Abstract
Background
Patients with hypertension and diabetes mellitus are susceptible to dementia, but regular therapy fails to reduce the risk of dementia. Glucagon‐like peptide‐1 receptor agonists have neuroprotective effects in experimental studies. We aimed to assess the effect of liraglutide, a glucagon‐like peptide‐1 receptor agonist, on cognitive function and whether its effect was associated with metabolic changes in patients with type 2 diabetes mellitus.
Methods and Results
Fifty patients with type 2 diabetes mellitus were recruited in this prospective study. All patients underwent cognitive assessment and brain activation monitoring by functional near‐infrared spectroscopy. At 12 weeks, patients in the glucagon‐like peptide‐1 group acquired better scores in all cognitive tests and showed remarkable improvement in memory and attention (P=0.040) test compared with the control group after multivariable adjustment. Compared with the control group, liraglutide significantly increased activation of the dorsolateral prefrontal cortex and orbitofrontal cortex brain regions (P=0.0038). After liraglutide treatment, cognitive scores were significantly correlated with changes in these activating brain regions (P<0.05), but no correlation was observed between the changes in cognitive function and changes of body mass index, blood pressure, and glycemic levels.
Conclusions
We concluded that liraglutide improves cognitive decline in patients with type 2 diabetes mellitus. This beneficial effect is independent of its hypoglycemic effect and weight loss. The optimal intervention should be targeted to cognitive decline in the early stages of dementia.
Registration
URL: https://www.ClinicalTrials.gov; Unique identifier: NCT03707171.
Keywords: cognitive function, functional near‐infrared spectroscopy, liraglutide, metabolic changes, type 2 diabetes mellitus
Subject Categories: Metabolism; Diabetes, Type 2; Imaging; Complications
Nonstandard Abbreviations and Acronyms
- DLPFC
dorsolateral prefrontal cortex
- fNIRS
functional near‐infrared spectroscopy
- GLP‐1 RAs
glucagon‐like peptide‐1 receptor agonists
- MMSE
Mini‐Mental State Examination
- OFC
orbitofrontal cortex
- VFT
verbal fluency task
Clinical Perspective
What Is New?
We examined liraglutide‐related cognitive improvement and demonstrated its neuroprotective effect in patients with diabetes mellitus without severe obesity.
Liraglutide significantly improved brain activities and alleviated cognitive decline in patients with diabetes mellitus, compared with other oral antidiabetic drugs.
The neuroprotective effect of liraglutide was not related to metabolic improvement, including blood pressure, blood lipids, glycemia. and body weight in patients with diabetes mellitus.
What Are the Clinical Implications?
This study provided evidence of glucagon‐like peptide‐1 effects on the central nervous system in patients with diabetes mellitus.
Early liraglutide treatment might have a beneficial effect on cognitive decline in patients with diabetes mellitus.
Glucagon‐like peptide‐1 receptor agonists might be promising drugs to improve cognitive dysfunction, however, these findings need to be confirmed in randomized controlled trials.
Alzheimer disease (AD) is highly prevalent worldwide.1 Type 2 diabetes mellitus (T2DM), hypertension, and obesity have been recognized as major risk factors for cognitive decline and AD.2 Importantly, patients with hypertension with diabetes mellitus usually display features of cognitive decline, such as defects in verbal memory and fluency, attention, and executive function.3 The underlying mechanism linking metabolic disorders and cognitive decline remains elusive although numerous studies have suggested that vascular and/or metabolic dysfunction might be involved.4, 5 However, several large trials did not show that anti‐hypertensive or hyperglycemic therapy benefited the cognitive function in patients with diabetes mellitus or hypertension with high cardiovascular risk.6 During the past 2 decades, a novel class of antidiabetic drugs, glucagon‐like peptide‐1 (GLP‐1) receptor agonists (RAs), have been extensively used to improve hyperglycemia, promote weight loss, and to reduce cardiovascular events in the clinic.7, 8 Importantly, experimental studies have demonstrated that GLP‐1 RAs display potent protective effect against cognitive impairment.9, 10 Several small trials have found that liraglutide improves the cognitive decline in patients with obesity with diabetes mellitus, which might be associated with weight loss.11, 12 Furthermore, a recent large trial suggests that dulaglutide administration might reduce the cognitive impairment in patients with T2DM through ameliorating the cerebrovascular injury.13 However, it is unknown whether the effect of GLP‐1 RAs on the improvement of cognitive decline directly acts on the central nervous system or indirectly ameliorates metabolic abnormality.14
Currently, various cognitive tests are used as major tools to assess the fundamental cognitive deficit in clinical trials.11, 15, 16 However, these cognitive tests are easily influenced by age, education, adherence, and the assessors' qualifications. Over the last decade, several neuroimaging techniques, such as functional magnetic resonance imaging, positron emission tomography, and functional near‐infrared spectroscopy (fNIRS) have been used to characterize behavioral and cognitive processes in patients with AD.11, 15, 16, 17 These up‐to‐date techniques, which combine with cognitive tests, provide a more reliable assessment of cognitive impairment.
In this study, we aimed to evaluate whether administration of liraglutide can improve cognitive decline in patients with T2DM through fNIRS neuroimaging combined with in‐person cognitive tests, and investigate whether its neuroprotective effect is associated with metabolic improvement.
