Abstract
Background
Heart failure (HF) poses a major public health burden in the United States. We examined the burden of out‐of‐pocket healthcare costs on patients with HF and their families.
Methods and Results
In the Medical Expenditure Panel Survey, we identified all families with ≥1 adult member with HF during 2014 to 2018. Total out‐of‐pocket healthcare expenditures included yearly care‐specific costs and insurance premiums. We evaluated 2 outcomes of financial toxicity: (1) high financial burden—total out‐of‐pocket healthcare expense to postsubsistence income ratio of >20%, and (2) catastrophic financial burden with the ratio of >40%—a bankrupting expense defined by the World Health Organization. There were 788 families in the Medical Expenditure Panel Survey with a member with HF representing 0.54% (95% CI, 0.48%–0.60%) of all families nationally. The overall mean annual out‐of‐pocket healthcare expenses were $4423 (95% CI, $3908–$4939), with medications and health insurance premiums representing the largest categories of cost. Overall, 14% (95% CI, 11%–18%) of families experienced a high burden and 5% (95% CI, 3%–6%) experienced a catastrophic burden. Among the two‐fifths of families considered low income, 24% (95% CI, 18%–30%) experienced a high financial burden, whereas 10% (95% CI, 6%–14%) experienced a catastrophic burden. Low‐income families had 4‐fold greater risk‐adjusted odds of high financial burden (odds ratio [OR] , 3.9; 95% CI, 2.3–6.6), and 14‐fold greater risk‐adjusted odds of catastrophic financial burden (OR, 14.2; 95% CI, 5.1–39.5) compared with middle/high‐income families.
Conclusions
Patients with HF and their families experience large out‐of‐pocket healthcare expenses. A large proportion encounter financial toxicity, with a disproportionate effect on low‐income families.
Keywords: costs of care, financial hardship, financial toxicity, out‐of‐pocket
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
One in 7 families with a member with heart failure and 1 in 4 of low‐income families with a member with HF experience financial toxicity from out‐of‐pocket healthcare costs, spending over 20% of their postsubsistence income on healthcare expenses in a given year.
Health insurance premiums and medication costs represent the largest categories of out‐of‐pocket healthcare spending among families of patients with heart failure.
What Are the Clinical Implications?
Financial toxicity represents an additional challenge for families of patients with heart failure, with a disproportionate effect on low‐income families.
Heart failure (HF) affects over 5.4 million individuals in the United States,1 costing the US healthcare system nearly 31 billion dollars every year. With the aging population, the healthcare costs of HF are projected to increase to 50 billion by 2030.2 Patients with HF experience 4‐fold higher overall 2‐year healthcare expenditures compared with individuals without HF.3 The costs incurred by patients with HF represent those for inpatient care for decompensated HF, outpatient care for longitudinal management of disease, and chronic therapy.4
In the contemporary era, one area of rapidly growing cost in HF is the advent of new medical therapies.5 The expenses for chronic therapy are expected to continue to increase, beginning with the emergence of neprilysin inhibitors in 2015. Despite the improvements in outcomes, these medications can represent a major cost burden for patients, with Medicare beneficiaries paying well over $1000 in out‐of‐pocket costs per year for these medications.6, 7 With clinical guidelines supporting a broader use of these drugs for their effects on patient outcomes, the financial implications of HF management, both to patient as out‐of‐pocket expenditures,8 and to the society as the overall cost of care are likely to continue to grow.
Despite growing knowledge of the burgeoning cost of HF to the healthcare system, the literature is limited on costs that directly affect patients. We aimed to examine the financial implications of out‐of‐pocket healthcare spending in patients with HF and their families in the contemporary era.
METHODS
The Medical Expenditure Panel Survey (MEPS), a yearly cross‐sectional survey of US families by the Agency of Healthcare Research and Quality, is a representative sample of the noninstitutionalized national US population,9 capturing information on demographic characteristics, medical conditions, healthcare expenditures, and insurance coverage and spending. All data and materials are publicly available online and can be accessed at https://meps.ahrq.gov/mepsweb/. For our study, we used the “household component” of MEPS, which is a detailed, serial survey of families on demographic characteristics, current health status and medical history, medical services used, amount and source of health expenditures, healthcare access, health insurance coverage, and socioeconomic status. Each year, a new panel of households is sampled based on respondents during the previous year's National Health Interview Survey. Approximately 15 000 families are sampled annually through a complex survey sampling design, with oversampling of Black, Hispanic, and Asian individuals. Overall response rates were ≈44% to 49% in the years 2014 to 2018.10 Families and individuals were given weights based on demographic proportions in the overall US population.11 To account for nonresponders, household analytic weights were first adjusted for individual and household nonresponse and then to national population totals. Estimates derived from MEPS are finally benchmarked for consistency against the National Health Interview Survey.12, 13 Because this study used only publicly available data, institutional review board approval was not required.
Study Population
We used the most recent 5 years of data spanning calendar years 2014 through 2018 for this study. We included all families with individuals 18 years old or older with HF, which was defined using International Classification of Diseases, Ninth Revision (ICD‐9) or Tenth Revision (ICD‐10) Clinical Modification (CM) codes (428 or I50, respectively) generated through coding transcribed interviews conducted by trained interviewers.11 Because the burden of healthcare costs is often shared within households,14 families were designated as the primary unit of measurement, as has been done in prior studies.15 Families were defined as 1 or more people related by birth, marriage, or adoption, and residing together. Unrelated individuals living in the same household were treated as separate families.16
Study Covariates
We identified demographic features of the index individuals with HF included in the survey including age, sex, and race/ethnicity (categorized as non‐Hispanic White, non‐Hispanic Black, Hispanic, and other, which included Asian, American Indian, and those of multiple racial origins). Educational attainment was categorized as less than high school, high school or Graduate Equivalency Degree, and some college or higher. Health insurance status was categorized into private insurance, public insurance, and uninsured; and census region was categorized as Northeast, Midwest, South, and West.
Medical comorbidities were based on standardized questionnaires that evaluated whether a medical provider had diagnosed an individual with a given condition, encoded as Clinical Classifications Software/ICD‐9‐CM/ICD‐10‐CM codes and/or self‐report (Table S1). For this study, we included atherosclerotic cardiovascular disease (ASCVD, inclusive of coronary artery, cerebrovascular, and peripheral artery disease), arthritis, cancer, asthma, chronic obstructive pulmonary disease, hepatitis, and chronic kidney disease. Additionally, we identified risk factors of cardiovascular disease including hypertension, diabetes mellitus, obesity, dyslipidemia, prior or active smoking, and measures of physical activity to identify insufficient physical activity, defined as less than moderate physical activity 5 times per week.17 We also defined a composite cardiovascular risk factor profile based on binary variables of current smoking status, diabetes mellitus status, hypertension status, insufficient physical activity, dyslipidemia, and obesity, and categorized as optimal (0–1 factors), average (2–3 factors), or poor (≥4 factors).
