analysis. PLoS One. 2020;15:e0233894. DOI: 10.1371/journal.pone.0233894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ando T, Takagi H, ALICE (All‐Literature Investigation of Cardiovascular Evidence) Group . The prognostic impact of new‐onset persistent left bundle branch block following transcatheter aortic valve implantation: a meta‐analysis. Clin Cardiol. 2016;39:544–550. DOI: 10.1002/clc.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faroux L, Chen S, Muntané‐Carol G, Regueiro A, Philippon F, Sondergaard L, Jørgensen TH, Lopez‐Aguilera J, Kodali S, Leon M, et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta‐analysis. Eur Heart J. 2020;41:2771–2781. DOI: 10.1093/eurheartj/ehz924. [DOI] [PubMed] [Google Scholar]
- 106.Fu J, Popal MS, Li Y, Li G, Qi Y, Fang F, Kwong JS, You B, Meng X, Du J. Transcatheter versus surgical aortic valve replacement in low and intermediate risk patients with severe aortic stenosis: systematic review and meta‐analysis of randomized controlled trials and propensity score matching observational studies. J Thorac Dis. 2019;11:1945–1962. DOI: 10.21037/jtd.2019.04.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haddad A, Arwani R, Altayar O, Sawas T, Murad MH, de Marchena E . Transcatheter aortic valve replacement in patients with pure native aortic valve regurgitation: a systematic review and meta‐analysis. Clin Cardiol. 2019;42:159–166. DOI: 10.1002/clc.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanjanahattakij N, Horn B, Vutthikraivit W, Biso SM, Ziccardi MR, Lu ML, Rattanawong P. Comparing outcomes after transcatheter aortic valve replacement in patients with stenotic bicuspid and tricuspid aortic valve: a systematic review and meta‐analysis. Clin Cardiol. 2018;41:896–902. DOI: 10.1002/clc.22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khan SU, Lone AN, Saleem MA, Kaluski E. Transcatheter vs surgical aortic‐valve replacement in low‐to intermediate‐surgical‐risk candidates: a meta‐analysis and systematic review. Clin Cardiol. 2017;40:974–981. DOI: 10.1002/clc.22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khan MR, Kayani WT, Manan M, Munir A, Hamzeh I, Virani SS, Birnbaum Y, Jneid H, Alam M. Comparison of surgical versus transcatheter aortic valve replacement for patients with aortic stenosis at low‐intermediate risk. Cardiovasc Diagn Ther. 2020;10:135–144. DOI: 10.21037/cdt.2020.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khatri PJ, Webb JG, Rodés‐Cabau J, Fremes SE, Ruel M, Lau K, Guo H, Wijeysundera HC, Ko DT. Adverse effects associated with transcatheter aortic valve implantation: a meta‐analysis of contemporary studies. Ann Intern Med. 2013;158:35–46. DOI: 10.7326/0003-4819-158-1-201301010-00007. [DOI] [PubMed] [Google Scholar]
- 112.Lee HA, Su IL, Chen SW, Wu VC, Chen DY, Chu PH, Chou AH, Cheng YT, Lin PJ, Tsai FC. Direct aortic route versus transaxillary route for transcatheter aortic valve replacement: a systematic review and meta‐analysis. PeerJ. 2020;8:e9102. DOI: 10.7717/peerj.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li YM, Tsauo JY, Jia KY, Liao YB, Xia F, Zhao ZG, Chen M, Peng Y. Transcatheter and surgical aortic valve replacement in patients with previous cardiac surgery: a meta‐analysis. Front Cardiovasc Med. 2021;7: 612155. DOI: 10.3389/fcvm.2020.612155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lou Y, Gao Y, Yu Y, Li Y, Xi Z, Swe KN, Zhou Y, Nie X, Liu W. Efficacy and safety of transcatheter vs. surgical aortic valve replacement in low‐to‐intermediate‐risk patients: a meta‐analysis. Front Cardiovasc Med. 2020;7:590975. DOI: 10.3389/fcvm.2020.590975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krasopoulos G, Falconieri F, Benedetto U, Newton J, Sayeed R, Kharbanda R, Banning A. European real world trans‐catheter aortic valve implantation: systematic review and meta‐analysis of European national registries. J Cardiothorac Surg. 2016;11:1–9. DOI: 10.1186/s13019-016-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Z, Kidney E, Bem D, Bramley G, Bayliss S, de Belder MA , Cummins C, Duarte R. Transcatheter aortic valve implantation for aortic stenosis in high surgical risk patients: a systematic review and meta‐analysis. PLoS One. 2018;13:e0196877. DOI: 10.1371/journal.pone.0196877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, Du Y, Fu M, Ma Y, Wang D, Zhang J, Liu W, Zhao Y, Zhou Y. Clinical outcomes of transcatheter aortic valve replacement in nonagenarians: a systematic review and meta‐analysis. J Interv Cardiol. 2019;2019:1–10. DOI: 10.1155/2019/5819232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wagner G, Steiner S, Gartlehner G, Arfsten H, Wildner B, Mayr H, Moertl D. Comparison of transcatheter aortic valve implantation with other approaches to treat aortic valve stenosis: a systematic review and meta‐analysis. Syst Rev. 2019;8:1–2. DOI: 10.1186/s13643-019-0954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang D, Huang L, Zhang Y, Cheng Z, Zhang X, Ren P, Hong Q, Kang D. Transcatheter aortic valve implantation versus surgical aortic valve replacement for treatment of severe aortic stenosis: comparison of results from randomized controlled trials and real‐world data. Braz J Cardiovasc Surg. 2020;35:346–367. DOI: 10.21470/1678-9741-2019-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams ML, Flynn CD, Mamo AA, Tian DH, Kappert U, Wilbring M, Folliguet T, Fiore A, Miceli A, D’Onofrio A, et al. Long‐term outcomes of sutureless and rapid‐deployment aortic valve replacement: a systematic review and meta‐analysis. Ann Cardiothorac Surg. 2020;9:265. DOI: 10.21037/acs-2020-surd-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xie X, Shi X, Xun X, Rao L. Efficacy and safety of transcatheter aortic valve implantation for bicuspid aortic valves: a systematic review and meta‐analysis. Ann Thorac Cardiovasc Surg. 2016;22:203–215. DOI: 10.5761/atcs.ra.16-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Siemieniuk RA, Agoritsas T, Manja V, Devji T, Chang Y, Bala MM, Thabane L, Guyatt GH. Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: systematic review and meta‐analysis. BMJ. 2016;354. DOI: 10.1136/bmj.i5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new‐generation devices: a systematic review. Eur Heart J. 2018;39:2003–2013. DOI: 10.1093/eurheartj/ehx785. [DOI] [PubMed] [Google Scholar]
- 124.Regueiro A, Abdul‐Jawad Altisent O, Del Trigo M, Campelo‐Parada F, Puri R, Urena M, Philippon F, Rodés‐Cabau J. Impact of new‐onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement: a systematic review and meta‐analysis. Circ Cardiovasc Interv. 2016;9:e003635. DOI: 10.1161/CIRCINTERVENTIONS.115.003635. [DOI] [PubMed] [Google Scholar]
- 125.Quintana RA, Monlezun DJ, DaSilva‐DeAbreu A, Sandhu UG, Okwan‐Duodu D, Ramírez J, Denktas AE, Jneid H, Paniagua D. One‐year mortality in patients undergoing transcatheter aortic valve replacement for stenotic bicuspid versus tricuspid aortic valves: a meta‐analysis and meta‐regression. J Interv Cardiol. 2019;2019:1–12. DOI: 10.1155/2019/8947204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Panchal HB, Barry N, Bhatheja S, Albalbissi K, Mukherjee D, Paul T. Mortality and major adverse cardiovascular events after transcatheter aortic valve replacement using Edwards valve versus CoreValve: a meta‐analysis. Cardiovasc Revasc Med. 2016;17:24–33. DOI: 10.1016/j.carrev.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 127.Nagaraja V, Raval J, Eslick GD, Ong AT. Transcatheter versus surgical aortic valve replacement: a systematic review and meta‐analysis of randomised and non‐randomised trials. Open Heart. 2014;1:e000013. DOI: 10.1136/openhrt-2013-000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malik AH, Zaid S, Ahmad H, Goldberg J, Dutta T, Undemir C, Cohen M, Aronow WS, Lansman SL. A meta‐analysis of 1‐year outcomes of transcatheter versus surgical aortic valve replacement in low‐risk patients with severe aortic stenosis. J Geriatr Cardiol. 2020;17:43–50. DOI: 10.11909/j.issn.1671-5411.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma X, Zhao D, Li J, Wei D, Zhang J, Yuan P, Kong X, Ma J, Ma H, Sun L, et al. Transcatheter aortic valve implantation in the patients with chronic liver disease: a mini‐review and meta‐analysis. Medicine. 2020;99:e19766. DOI: 10.1097/MD.0000000000019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gozdek M, Zieliński K, Pasierski M, Matteucci M, Fina D, Jiritano F, Meani P, Raffa GM, Malvindi PG, Pilato M, et al. Transcatheter aortic valve replacement with self‐expandable ACURATE neo as compared to balloon‐expandable SAPIEN 3 in patients with severe aortic stenosis: meta‐analysis of randomized and propensity‐matched studies. J Clin Med. 2020;9:397. DOI: 10.3390/jcm9020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arora S, Misenheimer JA, Jones W, Bahekar A, Caughey M, Ramm CJ, Caranasos TG, Yeung M, Vavalle JP. Transcatheter versus surgical aortic valve replacement in intermediate risk patients: a meta‐analysis. Cardiovasc Diagn Ther. 2016;6:241–249. DOI: 10.21037/cdt.2016.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saint Croix GR, Lacy SC, Hrachian H, Beohar N. Clinical impact of preexisting right bundle branch block after transcatheter aortic valve replacement: a systematic review and meta‐analysis. J Interv Cardiol. 2020;2020:21. DOI: 10.1155/2020/1789516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Franzoni I, Latib A, Maisano F, Costopoulos C, Testa L, Figini F, Giannini F, Basavarajaiah S, Mussardo M, Slavich M, et al. Comparison of incidence and predictors of left bundle branch block after transcatheter aortic valve implantation using the CoreValve versus the Edwards valve. Am J Cardiol. 2013;112:554–559. DOI: 10.1016/j.amjcard.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 134.Bisson A, Bodin A, Herbert J, Lacour T, Saint Etienne C, Pierre B, Clementy N, Deharo P, Babuty D, Fauchier L. Pacemaker implantation after balloon‐or self‐expandable transcatheter aortic valve replacement in patients with aortic stenosis. J Am Heart Assoc. 2020;9:e015896. DOI: 10.1161/JAHA.120.015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Puls M, Sobisiak B, Bleckmann A, Jacobshagen C, Danner BC, Huenlich M, Beissbarth T, Schoendube F, Hasenfuss G, Seipelt R, et al. Impact of frailty on short‐and long‐term morbidity and mortality after transcatheter aortic valve implantation: risk assessment by Katz Index of activities of daily living. EuroIntervention. 2014;10:609–619. DOI: 10.4244/EIJY14M08_03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S12
Figures S1–S25
References 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135


