Abstract
Background
Myocardial bridging (MB) may represent a cause of myocardial ischemia in patients with non‐obstructive coronary artery disease (NOCAD). Herein, we assessed the interplay between MB and coronary vasomotor disorders, also evaluating their prognostic relevance in patients with myocardial infarction and non‐obstructive coronary arteries (MINOCA) or stable NOCAD.
Methods and Results
We prospectively enrolled patients with NOCAD undergoing intracoronary acetylcholine provocative test. The incidence of major adverse cardiac events, defined as the composite of cardiac death, non‐fatal myocardial infarction, and rehospitalization for unstable angina, was assessed at follow‐up. We also assessed angina status using Seattle Angina Questionnaires summary score. We enrolled 310 patients (mean age, 60.6±11.9; 136 [43.9%] men; 169 [54.5%] stable NOCAD and 141 [45.5%] MINOCA). MB was found in 53 (17.1%) patients. MB and a positive acetylcholine test coexisted more frequently in patients with MINOCA versus stable NOCAD. MB was an independent predictor of positive acetylcholine test and MINOCA. At follow‐up (median, 22 months; interquartile range, 13–32), patients with MB had a higher rate of major adverse cardiac events, mainly driven by a higher rate of hospitalization attributable to angina, and a lower Seattle Angina Questionnaires summary score (all P<0.001) compared with patients without MB. In particular, the group of patients with MB and a positive acetylcholine test had the worst prognosis.
Conclusions
Among patients with NOCAD, coronary spasm associated with MB may predict a worse clinical presentation with MINOCA and a higher rate of hospitalization attributable to angina at long‐term follow‐up with a low rate of hard events.
Keywords: acute coronary syndrome, coronary spasm, MINOCA, myocardial bridging, myocardial ischemia, prognosis
Subject Categories: Prognosis, Clinical Studies, Coronary Circulation, Endothelium/Vascular Type/Nitric Oxide, Ischemia
Nonstandard Abbreviations and Acronyms
- MACE
major adverse cardiac events
- MB
myocardial bridging
- MINOCA
myocardial infarction and non‐obstructive coronary arteries
- NOCAD
non‐obstructive coronary artery disease
- SAQ
Seattle Angina Questionnaire
Clinical Perspective
What Is New?
Myocardial bridging is an independent predictor for a positive response to acetylcholine test and for myocardial infarction and non‐obstructive coronary arteries as clinical presentation.
The coexistence of myocardial bridging and a positive acetylcholine test is associated with a worse cardiovascular outcome.
What Are the Clinical Implications?
Performing acetylcholine provocative test may be useful to guide management and prognostic stratification of patients with myocardial bridging.
Myocardial bridging (MB) is a congenital variant in which a portion of an epicardial coronary artery takes an intramuscular course determining a dynamic compression during systole.1 As coronary blood flow occurs primarily during diastole, this phenomenon has been long considered a benign condition.1, 2 However, the presence of MB has been associated with the occurrence of angina, and suggested as a possible cause of acute coronary syndrome and sudden cardiac death.1, 2 Several mechanisms underlying myocardial ischemia in patients with MB have been suggested, as the result of the interplay between heart rate and diastolic perfusion time, transmural perfusion gradients, diffuse and focal coronary artery disease, localized coronary vasoconstriction, and Venturi‐like effect.3, 4, 5, 6, 7, 8 Of importance, it has been hypothesized that the longstanding compression‐relaxation effect of MB on the coronary arteries may induce endothelial dysfunction and an enhanced local vascular reactivity to systemic vasoconstrictor stimuli.9 Accordingly, previous studies demonstrated that patients with MB and non‐obstructive coronary artery disease (NOCAD) undergoing intracoronary provocative test have a higher occurrence of epicardial spasm compared with patients without MB.8, 10, 11, 12 However, these studies mainly enrolled patients with stable angina, thus the role of MB and vasomotor disorders in patients with myocardial infarction and non‐obstructive coronary arteries (MINOCA) has never been investigated.8, 10, 11, 12 Of note, coronary spasm, at both epicardial and microvascular level, has been demonstrated as an important cause of MINOCA.13, 14, 15, 16 In this study, we tested the hypothesis that the occurrence of epicardial or microvascular spasm in patients with MB may represent a mechanism involved in the pathogenesis of MINOCA.
Therefore, in our study we aimed at assessing the clinical correlates and the prognostic value of MB in patients with NOCAD undergoing provocative testing with acetylcholine. Moreover, we assessed the relationship between MB and the response to invasive provocative test in these patients, evaluating also their impact on prognosis.