Methods
Study Design and Participants
The data that support the findings of this study are available from the corresponding author upon reasonable request. This study is a 12‐week prospective, parallel assignment, open‐label, phase III study. Between October 2018 and May 2019, 50 patients with T2DM (diagnosed on the basis of the American Diabetes Association criteria) aged 18 to 65 years with a glycated hemoglobin (HbA1c) value of >7.0% who were treated with oral antidiabetic drugs or insulin for at least 3 months were enrolled in the study at Daping Hospital, Army Medical University, Chongqing, China.
At week 0 (baseline), patients who were overweight/obese (body mass index [BMI]: overweight, ≥25 kg/m2; obese, ≥28 kg/m2) were allocated to the GLP‐1 group (liraglutide at an initial dose of 0.6 mg/day and a maximum dose of 1.8 mg/day adjusted once a week when hyperglycemia was uncontrolled) or the control group (treated with oral antidiabetic drugs alone or combined with insulin). Previous treatments in both groups were continued with titration of the oral antidiabetic drug dose and/or combined with other oral antidiabetic drugs or insulin.
The withdrawal criteria included: (1) a fasting plasma glucose concentration >11.1 mmol/L after week 6; (2) intolerance to liraglutide (ie, nausea, vomiting, and other gastrointestinal adverse events). This study was conducted according to the principles of the Declaration of Helsinki. All protocols and experimental procedures were approved by the ethics committee at Daping Hospital. Written informed consent was obtained from all patients before initiation of any trial‐related activities. This study was registered on ClinicalTrials.gov (NCT03707171).
The exclusion criteria included: (1) T2DM with acute diabetic complications; (2) type 1 diabetes mellitus; (3) other diseases affecting cognitive function (eg, congenital dementia, brain trauma, epilepsy, severe hypoglycemic coma, cerebrovascular disease, ischemic heart disease, renal dysfunction); (4) alcohol abuse, mental illness, and psychoactive substance abuse; (5) history of thyroid disease; (6) any surgical or medical conditions that could significantly influence the absorption, distribution, metabolism, or excretion of interventional drugs; (7) unwillingness to provide informed consent.
Study Outcomes
The primary outcome was the change in cognitive function from baseline to 12 weeks. The secondary outcomes included changes in HbA1c, fasting plasma glucose, BMI, lipid profile, waist circumference, and blood pressure.
Sample Size Estimation
The sample size in this study was based on previous reports of cognitive improvement induced by GLP‐1 RA as evaluated by the Mini‐Mental State Examination (MMSE).11, 15, 16 We assumed at least a between‐group difference of 1.2 and an SD of 1.0 in the change in MMSE score from baseline to 12 weeks. Assuming a dropout rate of 20%, a total sample of 30 patients was targeted. Finally, 50 patients were enrolled in the study.
Biochemical and Anthropometric Measurements
Fasting plasma glucose, HbA1c, total cholesterol, triglycerides, high‐density lipid cholesterol (HDL‐c),and low‐density lipid cholesterol were measured after overnight fasting for 8 hours. Anthropometric measurements, including blood pressure, waist circumference, height, and body weight, were performed by trained assessors. Office blood pressure measurements were performed on both arms in the sitting position after 10 minutes of resting. BMI was defined as body weight (kg) divided by the square of body height (meters).
Cognitive Function Assessment
We used neuropsychological cognition tests to evaluate individual cognition. Those tests were widely used in previous neurological and chronic disease‐associated cognition assessment.18, 19, 20 A well‐trained examiner who was masked to the participants' treatments conducted the cognitive tests at baseline and again at 12 weeks. The general cognition evaluation was performed using the MMSE. The following tests were used to evaluate cognitive subdomains: (1) memory evaluation: Digit Span Test (including forward and backward), Rey Auditory Verbal Learning Test (including total learning, long‐delay free recall, and recognition); (2) executive function and attention: Trail Making Test, Clock Drawing Test; (3) verbal function: Animal Naming Test. Memory and Executive Screening is also a measurement of objective cognitive function used to evaluate both memory and executive function. All tests required 50 minutes to complete in a fixed order.
fNIRS Data Acquisition and Analysis
fNIRS permits real‐time, in‐situ measurement of changes in cortical oxyhemoglobin and deoxyhemoglobin that comprise brain activity and cerebral blood flow in different body positions with varying functional tests. Participants were seated in a light‐ and sound‐attenuated room. We performed a verbal fluency task (VFT), which is an experimental paradigm that is widely used in fNIRS studies for cognitive evaluation.17 The VFT is a task used to generate as many phrases that begin with a specified word as possible within a certain time frame. Each block of the VFT consisted of a 30‐second pre‐task baseline and a 20‐second VFT, then followed by a 30‐second resting period. The designated word was selected and presented in a random order from the following 2 groups of 4 words: “Da,” “Xiao,” “Chang,” “Gao”, or “Ren,” “Tian,” “Ri,” and “Zhong” (Figure S1). The fNIRS signals were recorded using a multi‐channel, continuous‐wave NIRScout system (NIRx Medical Technologies LLC, Minneapolis, MN) with 8 emitters and 7 detectors (yielding 20 channels) placed over the prefrontal cortex region (Figure S2). The distance between the emitter and the detector was 3 cm. The wavelengths used for oxyhemoglobin and deoxyhemoglobin detection were 760 and 850 nm, respectively, with a sampling frequency of 7.81 Hz. The recorded fNIRS signals were assessed with NIRS‐SPM software using MATLAB (2013a, MathWorks, Natick, MA). A band‐pass filter with cut‐off frequencies of 0.01 to 0.5 Hz was applied to remove artefacts arising from heartbeat and respiration. Optical density data were converted to oxyhemoglobin and deoxyhemoglobin concentration changes throughout the whole experiment according to the Modified Beer‐Lambert Law. We mainly focused on the oxyhemoglobin signals because they had a better signal‐to‐noise ratio and were more sensitive to changes in cerebral blood flow.17 Block average was performed to obtain the average response of each patient during the VFT from the 20 channels at baseline and after 12 weeks treatment.
