Abstract
Insulin stimulates glucose uptake into muscle and fat cells by promoting the translocation of glucose transporter 4 (GLUT4) to the cell surface. Phosphatidylinositide 3-kinase (PI3K) has been implicated in this process. However, the involvement of protein kinase B (PKB)/Akt, a downstream target of PI3K in regulation of GLUT4 translocation, has been controversial. Here we report that microinjection of a PKB substrate peptide or an antibody to PKB inhibited insulin-stimulated GLUT4 translocation to the plasma membrane by 66 or 56%, respectively. We further examined the activation of PKB isoforms following treatment of cells with insulin or platelet-derived growth factor (PDGF) and found that PKBβ is preferentially expressed in both rat and 3T3-L1 adipocytes, whereas PKBα expression is down-regulated in 3T3-L1 adipocytes. A switch in growth factor response was also observed when 3T3-L1 fibroblasts were differentiated into adipocytes. While PDGF was more efficacious than insulin in stimulating PKB phosphorylation in fibroblasts, PDGF did not stimulate PKBβ phosphorylation to any significant extent in adipocytes, as assessed by several methods. Moreover, insulin, but not PDGF, stimulated the translocation of PKBβ to the plasma membrane and high-density microsome fractions of 3T3-L1 adipocytes. These results support a role for PKBβ in insulin-stimulated glucose transport in adipocytes.
The ability of insulin to promote glucose storage in muscle and adipose tissue is crucial to the maintenance of glucose homeostasis. An impairment in the ability of insulin to stimulate glucose uptake in these tissues, a condition termed insulin resistance, contributes to the development of type 2 (non-insulin-dependent) diabetes, hypertension, and cardiovascular disease (25). The primary mechanism of insulin-stimulated glucose uptake is through the translocation of glucose transporter 4 (GLUT4) from an intracellular site to the cell surface (26). Defects in the insulin signal transduction pathways that regulate glucose transport have been considered likely causes of insulin resistance (28).
While the insulin signaling pathways responsible for triggering GLUT4 translocation are yet to be defined, rapid progress has been made. Activation of the insulin receptor results in the tyrosyl phosphorylation of insulin receptor substrate (IRS) proteins, docking proteins that recruit src homology 2-containing signaling proteins via phosphotyrosine moieties. Several lines of evidence suggest the involvement of IRS proteins in insulin-stimulated GLUT4 translocation. Disruption of IRS-1 and IRS-2 in mice causes mild insulin resistance and type 2 diabetes, respectively (6, 60). Overexpression of IRS-1 in rat adipocytes mimics the effect of insulin on GLUT4 translocation (43), while reduction of IRS-1 by an antisense ribozyme (43) or chronic insulin treatment (44) decreases insulin responsiveness. One of the molecules recruited by IRS proteins thought to be required for insulin-stimulated GLUT4 translocation is phosphatidylinositide 3-kinase (PI3K). Two inhibitors of PI3K, wortmannin and LY294002, both inhibit insulin-stimulated GLUT4 translocation (14, 17, 42). Furthermore, introduction of a dominant negative p85 regulatory subunit into adipocytes significantly impairs insulin-stimulated GLUT4 translocation either when microinjected (31) or when overexpressed (47). Overexpression of constitutively active p110 catalytic subunit stimulates GLUT4 translocation to the plasma membrane (PM) in the absence of insulin (38, 53). Hence, these experiments collectively suggest that PI3K is necessary for insulin-stimulated GLUT4 translocation.
Several protein serine/threonine kinases have recently been identified as downstream targets of PI3K. These include protein kinase B (PKB; cellular homolog of v-AKT, also termed RAC-PK) (11, 20, 22), PKCζ (8, 50), and PKCλ (32). Several studies have examined the role of PKB in insulin-stimulated GLUT4 translocation; however, the results have been somewhat contradictory. Constitutively active PKBα has been expressed in either 3T3-L1 adipocytes (30) or rat adipocytes (18, 54) and found to promote GLUT4 translocation to the plasma membrane. Similarly, constitutively active PKBα increased glucose uptake in L6 myotubes (23, 57). Studies utilizing dominant negative PKB have produced conflicting results. In support of a role for PKB in insulin action, Cong et al. (18) found that a kinase-inactive (K179A) PKBα mutant inhibited insulin-stimulated GLUT4 translocation by 20% when transfected into rat adipocytes. However, Hajduch et al. (23) found that this same construct had no significant effect in L6 myotubes. Similarly, two recent studies (29, 32) found that a double-phosphorylation site mutant of PKBα behaved as a dominant negative mutant with respect to 3T3-L1 adipocyte PKB activity measured in vitro but had no significant effect on insulin-stimulated GLUT4 translocation. In contrast, a kinase-inactive, phosphorylation-deficient mutant of PKBα was found to inhibit insulin-stimulated GLUT4 translocation in L6 myoblasts (59). Part of the difficulty in reconciling these results lies in the complexity of PKB regulation, which is only beginning to be deciphered. The use of different cell types, some of which may not be bona fide insulin-responsive cells, has also complicated the interpretation. In addition, chronic overexpression of mutant forms of PKB may enable the cell to adapt via alternate pathways. Another potential problem is isoform specificity. Three isoforms of PKB have been identified; however, these were not distinguished in most studies examining the role of PKB in insulin-stimulated glucose uptake. While all of the PKB mutants studied were based on PKBα, PKBβ was recently identified as the isoform that associates with GLUT4 vesicles in an insulin-dependent manner in rat adipocytes (12). Interestingly, PKBβ appears to be functionally distinct from PKBα and PKBγ, as stress activates PKBα and PKBγ, but not PKBβ (48).
To further assess the role of PKB in insulin stimulation of GLUT4 translocation, we have utilized a different approach from previous studies. Microinjection of specific substrate peptides and antibodies was used to acutely inhibit endogenous PKB action. Microinjection of a PKB substrate peptide (KRPRAATF) caused significant inhibition of insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes. An antibody directed against the N terminus of PKBα, which also recognizes PKBβ, produced similar effects. We further show that PKBβ expression is switched on during adipocyte differentiation whereas PKBα is down-regulated. Concomitant with this change, we also observed a switch in growth factor responsiveness during differentiation in that the ability of platelet-derived growth factor (PDGF) to stimulate PKB phosphorylation is switched off in adipocytes at the expense of a robust activation by insulin. Taken together, our results strongly support PKBβ as the isoform involved in mediating the metabolic effects of insulin in adipocytes.
MATERIALS AND METHODS
Antibodies.
Rabbit antibodies directed against the N terminus of PKBα (residues 3 to 22) were purchased from Santa Cruz Biotechnology (N19; Santa Cruz, Calif.). Sheep antibodies against PKBα (residues 466 to 480), PKBβ (residues 455 to 469, as described in reference 58), and PKBγ (residues 116 to 128) were purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Rabbit PKBβ antibodies were raised against a C-terminal peptide of PKBβ (CDQTHFPQFSYSASIRE). Antibodies specific for PKB phosphorylated at Ser473 or Thr308 were purchased from New England Biolabs (Beverly, Mass.). Monoclonal antihemagglutinin (anti-HA) antibodies (HA11, clone 16B12) were purchased from BabCo (Berkeley, Calif.). Polyclonal anti-GLUT4 antibodies (R017) were raised in rabbits against a 17-amino-acid peptide comprising the C terminus of GLUT4.
Peptide synthesis.
Peptides were synthesized with an Applied Biosystems 430A Peptide synthesizer coupled with the FastMoc strategy. Purity was checked by reverse-phase high-pressure liquid chromatography, and their integrity was confirmed by amino acid analysis and matrix-assisted laser desorption ionization mass spectrometry. Two peptides were examined. KRPRAATF was a peptide closely related to that reported by Alessi et al. (1) as a specific substrate for PKB, except that we included a lysine to increase its solubility in aqueous solutions. KRPRAAAF was the same peptide, except that alanine was substituted for threonine. The first peptide was found to be a good substrate for PKB in in vitro assays. The alanine-substituted peptide partially inhibited PKB phosphorylation of the substrate peptide only at the highest concentration tested (1 mg/ml) (data not shown).
Cell culture.
3T3-L1 fibroblasts (American Type Culture Collection) were grown and differentiated into adipocytes as described elsewhere (55). Briefly, 3T3-L1 fibroblasts were grown and passaged in Dulbecco’s modified Eagle medium supplemented with 10% newborn calf serum. Cells were differentiated 1 to 2 days postconfluence. The differentiation medium contained 10% fetal calf serum (FCS), 250 nM dexamethasone, 500 nM isobutyl methylxanthine, and 500 nM insulin. After 3 days, the differentiation medium was replaced with postdifferentiation medium containing 10% FCS and 250 nM insulin. Cells were fed every 3 days postdifferentiation in Dulbecco’s modified Eagle medium supplemented with 10% FCS. Unless otherwise stated, fibroblasts were used at confluence and adipocytes were used at 8 to 15 days after the initiation of differentiation. 3T3-L1 fibroblasts stably expressing HA-PKBα or HA-PKBβ have been described elsewhere (52).