Methods
Study Population
We prospectively enrolled consecutive patients admitted to the Department of Cardiovascular Sciences of Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome, Italy, undergoing clinically indicated coronary angiography for suspected myocardial ischemia with angiographic evidence of NOCAD (angiographically normal coronary arteries or diffuse atherosclerosis with stenosis <50% or fractional flow reserve >0.80) and undergoing an intracoronary provocative test with acetylcholine from September 2015 to December 2019. There was no overlap in the enrollment of patients with a previous study published by our group.13 We enrolled both patients admitted with suspected stable angina and patients with suspected MINOCA. Patients with stable angina were defined as patients admitted with a stable pattern of typical chest pain at rest, on exertion, or a combination of both, without signs of myocardial infarction (MI). Patients with MINOCA were diagnosed based on their reporting of one or more episodes of chest pain at rest, typical enough to suggest a cardiac ischaemic origin in the previous 24 hours, associated with ST‐segment and/or T wave abnormalities on the ECG and detection of raise and fall of serum troponin‐T levels with at least 1 value exceeding the 99th percentile of a normal reference population with an upper limit of 0.014 μg/L.17 Among patients presenting with MINOCA, we excluded those with obvious causes of MI other than suspected coronary vasomotor abnormalities and in whom provocative testing was not performed. In particular, we excluded 66 patients with a diagnosis of Takotsubo syndrome confirmed by left ventricle angiography, 40 patients with a suspected diagnosis of myocarditis (diagnosis based on the presence of signs and symptoms of inflammatory activation associated with wall motion abnormalities at left ventricular angiography and echocardiogram suggesting a non‐epicardial pattern confirmed by subsequent cardiac magnetic resonance imaging), 109 patients with type 2 MI with mechanism other than suspected vasospasm (eg, pulmonary embolism, evidence of coronary thrombosis on an unstable plaque confirmed by optical coherence tomography, cardiotoxic drug administration, hypertensive crisis, or severe valvulopathies). Finally, 310 patients who underwent acetylcholine intracoronary provocative test were included in the analysis (Figure 1). The study protocol complied with the Declaration of Helsinki and the study was approved by the institutional review committee. All patients gave written informed consent to coronary angiography and provocative tests and to be included in the follow‐up study.
Figure 1. Myocardial bridging and coronary spasm among patients with NOCAD with myocardial ischemia undergoing acetylcholine provocative test.
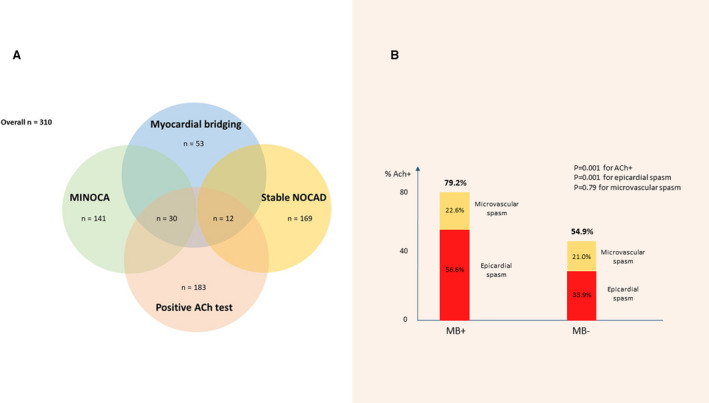
A, Incidence of myocardial bridging, positive acetylcholine provocative test, MINOCA, and stable non‐obstructive coronary artery disease among patients with myocardial ischemia and non‐obstructive coronary artery disease enrolled in our study. B, Incidence of a positive response at acetylcholine test, epicardial spasm or microvascular spasm according to the presence or absence of myocardial bridging. MB+ indicates myocardial bridging presence; MB−, myocardial bridging absence; MINOCA, myocardial infarction and non‐obstructive coronary arteries; and NOCAD, non‐obstructive coronary artery disease.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Coronary Angiography and Invasive Provocative Test Protocol
Coronary angiography was performed through radial or femoral artery approach. To fully expose all segments of the coronary arteries, at least 2 perpendicular projections for right coronary artery and 4 projections for left coronary artery were taken. MB was angiographically defined as a >50% reduction in the luminal diameter of the coronary artery in systole, which return normal or near normal in diastole; the presence of MB was assessed after intracoronary nitroglycerine administration at the end of the intracoronary acetylcholine provocative test.6 Intracoronary acetylcholine provocative test was performed immediately after coronary angiography. The decision of testing with provocative test left coronary artery or right coronary artery as first was left to the discretion of the physicians; both left coronary artery and right coronary artery were tested if the first test was negative. The acetylcholine provocative test was performed as previously described13 and procedural details are reported in Data S1.
The test was considered positive for epicardial coronary spasm in the presence of focal or diffuse epicardial coronary diameter reduction ≥90% in comparison with the relaxed state following intracoronary nitroglycerine administration given to relieve the spasm, associated with the reproduction of the patient's symptoms and ischemic ECG shifts.18 Microvascular spasm was diagnosed when typical ischemic ST‐segment changes and angina developed in the absence of epicardial coronary constriction ≥90% diameter reduction.19 Patients who experienced no angina, spasm, or ST‐segment shifts were considered to have a negative test response (normal coronary vasoreactivity).18 Furthermore, patients who experienced ischemic ECG shifts without angina were considered to have a negative test response.18
Clinical Outcome and Patient Follow‐Up
We defined major adverse cardiac events (MACE) as the composite of cardiac death, non‐fatal MI, and hospitalization because of unstable angina. We only counted the number of patients with the first occurrence of an MACE event during the follow‐up period. Cardiac death included sudden death or death preceded by typical chest pain; non‐fatal MI was defined as typical chest pain at rest associated with ST‐segment and/or T‐wave abnormalities on the ECG and detection of increased serum troponin‐T levels.
We also recorded the occurrence of episodes of angina (requiring or not hospitalization) during the follow‐up period and collected the Seattle Angina Questionnaires summary score at 1‐year.20
All patients received a clinical follow‐up by telephonic interview and/or clinical check at 6, 12, 24, 36,48, and 60 months, and of importance, all patients with a positive response at provocative testing were discharged from the hospital after the index admission with an optimal medical treatment including calcium‐channel blockers and statins up‐titrated at the highest tolerated doses.
Statistical Analysis
Data distribution was assessed according to the Kolmogorov‐Smirnov test. Continuous variables were compared using an unpaired Student t‐test or Mann–Whitney U test, as appropriate, and data were expressed as mean±SD or as median (interquartile range). Categorical data were evaluated using the χ2 test or Fisher exact test as appropriate. A multivariable logistic regression analysis for the occurrence of acetylcholine positive test or for MINOCA as clinical presentation in the overall population was performed including all variables with a P value of <0.05 at the univariate analysis.