Statistical Analyses
Numerical results are presented as mean±SD, median with interquartile range, or n (%). The Kolmogorov–Smirnov test or the Shapiro–Wilk test was used to determine whether each variable had a normal distribution. The baseline characteristics of patients were compared between groups using the Chi‐squared test for categorical variables, the independent 2‐sample t‐test for continuous variables with a normal distribution, and the Mann–Whitney non‐parametric U test for data with a non‐normal distribution. The paired t‐test was used to compare the cognitive assessments of patients at baseline and at 12 weeks after treatment in both groups. The independent 2‐sample t‐test was used to compare the cognitive assessments between groups after 12 weeks treatment. To determine the influence of metabolic parameters on cognitive tests between groups at 12 weeks, multivariable adjustment, including adjustment for age, sex, insulin usage, educational background, duration of T2DM, BMI, HbA1c, total cholesterol, triglycerides, HDL‐c, LDL‐c, and systolic and diastolic blood pressure, and corresponding 95% CIs were estimated using covariance analysis and the Univariate General Linear Model. The Wilcoxon matched‐pairs signed‐rank test was conducted to investigate the effects of liraglutide on brain activity during cognitive tasks at baseline and 12 weeks after treatment. The Mann–Whitney non‐parametric U test was used to analyze differences in mean oxyhemoglobin concentration indifferent channels between the control and GLP‐1 groups. Spearman non‐parametric correlation analysis was performed to assess the relationship between mean oxyhemoglobin concentration changes in significant channels (Ch13 and Ch15), BMI, blood pressure, fasting blood glucose (FBG), HbA1c, and cognitive assessments. Numerical statistical analyses were conducted using SPSS software, version 13.0 (SPSS, Inc.) or GraphPad Prism software, version 5.0 (GraphPad Software), and a 2‐sided P value of <0.05 was considered statistically significant.
Results
Baseline Characteristics
Fifty patients with T2DM were allocated to either the GLP‐1 group or control group in a 1:1 ratio. After the initial assessment, 2 patients in the control group and 1 patient in the GLP‐1 group quit the trial. A total of 47 patients completed the trial (Figure S3).
At baseline, all general characteristics, including age, sex, education, duration of T2DM, FBG, HbA1c, blood pressure, type of hypoglycemic drug (excluding metformin), and blood lipid concentrations were matched in the 2 groups (Table 1). Patients in the GLP‐1 group had a higher BMI and higher triglycerides and HDL‐c concentrations compared with the control group. There were no differences in cognitive performance at baseline between the 2 groups (Table 2).
Table 1.
Baseline Characteristics of the Patients With Type 2 Diabetes Mellitus
| Control Group (n=23) | GLP‐1 Group (n=24) | |
|---|---|---|
| Age, y | 59.5±7.4 | 55.0±11.9 |
| Male sex, n (%) | 9 (39.13) | 14 (58.33) |
| College degree and above, n (%) | 6 (26.09) | 10 (41.67) |
| Duration of type 2 diabetes mellitus, y | 9.00 [4.00, 12.75] | 9.50 [0.88, 17.00] |
| Body mass index, kg/m2 | 24.7±1.8 | 28.1±2.2* |
| Fasting blood glucose, mmol/L | 8.56±3.33 | 9.63±3.50 |
| HbA1c, % | 8.71±2.14 | 9.54±1.99 |
| Blood pressure, mm Hg | ||
| Systolic | 128±11 | 129±11 |
| Diastolic | 80±8 | 81±8 |
| Patients receiving (drug class) | ||
| Insulin | 15 (65.22) | 17 (70.83) |
| Metformin | 5 (21.74) | 13 (54.17)* |
| Sulfonylureas (2nd generation) | 3 (13.04) | 1 (4.17) |
| Thiazolidinediones | 0 (0.00) | 2 (8.33) |
| α‐glucosidase inhibitors | 3 (13.04) | 5 (20.83) |
| Glinides | 0 (0.00) | 1 (4.17) |
| TC, mmol/L | 5.09±1.49 | 4.58±1.35 |
| Triglycerides , mmol/L | 1.75±1.10 | 3.14±2.28* |
| HDL‐c, mmol/L | 1.23±0.27 | 0.95±0.14* |
| LDL‐c, mmol/L | 2.81±0.95 | 2.80±0.87 |
Data are mean±SD, median with interquartile range, or n (%). The body mass index is the weight in kilograms divided by the square of the height in meters. GLP‐1 indicates glucagon‐like peptide‐1; HbA1c, glycated hemoglobin; HDL‐c, high‐density lipid cholesterol; LDL‐c, low‐density lipid cholesterol; and TC, total cholesterol.
P<0.05 GLP‐1 group vs control group.
Table 2.