Microinjection.
Microinjection was performed as previously described (35). Cells grown to confluence and differentiated on coverslips were transferred to Krebs-Ringer bicarbonate buffer (111 mM NaCl, 4.87 mM KCl, 1.15 mM CaCl2, 1.22 mM KH2PO4, 1.21 mM MgSO4, 25.7 mM NaHCO3, 10 mM HEPES, 2.5 mM glucose, 0.5% bovine serum albumin [BSA], 1 mM Na pyruvate, pH 7.4) for 45 min. They were microinjected over a 45-min period with a Zeiss automated injection system (Carl Zeiss, Oberkochen, Germany) coupled to an Eppendorf (Hamburg, Germany) microinjector. Micropipettes were prepared with a Sutter (Novato, Calif.) P-97 micropipette puller. Reagents for microinjection were dissolved in microinjection buffer (5 mM sodium phosphate [pH 7.2] and 100 mM KCl). Sodium azide in the antibody preparation was removed by dialysis in three changes of microinjection buffer. Cells were transferred into fresh medium and allowed to recover for 60 to 90 min following injection of peptide (5 mg/ml) or antibody (0.2 mg/ml), prior to stimulation with insulin (100 nM) and analysis of GLUT4 translocation by the PM lawn assay.
PM lawn assay.
GLUT4 translocation was determined by the PM lawn assay as described by Robinson and James (46) with modifications described by Marsh et al. (37). Briefly, 3T3-L1 cells grown on coverslips were washed in poly-l-lysine after cell treatment, hypotonically shocked with three washes in one-third intracellular buffer (70 mM KCl, 5 mM MgCl2, 3 mM EGTA, 1 mM dithiothreitol, 30 mM HEPES, pH 7.2), and sonicated with a probe sonicator (Microson, Farmington, N.Y.) at setting 0 in intracellular buffer to generate a lawn of PM fragments that remained attached to the coverslip. The fragments were then immunolabeled with rabbit anti-GLUT4 antibodies (R1159) (27) and Cy3-labeled goat anti-rabbit antibodies (Amersham, Little Chalfont, United Kingdom). Coverslips were visualized and imaged with a Bio-Rad Lasersharp MRC-500 confocal laser scanning immunofluorescence microscope. GLUT4 translocation in microinjected cells was compared to that in noninjected cells in the immediate vicinity on the same coverslip. Data was analyzed with Bio-Rad COMOS confocal imaging software. Six or more fields were analyzed for each condition within each experiment.
Treatment of cells, metabolic labeling, and preparation of extracts.
Differentiated 3T3-L1 adipocytes were serum starved by incubating them in Krebs-Ringer phosphate buffer (KRP) containing 12.5 mM HEPES (pH 7.4), 120 mM NaCl, 6 mM KCl, 1.2 mM Mg2SO4, 1 mM CaCl2, 1 mM NaPO4, and 0.1% (wt/vol) BSA for at least 2 h at 37°C prior to stimulation. Insulin (1 μM; Eli Lilly) and PDGF (50 ng/ml; Gibco) treatments were for 5 or 15 min. Where indicated, 100 nM wortmannin (Sigma) was added to KRP 25 min prior to addition of insulin. For metabolic labeling, cells were incubated in low (0.2 mM)-phosphate KRP containing 0.5 mCi of 32Pi (ICN) per ml in place of the KRP incubation and then treated as described above. After treatment, cells were washed three times with ice-cold HES buffer (20 mM HEPES [pH 7.4], 1 mM EDTA, 250 mM sucrose) and homogenized in HES buffer supplemented with protease inhibitors (10 μg of aprotinin per ml, 10 μg of leupeptin per ml, and 1 mM phenylmethylsulfonyl fluoride) and phosphatase inhibitors (1 mM sodium orthovanadate, 1 mM sodium pyrophosphate, 1 mM ammonium molybdate, and 10 mM sodium fluoride) by 15 passes through a 22-gauge needle. 3T3-L1 fibroblasts were harvested in the same manner, except that homogenization was done through a 27-gauge needle. Subcellular fractionation of 3T3-L1 adipocytes was performed as described in the work of Clark et al. (16).
Preparation of primary rat adipocytes.
Primary rat adipocytes were prepared from epididymal fat pads of male Wistar rats (100 to 125 g) by the collagenase digestion method (49). After incubation in KRP (containing 2% BSA) with agitation for 1 h at 37°C, cells were treated with or without 1 μM insulin for 15 min. Adipocytes were collected by centrifugation and homogenized in HES buffer with inhibitors by 15 passes through a 27-gauge needle.
Immunoprecipitation.
Cells were harvested in lysis buffer (50 mM Tris [pH 7.5], 100 mM NaCl, 1% Triton X-100, 25 mM β-glycerophosphate, protease inhibitors, and phosphatase inhibitors) and homogenized by 15 passes through a 22-gauge needle. After incubation on ice for 15 min, lysates were centrifuged at 12,800 × g for 15 min at 4°C. Immunoprecipitation was performed by adding the supernatants to Eppendorf tubes containing blocked, antibody-conjugated protein A-G beads (Pierce) and incubating them at 4°C with mixing for at least 2 h. Immunocomplexes were collected by centrifugation and washed twice with cold lysis buffer and once with low-salt buffer (10 mM NaCl, 10 mM Tris, pH 7.5). Proteins bound were eluted by addition of gel sample buffer.
To characterize PKB antibodies and for PKBβ immunodepletion experiments, immunoprecipitation was performed by adding 100 μg of cell lysate (in HES buffer) to radioimmunoprecipitation assay buffer (150 mM NaCl, 10 mM Tris [pH 8], 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) supplemented with protease inhibitors and phosphatase inhibitors and incubating the lysate with antibody-conjugated protein A-G beads at 4°C. For PKBβ depletion experiments, the supernatants were subjected to a second round of immunoprecipitation. To analyze the lysate after PKBβ depletion, an aliquot of the supernatant after immunoprecipitation was subjected to methanol-chloroform precipitation (7). The resulting protein pellets were resuspended in Laemmli sample buffer and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE).
Electrophoresis and immunoblotting.
Protein assays were performed using the Bradford assay reagent (Bio-Rad) or the bicinchoninic acid protein assay kit (Pierce), with BSA (fraction V; Pierce) as standard. SDS-PAGE was performed according to the method of Laemmli (34), using the SE400 system (Hoefer).
Two-dimensional gel electrophoresis (2-DE) with immobilized pH gradients was performed exactly as described in the work of Hill et al. (24), with nonlinear pH 3 to 10 immobilized pH gradients in the first dimension and SDS–7.5% PAGE gels in the second dimension. To prepare samples for 2-DE, cell lysates in HES buffer were precipitated with 4 volumes of methanol at −20°C for 1 h and then centrifuged for 10 min at 10,000 × g at room temperature. After the supernatants were removed, the pellets were air dried and resolubilized in 2D sample buffer (7 M urea, 2 M thiourea, 40 mM Tris, 4.4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 84 mM dithiothreitol, 1% Pharmalyte, 0.01% bromophenol blue, 1 mM phenylmethylsulfonyl fluoride, and phosphatase inhibitors as described above). Samples were sonicated in a sonicating water bath (Branson) for 1 min at room temperature and then centrifuged as before. The resulting supernatants were underlaid into oil-filled sample cups positioned at the anode, and isoelectric point focusing was performed for 53.5 kV · h at 20°C.
After electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes (Immobilon-P from Millipore) according to the method of Towbin et al. (56). Membranes were blocked in 5% skim milk powder in Tris-buffered saline–Tween (TBST; 50 mM Tris [pH 7.5], 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature and then incubated with the relevant primary antibody (diluted in blocking buffer or TBST) at 4°C overnight. After washing in TBST for 30 min with three changes of buffer, immunoreactive proteins were detected with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG), goat anti-mouse IgG (Amersham), or rabbit anti-sheep IgG (Sigma) as appropriate and SuperSignal chemiluminescence substrate (Pierce). Images were captured on film (Fuji) or a Lumi-Imager (Boehringer).
RESULTS
Effect of a PKB substrate peptide or a PKB antibody on insulin-stimulated GLUT4 translocation.