Survival curves of MACE for patients with or without MB were produced using the Kaplan–Meier method and were compared by log‐rank test. Univariable Cox regression analysis was applied to assess the relationship of individual variables with MACE. Cox regression was then applied to identify variables independently associated with MACE; to this aim, we included in the multivariable model only variables showing P≤0.05 at univariable analysis. All tests were 2‐sided, and a P value of <0.05 represented statistically significant differences. All analyses were performed using SPSS version 21 (SPSS Inc., Chicago, IL).
Results
Baseline Characteristics According to Provocative Test Response
We enrolled 310 patients (mean age, 60±11.9 years; 136 [43.9%] men) with suspected myocardial ischemia and NOCAD (169 [54.5%] with stable angina and 141 [45.5%] with MINOCA) undergoing acetylcholine provocative test. A positive provocative acetylcholine test occurred in 183 (59.0%) patients (117 [37.7%] epicardial spasm, 66 [21.3%] microvascular spasm). Patients with a positive provocative test, compared with patients with a negative test, had a higher prevalence of MINOCA as clinical presentation (98 [53.6%] versus 43 [33.9%], P=0.001) and MB (42 [23%] versus 11 [8.7%], P=0.01) (Figure 1A; Table S1). There were no differences in the rates of complication during acetylcholine test between MINOCA and patients with stable angina (14 [9.9%] versus 14 [8.3%], P=0.61).
Clinical Characteristics and Provocative Test Response According to the Presence of Myocardial Bridging
In the overall population, 53 (17.1%) patients were found to have MB on coronary angiography. MB was located in 46 (14.8%) patients within the left anterior descending coronary artery and in 7 (2.3%) patients within the left circumflex coronary artery. Compared with patients without MB, patients with MB had a higher prevalence of dyslipidemia (36 [67.0%] versus 122 [47.5%], P=0.007) and MINOCA as clinical presentation (34 [64.1%] versus 107 [41.6%] P=0.003). All other clinical, biochemical, and echocardiographic parameters were not significantly different between the 2 groups (Table 1).
Table 1.
Clinical, Echocardiographic and Angiographic Features in the Overall Population and According to the Presence or Absence of Myocardial Bridging
| Characteristics | Overall Population (n=310) | Presence of Myocardial Bridging (n=53) | Absence of Myocardial Bridging (n=257) | P Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y; median (IQR) | 60.6±11.9 | 60±12.8 | 60.7±11.8 | 0.70 |
| Male sex, n (%) | 136 (43.9) | 29 (54.7) | 107 (41.6) | 0.08 |
| Hypertension, n (%) | 206 (66.5) | 33 (62.2) | 173 (67.3) | 0.48 |
| Diabetes mellitus, n (%) | 61 (19.7) | 15 (28.3) | 46 (17.9) | 0.08 |
| Smoking habit, n (%) | 105 (33.9) | 20 (37.7) | 85 (33.1) | 0.51 |
| Dyslipidemia, n (%) | 158 (51.0) | 36 (67.0) | 122 (47.5) | 0.007 |
| Obesity, n (%) | 24 (7.7) | 3 (5.7) | 21 (8.2) | 0.53 |
| Family history of CAD, n (%) | 94 (30.3) | 16 (30.2) | 78 (30.3) | 0.98 |
| Clinical presentation, n (%) | 0.003 | |||
| MINOCA, n (%) | 141 (45.5) | 34 (64.1) | 107 (41.6) | |
| Stable angina, n (%) | 169 (54.5) | 18 (35.8) | 150 (58.3) | |
| Previous cardiovascular history, n (%) | 27 (8.7) | 2 (3.8) | 25 (9.7) | 0.16 |
| Laboratory data | ||||
| Hemoglobin (g/dL), median (IQR) | 13.2 (12.4; 14.2) | 13.3 (12.3; 15.1) | 13.1 (12.4; 14.1) | 0.34 |
| WBC (×103/L), median (IQR) | 7.1 (6.1; 7.9) | 7.2 (6.1; 8.1) | 7.0 (6.1; 7.8) | 0.96 |
| Serum creatinine on admission (mg/dL), median (IQR) | 0.83 (0.71; 0.96) | 0.82 (0.68; 0.96) | 0.83 (0.71; 0.97) | 0.77 |
| Troponin‐T peak (ng/mL), median (IQR) | 0.01 (0.01; 0.19) | 0.13 (0.01; 0.46) | 0.01 (0.01; 0.13) | 0.52 |
| CRP (mg/L), median (IQR) | 0.05 (0.05; 0.5) | 0.05 (0.05; 3.1) | 0.05 (0.05; 0.50) | 0.97 |
| Echocardiographic data | ||||
| EF on admission (%), median (IQR) | 61 (58; 64) | 61 (58; 63) | 61 (58; 64) | 0.79 |
| EF on admission <50%, n, (%) | 20 (6.5) | 2 (3.8) | 18 (7.0) | 0.38 |
| Diastolic dysfunction, n, (%) | 191 (61.6) | 29 (54.7) | 162 (63.0) | 0.26 |
| Angiographic data | ||||
| Myocardial bridging location | ||||
| LAD, n, (%) | 46 (14.8) | 46 (86.8) | 0 (0.0) | |
| LCx, n, (%) | 7 (2.2) | 7 (13.2) | 0 (0.0) | |
| RCA, n, (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Myocardial bridging segment | ||||
| Proximal, n (%) | 3 (1.0) | 3 (5.7) | 0 (0.0) | |
| Mid, n (%) | 38 (12.2) | 38 (71.7) | 0 (0.0) | |
| Distal, n (%) | 12 (3.9) | 12 (22.6) | 0 (0.0) | |
| Myocardial bridging length, mm (mean±SD) | 24.9±7.3 | 24.9±7.3 | … | |