Cognitive Assessments of the Patients at Baseline and at 12‐Weeks of Treatment in Both Groups
| Control Group (n=23) | GLP‐1 Group (n=24) | P Value | Adjusted‐P Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0‐wk | 12‐wk | P Value* | 0‐wk | 12‐wk | P Value† | |||
| Digit Span Test‐ forwards | 6.96±1.49 | 7.17±1.07 | 0.3647 | 6.71±1.20 | 7.63±1.28 | <0.001 | 0.198 | 0.505 |
| Digit Span Test‐ backwards | 3.91±1.00 | 4.26±1.39 | 0.2009 | 4.21±1.28 | 4.96±1.30 | 0.0064 | 0.082 | 0.889 |
| Total Learning | 23.48±6.86 | 25.70±6.27 | 0.0078 | 24.83±7.31 | 29.88±7.15 | <0.001 | 0.039 | 0.444 |
| Long‐Delay Free Recall | 6.10±2.61 | 6.67±3.52 | 0.3343 | 5.00±2.35 | 7.13±2.83 | 0.0236 | 0.692 | 0.842 |
| Recognition | 20.09±5.34 | 20.94±3.09 | 0.8914 | 19.58±3.34 | 22.00±1.81 | 0.0049 | 0.182 | 0.725 |
| Animal Naming Test | 17.91±7.01 | 17.39±4.67 | 0.7382 | 17.67±4.82 | 20.75±5.20 | <0.001 | 0.025 | 0.726 |
| Clock Drawing Test | 3.09±1.11 | 3.52±0.73 | 0.0706 | 2.96±1.00 | 3.42±0.78 | 0.0183 | 0.635 | 0.623 |
| Trail Making Test | 49.68±30.52 | 52.55±31.06 | 0.7336 | 44.78±20.77 | 39.29±18.22 | 0.0100 | 0.081 | 0.658 |
| Minimum Mental State Examination | 27.39±2.04 | 27.48±1.73 | 0.8171 | 27.92±1.86 | 28.96±1.00 | 0.0087 | 0.001 | 0.040 |
| Memory and executive screening—memory | 40.43±7.65 | 43.74±4.27 | 0.0222 | 41.00±5.88 | 44.92±5.15 | <0.001 | 0.399 | 0.499 |
| Memory and executive screening—executive | 46.04±7.45 | 47.26±6.20 | 0.3605 | 46.04±3.43 | 47.67±2.04 | 0.0072 | 0.762 | 0.385 |
| Memory and executive screening—total | 86.48±11.77 | 90.09±9.38 | 0.0817 | 87.04±8.16 | 92.58±5.94 | <0.001 | 0.279 | 0.892 |
Adjusted for data at week 12. Variables include age, sex, insulin usage, educational background, duration of type 2 diabetes mellitus, body mass index, glycated hemoglobin, total cholesterol, triglyceride, HDL‐c, high‐density lipid cholesterol; LDL, low‐density lipid cholesterol; and systolic and diastolic blood pressure. GLP‐1 indicates glucagon‐like peptide‐1.
cognitive tests comparison between 0‐week and 12‐week in Control group.
cognitive tests comparison between 0‐week and 12‐week in GLP‐1 group.
Effect of Liraglutide on Cognitive Function and Metabolic Parameters
After 12 weeks of treatment, patients in the GLP‐1 group demonstrated better performance in all cognitive tests, which included the total learning (P=0.039), Animal Naming Test (P=0.025), and MMSE (P=0.001) tests, compared with the control group. In contrast, only total learning and Memory and Executive Screening memory scores increased in the control group after treatment (Table 2). FBG and HbA1c concentrations significantly decreased in both groups after treatment, but no significant difference was observed between the 2 groups. Administration of liraglutide significantly decreased BMI (P=0.001) and increased HDL‐c concentration (P=0.0046) compared with the control group (Table S1). In the GLP‐1 group, patients maintained a higher MMSE score compared with the control group (P=0.040) after adjustment for age, sex, insulin usage, educational background, duration of T2DM, BMI, HbA1c, total cholesterol, triglycerides, HDL‐c, LDL, and systolic and diastolic blood pressure (Table 2).
Effect of Liraglutide on Cognition‐Associated Brain Function
At baseline and after liraglutide treatment for 12 weeks, we simultaneously evaluated brain activation using the VFT and fNIRS monitoring. Compared with baseline, liraglutide significantly increased brain activation (represented by the increment in oxyhemoglobin concentration) in the dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex (OFC) (channels 2, 8, 13, and 17; Figure 1). The effect of liraglutide on the mean oxyhemoglobin concentration in these brain regions was analyzed (Figure 1, Table S2). In contrast, brain activation was not different between baseline and after 12 weeks of treatment in the control group. Compared with the control group, it further showed that liraglutide significantly increased brain activation in the DLPFC (channel 13: GLP‐1 versus control: 0.2248 [0.1136, 0.4036] versus 0.0286 [−0.0674, 0.2650], respectively; P=0.0224) and brain activation in the OFC (channel 15: GLP‐1 versus control: 0.1248 [0.0353, 0.2254] versus 0.0352 [−0.0551, 0.0990], respectively; P=0.0038) (Figure 2). However, these activating effects on brain regions were absent in the control group.
Figure 1. The grand averaged oxyhemoglobin concentration in relevant channels during verbal fluency task between baseline and 12 weeks after glucagon‐like peptide‐1 treatment.