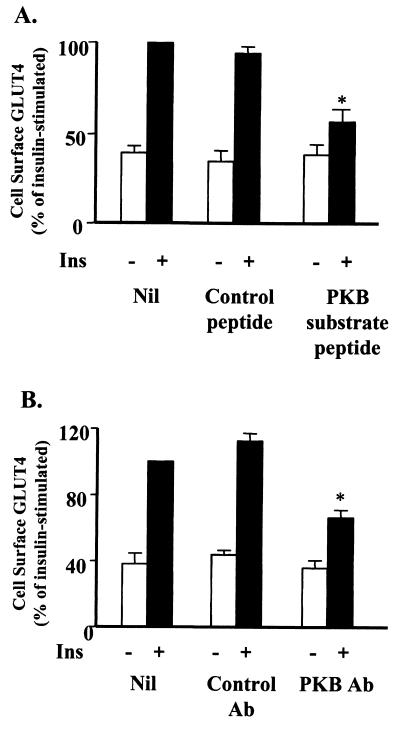
Previous studies investigating the role of PKB in insulin-stimulated glucose transport have utilized overexpression of either constitutively active (18, 23, 30, 54, 57) or dominant negative (18, 23, 29, 32, 59) mutants of PKBα. These studies have produced conflicting results, and so we undertook a different approach to assess the role of PKB in insulin-stimulated glucose transport. Firstly, we have used microinjection in 3T3-L1 adipocytes. This approach circumvents potential adaptive responses that may occur during chronic overexpression of mutants, because we routinely perform our analysis 60 to 90 min after microinjection. This technique in combination with the PM lawn assay provides a means for examining the effects of inhibitors in single cells. Using this system, we routinely observe a 2.5-fold increase in cell surface levels of GLUT4 in response to insulin in cells microinjected with control peptides or control antibodies and in neighboring noninjected cells. Microinjection was performed with 3T3-L1 adipocytes with a peptide (KRPRAATF) closely related to the peptide previously determined to be relatively specific for PKB (1), except that a lysine was included at the N terminus to increase its solubility. As shown in Fig. 1A, the substrate peptide inhibited insulin-stimulated GLUT4 translocation by (66.1 ± 7.8)% (n = 10). A control peptide in which alanine was substituted for the phosphorylation site threonine had no significant effect on GLUT4 translocation ([7.3 ± 3.7]%, n = 6). This suggests that the injected substrate peptide is interfering with the propagation of the signaling pathway leading to GLUT4 translocation, probably by competing with a physiological substrate of PKB.
FIG. 1.
Microinjection of PKB substrate peptide or an N-terminal PKB antibody inhibits insulin-stimulated GLUT4 translocation. 3T3-L1 adipocytes on coverslips were preincubated in Krebs-Ringer bicarbonate–HEPES buffer for 45 to 90 min. (A) Cells were then either not microinjected (Nil), microinjected with PKB substrate peptide (KRPRAATF), or microinjected with control peptide (KRPRAAAF) at 5 mg/ml. (B) 3T3-L1 adipocytes were either not injected (Nil) or microinjected with a purified N-terminal PKB antibody (PKB Ab) (N19; Santa Cruz) or purified rabbit IgG fraction (Control Ab) at 0.2 mg/ml. Bathing buffer was changed, and the cells were allowed to recover for 60 min. Cells were then stimulated or not with 100 nM insulin for 20 min prior to assessment of PM GLUT4 levels by the PM lawn assay as described in Materials and Methods. Results are from four or more experiments in which GLUT4 levels in six or more fields were determined for each condition within each experiment. ∗, P < 0.01 compared with insulin stimulation (paired t test).
To further assess the role of PKB in insulin-stimulated GLUT4 translocation, studies in which antibodies against PKB were microinjected into 3T3-L1 adipocytes were performed. An antibody directed against the N terminus of PKBα (N19 from Santa Cruz), which cross-reacts with PKBβ, inhibited insulin-stimulated GLUT4 translocation by (55.6 ± 7.8)% whereas an unrelated antibody had no significant effect on this process (Fig. 1B). We also examined the effect of a variety of C-terminal PKB antibodies, including the isoform-specific sheep PKBβ antibody described below. However, none of these antibodies had a significant effect on insulin-stimulated GLUT4 translocation in this system (data not shown). This may indicate that the C terminus is inaccessible in vivo or that antibody binding to this domain is less disruptive to PKB function.
Down-regulation of PDGF responsiveness in 3T3-L1 adipocytes.
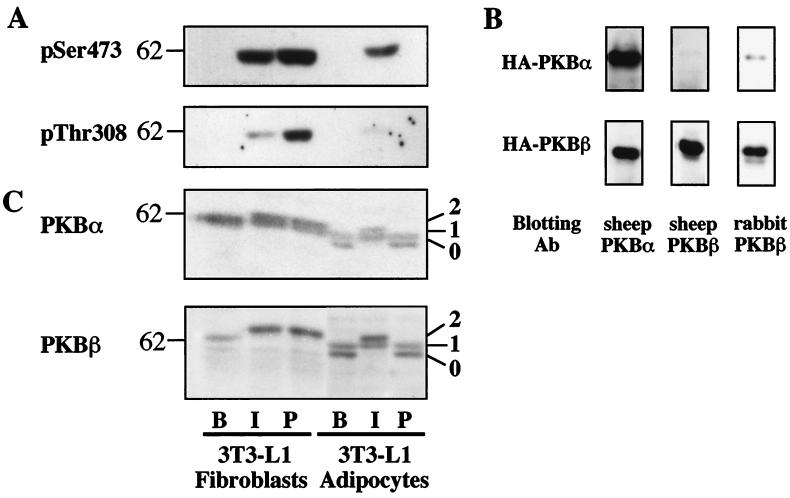
The microinjection data indicates that activation of PKB is necessary to fully mediate insulin-stimulated GLUT4 translocation in adipocytes. However, it has been reported that PDGF also activates PKB in adipocytes without any significant effect on GLUT4 translocation (10, 51, 54), raising the possibility that PKB activation may not be sufficient for insulin action. To explore this issue further, we have compared the effects of insulin with those of PDGF on the activation of PKB in 3T3-L1 adipocytes as well as their precursor cells, 3T3-L1 fibroblasts. As phosphorylation at Ser473 and Thr308 is essential for PKB activation, the effect of insulin and PDGF on PKB phosphorylation in 3T3-L1 cells was initially examined by immunoblotting with phosphospecific PKB antibodies (Fig. 2A). Treatment of 3T3-L1 fibroblasts with either insulin or PDGF for 15 min caused PKB phosphorylation on Ser473 and Thr308, with PDGF being more efficacious than insulin (Fig. 2A). The response to insulin compared with that to PDGF in 3T3-L1 adipocytes was quite different from that of fibroblasts. In this case, only insulin stimulated PKB phosphorylation, as detected by phosphospecific PKB antibodies (Fig. 2A).
FIG. 2.
Change in growth factor response accompanies differentiation of 3T3-L1 adipocytes. (A) Serum-starved 3T3-L1 fibroblasts or adipocytes were stimulated with 1 μM insulin (I) or 50 ng of PDGF per ml (P) for 15 min or left untreated (B). Cell lysates (30 μg) were analyzed by SDS-PAGE and immunoblotting with phospho-Ser473 (pSer473) or phospho-Thr308 (pThr308) PKB antibodies. (B) HA-PKBα or HA-PKBβ was immunoprecipitated from lysates (100 μg) of 3T3-L1 fibroblasts expressing either HA-PKBα or HA-PKBβ and analyzed by SDS-PAGE. Immunoblotting was performed with the sheep PKBα, the sheep PKBβ, or the rabbit PKBβ antibodies. (C) Cell lysates (30 μg) prepared as described for panel A were analyzed by SDS-PAGE and immunoblotting with the sheep PKBα or the sheep PKBβ antibody. Bands labeled as band 1 exhibited the same electrophoretic mobility.
In order to correlate these results with phosphorylation of specific PKB isoforms, the isoform specificity of PKB antibodies was examined by immunoblotting using stable cell lines of 3T3-L1 fibroblasts expressing HA-tagged forms of PKBα or PKBβ. HA-tagged PKB was immunoprecipitated from lysates with a monoclonal HA antibody and immunoblotted with a panel of PKB antibodies. The sheep PKBβ antibody immunoblotted HA-PKBβ but exhibited no detectable cross-reactivity with HA-PKBα (Fig. 2B). In contrast, the sheep PKBα antibody immunoblotted both HA-PKBα and HA-PKBβ, and the rabbit PKBβ antibody showed a slight cross-reactivity with HA-PKBα (Fig. 2B).