| Presence of non‐obstructive atherosclerosis | 150 (48.4) | 24 (45.3) | 126 (49.0) | 0.62 |
| Provocative test | ||||
| Positive, n (%) | 183 (59.0) | 42 (79.2) | 141 (54.9) | 0.001 |
| Type of positive response | ||||
| Epicardial spasm, n (%) | 117 (37.7) | 30 (56.6) | 87 (33.8) | 0.001 |
| Microvascular spasm, n (%) | 66 (21.3) | 12 (22.6) | 54 (21.0) | 0.79 |
| Coronary vasospasm only on MB segment, n (%) | 25 (8.0) | 25 (47.2) | … | |
| Coronary vasospasm on MB segment and other segments, n (%) | 4 (1.3) | 4 (7.5) | … | |
| Coronary vasospasm only on segments other than MB, n (%) | 1 (0.3) | 1 (1.9) | … | |
| High Ach dose (≥100 µg), n (%) | 190 (61.3) | 22 (41.5) | 168 (65.4) | 0.001 |
| Complications | 28 (9.0) | 4 (7.6) | 24 (9.3) | 0.68 |
| AF/SVT, n (%) | 8 (2.6) | 2 (3.8) | 6 (2.3) | 0.12 |
| Atrioventricular Block, n (%) | 19 (6.1) | 2 (3.8) | 17 (6.6) | 0.88 |
| VT/VF, n (%) | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0.89 |
| Therapy at discharge, n (%) | ||||
| Statin | 182 (58.7) | 32 (60.4) | 150 (58.4) | 0.79 |
| Calcium channel blockers | 206 (66.5) | 44 (83) | 162 (63) | 0.005 |
| β‐blockers | 93 (30) | 8 (15.1) | 85 (33.1) | 0.009 |
| Nitrates | 6 (1.9) | 3 (5.7) | 3 (1.2) | 0.06 |
| Cardioaspirin | 143 (46.1) | 27 (50.9) | 116 (45.1) | 0.44 |
| ACEi/ARBs | 216 (69.7) | 41 (77.4) | 175 (68.1) | 0.18 |
ACEI indicates angiotensin‐converting enzymes inhibitors; ACH, acetylcholine; AF, atrial fibrillation; ARBs, angiotensin receptor blockers; CAD, coronary artery disease; CRP, C‐reactive protein; EF, ejection fraction; IQR, interquartile range; LAD, left anterior descending; LCx, left circumflex; MB, myocardial bridging; MINOCA, myocardial infarction and non‐obstructive coronary arteries; RCA, right coronary artery; SVT, supraventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia; and WBC, white blood count.
Of importance, patients with MB had a higher occurrence of acetylcholine positive test (42 [79.2%] versus 141 [54.9%], P=0.001) compared with patients without MB, driven by a higher occurrence of epicardial spasm, while rate of microvascular spasm did not differ between the 2 groups (Figure 1B). Furthermore, among patients with a positive test, the presence of MB was associated with a higher rate of acetylcholine low (<100 μg) dose needed to achieve a positive test (28 [66.6%] versus 68 [47.9%], P=0.032) (Table 1). Of interest, MB and a positive acetylcholine test coexisted more frequently in patients with MINOCA as clinical presentation than in stable patients with NOCAD (30/141 [21.3%] versus 12/169 [7%], P=0.002).
Of note, among patients with MB, those with acetylcholine positive test had a longer MB segment compared with patients with acetylcholine negative test (26.3±7.5 mm versus 18.2±4.0 mm, P=0.001).
Finally, promptly reversible arrhythmic complications during provocative test occurred in 28 (9%) patients, without significant differences between patients with and without MB (Table 1).
Predictors of Positive Acetylcholine Provocative Test in the Overall Population
At univariate logistic regression analysis, dyslipidemia (odds ratio [OR], 1.60; 95% CI, 1.01–2.52; P=0.044), C‐reactive protein levels (OR, 1.06; 95% CI, 1.01–1.11, P=0.035), MINOCA as clinical presentation (OR, 2.39; 95% CI, 1.49–3.82; P<0.001) and the presence of MB (OR, 3.14; 95% CI, 1.55–6.37; P=0.001) were predictors of positive acetylcholine test. At multivariate logistic regression analysis, C‐reactive protein levels (OR, 1.06; 95% CI, 1.01–1.11; P=0.028), MINOCA as clinical presentation (OR, 2.20; 95% CI, 1.35–3.58; P=0.002), and the presence of MB (OR, 2.57; 95% CI, 1.24–5.33; P=0.011) remained independent predictors for acetylcholine positive test (Table S2).
Predictors of MINOCA as Clinical Presentation
We analyzed predictors of MINOCA as clinical presentation in the overall population and we demonstrated that only a positive acetylcholine test and the presence of MB (OR, 2.02; 95% CI, 1.25–3.26; P=0.004 and OR, 2.39; 95% CI, 1.27–4.49; P=0.007, respectively) were independent predictors of MINOCA (Table S3). Moreover, we performed a sensitivity analysis evaluating the predictive value of MB for MINOCA as clinical presentation in patients with a positive response and in patients with a negative response to acetylcholine test, demonstrating that the presence of MB was a predictor of MINOCA only in patients with a positive acetylcholine test, but not in patients with a negative acetylcholine test (Table S4), suggesting a strong relationship between MB and vasomotor disorders in MINOCA.