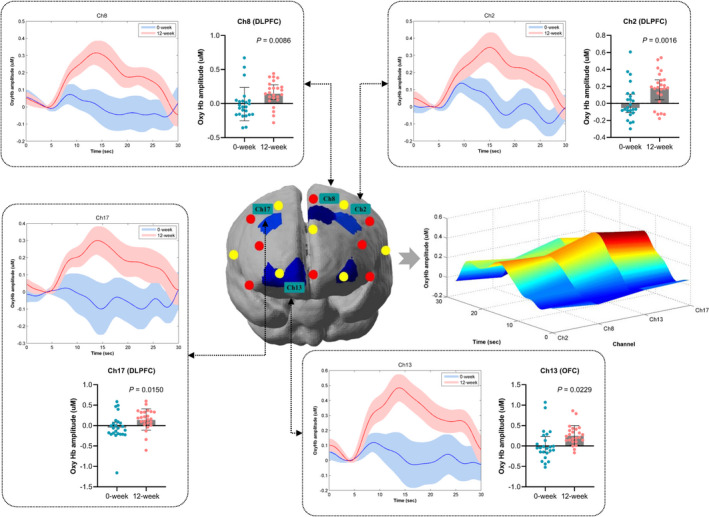
The thick curves showed the averaged oxyhemoglobin concentration over patients in significant channels (channels 2, 8, 13, and 17). The shaded area indicated the SEM. The extracted mean oxyhemoglobin concentration during verbal fluency task in relevant channels after glucagon‐like peptide‐1 treatment were expressed as median with interquartile range and were compared using the Wilcoxon matched‐pairs signed‐rank test. T‐map of mean oxyhemoglobin concentration changes for 12‐week vs 0‐week contrast in glucagon‐like peptide‐1 group with the distribution of the significant channels was presented in the middle row panel (P<0.05). The mean oxyhemoglobin concentration differences (between 0 and 12 weeks) as a function of significant channels and time of all patients in glucagon‐like peptide‐1 group was presented in the middle row right panel. Ch2, Ch8, Ch13, and Ch17 indicate Channels 2, 8, 13 and 17; DLFPC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; and OxyHb, Oxyhemoglobin.
Figure 2. The grand averaged oxyhemoglobin concentration in relevant channels during verbal fluency task at 12weeks between control and GLP‐1 groups, and the correlation of significant channels with metabolic factors and cognitive tests.
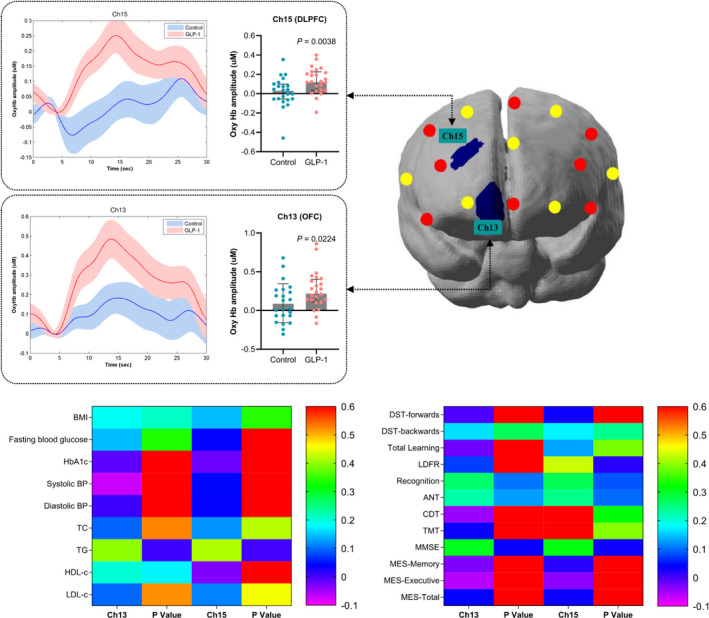
The thick curves showed the averaged oxyhemoglobin concentration over patients in significant channels (channels 13 and 15) in both groups. The shaded area indicated the SEM. T‐map of mean oxyhemoglobin concentration difference at 12 weeks of GLP‐1 group vs control group contrast with the distribution of the significant channels was presented in the upper row right panel (P<0.05). The comparisons of grand mean oxyhemoglobin concentration in significant channels at 12‐weeks between control and GLP‐1 groups. Data were expressed as median with interquartile range. The correlations of metabolic parameters and cognitive assessments with the mean oxyhemoglobin concentration in significant channels were presented by heat map in the lower row panel. ANT indicates Animal Naming Test; BMI, body mass index; BP, blood pressure; Ch13, and Ch15, Channels 13 and 15; CDT, Clock Drawing Test; DST, Digit Span Test; GLP‐1, glucagon‐like peptide‐1; HbA1c, glycated hemoglobin; HDL‐c, high‐density lipid cholesterol; LDFR, long‐delay free recall; LDL‐c, low‐density lipid cholesterol; MES, Memory and Executive Screening; MMSE, Mini‐Mental State Examination; OxyHb, Oxyhemoglobin; TC, total cholesterol; TG, triglycerides; and TMT, Trail Making Test.