Study Exposure
We used 2 main indicators of economic status for analyses of financial burden: (1) income and (2) insurance status. Income was defined as total annual family income, compiled from diverse data sources, including wages, social security, and veteran pay,18 and was operationalized into low income and middle/high income. Low income was defined as a family income below 200% of the federal poverty limit, and middle/high income was defined as >200% of the federal poverty limit, as used in previous studies.15, 19
We also operationalized insurance status as either private insurance, public insurance, or uninsured. Private insurance included all group or non‐group private insurance programs, and public insurance included Medicaid, Medicare, Tricare, or other state‐funded public insurance.16 Insurance provider was reported as a monthly field. If covered by both private and public insurance, the individual was defined based on the insurance type that provided coverage for the largest number of months in a given year. Patients were defined as uninsured if they did not have health insurance for even 1 month in a calendar year. Insurance status of the family was defined by the insurance coverage of the index individual with HF.
Study Outcomes
We defined a set of outcomes that assessed both the absolute out‐of‐pocket healthcare spending and out‐of‐pocket spending as a function of income. For each calendar year, total out‐of‐pocket healthcare expenses were defined as the sum of a family's yearly health insurance premiums and out‐of‐pocket spending on direct healthcare services including inpatient hospitalizations, outpatient care, medications, emergency department visits, and other healthcare spending including medical equipment. Copayment and deductible costs were included in these categories. These costs were ascertained through interview of participants, who were frequently encouraged to use objective information such as billing records when it was available to them.16 To ascertain which category of healthcare cost contributed the greatest extent to financial burden, we specifically determined the largest out‐of‐pocket cost category for each family.
We defined accepted measures of financial toxicity from healthcare costs based on the proportionate spending on healthcare relative to income. Consistent with definitions laid out by the World Health Organization and the World Bank, we computed an annual income measure that accounted for food‐related spending (or subsistence expenses).20 This annual postsubsistence income was defined as annual family income minus the annual income‐group specific food expenses, based on nomograms from the Bureau of Labor Statistics.21
We calculated the ratio of annual total out‐of‐pocket healthcare expenditures divided by annual household post‐subsistence income. High financial burden was defined as health expenses representing higher than 20% of annual postsubsistence income. Similarly, catastrophic financial burden was defined as healthcare expenses representing higher than 40% of postsubsistence income.22 These definitions represent situations where health insurance coverage is insufficient in preventing significant healthcare‐related out‐of‐pocket‐spending. In particular, a catastrophic financial burden represents a measure of health spending used by the World Health Organization to identify expenses that are likely to be financially ruinous.20 In order to generate a more conservative estimate, families were not included in subgroup analyses if their postsubsistence income was <$0, ≈5% (46 of 834) of families.15
Statistical Analysis
We used survey‐specific analytic methods that accounted for the multistage probability sampling design of the MEPS,23 explicitly accounting for the stratified sampling and clustering of the data within sampling units, as well as using the appropriate sampling weights to obtain national‐level estimates. We used the Stata 14 (College Station, TX) "svy" package for all analyses. Our approach followed the methodological best practices for survey data. A 2‐sided P value of <0.05 was deemed statistically significant.
First, we compared baseline characteristics, overall and by income groups, using analysis of variance for continuous variables and chi‐square tests for categorical variables. Then, we examined annual cost for each category of out‐of‐pocket health costs by each demographic, socioeconomic, and health status category. We then evaluated temporal trends in out‐of‐pocket healthcare expenses across the study years using survey linear regression of log‐transformed incomes. Next, we described the most expensive category of out‐of‐pocket expenses across income and insurance categories. All costs and incomes in our analyses were inflation adjusted to year 2018.24
Next, we calculated the proportion of high and catastrophic financial burden by age, sex, income category, and insurance status. Owing to small numbers of uninsured in our study population (n=15), we focused on the 2 groups of insurance: public and private. Logistic regression was used to determine odds ratios (ORs) of financial burden by socioeconomic subgroups, adjusted for age, sex, race/ethnicity, cardiovascular disease risk factor profile, ASCVD, chronic kidney disease, and cancer.
In the 5 cases (0.6%) where 2 members of the same family had HF, we included both members in the assessment of out‐of‐pocket costs. However, we randomly included 1 of the 2 individuals for defining characteristics in risk adjusted assessments of financial burden.
RESULTS
Population Characteristics of Patients With Heart Failure
There were 788 families with one or more members with HF, representing an estimated 1.7 million (95% CI, 1.5–1.9 million) or 0.54% (95% CI, 0.49%–0.61%) of US families nationally. Mean age of members with HF was 70 (95% CI, 69–71) years, 51% (95% CI, 46%–56%) were men, and 15% (95% CI, 12%–19%) were non‐Hispanic Black; 42% of families were from the South region. Overall, 37% (95% CI, 32%–42%) were represented in low‐income families. A total of 51% (95% CI, 45%–56%) had private insurance, 48% (95% CI, 43%–53%) had public insurance, and 2% (95% CI, 1%–3%) were uninsured. In terms of comorbidities, 49% (95% CI, 44%–55%) of index members with HF had 4 or more cardiovascular disease risk factors. Of all members with HF, 74% (95% CI, 69%–78%) also had ASCVD, 32% (95% CI, 27%–38%) had cancer, 33% (95% CI, 28%–39%) had chronic obstructive pulmonary disease, and 46% (95% CI, 41%–51%) had chronic kidney disease (Table 1). Only 15% of individuals with HF were employed (Figure S1), and 51% of families had no members who were employed.
Table 1.