The above data suggests that the sheep PKBβ antibody is isoform specific, at least in immunoblotting; however, the sheep PKBα antibody cross-reacts with PKBβ to a considerable extent. Nevertheless, we proceeded to examine the relative expression and the effect of growth factors on the electrophoretic mobility of PKBα and PKBβ by using the sheep PKB antibodies. As shown in Fig. 2C, both sheep PKBα and PKBβ antibodies labeled bands in 3T3-L1 cells of an average molecular mass of 60 kDa, consistent with the calculated molecular mass of 56 kDa. While a single immunoreactive band was detected in basal fibroblasts, an additional band with lower molecular mass (band 0) was detected in basal adipocytes, with either the PKBα or the PKBβ antibody (Fig. 2C). The band labeled with the PKBβ antibody was up-regulated during the differentiation process whereas the intensity of the band labeled by the PKBα antibody in fibroblasts was not significantly different from the combined intensity of the two bands in adipocytes (Fig. 2C). In agreement with results presented in Fig. 2A, both insulin and PDGF caused an electrophoretic mobility shift in PKB in fibroblasts, whereas only insulin caused a shift in adipocytes. Interestingly, the electrophoretic shift for both PKBα and PKBβ was much more pronounced in adipocytes than in fibroblasts. Moreover, in insulin-treated adipocytes, band 0 was almost quantitatively shifted to a position slightly above band 1 (labeled band 2) whereas the apparent mobility and intensity of band 1 were unchanged (Fig. 2C).
PKBβ is highly up-regulated during adipocyte differentiation.
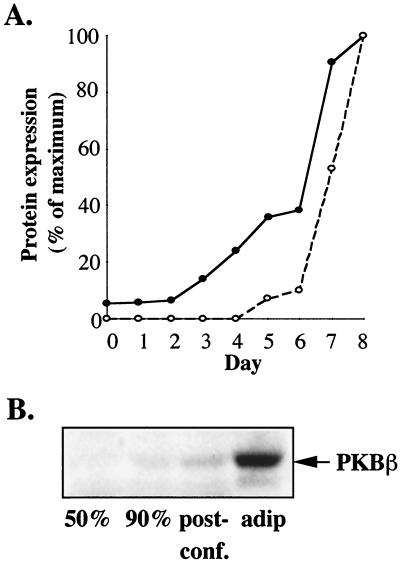
Key molecules mediating insulin regulation of metabolism are increased upon adipocyte differentiation. Thus, we further examined the change in PKBβ expression during the differentiation process and found that the increase in PKBβ expression during differentiation closely paralleled that observed for GLUT4 (Fig. 3A). The most dramatic increase in both PKBβ and GLUT4 occurred after day 6, when the differentiation medium was removed and cells were fed with medium supplemented only with 10% FCS (Fig. 3A). No GLUT4 expression was detected prior to initiation of differentiation (day 0, Fig. 3A). However, a low level of PKBβ was detected (day 0, Fig. 3A). Preliminary experiments suggested that there may be changes in PKBβ expression associated with cell confluency. This possibility was examined by immunoblotting lysates harvested from 3T3-L1 fibroblasts at different confluencies. As shown in Fig. 3B, no PKBβ was detected in fibroblasts at 50% confluence, but a low level of expression was observed when 3T3-L1 fibroblasts reached confluence. Differentiation into adipocytes, however, induced a much more significant up-regulation of PKBβ expression (Fig. 3B).
FIG. 3.
PKBβ expression is induced upon adipocyte differentiation. (A) 3T3-L1 cells were harvested on each day of the differentiation procedure and analyzed for the expression of PKBβ (●) or GLUT4 (○) by immunoblotting with the sheep PKBβ antibody or a rabbit anti-GLUT4 antibody (R017), respectively. Immunoreactive signal (obtained as Lumi-Imager units) was adjusted for total protein obtained per sample and then expressed as a percentage of the maximum. Results are representative of two separate experiments. (B) 3T3-L1 fibroblasts were harvested at subconfluence (50% or 90%) or 1 day after reaching confluence (post-conf.). 3T3-L1 adipocytes were harvested after completion of differentiation, at day 8 (adip). Cell lysates (30 μg) were analyzed for the level of PKBβ expression by SDS-PAGE and immunoblotting with the sheep PKBβ antibody.
PKBα is down-regulated in adipocytes.
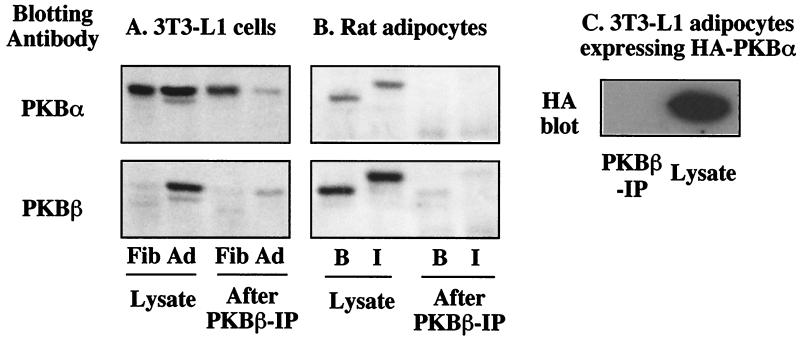
The above data clearly demonstrates an induction of PKBβ expression during adipocyte differentiation. However, while PKBα appears to be highly expressed in fibroblasts, its expression in adipocytes is not clear due to antibody cross-reactivity. Thus, to more clearly determine PKBα expression, we performed immunoblotting on cell lysates that had been depleted of PKBβ with the specific sheep PKBβ antibody. In agreement with Fig. 2C, prior to PKBβ immunodepletion, the PKBα antibody detected bands with similar intensities in fibroblasts and adipocytes, while a substantial induction of PKBβ was observed in adipocytes (Fig. 4A). After immunodepletion with the sheep PKBβ antibody, almost all of the immunoreactive PKBβ was removed from the adipocyte lysate (Fig. 4A). When the same samples were immunoblotted with the PKBα antibody, there was little difference in the fibroblasts before and after PKBβ immunodepletion, suggesting that PKBα is expressed at high levels in fibroblasts. However, there was a substantial reduction in immunolabeling with this antibody in adipocytes after PKBβ depletion, suggesting that most of the PKBα immunoreactivity is due to PKBβ in these cells and that PKBβ is the predominant isoform expressed in 3T3-L1 adipocytes (Fig. 4A). To determine if this is a general feature of adipocytes, we performed similar experiments with primary cultures of adipocytes isolated from rat fat. Prior to PKBβ immunodepletion, both the PKBα and the PKBβ antibodies detected a band which showed an electrophoretic mobility shift with insulin treatment (Fig. 4B). However, after PKBβ depletion, no PKBα was detected in rat adipocyte lysates, suggesting that these cells do not express significant levels of PKBα (Fig. 4B).
FIG. 4.
PKBβ is the predominant isoform in adipocytes. (A) Cell lysates (100 μg) from serum-starved 3T3-L1 fibroblasts (Fib) or 3T3-L1 adipocytes (Ad) were depleted of PKBβ by two consecutive rounds of immunoprecipitation with the sheep PKBβ antibody. Twenty micrograms of lysate (Lysate) and one-fifth of the immunoprecipitation supernatant (After PKBβ-IP) were analyzed by SDS-PAGE and immunoblotting with the sheep PKBα or the sheep PKBβ antibodies. (B) Cell lysates (100 μg) from isolated rat adipocytes treated with 1 μM insulin (I) for 15 min or left basal (B) were depleted of PKBβ and analyzed as described for panel A. (C) Immunoprecipitation with the sheep PKBβ antibody was performed on 100 μg of cell lysates prepared from 3T3-L1 adipocytes overexpressing HA-PKBα. The immunoprecipitate (PKBβ-IP) and 10 μg of lysate (Lysate) were analyzed by SDS-PAGE and immunoblotting for the presence of HA-PKBα, with a monoclonal HA antibody.
One trivial explanation for these observations is that PKBα and PKBβ hetero-oligomerize and therefore all of the PKBα is removed during PKBβ immunodepletion. However, this seems unlikely because interaction between PKB molecules has been shown to be isoform specific, as the Akt homology domain of PKBα coimmunoprecipitates PKBα but not PKBβ (21). Nevertheless, we addressed this possibility using 3T3-L1 adipocytes overexpressing HA-PKBα. As shown in Fig. 4C, no signal corresponding to HA-PKBα was detected in the PKBβ immunoprecipitate, demonstrating that, under these conditions, PKBα does not coimmunoprecipitate with PKBβ.