Of importance, considering only patients with MB, we assessed the predictors of MINOCA as clinical presentation to identify MB features associated with a more “malignant” clinical course. We demonstrated that among patients having MB, the presence of acetylcholine positive test was the only independent predictor of MINOCA as clinical presentation (OR, 4.37; 95% CI, 1.08–17.72; P=0.039).
Clinical Outcome According to the Presence of MB and Provocative Test Response
At a median follow‐up of 22 months (interquartile range, 13–32 months), MACE occurred in 25 patients (8.1%) (Table 2). MACE rate was higher in patients with MB compared with patients without MB (12 [22.6%] versus 13 [5.1%], P<0.001). Moreover, patients with MB had more frequent recurrence of angina (20 [37.7%] versus 50 [19.4%], P=0.004) and a lower Seattle Angina Questionnaires summary score (78 [68–84] versus 84 [78–88], P<0.001) compared with patients without MB (Table 2). At univariate Cox regression analysis a positive acetylcholine test (hazard rartio [HR], 2.76; CI, 1.03–7.34; P=0.043), the presence of MB (HR, 5.46; 95% CI, 2.48–11.99; P<0.001) and MINOCA as clinical presentation (HR, 5.48; 95% CI, 2.05–14.63; P=0.001), were predictors of MACE at follow‐up, but only the presence of MB (HR, 3.98; 95% CI, 1.78–8.93; P=0.001) and MINOCA (HR, 4.23; 95% CI, 1.56–11.48; P=0.005) as clinical presentation remained significant at multivariable Cox regression analysis (Table S5). Finally, comparisons of the Kaplan–Meier curves by log‐rank test showed that patients with MB had a worse MACE‐free survival compared with those without MB (P<0.001) (Figure 2A).
Table 2.
Clinical Outcome in the Overall Population and According to the Presence or Absence of Myocardial Bridging
| Characteristics | Overall Population (n=310) | Presence of Myocardial Bridging (n=53) | Absence of Myocardial Bridging (n=257) | P Value |
|---|---|---|---|---|
| MACE, n (%) | 25 (8.1) | 12 (22.6) | 13 (5.1) | <0.001 |
| Cardiovascular death, n (%) | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0.65 |
| MI occurrence, n (%) | 6 (1.9) | 2 (3.8) | 4 (1.6) | 0.29 |
| Hospitalization for unstable angina, n (%) | 18 (5.8) | 10 (18.9) | 8 (3.1) | <0.001 |
| Recurrent angina, n (%) | 70 (22.6) | 20 (37.7) | 50 (19.4) | 0.004 |
| SAQ summary score, median (IQR) | 82 (78; 88) | 78 (68; 84) | 84 (78; 88) | <0.001 |
| Follow‐up time (mo), median (IQR) | 22 (13; 32) | 18 (13; 28) | 23 (13; 34) | 0.09 |
IQR indicates interquartile range; MACE, major adverse cardiovascular event; MI, myocardial infarction; and SAQ, Seattle Angina Questionnaire.
Figure 2. Survival Kaplan–Meier curves for major adverse cardiac events according to the presence or absence of myocardial bridging (A), and according to the presence/absence of myocardial bridging and positive/negative response at acetylcholine provocative test (B).
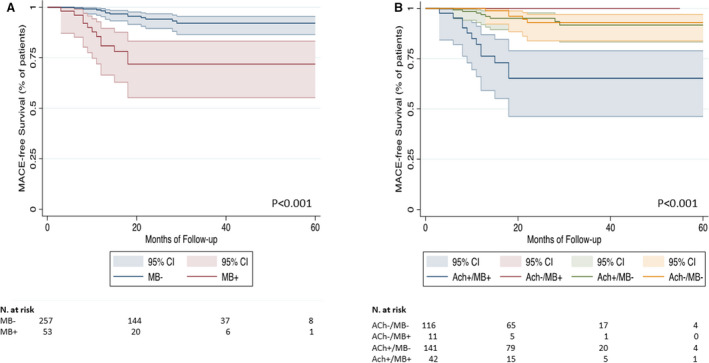
Ach indicates acetylcholine; MACE, major adverse cardiac events; MB+, myocardial bridging presence; and MB−, myocardial bridging absence.
Of interest, the rates of MACE and of recurrent angina were significantly higher among patients with MB and a positive acetylcholine test (P<0.001 for both MACE and recurrent angina), along with a lower Seattle Angina Questionnaires summary score (P<0.001) (Figure 3A and 3B). Moreover, comparisons of the Kaplan–Meier curves by log‐rank test showed that patients with MB and a positive acetylcholine test had also a lower MACE‐free survival and they represent the group with the worst prognosis (P<0.001, Figure 2B). Therapy at discharge according to presence or absence of MB and according to acetylcholine response is reported in Table S6.
Figure 3. Curves are compared by the log‐rank test.
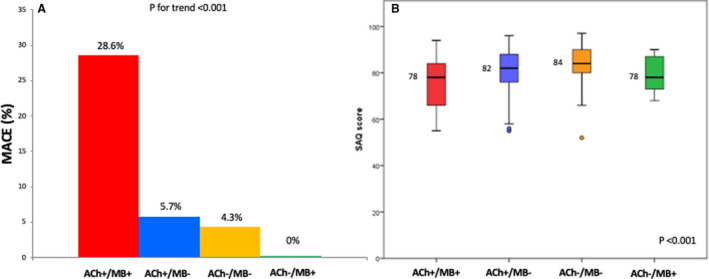
We had no patient loss at follow‐up. Occurrence of major adverse cardiac events at follow‐up (A) and Seattle Angina Questionnaires summary score at 1‐year (B) according to the presence/absence of myocardial bridging and positive/negative response at acetylcholine provocative test. Ach indicates acetylcholine; MACE, major adverse cardiac events; MB+, myocardial bridging presence; MB−, myocardial bridging absence; and SAQ. Seattle Angina Questionnaires.