Baseline Characteristics of Individuals With HF, Stratified by Income Level, From the MEPS 2014 to 2018
| Overall | Low Income | Middle/High Income | P Value | |
|---|---|---|---|---|
| Age (y), mean (95% CI) | 70 (69–71) | 69 (67–71) | 71 (69–72) | 0.19 |
| Sex, % (95% CI) | <0.01 | |||
| Male | 51 (46–56) | 39 (31–47) | 58 (52–65) | |
| Female | 49 (44–54) | 61 (53–69) | 42 (36–48) | |
| Race/ethnicity, % (95% CI) | <0.01 | |||
| Non‐Hispanic White | 73 (69–78) | 66 (59–72) | 78 (72–83) | |
| Non‐Hispanic Black | 15 (12–19) | 23 (18–29) | 11 (7–15) | |
| Hispanic | 6 (4–8) | 7 (5–11) | 5 (3–8) | |
| Other† | 5 (4–8) | 4 (2–7) | 6 (4–11) | |
| Education level, % (95% CI) | <0.01 | |||
| Less than high school | 13 (10–17) | 20 (16–27) | 8 (5–14) | |
| High school/graduate equivalency degree & equivalent | 44 (39–49) | 46 (38–55) | 42 (36–49) | |
| Some college or higher | 43 (38–49) | 33 (26–42) | 49 (42–56) | |
| Insurance type, % (95% CI) | <0.01 | |||
| Private | 51 (45–56) | 28 (22–35) | 64 (58–70) | |
| Public | 48 (43–53) | 70 (63–76) | 35 (29–41) | |
| Uninsured | 2 (1–3) | 2 (1–4) | 1 (0–3) | |
| Census region, % (95% CI) | 0.51 | |||
| Northeast | 15 (11–21) | 17 (11–26) | 14 (9–21) | |
| Midwest | 29 (24–35) | 26 (20–33) | 30 (24–38) | |
| South | 42 (37–48) | 45 (37–54) | 41 (34–48) | |
| West | 14 (11–18) | 12 (1–18) | 15 (11–20) | |
| Hypertension, % (95% CI) | 88 (85–91) | 93 (86–88) | 86 (80–90) | <0.01 |
| Diabetes mellitus, % (95% CI) | 46 (41–51) | 41 (34–49) | 48 (42–55) | 0.18 |
| Dyslipidemia, % (95% CI) | 77 (73–82) | 76 (69–82) | 78 (72–83) | 0.63 |
| Smoker, % (95% CI)* | 12 (9–17) | 21 (14–30) | 8 (5–13) | <0.01 |
| Obesity, % (95% CI)* | 49 (44–55) | 52 (44–60) | 47 (40–55) | 0.37 |
| Sufficient physical activity, % (95% CI) | 25 (22–29) | 23 (18–29) | 26 (22–31) | 0.40 |
| Cardiovascular risk factor profile, % (95% CI) | 0.24 | |||
| Optimal | 7 (5–10) | 6 (3–10) | 8 (5–12) | |
| Average | 44 (39–49) | 40 (33–48) | 46 (39–52) | |
| Poor | 49 (44–55) | 54 (47–62) | 47 (40–53) | |
| Atherosclerotic cardiovascular disease, % (95% CI) | 74 (69–78) | 75 (68–81) | 73 (67–78) | 0.70 |
| Arthritis, % (95% CI) | 71 (66–75) | 73 (67–79) | 70 (63–75) | 0.40 |
| Cancer, % (95% CI) | 32 (27–38) | 27 (20–34) | 36 (29–44) | 0.08 |
| Asthma, % (95% CI) | 22 (18–26) | 33 (26–40) | 15 (11–21) | <0.01 |
| Chronic obstructive pulmonary disease, % (95% CI) | 33 (28–39) | 42 (34–50) | 28 (22–35) | <0.01 |
| Hepatitis, % (95% CI) | 0 (0–1) | 0 (0–3) | 0 (0–2) | 0.87 |
| Chronic kidney disease, % (95% CI) | 46 (41–51) | 46 (38–55) | 46 (40–52) | 0.90 |
| Low income level, % (95% CI) | 37 (32–42) | NA | NA | NA |
HF indicates heart failure; and MEPS, Medical Expenditure Panel Survey.
2017 year does not have data on obesity, and 2018 year does not have data on smoking status.
Other includes Asian, American Indian, and those of multiple racial origins.
Out‐of‐Pocket Healthcare Costs
The mean family out‐of‐pocket expenditures was $4423 (95% CI, $3908–$4939) (Table 2), with premiums of $1614 (95% CI, $1346–$1882), and mean out‐of‐pocket expenditures of $185 (95% CI, $93–$278) for inpatient care, $584 (95% CI, $453–$715) for outpatient care, $1078 (95% CI, $896–$1262) for medications, and $39 (95% CI, $24–$54) for emergency care (Table 3).
Table 2.
Total Out‐of‐Pocket Healthcare Costs of Care for Families With 1 or More Members With HF, From the MEPS 2014 to 2018
| Characteristic | No. in National Population ×1000 Families (95% CI) | OOP Expenditures, $ (95% CI) | Total OOP Health Services Cost, $ (95% CI) |
|---|---|---|---|
| Overall | 1701 (1501–1900) | 4423 (3908–4939) | 2809 (2409–3209) |
| Age, y | |||
| 18–64 | 501 (416–586) | 3455 (2792–4117) | 1861 (1487–2235) |
| ≥ 65 | 1199 (1025–1374) | 4828 (4146–5509) | 3205 (2661–3749) |
| Sex | |||
| Male | 868 (735–1001) | 5326 (4456–6196) | 3389 (2697–4080) |
| Female | 832 (705–960) | 3482 (3047–3916) | 2204 (1830–2579) |
| Race/ethnicity | |||
| Non‐Hispanic White | 1249 (1071–1428) | 4931 (4265–5597) | 3251 (2723–3780) |
| Non‐Hispanic Black | 260 (203–317) | 2601 (2030–3173) | 1275 (999–1551) |
| Hispanic | 98 (60–136) | 3725 (2714–4737) | 2393 (1566–3219) |
| Other† | 93 (52–134) | 3432 (2033–4830) | 1594 (942–2247) |
| Family income | |||
| Low income | 631 (520–742) | 2698 (1875–3521) | 2140 (1374–2906) |
| Middle/high income | 1069 (909–1229) | 5442 (4860–6024) | 3204 (2764–3645) |
| Education level | |||
| Less than high school | 197 (132–262) | 3198 (2104–4292) | 2181 (1299–3062) |
| High school/graduate equivalency degree & equivalent | 669 (560–777) | 3672 (3029–4315) | 2305 (1770–2839) |
| Some college or higher | 662 (549–775) | 5382 (4325–6439) | 3305 (2483–4127) |
| Insurance type | |||
| Private | 860 (719–1001) | 6073 (5193–6954) | 3479 (2772–4186) |