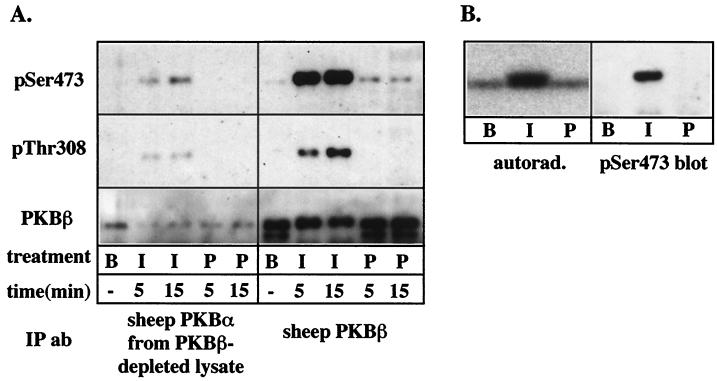
Insulin but not PDGF stimulates PKBβ phosphorylation in 3T3-L1 adipocytes.
The above data suggests that PKBβ is the most pertinent isoform for the insulin-specific PKB phosphorylation in 3T3-L1 adipocytes observed in Fig. 2A. To verify this conclusion, we next examined the phosphorylation of PKB isoforms following immunoprecipitation with isoform-specific antibodies. To examine the extent of PKBα phosphorylation, it was necessary to first deplete lysates of PKBβ prior to immunoprecipitation with the PKBα antibody. In these experiments, we also examined the effect of insulin and PDGF after 5 min of stimulation, in order to assess if PDGF has a transient effect on PKB activity. Insulin, but not PDGF, stimulated the phosphorylation of immunoprecipitated PKBα in 3T3-L1 adipocytes as detected by phospho-PKB antibodies (Fig. 5A). However, this signal most likely represents residual PKBβ that was immunoprecipitated by the PKBα antibody (Fig. 5A). A marked increase in PKBβ phosphorylation was observed in response to insulin, while PDGF had little effect on PKBβ phosphorylation at 5 or 15 min of stimulation (Fig. 5A). PKBβ phosphorylation was also directly examined by immunoprecipitation from 3T3-L1 adipocytes metabolically labeled with 32P (Fig. 5B). A low level of constitutive phosphorylation was observed under basal conditions, and insulin stimulation caused a threefold increase in PKBβ phosphorylation (Fig. 5B). Insulin-stimulated phosphorylation was accompanied by an electrophoretic mobility shift, and the shifted band was specifically detected by phospho-Ser473 PKB antibodies (Fig. 5B). In agreement with previous results (Fig. 2 and 5A), PDGF had no significant effect on PKBβ phosphorylation in 3T3-L1 adipocytes.
FIG. 5.
Insulin but not PDGF stimulates phosphorylation of PKBβ in 3T3-L1 adipocytes. (A) PKB isoforms were immunoprecipitated from 100 μg of 3T3-L1 lysates prepared from unstimulated cells (B) or cells treated with 1 μM insulin (I) or 50 ng of PDGF per ml (P) for 5 or 15 min. In the case of the PKBα immunoprecipitation, PKBβ was first depleted from the cell lysate by two consecutive rounds of immunoprecipitation. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with phospho-Ser473 (pSer473) or phospho-Thr308 (pThr308) PKB antibodies or the sheep PKBβ antibody (PKBβ). (B) PKBβ was immunoprecipitated from 32P-labeled 3T3-L1 adipocytes treated with 1 μM insulin (I) or 50 ng of PDGF per ml (P) for 15 or left basal (B), by using the sheep PKBβ antibody, and analyzed by SDS-PAGE. The polyvinylidene difluoride membrane was subjected to autoradiography (autorad.) and then immunoblotted with the phospho-Ser473 antibody (pSer473).
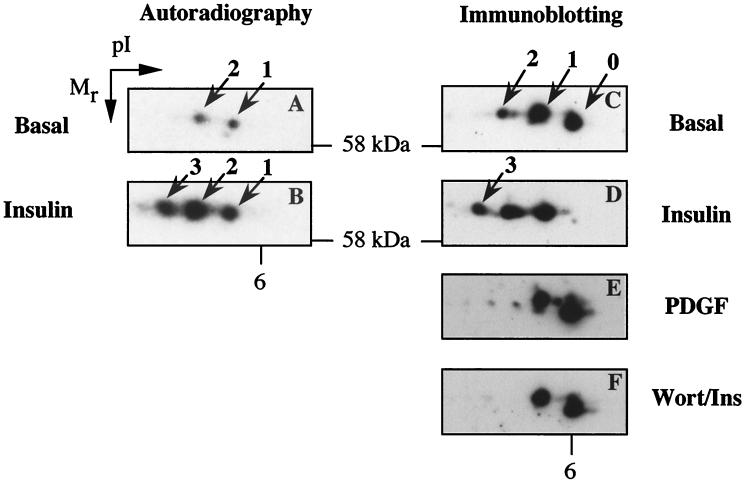
2-DE was used to further examine PKBβ phosphorylation in adipocytes. PKBβ was immunoprecipitated from 32P-labeled 3T3-L1 adipocytes and then subjected to 2-DE and autoradiography. Two spots of similar molecular masses but different pIs were detected in basal adipocytes (spots 1 and 2, Fig. 6A). The observed pI of these phosphospots was 5.6 to 5.8, which agrees with that calculated for PKBβ (5.9). The presence of the two phospho-PKBβ spots is consistent with two sites of constitutive phosphorylation, where spots 1 and 2 represent mono- and dual-phosphorylated forms, respectively. Insulin stimulation resulted in an increase in the intensity of spots 1 and 2, as well as the 32P labeling of an additional spot (spot 3) of more acidic pI (Fig. 6B). To determine the relative abundance of differentially phosphorylated forms of PKBβ, we performed 2D immunoblotting. In addition to spots 1 and 2, a spot at pI 6 in 2D blots of basal adipocyte lysate which represents unphosphorylated PKBβ was detected (spot 0, Fig. 6C). A close examination of Fig. 6C shows that, under basal conditions, the majority of PKBβ is either unphosphorylated (spot 0) or monophosphorylated (spot 1), with only a minor proportion being dual phosphorylated (spot 2). Insulin stimulation caused the complete disappearance of spot 0 with a significant increase in the intensity of spot 2 and the appearance of spot 3 (Fig. 6D). The effect of PDGF stimulation on the 2D pattern of PKBβ spots was also examined. Consistent with previous results (Fig. 2 and 5), stimulation of 3T3-L1 adipocytes with 50 ng of PDGF per ml for 15 min did not result in the leftward shift of 2D PKBβ spots observed in response to insulin (compare Fig. 6C to E). In addition, pretreatment of cells with 100 nM wortmannin completely inhibited the insulin effect on the PKBβ 2D pattern (Fig. 6F), consistent with the reported PI3K dependence of insulin-stimulated PKB activation.
FIG. 6.
Analysis of insulin-stimulated PKBβ phosphorylation in 3T3-L1 adipocytes by 2-DE. 3T3-L1 adipocytes were 32P labeled and then stimulated without (A) or with (B) 1 μM insulin for 15 min. PKBβ was immunoprecipitated from cell lysates by using the rabbit anti-PKBβ antibody and analyzed by 2-DE and autoradiography. Lysates (150 μg) prepared from untreated 3T3-L1 adipocytes (C) or adipocytes treated with 1 μM insulin for 15 min (D), 50 ng of PDGF per ml for 15 min (E), or 100 nM wortmannin for 40 min with 1 μM insulin added for the last 15 min (F) were analyzed by 2-DE and immunoblotting with the rabbit anti-PKBβ antibody. Immunoblots of the polyvinylidene difluoride membranes from panels A and B yielded results similar to those for panels C and D.
Insulin stimulates PKBβ translocation to the PM and HDM fractions.