Correlation Between Metabolic Changes and Cognition‐Associated Brain Function
To determine whether brain activation was associated with cognitive performance in these regions, we analyzed the correlation between mean oxyhemoglobin concentration changes in activated brain regions and metabolic parameters, as well as cognitive performance (Figure 2, Table S3). No significant correlation between mean oxyhemoglobin concentration changes in activated brain regions and BMI or FBG in patients were observed after liraglutide treatment. Importantly, MMSE scores were significantly correlated with mean changes of oxyhemoglobin concentration in these activated brain regions (channel 13, r=0.3056, P=0.0367; channel 15, r=0.3120, P=0.0328) in patients after liraglutide treatment. Similarly, mean changes of oxyhemoglobin concentration in channel 15 were highly correlated with long‐delay free recall score (r=0.4303, P=0.0176) in patients after liraglutide treatment.
Discussion
Main Findings
The novel findings in this study included: (1) treatment with liraglutide for 12 weeks remarkably improved cognitive function in patients with T2DM compared with regular hypoglycemic treatment; (2) Relative to control group, liraglutide significantly activated certain brain regions, including the DLPFC and the OFC, which were highly associated with improvements in cognitive performance; (3) The neuroprotective effect of liraglutide was not related with changes of blood pressure, glycemia, and body weight in patients with T2DM. These findings clearly suggest that the beneficial effects of liraglutide were independent of metabolic improvement.
Interpretation of Study Findings and Comparison With Existing Literature
Although epidemiological evidence indicates that diabetes mellitus, obesity, and hypertension increase the risk of dementia, remission of cardiometabolic risk factors through medical treatment failed to ameliorate cognitive decline in patients, as shown by several large and prospective clinical trials.21, 22, 23, 24 In these trials, patients had higher cardiovascular risk and multiple complications, thus cardiovascular outcomes were a primary end point and cognitive function was a secondary end point. GLP‐1 RAs are a new class of hypoglycemic drug that can stimulate insulin release, reduce glucagon release, slow gastric emptying, and induce satiety.25 Importantly, plenty of experimental studies have demonstrated that GLP‐1 administration or activation of the GLP‐1 receptor have neuroprotective actions, including lowering of amyloid‐β plaque loads in the brain, preventing age‐dependent tau hyperphosphorylation, and improving cognitive performance in animal models of AD.9, 26 However, the beneficial effect of GLP‐1 RAs on cognitive impairment is controversial in patients with AD. In a randomized trial, Gejl et al reported that liraglutide did not improve cognitive function although brain glucose transport was enhanced in patients with AD after treatment.16, 27 Another study using exenatide was prematurely terminated because of a lack of clear conclusions in patients thought to have AD.15 However, some positive results in patients with diabetes mellitus with obesity were reported in several small trials. Zhang et al found that treatment with GLP‐1 RAs improved cognitive impairment and olfactory dysfunction, which was partially associated with weight loss in patients with obesity and T2DM.11 Vadini et al showed that liraglutide could slow the decline in memory in patients with severe obesity with pre‐ or early diabetes mellitus, which was related to weight loss.12 It is worthy to note that cognitive improvement was a primary outcome in these studies. In this study, we showed that liraglutide remarkably improved cognitive decline, even after adjusting for BMI and blood glucose concentrations in patients with T2DM. The effects of liraglutide were not associated with a reduction in glycemia or body weight. One prominent difference between our study and the others was that our patients treated with liraglutide had a higher HbA1c value (mean, 9.6%) and a lower BMI (mean, 28 kg/m2) compared with patients enrolled in Zhang's (HbA1c, 8.1%; BMI, 32 kg/m2) and Vadini's (HbA1c, 5.9%; BMI, 36.7 kg/m2) reports. Therefore, these studies cannot exclude the effect of GLP‐1 RA‐mediated weight loss on cognitive improvement in patients with obesity and T2DM. Recently, a large clinical trial reported that dulaglutide might reduce cognitive decline in patients with T2DM with high cardiovascular risk, implying that this effect may be derived from its cardiovascular benefits.13 By contrast, our patients with liraglutide treatment had lower cardiovascular risk. Therefore, our findings could reflect a direct neuroprotective effect of liraglutide because GLP‐1 receptor is found in many brain regions associated with memory and learning.28 Meanwhile, the brain insulin resistance was suggested contributing to the AD development, and intranasal insulin administration has been shown to improve memory in human studies.29, 30 In this study, the dosage of insulin was comparable in both groups, but we did not find its beneficial effect on cognitive improvement in patients with T2DM. Currently, there are some controversial results on this issue, because insulin could cause hypoglycemia related cognitive damage in patients with T2DM.31, 32 Importantly, GLP‐1 RA activation was found to increase in the cAMP levels leading to the modulation of downstream kinases that are related to growth factor signaling, which may thereby restore brain insulin resistance and reduce levels of amyloid‐β peptide.26
Strengths and Limitations
We used a neuroimaging technique for further validating our assumption. At present, the major assessments of cognitive status are neuropsychological tests. However, these methods are subject to interference by subjective judgments and personal qualities. Over the past decade, functional magnetic resonance imaging and positron emission tomography have been used to characterize behavioral and cognitive processes in patients with AD.11, 15, 16, 33 However, functional magnetic resonance imaging is limited to postural changes and cannot be used to obtain real‐time information about brain activation during execution of cognitive tasks. fNIRS is a non‐invasive neuroimaging technique that has a spatial resolution similar to functional magnetic resonance imaging with a high temporal resolution and portability.17 This approach permits in‐situ measurement of brain activity and cerebral blood flow in different body positions using varying functional tests. In this study, we evaluated the effect of liraglutide on cognitive status using the VFT combined with real‐time fNIRS. It clearly showed that liraglutide activated the DLPFC and OFC brain regions, which have long been recognized to participate in regulation of appetite and eating behaviors, especially for reward evaluation.34 Activation of the DLPFC and the OFC was also reported to be associated with better cognitive performance in patients with AD.35, 36 In this study, improvements in MMSE scores also correlated with activation of these brain regions, as shown by fNIRS analysis. Thus, we provide a novel modality for assessing brain function in clinical studies. We validate that the beneficial effect of liraglutide on cognitive impairment is independent of metabolic improvements in patients with T2DM. This study has several limitations. First, our study was not a randomized controlled clinical trial. Second, it is unclear whether other GLP‐1 RAs have similar effects as liraglutide in patients with T2DM. Third, repeating cognitive tests could increase the deviation in memorization, which is often difficult to avoid in this type of study.