| Public | 815 (691–939) | 2666 (2177–3155) | 2089 (1630–2549) |
| Uninsured | 26 (9–42) | 4919 (2132–7706) | 3209 (1389–5028) |
| Census region | |||
| Northeast | 256 (162–350) | 3966 (3130–4803) | 2180 (1378–2982) |
| Midwest | 489 (375–602) | 4740 (3942–5537) | 3006 (2342–3671) |
| South | 718 (599–837) | 4395 (3475–5315) | 2873 (2199–3547) |
| West | 238 (174–302) | 4351 (2727–5976) | 2888 (1590–4186) |
| Hypertension | |||
| No hypertension | 199 (139–258) | 4739 (3910–5568) | 2881 (2065–3697) |
| Hypertension | 1502 (1318–1686) | 4382 (3804–4959) | 2800 (2341–3258) |
| Diabetes mellitus | |||
| No diabetes mellitus | 922 (777–1067) | 3841 (3280–4402) | 2522 (2056–2988) |
| Diabetes mellitus | 779 (659–899) | 5113 (4182–6043) | 3149 (2430–3868) |
| Dyslipidemia | |||
| No dyslipidemia | 383 (295–471) | 4133 (3164–5101) | 2461 (1633–3288) |
| Dyslipidemia | 1317 (1146–1488) | 4508 (3880–5136) | 2910 (2432–3389) |
| Smoker* | |||
| Nonsmoker | 1195 (1032–1357) | 4632 (4029–5234) | 2939 (2473–3405) |
| Current smoker | 169 (111–227) | 2265 (1354–3177) | 1438 (900–1975) |
| Obesity* | |||
| No obesity | 692 (579–805) | 4870 (4030–5711) | 2965 (2200–3731) |
| Obesity | 669 (553–785) | 4074 (3283–4864) | 2609 (2063–3156) |
| Physical activity | |||
| Sufficient | 428 (356–499) | 3058 (3302–4613) | 2324 (1826–2822) |
| Insufficient | 1273 (1110–1437) | 4580 (3964–5196) | 2972 (2497–3447) |
| Cardiovascular risk factor profile | |||
| Optima | 121 (79–163) | 4664 (3341–5987) | 2554 (1530–3578) |
| Average | 740 (620–860) | 4026 (3422–4630) | 2547 (2038–3055) |
| Poor | 839 (704–975) | 4739 (3890–5588) | 3077 (2406–3748) |
| ASCVD | |||
| No ASCVD | 444 (357–530) | 4580 (3355–5805) | 2954 (1905–4003) |
| ASCVD | 1257 (1085–1429) | 4368 (3802–4934) | 2758 (2340–3176) |
| Arthritis | |||
| No arthritis | 491 (395–588) | 4900 (3734–6066) | 2909 (2108–3710) |
| Arthritis | 1209 (1046–1373) | 4230 (3707–4752) | 2768 (2330–3207) |
| Cancer | |||
| No cancer | 1148 (984–1312) | 4431 (3719–5143) | 2743 (2201–3285) |
| Cancer | 552 (444–661) | 4408 (3709–5107) | 2946 (2371–3522) |
| Asthma | |||
| No asthma | 1330 (1156–1504) | 4884 (4266–5502) | 3134 (2644–3625) |
| Asthma | 371 (287–455) | 2772 (2177–3367) | 1642 (1289–1995) |
| COPD | |||
| No COPD | 1139 (994–1284) | 4764 (4072–5455) | 2963 (2406–3520) |
| COPD | 561 (431–692) | 3733 (3079–4387) | 2497 (2002–2991) |
| Hepatitis | |||
| No hepatitis | 1695 (1495–1895) | 4436 (3918–4954) | 2817 (2415–3218) |
| Hepatitis | 5 (−2 to 13) | 348 (−63 to 759) | 348 (−63 to 759) |
| CKD | |||
| No CKD | 918 (792–1044) | 4386 (3676–5096) | 2832 (2266–3399) |
| CKD | 782 (642–923) | 4467 (3744–5191) | 2782 (2234–3329) |
ASCVD indicates atherosclerotic cardiovascular disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; MEPS, Medical Expenditure Panel Survey; and OOP, out‐of‐pocket.
2017 year does not have data on obesity, and 2018 year does not have data on smoking status.
Other includes Asian, American Indian, and those of multiple racial origins
Table 3.
Categories of Out‐of‐Pocket Healthcare Costs of Care for Families With 1 or More Members With HF, From the MEPS 2014 to 2018
| Characteristic | Premiums, $ (95% CI) | Inpatient, $ (95% CI) | Outpatient, $ (95% CI) | Medication, $ (95% CI) | Emergency, $ (95% CI) | Other, $ (95% CI) |
|---|---|---|---|---|---|---|
| Overall | 1614 (1346–1882) | 185 (93–278) | 584 (453–715) | 1078 (896–1262) | 39 (24–54) | 922 (601–1244) |
| Age, y | ||||||
| 18–64 | 1594 (1201–1987) | 115 (41–189) | 447 (289–604) | 939 (659–1219) | 42 (18–67) | 318 (213–423) |
| ≥ 65 | 1623 (1274–1971) | 215 (88–342) | 641 (480–803) | 1137 (906–1367) | 38 (19–56) | 1175 (720–1629) |
| Sex | ||||||
| Male | 1937 (1489–2385) | 279 (112–447) | 661 (479–842) | 1169 (858–1480) | 44 (25–63) | 1236 (642–1830) |
| Female | 1277 (993–1561) | 87 (40–135) | 504 (332–676) | 985 (804–1165) | 33 (11–56) | 595 (406–785) |
| Race/ethnicity | ||||||
| Non‐Hispanic White | 1680 (1340–2020) | 203 (83–323) | 667 (494–840) | 1229 (992–1466) | 41 (23–60) | 1111 (676–1545) |
| Non‐Hispanic Black | 1326 (881–1771) | 53 (23–84) | 264 (178–349) | 652 (485–818) | 21 (10–31) | 285 (122–449) |
| Hispanic | 1333 (823–1843) | 395 (−20 to 811) | 604 (246–963) | 727 (348–1106) | 27 (5–48) | 639 (225–1053) |
| Other† | 1837 (722–2952) | 95 (−11 to 202) | 338 (169–508) | 624 (280–968) | 66 (−16 to 148) | 470 (210–731) |
| Family income | ||||||
| Low income | 558 (378–739) | 91 (39–144) | 315 (205–425) | 775 (402–1147) | 21 (7–36) | 938 (286–1590) |
| Middle/high income | 2238 (1843–2632) | 241 (101–381) | 743 (557–929) | 1258 (984–1532) | 49 (27–71) | 913 (686–1140) |
| Education level | ||||||
| Less than high school | 1017 (455–1580) | 121 (−5 to 247) | 474 (205–742) | 1133 (397–1868) | 26 (10–42) | 428 (181–674) |