We performed a subgroup analysis considering only patients with MINOCA demonstrating that a positive acetylcholine test (HR, 5.38; 95% CI, 1.08–26.93; P=0.037) and the presence of MB (HR, 3.17; 95% CI, 1.07–9.41; P=0.04) were the only independent predictors of MACE. At the same time, a positive acetylcholine test (HR, 2.88; 95% CI, 1.11–7.42; P=0.029) and the presence of MB (HR, 2.29; 95% CI, 1.19–4.39; P=0.013) were the only independent predictors of recurrent angina.
Discussion
In our study, we assessed the relationship between MB and coronary spasm in patients with non‐obstructive coronary artery disease undergoing provocative testing with acetylcholine and their impact on the outcomes. We demonstrate that: (1) MB is an independent predictor for a positive response to acetylcholine test; (2) MB is an independent predictor of MINOCA as initial clinical presentation; (3) the coexistence of MB and a positive acetylcholine test is present in 21% of patients with MINOCA with suspected vasomotor abnormalities, thus suggesting that in this cohort coronary spasm is the mechanism of instability; (4) not all MB are born equal as a positive response to acetylcholine test identifies a subset with a worse outcome while, in sharp contrast, patients with MB but without a positive acetylcholine test have an excellent outcome.
MB is a well‐known cause of myocardial ischemia in patients with NOCAD.1, 4, 5, 6, 21 In keeping with previous studies, we found that MB is present in ≈17% of patients undergoing coronary angiography for suspected myocardial ischemia.1, 3, 22, 23 Moreover, we demonstrated that patients with MB had more frequently a positive response to acetylcholine test compared with those without MB. Previous studies demonstrated an enhanced local vascular reactivity to vasoconstrictor stimuli in MB coronary segments.24, 25, 26, 27 Accordingly, a recent study by Sara et al showed that patients with MB may present endothelial dysfunction at both epicardial and microvascular level during intracoronary acetylcholine infusion,27 and another study by Nam et al demonstrated that MB is associated with a higher incidence of epicardial spasm during acetylcholine provocative test compared with patients without MB.12 Previous studies evaluating the relationship between vasomotor disorders and MB, however, enrolled stable patients with NOCAD, not considering patients with MINOCA.8, 10, 11, 12, 27 Thus, a novelty of our study is the enrollment not only of patients with stable NOCAD, but also patients with MINOCA, allowing us to assess the relationship between vasomotor disorders and MB, along with their clinical relevance, both in the stable and in the acute setting. Of importance, we found that the presence of MB was an independent predictor of MINOCA as clinical presentation and the coexistence of MB and a positive response at acetylcholine provocative test was detected in 21% of our study population presenting with MINOCA. Of note, in our study only patients with MINOCA with suspected vasomotor disorders underwent acetylcholine provocative test, and of consequence, these results cannot be extended to all patients presenting with MINOCA. Another novelty of our study is its longitudinal design, which allowed us to demonstrate that the presence of MB was an independent predictor of a worse outcome including a higher rate of MACEs and of angina recurrence as well as a worse quality of life. Importantly, this negative impact on the outcomes was confined to those patients with associated coronary spasm. It is worth noting that the higher occurrence of MACEs among patients with MB and coronary spasm is mainly driven by a higher rate of rehospitalization for unstable angina. We have recently demonstrated that invasive provocative test in patients with MINOCA is safe and may be useful to detect the presence of vasomotor disorders as cause of MINOCA, allowing physicians to get a proper diagnosis and to begin a therapy based on the underlying pathogenic mechanism.13 In this study we expand this notion, suggesting that vasomotor disorders occurring in patients with MB may represent an important and frequently underdiagnosed cause of MINOCA. Therefore, the detection of MB at coronary angiography in patients with NOCAD should be considered as a hint to perform an invasive provocative test to unmask the presence of functional alterations of coronary circulation, especially in patients with an acute presentation (Figure 4).
Figure 4. Clinical and prognostic implications of the interplay between myocardial bridging and coronary spasm in patients with myocardial ischemia and non‐obstructive coronary artery disease.
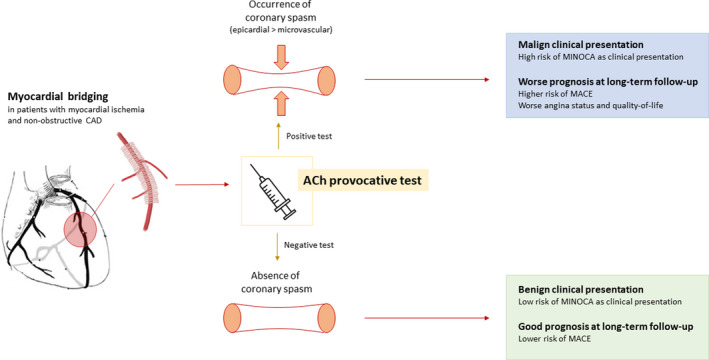
CAD indicates coronary artery disease; MACE, major adverse cardiac events; and MINOCA, myocardial infarction and non‐obstructive coronary arteries.
The study from Nam et al12 demonstrated that patients with MB and with a positive response at acetylcholine provocative test had a higher rate of recurrent angina compared with patients with MB and without induced coronary spasm. However, this study enrolled only patients with stable NOCAD. Our study enrolled both patients with stable NOCAD and MINOCA allowing us to assess the prognostic value of MB and vasomotor disorders in patients with a higher risk of developing cardiac events.