Perspective
Overall, our study has demonstrated that the improvement of cognitive decline by liraglutide is independent of its blood pressure and metabolic changes in patients with diabetes mellitus. The optimal treatment for cognitive impairment should be targeted in the patients with mild cognitive impairment. In addition, cognitive tests integrating the fNIRS are reliable approaches to evaluate brain function in clinical practice. If randomized controlled trials confirm these findings in the future, GLP‐1 RAs might be a promising drug to improve the patients with cognitive dysfunction.
Sources of Funding
This project has received funding from the National Natural Science Foundation of China and Third Military Medical University for funding of the Triple A program, led by Zhu. This study was supported by grants from the National Key Research and Development Project (2018YFA0800601) and the National Natural Science Foundation of China (81721001, 81870614, 31701023, 81900380). The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S3
Acknowledgments
We thank Dr Hongbo Jia (Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou, China) for his critical comments.
(J Am Heart Assoc. 2021;10:e020734. DOI: 10.1161/JAHA.120.020734.)
For Sources of Funding and Disclosures, see page 9.
References
- 1.Alzheimer's Association . 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. DOI: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Adams ML, Grandpre J, Katz DL, Shenson D. Cognitive impairment and cardiovascular disease: a comparison of risk factors, disability, quality of life, and access to health care. Public Health Rep. 2020;135:132–140. DOI: 10.1177/0033354919893030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW. Diabetes and cognitive impairment. Curr Diab Rep. 2016;16:87. DOI: 10.1007/s11892-016-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591–604. DOI: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14:329–340. DOI: 10.1016/S1474-4422(14)70249-2. [DOI] [PubMed] [Google Scholar]
- 6.Biessels GJ, Verhagen C, Janssen J, van den Berg E, Zinman B, Rosenstock J, George JT, Passera A, Schnaidt S, Johansen OE, et al. Effect of linagliptin on cognitive performance in patients with type 2 diabetes and cardiorenal comorbidities: the CARMELINA randomized trial. Diabetes Care. 2019;42:1930–1938. DOI: 10.2337/dc19-0783. [DOI] [PubMed] [Google Scholar]
- 7.Areosa Sastre A, Vernooij RW, Gonzalez‐Colaco Harmand M, Martinez G. Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev. 2017;6:CD003804. DOI: 10.1002/14651858.CD003804.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon‐like peptide‐1 receptor agonists on weight loss: systematic review and meta‐analyses of randomised controlled trials. Br Med J. 2012;344:d7771. DOI: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, et al. Glucagon‐like peptide‐1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. DOI: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 10.Ma DL, Chen FQ, Xu WJ, Yue WZ, Yuan G, Yang Y. Early intervention with glucagon‐like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. J Neurochem. 2015;135:301–308. DOI: 10.1111/jnc.13248. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Zhang B, Wang X, Zhang X, Yang QX, Qing Z, Zhang W, Zhu D, Bi Y. Olfactory dysfunction mediates adiposity in cognitive impairment of type 2 diabetes: insights from clinical and functional neuroimaging studies. Diabetes Care. 2019;42:1274–1283. DOI: 10.2337/dc18-2584. [DOI] [PubMed] [Google Scholar]
- 12.Vadini F, Simeone PG, Boccatonda A, Guagnano MT, Liani R, Tripaldi R, Di Castelnuovo A, Cipollone F, Consoli A, Santilli F. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes. 2020;44:1254–1263. DOI: 10.1038/s41366-020-0535-5. [DOI] [PubMed] [Google Scholar]
- 13.Cukierman‐Yaffe T, Gerstein HC, Colhoun HM, Diaz R, García‐Pérez L‐E, Lakshmanan M, Bethel A, Xavier D, Probstfield J, Riddle MC, et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 2020;19:582–590. DOI: 10.1016/S1474-4422(20)30173-3. [DOI] [PubMed] [Google Scholar]
- 14.Grieco M, Giorgi A, Gentile MC, d'Erme M, Morano S, Maras B, Filardi T. Glucagon‐like peptide‐1: a focus on neurodegenerative diseases. Front Neurosci. 2019;13:1112. DOI: 10.3389/fnins.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullins RJ, Mustapic M, Chia CW, Carlson O, Gulyani S, Tran J, Li Y, Mattson MP, Resnick S, Egan JM, et al. A pilot study of exenatide actions in Alzheimer's disease. Curr Alzheimer Res. 2019;16:741–752. DOI: 10.2174/1567205016666190913155950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gejl M, Gjedde A, Egefjord L, Møller A, Hansen SB, Vang K, Rodell A, Brændgaard H, Gottrup H, Schacht A, et al. In Alzheimer's disease, 6‐month treatment with GLP‐1 analog prevents decline of brain glucose metabolism: randomized, placebo‐controlled, double‐blind clinical trial. Front Aging Neurosci. 2016;8:108. DOI: 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang D, Hong KS, Yoo SH, Kim CS. Evaluation of neural degeneration biomarkers in the prefrontal cortex for early identification of patients with mild cognitive impairment: an fNIRS study. Front Hum Neurosci. 2019;13:317. DOI: 10.3389/fnhum.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Zhang B, Wang X, Zhang X, Yang QX, Qing Z, Lu J, Bi Y, Zhu D. Altered odor‐induced brain activity as an early manifestation of cognitive decline in patients with type 2 diabetes. Diabetes. 2018;67:994–1006. DOI: 10.2337/db17-1274. [DOI] [PubMed] [Google Scholar]