| High school/graduate equivalency degree & equivalent | 1367 (1049–1685) | 126 (64–187) | 447 (312–583) | 1144 (762–1526) | 58 (26–90) | 529 (275–784) |
| Some college or higher | 2077 (1532–2622) | 232 (50–414) | 766 (515–1017) | 997 (789–1204) | 22 (9–35) | 1288 (582–1994) |
| Insurance type | ||||||
| Private | 2594 (2148–3041) | 235 (65–405) | 755 (533–977) | 1373 (1038–1707) | 48 (22–74) | 1069 (462–1675) |
| Public | 577 (369–785) | 107 (55–160) | 411 (299–523) | 771 (551–991) | 29 (15–42) | 771 (438–1104) |
| Uninsured | 1710 (53–3368) | 1003 (−144 to 2150) | 328 (143–514) | 997 (479–1514) | 65 (−22 to 152) | 816 (124–1507) |
| Census region | ||||||
| Northeast | 1786 (1174–2398) | 319 (−42 to 679) | 347 (195–500) | 1128 (322–1934) | 52 (11–93) | 334 (156–512) |
| Midwest | 1733 (1317–2149) | 159 (54–264) | 610 (369–851) | 1140 (866–1413) | 34 (12–56) | 1064 (589–1538) |
| South | 1522 (1034–2010) | 150 (5–295) | 664 (435–893) | 1118 (901–1335) | 41 (15–66) | 900 (320–1479) |
| West | 1463 (876–2051) | 202 (7–398) | 543 (201–886) | 781 (357–1205) | 28 (−6 to 61) | 1334 (235–2433) |
| Hypertension | ||||||
| No hypertension | 1858 (1099–2617) | 157 (62–252) | 729 (455–1003) | 1365 (703–2027) | 22 (4–39) | 608 (369–848) |
| Hypertension | 1582 (1294–1870) | 189 (88–290) | 565 (424–705) | 1041 (804–1278) | 41 (24–58) | 964 (601–1327) |
| Diabetes mellitus | ||||||
| No diabetes mellitus | 1319 (1041–1596) | 204 (51–357) | 570 (379–761) | 894 (713–1074) | 35 (17–52) | 820 (500–1139) |
| Diabetes mellitus | 1964 (1472–2456) | 163 (77–249) | 601 (451–750) | 1298 (943–1652) | 43 (18–69) | 1044 (454–1633) |
| Dyslipidemia | ||||||
| No dyslipidemia | 1672 (1223–2121) | 106 (51–161) | 539 (315–763) | 870 (487–1254) | 29 (7–51) | 916 (254–1578) |
| Dyslipidemia | 1597 (1277–1917) | 208 (91–326) | 597 (443–751) | 1139 (864–1414) | 42 (23–60) | 924 (560–1288) |
| Smoker* | ||||||
| Nonsmoker | 1693 (1364–2022) | 240 (109–370) | 622 (457–787) | 1167 (919–1415) | 37 (23–52) | 873 (557–1189) |
| Current smoker | 828 (210–1446) | 27 (1–53) | 285 (93–478) | 898 (435–1360) | 38 (−8 to 84) | 189 (24–354) |
| Obesity* | ||||||
| No obesity | 1905 (1563–2246) | 227 (35–419) | 526 (400–653) | 1000 (638–1363) | 44 (20–67) | 1068 (496–1840) |
| Obesity | 1464 (939–1990) | 85 (46–124) | 561 (334–788) | 1178 (814–1542) | 24 (9–40) | 761 (492–1031) |
| Physical activity | ||||||
| Sufficient | 1634 (1247–2021) | 184 (61–308) | 546 (330–761) | 849 (579–1119) | 48 (19–78) | 697 (489–904) |
| Insufficient | 1608 (1276–1940) | 186 (77–295) | 597 (444–749) | 1156 (941–1371) | 35 (18–52) | 998 (603–1393) |
| Cardiovascular risk factor profile | ||||||
| Optima | 2110 (1226–2994) | 135 (29–241) | 543 (226–861) | 1172 (302–2042) | 16 (−5 to 36) | 688 (360–1016) |
| Average | 1479 (1196–1763) | 270 (77–462) | 604 (425–783) | 775 (630–920) | 39 (19–60) | 858 (483–1234) |
| Poor | 1662 (1208–2115) | 118 (51–185) | 572 (382–762) | 1333 (942–1724) | 41 (18–65) | 1013 (465–1560) |
| ASCVD | ||||||
| No ASCVD | 1626 (1193–2059) | 171 (50–292) | 607 (337–877) | 996 (637–1355) | 34 (12–57) | 1146 (236–2056) |
| ASCVD | 1610 (1280–1940) | 191 (76–305) | 576 (432–720) | 1108 (844–1372) | 40 (22–59) | 843 (554–1133) |
| Arthritis | ||||||
| No arthritis | 1991 (1316–2666) | 127 (47–207) | 605 (356–855) | 965 (700–1229) | 28 (14–42) | 1184 (508–1861) |
| Arthritis | 1461 (1218–1704) | 209 (84–334) | 575 (427–723) | 1125 (896–1354) | 43 (23–64) | 816 (490–1142) |
| Cancer | ||||||
| No cancer | 1688 (1329–2046) | 139 (74–204) | 574 (401–748) | 994 (750–1238) | 40 (20–60) | 995 (555–1435) |
| Cancer | 1462 (1098–1825) | 282 (44–520) | 604 (436–772) | 1254 (1021–1487) | 35 (14–56) | 771 (383–1159) |
| Asthma | ||||||
| No asthma | 1749 (1436–2063) | 226 (109–344) | 649 (493–805) | 1112 (867–1356) | 43 (24–61) | 1105 (700–1510) |
| Asthma | 1130 (691–1568) | 39 (15–63) | 350 (228–473) | 961 (750–1171) | 24 (8–40) | 268 (141–395) |
| COPD | ||||||
| No COPD | 1801 (1449–2152) | 171 (76–266) | 662 (487–838) | 919 (753–1085) | 40 (23–56) | 1172 (711–1634) |
| COPD | 1236 (860–1613) | 216 (20–411) | 425 (295–555) | 1404 (1007–1800) | 37 (7–67) | 415 (245–586) |
| Hepatitis | ||||||
| No hepatitis | 1619 (1350–1888) | 186 (93–279) | 585 (454–717) | 1082 (898–1265) | 39 (24–54) | 925 (603–1248) |
| Hepatitis | NA | NA | 185 (−35 to 404) | 70 (−11 to 151) | NA | 93 (−18 to 204) |
| CKD | ||||||
| No CKD | 1553 (1248–1858) | 153 (78–229) | 610 (430–791) | 932 (746–1117) | 45 (22–68) | 1092 (596–1589) |
| CKD | 1686 (1234–2138) | 223 (51–395) | 553 (391–715) | 1251 (845–1658) | 31 (13–49) | 723 (397–1049) |
ASCVD indicates atherosclerotic cardiovascular disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; MEPS, Medical Expenditure Panel Survey; and NA, not applicable.