The causes of MINOCA in patients with MB in the absence of coronary spasm cannot be deduced from our study. Other mechanisms linking MB to MINOCA are multiple, including transient thrombosis associated with subangiographic plaque disruption, hemodynamically significant bridging or spontaneous coronary artery dissection.14, 28, 29, 30 Nevertheless, our data suggest that only in the case in which MB‐associated MINOCA is mediated by coronary spasm this is associated with a worse medium‐long term outcome. In contrast with our findings, a study by Brolin et al31 demonstrated no causal link between MB detected at computed tomography and MINOCA as clinical presentation. Differences in study design, diagnostic techniques, and sample size may in part explain the different results compared with our study and suggesting that further studies are needed to clarify the role of MB in the pathogenesis of MINOCA. Moreover, as reported above, in our study only patients with MINOCA with suspected vasomotor disorders underwent acetylcholine provocative test.
Our study has several limitations. First, it is a single‐center study; second, MB was only diagnosed at coronary angiography; coronary angiography is the most common diagnostic tool used to detect the presence of MB, however, recent imaging method techniques such as intravascular ultrasonography, optical coherence tomography, fractional flow reserve, and multidetector computed tomography may be helpful to accurately assess the anatomical and physiological characteristics of MB as well as its hemodynamic significance in addition to coronary angiography,19, 30, 32 therefore, MB cases may be missed or underestimated. However, because the angiographic confirmation could be achieved maximally at "MB augmentation view" in the right anterior oblique cranial view and anterior‐posterior cranial view after intracoronary nitroglycerine injection, by at least 2 different operators, we think that the missing or underestimated MB would be extremely rare in our study population. Third, we did not measure coronary flow reserve and, therefore, its potential relationship with the response to vasoconstrictor stimuli and with MB. Moreover, because MB are dynamic stenosis, an invasive assessment during exercise or situations of increased inotropism could have helped in identifying the hemodynamic relevance of MB during these conditions; it remains unknown if the excess of events is patients with MB and positive acetylcholine provocative tests is also the result of ischemia caused by the dynamic stenosis. Furthermore, only patients with MINOCA with suspected vasomotor disorders underwent acetylcholine provocative test, while patients with MINOCA with other pathogenic mechanisms (ie, plaque rupture/erosion, coronary microembolism, coronary dissection) were not included in our study. As a consequence, our findings cannot be extended to all patients with MINOCA. Finally, other limitations are represented by the lack of an independent clinical event committee for MACE adjudication and by the lack of a core laboratory analysis for MB assessment.
Our study has therapeutic implications. Indeed, β‐blockers and calcium‐channel blockers represent the first‐line medical therapy for flow‐limiting MB.5, 6 However, the use of β‐blockers has been shown to favor the occurrence of coronary spasm.28 Therefore, in the era of precision medicine, performing acetylcholine provocative test may be useful to guide management of patients with MB (Figure 4), prompting the introduction of calcium‐channel blockers rather than β‐blockers in patients with evidence of vasospasm who have a worse outcome.
Sources of Funding
None.
Disclosures
None.
Supporting information
Data S1
Tables S1–S6
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020535
For Sources of Funding and Disclosures, see page 10.
References
- 1.Alegria JR, Herrmann J, Holmes DR Jr, Lerman A, Rihal CS. Myocardial bridging. Eur Heart J. 2005;26:1159–1168. DOI: 10.1093/eurheartj/ehi203. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi T, Ishikawa K. Myocardial bridge: harmless or harmful. Intern Med. 2004;43:1097–1098. DOI: 10.2169/internalmedicine.43.1097. [DOI] [PubMed] [Google Scholar]
- 3.Kim PJ, Hur G, Kim SY, Namgung J, Hong SW, Kim YH, Lee WR. Frequency of myocardial bridges and dynamic compression of epicardial coronary arteries: a comparison between computed tomography and invasive coronary angiography. Circulation. 2009;119:1408–1416. DOI: 10.1161/CIRCULATIONAHA.108.788901. [DOI] [PubMed] [Google Scholar]
- 4.Gould KL, Johnson NP. Myocardial bridges: lessons in clinical coronary pathophysiology. JACC Cardiovasc Imaging. 2015;8:705–709. [DOI] [PubMed] [Google Scholar]
- 5.Corban MT, Hung OY, Eshtehardi P, Rasoul‐Arzrumly E, McDaniel M, Mekonnen G, Timmins LH, Lutz J, Guyton RA, Samady H. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol. 2014;63:2346–2355. DOI: 10.1016/j.jacc.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourassa MG, Butnaru A, Lespérance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351–359. DOI: 10.1016/S0735-1097(02)02768-7. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa Y, Akasaka Y, Suzuki K, Fujiwara M, Ogawa T, Yamazaki K, Niino H, Tanaka M, Ogata K, Morinaga S, et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation. 2009;120:376–383. DOI: 10.1161/CIRCULATIONAHA.108.820720. [DOI] [PubMed] [Google Scholar]