- 19.Valls‐Pedret C, Sala‐Vila A, Serra‐Mir M, Corella D, de la Torre R, Martínez‐González MÁ, Martínez‐Lapiscina EH, Fitó M, Pérez‐Heras A, Salas‐Salvadó J, et al. Mediterranean diet and age‐related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175:1094–1103. DOI: 10.1001/jamainternmed.2015.1668. [DOI] [PubMed] [Google Scholar]
- 20.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. DOI: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 21.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. DOI: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 22.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–666. DOI: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 23.Richard E, Jongstra S, Soininen H, Brayne C, Moll van Charante EP, Meiller Y, van der Groep B, Beishuizen CRL, Mangialasche F, Barbera M, et al. Healthy ageing through internet counselling in the elderly: the HATICE randomised controlled trial for the prevention of cardiovascular disease and cognitive impairment. BMJ Open. 2016;6:e010806. DOI: 10.1136/bmjopen-2015-010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran C, Callisaya ML, Srikanth V, Arvanitakis Z. Diabetes therapies for dementia. Curr Neurol Neurosci Rep. 2019;19:58. DOI: 10.1007/s11910-019-0973-4. [DOI] [PubMed] [Google Scholar]
- 25.Patti AM, Rizvi AA, Giglio RV, Stoian AP, Ligi D, Mannello F. Impact of glucose‐lowering medications on cardiovascular and metabolic risk in type 2 diabetes. J Clin Med. 2020;9:912. DOI: 10.3390/jcm9040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClean PL, Parthsarathy V, Faivre E, Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci. 2011;31:6587–6594. DOI: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gejl M, Brock B, Egefjord L, Vang K, Rungby J, Gjedde A. Blood‐brain glucose transfer in Alzheimer's disease: effect of GLP‐1 analog treatment. Sci Rep. 2017;7:17490. DOI: 10.1038/s41598-017-17718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia XT, Ye‐Tian, Yuan‐Li, Zhang GJ, Liu ZQ, Di ZL, Ying XP, Fang Y, Song EF, Qi JS, et al. Exendin‐4, a glucagon‐like peptide 1 receptor agonist, protects against amyloid‐beta peptide‐induced impairment of spatial learning and memory in rats. Physiol Behav. 2016;159:72–79. DOI: 10.1016/j.physbeh.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early‐stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;45:1269–1270. DOI: 10.3233/JAD-159002. [DOI] [PubMed] [Google Scholar]
- 30.Arnold SE, Arvanitakis Z, Macauley‐Rambach SL, Koenig AM, Wang H‐Y, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168–181. DOI: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He C, Gao P, Cui Y, Li Q, Li Y, Lu Z, Ma H, Zhao YU, Li LI, Sun F, et al. Low‐glucose‐sensitive TRPC6 dysfunction drives hypoglycemia‐induced cognitive impairment in diabetes. Clin Transl Med. 2020;10:e205. DOI: 10.1002/ctm2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palleria C, Leporini C, Maida F, Succurro E, De Sarro G, Arturi F, Russo E. Potential effects of current drug therapies on cognitive impairment in patients with type 2 diabetes. Front Neuroendocrinol. 2016;42:76–92. DOI: 10.1016/j.yfrne.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Beishon L, Haunton VJ, Panerai RB, Robinson TG. Cerebral hemodynamics in mild cognitive impairment: a systematic review. J Alzheimers Dis. 2017;59:369–385. DOI: 10.3233/JAD-170181. [DOI] [PubMed] [Google Scholar]
- 34.Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M. Peptide hormones regulating appetite—focus on neuroimaging studies in humans. Diabetes Metab Res Rev. 2011;27:104–112. DOI: 10.1002/dmrr.1154. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Liang P, Lu S, Li K, Zhong N. The role of the DLPFC in inductive reasoning of MCI patients and normal agings: an fMRI study. Sci China C Life Sci. 2009;52:789–795. DOI: 10.1007/s11427-009-0089-1. [DOI] [PubMed] [Google Scholar]
- 36.Staffaroni AM, Melrose RJ, Leskin LP, Riskin‐Jones H, Harwood D, Mandelkern M, Sultzer DL. The functional neuroanatomy of verbal memory in Alzheimer's disease: [F‐18]‐Fluoro‐2‐deoxy‐D‐glucose positron emission tomography (FDG‐PET) correlates of recency and recognition memory. J Clin Exp Neuropsychol. 2017;39:682–693. DOI: 10.1080/13803395.2016.1255312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S3