2017 year does not have data on obesity, and 2018 year does not have data on smoking status.
Other includes Asian, American Indian, and those of multiple racial origins.
Certain characteristics of the index patient with HF were associated with total out‐of‐pocket expenditures in these descriptive analyses. Age over 65 years, male sex, non‐Hispanic White race/ethnicity, and presence of diabetes mellitus were associated with larger out‐of‐pocket expenses, compared with age <65 years, female sex, non‐Hispanic Black race/ethnicity, and absence of diabetes mellitus, respectively. Middle/high income individuals with HF had greater total out‐of‐pocket health expenditures than low‐income individuals with HF, at $5442 (95% CI, $4860–$6024) versus $2698 (95% CI, $1875–$3521) (P<0.01). Finally, individuals with private insurance had greater out‐of‐pocket healthcare expenditures than individuals with public insurance ($6073 [95% CI, $5193–$6954]) for private insurance, $2666 (95% CI, $2177–$3155) for public insurance (P<0.01).
In analyses of temporal trends between 2014 and 2018, there were no significant differences in family income, premium costs, or out‐of‐pocket healthcare costs by year among families, either overall nor by income strata (Table 4).
Table 4.
Yearly Trends of Out‐of‐Pocket Healthcare Costs of Care for Families With 1 or More Members With HF, Stratified by Income Level, From the MEPS 2014 to 2018
| 2014 | 2015 | 2016 | 2017 | 2018 | P Value | |
|---|---|---|---|---|---|---|
| Overall | ||||||
| No. in national population ×1000 families (95% CI) | 1808 (1407–2209) | 1884 (1488–2281) | 1350 (1090–1610) | 1666 (1350–1981) | 1661 (1331–1992) | |
| Family income, $ (95% CI) | 62 351 (46 378–78 324) | 57 170 (48 996–65 344) | 59 019 (47 466–70 571) | 66 999 (55 837–78 161) | 57 909 (46 571–69 346) | 0.83 |
| Total OOP expenditures, $ (95% CI) | 4276 (3073–5478) | 4832 (3785–5878) | 3768 (3026–4510) | 4263 (3348–5178) | 4793 (3289–6297) | 0.65 |
| Insurance premiums, $ (95% CI) | 1775 (926–2624) | 1629 (1226–2033) | 1596 (1060–2132) | 1332 (939–1724) | 1750 (1264–2237) | 0.44 |
| OOP health service, $ (95% CI) | 2501 (1803–3199) | 3202 (2271–4134) | 2172 (1584–2759) | 2931 (2214–3648) | 3043 (1687–4400) | 0.85 |
| Middle/high income | ||||||
| No. in national population ×1000 families (95% CI) | 1199 (885–1512) | 1164 (841–1487) | 902 (649–1156) | 1143 (878–1409) | 939 (712–1166) | NA |
| Family income, $ (95% CI) | 84 281 (63 773–104 789) | 77 868 (68 069–87 667) | 78 264 (63 140–93 387) | 88 184 (74 199–102 169) | 86 001 (69 983–102 018) | 0.63 |
| Total OOP expenditures, $ (95% CI) | 5457 (3917–6998) | 5731 (4289–7174) | 5029 (4099–5959) | 5017 (3861–6173) | 5976 (4936–7016) | 0.74 |
| Insurance premiums, $ (95% CI) | 2508 (1338–3678) | 2230 (1589–2871) | 2178 (1416–2941) | 1616 (1093–2139) | 2715 (1891–3540) | 0.53 |
| OOP health service, $ (95% CI) | 2949 (2031–3867) | 3501 (2271–4731) | 2850 (2074–3626) | 3401 (2505–4297) | 3261 (2721–3801) | 0.35 |
| Low income | ||||||
| No. in national population ×1000 families (95% CI) | 610 (403–816) | 720 (480–960) | 448 (310–586) | 523 (359–686) | 722 (470–976) | NA |
| Family income, $ (95% CI) | 19 233 (16 339–22 128) | 23 710 (19 924–27 497) | 20 243 (15 963–24 523) | 20 660 (17 440–23 879) | 21 425 (18 191–24 658) | 0.88 |
| Total OOP expenditures, $ (95% CI) | 1952 (928–2977) | 3377 (1781–4974) | 1227 (742–1713) | 2613 (1366–3860) | 3457 (433–6081) | 0.96 |
| Insurance premiums, $ (95% CI) | 334 (61–606) | 658 (215–1101) | 423 (−45 to 890) | 710 (345–1074) | 496 (246–746) | 0.90 |
| OOP health service, $ (95% CI) | 1619 (764–2474) | 2719 (1144–4295) | 805 (590–1019) | 1904 (714–3093) | 2760 (13–5507) | 0.81 |
HF indicates heart failure; MEPS, Medical Expenditure Panel Survey; NA, not applicable; and OOP, out‐of‐pocket.
Category of Highest Out‐of‐Pocket Healthcare Expenses
The most expensive out‐of‐pocket category of spending in HF was medications in 36% (95% CI, 32%–41%) and health insurance premiums in 32% (95% CI, 28%–37%) of families, with outpatient and inpatient care expenses representing the most expensive category in 12% (95% CI, 9%–15%) and 4% (95% CI, 2%–6%) of families, respectively (Table S2).