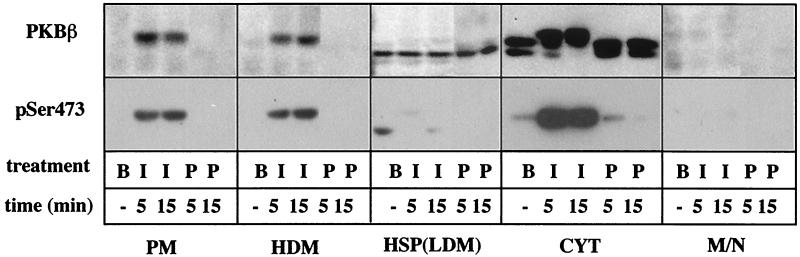
Apart from phosphorylation, membrane translocation is thought to be required for activation of PKB. The current model of PKB activation suggests that PKB is recruited to the PM via its pleckstrin homology domain by the 3′-phosphoinositides generated by the receptor-activated PI3K, where it is phosphorylated at Ser473 and Thr308 by 3′-phosphoinositide-dependent kinase 1 (PDK1) and PDK2 (reviewed in references 2 and 40). We and others have previously reported that insulin and PDGF stimulate PI3K in adipocytes but that the subcellular distribution of the enzyme is different in each case (16, 41, 45). With PDGF, we observed a significant increase in PI3K activity in the PM fraction, whereas with insulin, it was enriched in a high-speed pellet (HSP) fraction associated with IRS-1 and/or -2 (16). Thus, we next studied the distribution of PKBβ among these fractions. Under basal conditions, almost all the immunoreactive PKBβ was found in the cytosol (Fig. 7). In agreement with previous results, basal PKBβ in the cytosol fraction was resolved into two bands, corresponding to bands 0 and 1 in Fig. 2A. A band detected in the HSP fraction in the basal state exhibited apparent electrophoretic mobility similar to that of band 0; however, in contrast to bands in the cytosol, insulin did not induce an electrophoretic mobility shift of the HSP band (Fig. 7). Insulin induced the translocation of PKBβ to the PM and high-density microsome (HDM) fractions, while PDGF was without effect. This translocation does not represent the association of PKBβ with large protein complexes because we find that it floats up through sucrose upon sedimentation, consistent with a membrane-associated protein (data not shown). Insulin-stimulated translocation of PKBβ was restricted to band 2, the activated form of PKBβ, as confirmed by immunoreactivity to the phospho-Ser473 PKB antibody (Fig. 7). Interestingly, PKBβ in the cytosol fraction underwent an almost quantitative shift from band 0 to band 2 within 5 min of insulin stimulation, coincident with increased Ser474 phosphorylation (Fig. 7). This suggests that either PKBβ was activated in the cytosol or it is activated at the PM and rapidly translocates from this site to the cytosol.
FIG. 7.
Insulin, but not PDGF, stimulates the translocation of PKBβ to membrane fractions in 3T3-L1 adipocytes. 3T3-L1 adipocytes were stimulated with insulin (I) or PDGF (P) for 5 or 15 min or left basal (B). Cells were homogenized and subfractionated by differential centrifugation as described in Materials and Methods, to generate the PM, HDM, HSP (also termed LDM), cytosol (CYT), and the mitochondrial-nuclear (M/N) fractions. Twenty micrograms of each fraction was analyzed by SDS-PAGE and immunoblotting with the sheep PKBβ (PKBβ) or the phospho-Ser473 PKB (pSer473) antibody.
Recent studies reporting the insulin-induced association of PKBβ with GLUT4-containing vesicles (12) and phosphorylation of its components (33) suggest that PKBβ may directly mediate GLUT4 translocation. In contrast to the report by Calera et al. (12) for rat adipocytes, we did not detect PKBβ in membrane fractions in the basal state, nor did we observe significant insulin-induced translocation of PKBβ to the HSP fraction (also termed the low-density microsome [LDM] fraction) in 3T3-L1 adipocytes (Fig. 7). To further assess if PKBβ directly interacts with GLUT4 vesicles, we examined the ability of insulin or PDGF to stimulate GLUT4 translocation in 3T3-L1 fibroblasts overexpressing both GLUT4 and PKBβ. Activation of PKBβ by either insulin or PDGF did not alter the subcellular location of GLUT4 in these cells (data not shown), suggesting that activation of PKBβ is not sufficient to stimulate GLUT4 translocation in this system.
DISCUSSION
In the present study, we have provided further evidence to support a role for the serine/threonine kinase PKB in insulin-stimulated glucose transport in adipocytes. We show that a PKB substrate peptide or an antibody to PKB inhibited insulin-stimulated GLUT4 translocation by 66 and 56%, respectively. Moreover, we have obtained evidence to implicate the PKBβ isoform in insulin action: (i) the expression of PKBβ is substantially increased during adipocyte differentiation whereas the expression of PKBα is markedly reduced during this process, (ii) primary cultures of rat adipocytes have no detectable immunoreactive PKBα, and (iii) insulin but not PDGF stimulates PKBβ phosphorylation and translocation to membranes in 3T3-L1 adipocytes.
Previous studies have reported contradictory findings concerning the role of PKB in insulin-stimulated glucose transport (18, 23, 29, 30, 32, 54, 57, 59). We believe the present study provides strong support for the involvement of PKB in insulin action and clarifies some potential areas of contradiction that have arisen. First, as discussed in more detail below, our study implicates a role only for PKBβ and not for PKBα in insulin regulation of glucose transport in adipocytes. This may be critical, as many previous studies have used reagents that either specifically target PKBα or, as a minimum, have questionable specificity for different PKB isoforms. Second, we have employed a strategy involving microinjection of either antibodies or substrate peptides and then examination of the consequences of these reagents for GLUT4 trafficking 60 to 90 min later. This likely overcomes potential cellular adaptive mechanisms which could occur in response to expression of cDNAs over a much longer time course.
Role of PKBα versus PKBβ.
Initial comparison of the relative expression of PKBα and PKBβ in 3T3-L1 fibroblasts with that in adipocytes by immunoblotting suggested that, while PKBβ was up-regulated, PKBα expression was not significantly altered by adipocyte differentiation (Fig. 2C). However, as the PKBα antibody was found to cross-react with PKBβ (Fig. 2B), part of the PKBα immunoreactivity in 3T3-L1 adipocytes likely represented PKBβ, which is highly expressed in these cells. We tested this hypothesis by depleting PKBβ from cell lysates prior to immunoblotting with the PKBα antibody and found that, indeed, most of the PKBα immunoreactivity in adipocytes was removed by PKBβ depletion (Fig. 4A). In contrast, substantial PKBα immunoreactivity remained after PKBβ depletion of fibroblast lysates (Fig. 4A). Consistent with our findings in 3T3-L1 adipocytes, we also find that there is very little PKBα in rat adipocytes, with PKBβ being the major isoform (Fig. 4B). Based on the present studies, we predict that previous reports of insulin-activated PKBα activity in rat adipocytes may be due to antibody cross-reactivity.
These results suggest that there is a switch in isoform expression during differentiation of fibroblasts into adipocytes. As PKBα and PKBβ are 81% identical in amino acid sequence, the specific change in isoform expression upon adipocyte differentiation is likely to be functional. Fibroblasts represent a highly proliferative cell type, and so it seems likely that PKBα is important in the regulation of cell growth and proliferation, and its down-regulation upon differentiation is appropriate as adipocytes are terminally differentiated and not proliferative. On the other hand, the induction of PKBβ expression during adipocyte differentiation parallels that observed for GLUT4 (Fig. 3A), implying a role for PKBβ in metabolic regulation in adipocytes. Further support comes from our observation that rat adipocytes express high levels of PKBβ (Fig. 4B) and from the reported high PKBβ mRNA expression in brown adipose tissue (3). An induction of PKBβ mRNA was also observed upon differentiation of Sol8 muscle cells (13) and C2C12 myotubes (3), supporting a role for PKBβ in insulin regulation of metabolism in muscle cells in addition to adipocytes. In our study, no PKBβ expression was observed in subconfluent, highly proliferative fibroblasts. A slight increase in PKBβ expression was observed when cells became confluent in culture, followed by a more dramatic induction when fibroblasts differentiated into adipocytes (Fig. 3). Taken together, these observations suggest that the expression of PKBβ is normally restricted to terminally differentiated, nonproliferative cells, where PKBβ plays a role in metabolic regulation. The inappropriate expression of PKBβ in undifferentiated cells can lead to uncontrolled cell proliferation as demonstrated by the transforming ability of PKBβ when overexpressed in NIH 3T3 cells (15). Overexpression of constitutively active PKBα in 3T3-L1 fibroblasts leads to spontaneous differentiation into adipocytes (36). To further address the roles of different PKB isoforms, it may be of interest to compare the effects of overexpressing constitutively active PKBβ or PKBγ to that observed for PKBα in 3T3-L1 fibroblasts.
Activation of PKBβ is insulin specific in 3T3-L1 adipocytes.
Several recent studies have compared the signaling of insulin with that of PDGF in adipocytes, based on the hypothesis that the inability of PDGF to induce glucose uptake reflects its inability to activate a relevant downstream signaling pathway(s). Indeed, several groups have reported differences between the subcellular location of PI3K activated by insulin and that of PI3K activated by PDGF (16, 41, 45). Furthermore, PDGF was found to have little effect on the levels of phosphatidylinositol 3,4,5-trisphosphate (PIP3) in 3T3-L1 adipocytes, whereas insulin induced a significant increase (19). These results suggest that, while PI3K is activated by recruitment to activated PDGF receptors at the PM in 3T3-L1 adipocytes, it may not have access to appropriate substrates and thus is unable to produce a rise in PIP3. In agreement with the lack of PDGF-induced PIP3 production, we found no effect of PDGF on PKBβ phosphorylation in 3T3-L1 adipocytes by electrophoretic mobility shift, immunoprecipitation in conjunction with 32P labeling or immunoblotting with phosphospecific antibodies, or isoelectric point shifts analyzed by 2-DE. Furthermore, PDGF did not stimulate membrane translocation of PKBβ, as observed with insulin, nor did it have a transient effect on PKBβ phosphorylation (Fig. 5A). The insulin specificity further supports a role for PKBβ in the regulation of glucose metabolism in adipocytes.