- 8.Kim JW, Park CG, Suh SY, Choi CU, Kim EJ, Rha SW, Seo HS, Oh DJ. Comparison of frequency of coronary spasm in Korean patients with versus without myocardial bridging. Am J Cardiol. 2007;100:1083–1086. DOI: 10.1016/j.amjcard.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Ciampricotti R, el Gamal M . Vasospastic coronary occlusion associated with a myocardial bridge. Cathet Cardiovasc Diagn. 1988;14:118–120. DOI: 10.1002/ccd.1810140213. [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Kitahara H, Shoji T, Tokimasa S, Nakayama T, Sugimoto K, Fujimoto Y, Kobayashi Y. Relation between severity of myocardial bridge and vasospasm. Int J Cardiol. 2017;248:34–38. DOI: 10.1016/j.ijcard.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Teragawa H, Fukuda Y, Matsuda K, Hirao H, Higashi Y, Yamagata T, Oshima T, Matsuura H, Chayama K. Myocardial bridging increases the risk of coronary spasm. Clin Cardiol. 2003;26:377–383. DOI: 10.1002/clc.4950260806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nam P, Choi BG, Choi SY, Byun JK, Mashaly A, Park Y, Jang WY, Kim W, Choi JY, Park EJ, et al. The impact of myocardial bridge on coronary artery spasm and long‐term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis. 2018;270:8–12. DOI: 10.1016/j.atherosclerosis.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, Lanza GA, Crea F. Patients with acute myocardial infarction and non‐obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–98. DOI: 10.1093/eurheartj/ehx667. [DOI] [PubMed] [Google Scholar]
- 14.Scalone G, Niccoli G, Crea F. Editor's Choice‐ Pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J Acute Cardiovasc Care. 2019;8:54–62. DOI: 10.1177/2048872618782414. [DOI] [PubMed] [Google Scholar]
- 15.Crea F, Montone RA, Niccoli G. Myocardial infarction with non‐obstructive coronary arteries: dealing with pears and apples. Eur Heart J. 2020;41:879–881. DOI: 10.1093/eurheartj/ehz561. [DOI] [PubMed] [Google Scholar]
- 16.Montone RA, Meucci MC, De Vita A, Lanza GA, Niccoli G. Coronary provocative tests in the catheterization laboratory: pathophysiological bases, methodological considerations and clinical implications. Atherosclerosis. 2021;318:14–21. DOI: 10.1016/j.atherosclerosis.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237–269. DOI: 10.1161/CIR.0000000000000617. [DOI] [Google Scholar]
- 18.Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group (COVADIS) . International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. DOI: 10.1093/eurheartj/ehv351. [DOI] [PubMed] [Google Scholar]
- 19.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group (COVADIS) . International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. DOI: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 20.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7:640–647. DOI: 10.1161/CIRCOUTCOMES.114.000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. DOI: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irvin RG. The angiographic prevalence of myocardial bridging in man. Chest. 1982;81:198–202. DOI: 10.1378/chest.81.2.198. [DOI] [PubMed] [Google Scholar]
- 23.Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Iliceto S. Left anterior descending artery myocardial bridging: a clinical approach. J Am Coll Cardiol. 2016;68:2887–2899. DOI: 10.1016/j.jacc.2016.09.973. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann J, Higano ST, Lenon RJ, Rihal CS, Lerman A. Myocardial bridging is associated with alteration in coronary vasoreactivity. Eur Heart J. 2004;25:2134–2142. DOI: 10.1016/j.ehj.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Kim JW, Seo HS, Na JO, Suh SY, Choi CU, Kim EJ, Rha SW, Park CG, Oh DJ. Myocardial bridging is related to endothelial dysfunction but not to plaque as assessed by intracoronary ultrasound. Heart. 2008;94:765–769. DOI: 10.1136/hrt.2007.115980. [DOI] [PubMed] [Google Scholar]
- 26.Javadzadegan A, Moshfegh A, Qian Y, Kritharides L, Yong ASC. Myocardial bridging and endothelial dysfunction—computational fluid dynamics study. J Biomech. 2019;85:92–100. DOI: 10.1016/j.jbiomech.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Sara JDS, Corban MT, Prasad M, Prasad A, Gulati R, Lerman LO, Lerman A. Prevalence of myocardial bridging associated with coronary endothelial dysfunction in patients with chest pain and non‐obstructive coronary artery disease. EuroIntervention. 2020;15:1262–1268. DOI: 10.4244/EIJ-D-18-00920. [DOI] [PubMed] [Google Scholar]
- 28.Wu S, Liu W, Zhou Y. Spontaneous coronary artery dissection in the presence of myocardial bridge causing myocardial infarction: an insight into mechanism. Int J Cardiol. 2016;206:77–78. DOI: 10.1016/j.ijcard.2016.01.085. [DOI] [PubMed] [Google Scholar]
- 29.Escaned J, Cortés J, Flores A, Goicolea J, Alfonso F, Hernández R, Fernández‐Ortiz A, Sabaté M, Bañuelos C, Macaya C. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol. 2003;42:226–233. DOI: 10.1016/S0735-1097(03)00588-6. [DOI] [PubMed] [Google Scholar]
- 30.Klues HG, Schwarz ER, vom Dahl J , Reffelmann T, Reul H, Potthast K, Schmitz C, Minartz J, Krebs W, Hanrath P. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation. 1997;96:2905–2913. DOI: 10.1161/01.CIR.96.9.2905. [DOI] [PubMed] [Google Scholar]
- 31.Brolin EB, Brismar TB, Collste O, Y‐Hassan S, Henareh L, Tornvall P, Cederlund K. Prevalence of myocardial bridging in patients with myocardial infarction and nonobstructed coronary arteries. Am J Cardiol. 2015;116:1833–1839. DOI: 10.1016/j.amjcard.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Tsujita K, Maehara A, Mintz GS, Doi H, Kubo T, Castellanos C, Liu J, Yang J, Oviedo C, Franklin‐Bond T, et al. Comparison of angiographic and intravascular ultrasonic detection of myocardial bridging of the left anterior descending coronary artery. Am J Cardiol. 2008;102:1608–1613. DOI: 10.1016/j.amjcard.2008.07.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S6