Medications represented the largest category of health spending in nearly twice as many low‐income families compared with middle/high‐income families (50% [95% CI, 43%–57%] of low‐income families versus 28% [95% CI, 23%–34%] of middle/high income families) (Figure 1A). In contrast, 43% (95% CI, 37%–50%) of middle/high income families spent most out‐of‐pocket costs on health insurance premiums, compared with 13% (95% CI, 10%–18%) of low‐income families.
Figure 1. Highest category of OOP spending by income level (A) and insurance status (B) among families with prevalent HF in the United States, MEPS 2014 to 2018.
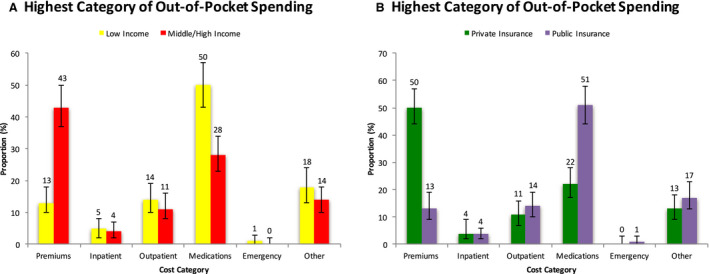
Error bars represent 95% CIs. HF indicates heart failure; MEPS, Medical Expenditure Panel Survey; and OOP, out‐of‐pocket.
Across insured groups, premiums were the greatest category of healthcare spending in patients with private insurance, whereas medications were the greatest category in the publicly insured (Figure 1B). Among families with either private or public insurance, inpatient costs was the great category of spending in only 4% of families (95% CI, 2%–9% for private insurance, and 95% CI, 2%–6% for public insurance). Among families without insurance, inpatient costs were the most expensive out‐of‐pocket cost in 22% (95% CI, 5%–58%) of families whereas medication cost was the most expensive out‐of‐pocket cost in 37% (95% CI, 13%–71%) of families.
High and Catastrophic Healthcare Burden
Overall, an estimated 236 471 (95% CI, 178 833–294 109) families or 14% (95% CI, 11%–17%) of families with a member with HF experienced high financial burden from out‐of‐pocket healthcare expenditures, while 75 997 (95% CI, 44 861–107 132) families or 5% (95% CI, 3%–6%) experienced catastrophic financial burden in the United States (Figure 2).
Figure 2. Proportion of high and catastrophic financial burden in the overall population, and by subgroups, among families with a member with HF in the United States, MEPS 2014 to 2018.
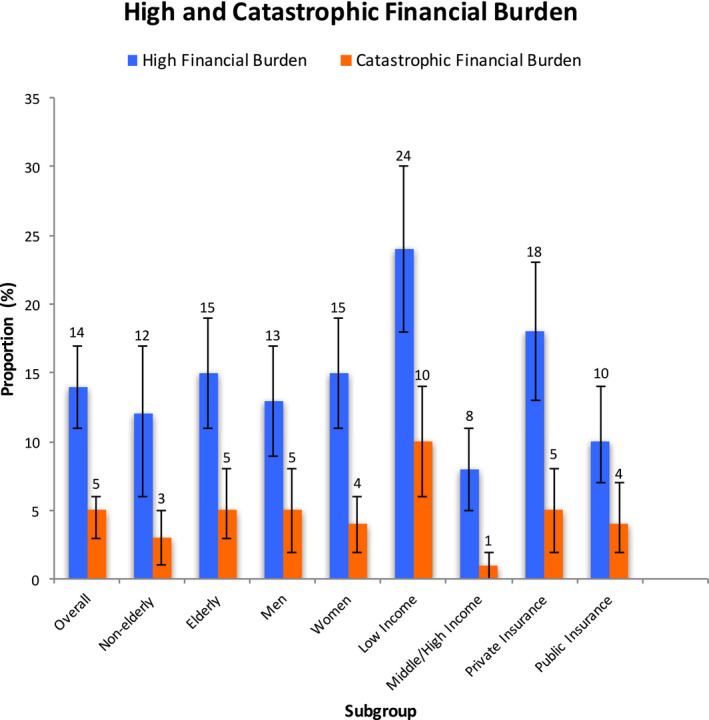
HF indicates heart failure; and MEPS, Medical Expenditure Panel Survey. Error bars represent 95% CIs.
Among low‐income families, 24% (95% CI, 18%–30%) experienced high financial burden and 10% (95% CI, 6%–14%) experienced catastrophic financial burden from out‐of‐pocket healthcare costs. In contrast, among middle/high income families, only 8% (95% CI, 5%–11%) experienced high financial burden and 1% (95% CI, 0%–2%) experienced catastrophic financial burden.
In regression analyses adjusted for demographic factors, cardiovascular disease risk factors and comorbidities, low‐income families experienced significantly higher odds of high (OR, 3.9; 95% CI, 2.3–6.6; P<0.01) and catastrophic financial burden (OR 14.2; 95% CI, 5.1–39.5; P<0.01) compared with middle/high‐income families. Privately insured individuals more frequently experienced high financial burden from healthcare costs than those with public insurance (OR, 1.9; 95% CI, 1.2–3.2; P=0.01) but did not differ in proportion experiencing catastrophic financial burden.
DISCUSSION
Patients with HF and their families experience significant financial toxicity from their out‐of‐pocket healthcare costs. Among all families with a member with HF, during 2014 to 2018, 1 in 7 experienced high financial burden. This effect was more severe among low‐income families, where 1 in 4 experienced high financial burden. Furthermore, 1 in 10 low‐income families had out‐of‐pocket spending in a single year that would represent a catastrophic financial burden, exceeding a threshold of healthcare expenses that is considered financially ruinous based on internationally accepted definitions. Financial challenges with HF were also experienced by middle/high‐income families, almost 1 in 10 of whom had high financial burden due to out‐of‐pocket healthcare costs. Medication costs and insurance premium costs were most burdensome, representing the highest categories of spending in low‐income and middle/high‐income families, respectively.