Three previous studies have reported variable effects of PDGF on PKB in 3T3-L1 adipocytes (10, 51, 54). Two studies utilized phospho-Ser473 PKB antibodies and found a small PDGF-induced PKB phosphorylation (∼20% of insulin effect). We were unable to detect PDGF-stimulated PKB phosphorylation in 3T3-L1 adipocytes treated with 50 ng of PDGF per ml with the same antibody in immunoblotting of cell lysates (Fig. 2A), but very weak PDGF-induced PKBβ phosphorylation was sometimes observed in immunoprecipitates (Fig. 5A). Tanti et al. observed a significant PDGF-stimulated activation of PKB (to 50% of the insulin effect) in 3T3-L1 adipocytes by immunoprecipitation-coupled kinase assays (54). The reason for the discrepancy between these studies is not clear. One possible explanation is differences in the integrity of 3T3-L1 adipocyte cultures. As PKBα is the predominant isoform in 3T3-L1 fibroblasts and is strongly activated by PDGF in these cells, an increased level of fibroblast contamination of adipocyte cultures may give rise to a PDGF response on PKB. This may be further amplified in the case of immunoprecipitation with an antibody with a high affinity for PKBα.
Regulation of PKBβ activation in 3T3-L1 adipocytes.
A synthesis of our data concerning activation of PKBβ in 3T3-L1 adipocytes suggests that both unphosphorylated (band 0 and spots 0) and phosphorylated (band 1 and spots 1 and 2) PKBβ are present in basal adipocytes. Furthermore, separation of phospho-PKB into two spots with different pI suggests that there are two populations of differentially phosphorylated forms of PKBβ in basal 3T3-L1 adipocytes. Constitutive phosphorylation of PKBβ in 3T3-L1 adipocytes does not occur on either Ser474 or Thr309, because no significant signal was detected with phospho-PKB antibodies in basal adipocytes (Fig. 2A and 5A). Two constitutive phosphorylation sites have been reported for PKBβ transfected into HEK-293 cells, and these are Ser125 and Thr451 (39). Thus one interpretation of our results is that ∼50% of PKBβ is constitutively phosphorylated, possibly on either Ser125 or Thr451 in the basal state in 3T3-L1 adipocytes (spot 1), while a small amount is dual phosphorylated on both residues, resulting in a more acidic pI (spot 2). Insulin caused the total disappearance of nonphosphorylated PKBβ (spot 0), with an increase in the immunoreactivity of spots 2 and 3, concomitant with insulin-induced phosphorylation (Fig. 6A to D). A recent study suggests that constitutive phosphorylation at Thr450 primers PKBα for subsequent growth factor stimulation when expressed in NIH 3T3 cells (9). Thus, one possibility is that insulin stimulates phosphorylation of basally unphosphorylated PKBβ (spot 0) on one or both of the constitutive sites (Ser125 and Thr451) but that only basally phosphorylated PKBβ (spots 1 and 2) becomes phosphorylated on activating sites (Ser474 and Thr309). As the present data does not provide information on the sites of phosphorylation of the differentially charged PKBβ isoforms, further investigation is required to confirm this hypothesis in 3T3-L1 adipocytes. Interestingly, only a single band which exhibits an electrophoretic mobility that is intermediate between bands 0 and 1 was observed in rat adipocytes. Upon insulin stimulation, the entire band shifts to a position corresponding to band 2. The phosphorylation state of the basal band in rat adipocytes is yet to be determined and should provide valuable data on the role of constitutive phosphorylation.
Current models of growth factor-stimulated PKB activation suggest that the products of PI3K recruit both PDK1 and PKB to the PM, where PDK1 phosphorylates and activates PKB (2, 4, 40). The requirement for membrane translocation in PKB activation is supported by the finding that forced translocation to the PM by myristoylation-palmitylation is sufficient to induce PKB phosphorylation at Thr308 and Ser473 (5). In the present study, we observed specific translocation of phosphorylated PKBβ to the PM and HDM fractions in response to insulin treatment of 3T3-L1 adipocytes. In agreement with the inability of PDGF to induce PKBβ phosphorylation, no membrane translocation was observed in response to PDGF stimulation. Interestingly, while some PKBβ remained associated with the PM and the HDM, a major portion of activated PKBβ was observed in the cytosol within 5 min of insulin stimulation (Fig. 7). These results suggest that either PKBβ can be activated in the cytosol or, more likely, following activation at the membrane, most PKBβ returns to the cytosol where it is able to phosphorylate its physiological substrates. Recent reports of insulin-induced association of PKBβ with GLUT4-containing vesicles (12) and phosphorylation of its components (33) suggest that PKBβ may directly regulate the GLUT4 translocation machinery. We did not observe any significant recruitment of PKBβ to the HSP-LDM fraction which contains the insulin-responsive pool of GLUT4 in 3T3-L1 adipocytes. However, it is possible that our assay is not sensitive enough to detect a low level of PKBβ recruitment to this fraction. Thus, we further tested the hypothesis that PKBβ directly mediates GLUT4 translocation by examining the effect of activating PKBβ by insulin or PDGF in 3T3-L1 fibroblasts expressing both PKBβ and GLUT4. Neither insulin nor PDGF stimulated GLUT4 translocation in these cells, suggesting that either another signaling pathway is required or fibroblasts lack the expression of downstream signaling molecules or machinery required for GLUT4 translocation.
In summary, our data implicates an important role for PKB in insulin action in adipocytes. We cannot exclude a role for alternate parallel pathways such as PKCλ and/or PKCζ. Using microinjection of either a PKB substrate peptide or a PKB antibody, we observed a ∼60% inhibition of insulin-stimulated GLUT4 translocation to the PM. Hence, it is conceivable that the residual insulin action is due to a PKB-independent pathway. Further effort is required to identify this alternate pathway and to identify downstream targets of PKBβ in adipocytes.
ACKNOWLEDGMENTS
The first two authors contributed equally to this paper.
This work was supported by grants from the National Health and Medical Research Council of Australia and the Juvenile Diabetes Foundation International. D.E.J. is an NHMRC Principal Research Fellow. CMCB is a Special Research Centre of the Australian Research Council.
We thank Laura Martin, Ning-Xia Fang, Timo Meerloo, and Teresa Munchow for technical assistance and Emme Lin for discussions on the microinjection studies.
REFERENCES
- 1.Alessi D R, Caudwell F B, Andjelkovic M, Hemmings B A, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 3.Altomare D A, Lyons G E, Mitsuuchi Y, Cheng J Q, Testa J R. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene. 1998;16:2407–2411. doi: 10.1038/sj.onc.1201750. [DOI] [PubMed] [Google Scholar]
- 4.Anderson K E, Coadwell J, Stephens L R, Hawkins P T. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998;8:684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- 5.Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 6.Araki E, Lipes M A, Patti M E, Bruning J C, Haag B, 3rd, Johnson R S, Kahn C R. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 7.Arand M, Friedberg T, Oesch F. Monitoring sodium dodecyl sulfate contamination. In: Celis J E, editor. Cell biology: a laboratory handbook. Vol. 3. London, United Kingdom: Academic Press; 1994. pp. 276–278. [Google Scholar]
- 8.Bandyopadhyay G, Standaert M L, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese R V. Activation of protein kinase C (α, β, and ζ) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-ζ in glucose transport. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 9.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 10.Brady M J, Bourbonais F J, Saltiel A R. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3-L1 cells. J Biol Chem. 1998;273:14063–14066. doi: 10.1074/jbc.273.23.14063. [DOI] [PubMed] [Google Scholar]
- 11.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 12.Calera M R, Martinez C, Liu H, Jack A K, Birnbaum M J, Pilch P F. Insulin increases the association of Akt-2 with Glut4-containing vesicles. J Biol Chem. 1998;273:7201–7204. doi: 10.1074/jbc.273.13.7201. [DOI] [PubMed] [Google Scholar]
- 13.Calera M R, Pilch P F. Induction of akt-2 correlates with differentiation in sol8 muscle cells. Biochem Biophys Res Commun. 1998;251:835–841. doi: 10.1006/bbrc.1998.9566. [DOI] [PubMed] [Google Scholar]
- 14.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng J Q, Altomare D A, Klein M A, Lee W C, Kruh G D, Lissy N A, Testa J R. Transforming activity and mitosis-related expression of the AKT2 oncogene: evidence suggesting a link between cell cycle regulation and oncogenesis. Oncogene. 1997;14:2793–2801. doi: 10.1038/sj.onc.1201121. [DOI] [PubMed] [Google Scholar]
- 16.Clark S F, Martin S, Carozzi A J, Hill M M, James D E. Intracellular localization of phosphatidylinositide 3-kinase and insulin receptor substrate-1 in adipocytes: potential involvement of a membrane skeleton. J Cell Biol. 1998;140:1211–1225. doi: 10.1083/jcb.140.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke J F, Young P W, Yonezawa K, Kasuga M, Holman G D. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J. 1994;300:631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cong L N, Chen H, Li Y, Zhou L, McGibbon M A, Taylor S I, Quon M J. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 19.Conricode K M. Involvement of phosphatidylinositol 3-kinase in stimulation of glucose transport by growth factors in 3T3-L1 adipocytes. Biochem Mol Biol Int. 1995;36:835–843. [PubMed] [Google Scholar]
- 20.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 21.Datta K, Franke T F, Chan T O, Makris A, Yang S I, Kaplan D R, Morrison D K, Golemis E A, Tsichlis P N. AH/PH domain-mediated interaction between Akt molecules and its potential role in Akt regulation. Mol Cell Biol. 1995;15:2304–2310. doi: 10.1128/mcb.15.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 23.Hajduch E, Alessi D R, Hemmings B A, Hundal H S. Constitutive activation of protein kinase Bα by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes. 1998;47:1006–1013. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 24.Hill M M, Clark S F, James D E. Insulin-regulatable phosphoproteins in 3T3-L1 adipocytes form detergent-insoluble complexes not associated with caveolin. Electrophoresis. 1997;18:2629–2637. doi: 10.1002/elps.1150181419. [DOI] [PubMed] [Google Scholar]
- 25.Hunter S J, Garvey W T. Insulin action and insulin resistance: diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am J Med. 1998;105:331–345. doi: 10.1016/s0002-9343(98)00300-3. [DOI] [PubMed] [Google Scholar]
- 26.James D E, Strube M, Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989;338:83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- 27.Kelada A S, Macaulay S L, Proietto J. Cyclic AMP acutely stimulates translocation of the major insulin-regulatable glucose transporter GLUT4. J Biol Chem. 1992;267:7021–7025. [PubMed] [Google Scholar]
- 28.Kellerer M, Lammers R, Haring H U. Insulin signal transduction: possible mechanisms for insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:97–106. doi: 10.1055/s-0029-1212082. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 31.Kotani K, Carozzi A J, Sakaue H, Hara K, Robinson L J, Clark S F, Yonezawa K, James D E, Kasuga M. Requirement for phosphoinositide 3-kinase in insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1995;209:343–348. doi: 10.1006/bbrc.1995.1509. [DOI] [PubMed] [Google Scholar]
- 32.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase Cλ for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kupriyanova T A, Kandror K V. Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. J Biol Chem. 1999;274:1458–1464. doi: 10.1074/jbc.274.3.1458. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Macaulay S L, Hewish D R, Gough K H, Stoichevska V, MacPherson S F, Jagadish M, Ward C W. Functional studies in 3T3L1 cells support a role for SNARE proteins in insulin stimulation of GLUT4 translocation. Biochem J. 1997;324:217–224. doi: 10.1042/bj3240217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magun R, Burgering B M, Coffer P J, Pardasani D, Lin Y, Chabot J, Sorisky A. Expression of a constitutively activated form of protein kinase B (c-Akt) in 3T3-L1 preadipose cells causes spontaneous differentiation. Endocrinology. 1996;137:3590–3593. doi: 10.1210/endo.137.8.8754791. [DOI] [PubMed] [Google Scholar]
- 37.Marsh B J, Alm R A, McIntosh S R, James D E. Molecular regulation of GLUT4 targeting in 3T3-L1 adipocytes. J Cell Biol. 1995;130:1081–1091. doi: 10.1083/jcb.130.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin S S, Haruta T, Morris A J, Klippel A, Williams L T, Olefsky J M. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1996;271:17605–17608. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- 39.Meier R, Alessi D R, Cron P, Andjelkovic M, Hemmings B A. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 40.Meier R, Hemmings B A. Regulation of protein kinase B. J Recept Signal Transduct Res. 1999;19:121–128. doi: 10.3109/10799899909036639. [DOI] [PubMed] [Google Scholar]
- 41.Nave B T, Haigh R J, Hayward A C, Siddle K, Shepherd P R. Compartment-specific regulation of phosphoinositide 3-kinase by platelet-derived growth factor and insulin in 3T3-L1 adipocytes. Biochem J. 1996;318:55–60. doi: 10.1042/bj3180055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 43.Quon M J, Butte A J, Zarnowski M J, Sesti G, Cushman S W, Taylor S I. Insulin receptor substrate 1 mediates the stimulatory effect of insulin on GLUT4 translocation in transfected rat adipose cells. J Biol Chem. 1994;269:27920–27924. [PubMed] [Google Scholar]
- 44.Rice K M, Garner C W. Correlation of the insulin receptor substrate-1 with insulin-responsive deoxyglucose transport in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1994;198:523–530. doi: 10.1006/bbrc.1994.1077. [DOI] [PubMed] [Google Scholar]
- 45.Ricort J M, Tanti J F, Van Obberghen E, Le Marchand-Brustel Y. Different effects of insulin and platelet-derived growth factor on phosphatidylinositol 3-kinase at the subcellular level in 3T3-L1 adipocytes. A possible explanation for their specific effects on glucose transport. Eur J Biochem. 1996;239:17–22. doi: 10.1111/j.1432-1033.1996.0017u.x. [DOI] [PubMed] [Google Scholar]
- 46.Robinson L J, James D E. Insulin-regulated sorting of glucose transporters in 3T3-L1 adipocytes. Am J Physiol. 1992;263:E383–393. doi: 10.1152/ajpendo.1992.263.2.E383. [DOI] [PubMed] [Google Scholar]
- 47.Sharma P M, Egawa K, Huang Y, Martin J L, Huvar I, Boss G R, Olefsky J M. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J Biol Chem. 1998;273:18528–18537. doi: 10.1074/jbc.273.29.18528. [DOI] [PubMed] [Google Scholar]
- 48.Shaw M, Cohen P, Alessi D R. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336:241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpson I A, Yver D R, Hissin P J, Wardzala L J, Karnieli E, Salans L B, Cushman S W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983;763:393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- 50.Standaert M L, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese R V. Protein kinase C-ζ as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 51.Staubs P A, Nelson J G, Reichart D R, Olefsky J M. Platelet-derived growth factor inhibits insulin stimulation of insulin receptor substrate-1-associated phosphatidylinositol 3-kinase in 3T3-L1 adipocytes without affecting glucose transport. J Biol Chem. 1998;273:25139–25147. doi: 10.1074/jbc.273.39.25139. [DOI] [PubMed] [Google Scholar]
- 52.Summers S A, Whiteman E L, Cho H, Lipfert L, Birnbaum M J. Differentiation-dependent suppression of platelet-derived growth factor signaling in cultured adipocytes. J Biol Chem. 1999;274:23858–23867. doi: 10.1074/jbc.274.34.23858. [DOI] [PubMed] [Google Scholar]
- 53.Tanti J F, Gremeaux T, Grillo S, Calleja V, Klippel A, Williams L T, Van Obberghen E, Le Marchand-Brustel Y. Overexpression of a constitutively active form of phosphatidylinositol 3-kinase is sufficient to promote Glut 4 translocation in adipocytes. J Biol Chem. 1996;271:25227–25232. doi: 10.1074/jbc.271.41.25227. [DOI] [PubMed] [Google Scholar]
- 54.Tanti J F, Grillo S, Gremeaux T, Coffer P J, Van Obberghen E, Le Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 55.Tordjman K M, Leingang K A, James D E, Mueckler M M. Differential regulation of two distinct glucose transporter species expressed in 3T3-L1 adipocytes: effect of chronic insulin and tolbutamide treatment. Proc Natl Acad Sci USA. 1989;86:7761–7765. doi: 10.1073/pnas.86.20.7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering B M, Coffer P J, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 58.Walker K S, Deak M, Paterson A, Hudson K, Cohen P, Alessi D R. Activation of protein kinase B β and γ isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B α. Biochem J. 1998;331:299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q, Somwar R, Bilan P J, Liu Z, Jin J, Woodgett J R, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Withers D J, Gutierrez J S, Towery H, Burks D J, Ren J M, Previs S, Zhang Y, Bernal D, Pons S, Shulman G I, Bonner-Weir S, White M F. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]