These data complement an evaluation of financial burden among patients with ASCVD.15 In contrast to ASCVD, the average out‐of‐pocket spending in HF is 2‐fold higher. Moreover, medications accounted for the highest out‐of‐pocket cost for nearly a third of patients with HF, nearly 1.5‐fold higher than those with ASCVD. Further, although they did not account for food‐related costs, Bernard and Fang found that annual proportion of high financial burden in families with noncardiovascular chronic diseases between 2010 and 2015 was 9.5%, about two‐thirds the risk of families with HF.25 These differences may be because of chronic use of multiple medications used to improve outcomes in patients with HF, particularly among those with reduced ejection fraction, including more expensive therapies such as sacubitril/valsartan,26 that has become a first‐line therapy,27 but with an out‐of‐pocket cost of $1000 among insured Medicare beneficiaries.6 Moreover, among Medicare beneficiaries, only about 1 in 10 eligible patients were using sacubitril/valsartan during 2015 to 2018,28 potentially forecasting an even larger financial toll with greater use of therapy over time. These financial considerations are more critical now with the emergence of novel therapies with a role in HF, including sodium glucose cotransporter 2 inhibitors, which have been shown to have mortality benefit,29 other drugs with broad indications aimed at reducing risk of HF hospitalization,30, 31 and those with more targeted effects.32, 33, 34 Of note, although the cost‐related challenges are presented as annual expenses, nearly all patients would incur similar high costs year after year over the course of their lives, given the chronic nature of HF.35
Although health insurance coverage has increased in the population since the introduction of the Affordable Care Act,22 and the vast majority of patients with HF have insurance coverage, we find evidence that insurance continues to provide inadequate coverage for patients in this population with substantial healthcare needs. In particular, privately insured individuals were twice as likely to suffer a high financial burden compared with those with Medicare or other public insurance. This was driven by health insurance premiums in this group, which continue to be a challenge in the United States.36 Conversely, individuals with public insurance lack broad coverage for medications costs,37 and this was the highest category of spending in this population. These areas may allow for dedicated interventions, particularly among low‐income individuals, through maximum spending limits that are scaled to income.
This study evinces the burden of healthcare costs among low‐income families of patients with HF, who represent nearly two‐fifths of families in our study, and who have 14‐fold greater odds of catastrophic financial burden after adjustment for cardiovascular disease risk factors and comorbidities, compared with families with middle/high income. HF patients with low income already face higher risk of cardiovascular mortality,38 and although it is unclear how financial challenges contribute to patient outcomes, Pool et al have shown that significant wealth shocks are associated with mortality.39 In addition, 1 area of special concern in the low‐income population is medication costs, which was the costliest expense in about half of low‐income families in this study. Safety net programs currently appear insufficient among low‐income patients with HF. Given the continued advances in HF treatment that can lead to greater financial strain on low‐income patients, policies that improve medication affordability are needed.
A notable observation is that inpatient costs represented substantial out‐of‐pocket costs in only a small proportion of patients. The relatively low out‐of‐pocket costs for hospitalizations may suggest that a majority of patients do not experience a hospitalization in a given year40 and may also highlight the success of the current insurance mechanisms that are designed to defray costs associated with expensive hospitalizations. Among patients with no insurance, inpatient costs were the costliest category in 22% of families, compared with only 4% of patients with private or public insurance. Although this number should be interpreted with caution owing to the small sample size, this would suggest that insurance status may guard against financial burden due to hospitalizations for HF. These observations are particularly relevant as they contextualize studies that suggest inpatient costs as the highest cost category, because most studies focus on overall costs as opposed to out‐of‐pocket spending.4
The following limitations should be considered in context of the study findings. First, we were unable to categorize HF as reduced or preserved ejection fraction, and there may be significant differences in costs based on variation in treatment for these different conditions. Second, data collected for diagnosis of HF and on costs were based on transcription of participant interviews and not based on direct transcribing of medical or financial records. However, participants were frequently encouraged to use objective information such as these records when it was available to them.16 Whether such objective data were used in a given interview are not captured. Of note, MEPS is based on interviews by trained interviewers and the observations from MEPS frequently guide national health policy. Third, although income data were compiled from diverse areas, such as wages, social security, and veterans payments,18 crucial areas that were unmeasured include savings, financial support from relatives, or in this age, crowdsourcing revenue for medical care,41 each of which can significantly mitigate financial burden. However, these mechanisms in themselves would only further highlight the strain of healthcare costs on resources. In addition, there may be important liabilities apart from food‐related expenses that contributed to financial toxicity; however, we focused on postsubsistence income as it is the measure used by the World Health Organization to evaluate financial burden from healthcare spending.20 Fourth, our subpopulation of adults with HF was only a subset of all HF patients nationally and is prone to sampling error. However, it was reassuring that the demographic characteristics of the adult patients with HF in MEPS was similar to other outpatient and inpatient cohorts that include patients with HF.42, 43 Fifth, we were unable to account for risk factors or comorbidities in other members of the family, which could contribute to total out‐of‐pocket spending. However, we found that on average, 73% of the total family out‐of‐pocket costs were attributable to the index individual with HF in each family. Furthermore, although the out‐of‐pocket costs may not be directly attributable to HF, these costs nevertheless exert a financial burden on families. Finally, category‐based spending is likely determined by choice of insurance, which may reflect patient preferences for fixed premium payments or direct medical spending, such as for medications. However, data on medication use and information on insurance drug benefits were limited.
CONCLUSIONS
Patients with HF and their families have large out‐of‐pocket healthcare expenses, driven by prescription drug and health insurance premium costs. A large proportion of HF patients encounter financial toxicity, with a disproportionate effect on low‐income families. Further policies should aim to mitigate direct, out‐of‐pocket healthcare expenditures through decreasing medication costs and improving quality of health insurance coverage.
Sources of Funding
Dr Khera received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under the award K23HL153775‐01A1. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
Dr Nasir is on the advisory board of Amgen, Novartis, Medicine Company, and his research is partly supported by the Jerold B. Katz Academy of Translational Research. Dr Krumholz works under contract with the Centers for Medicare & Medicaid Services to support quality measurement programs, was a recipient of a research grant from Johnson & Johnson, through Yale University, to support clinical trial data sharing; was a recipient of a research agreement, through Yale University, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; receives payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation, from the Martin Baughman Law Firm for work related to the Cook Celect IVC filter litigation, and from the Siegfried and Jensen Law Firm for work related to Vioxx litigation; chairs a Cardiac Scientific Advisory Board for UnitedHealth; was a member of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science, the Advisory Board for Facebook, and the Physician Advisory Board for Aetna; and is the co‐founder of HugoHealth, a personal health information platform, and cofounder of Refactor Health, a healthcare AI‐augmented data management company. Dr Nasir is on the advisory board of Amgen, Novartis, Medicine Company, and his research is partly supported by the Jerold B. Katz Academy of Translational Research. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figure S1
(J Am Heart Assoc. 2021;10:e022164. DOI: 10.1161/JAHA.121.022164.)
This study was presented at the American College of Cardiology Scientific Sessions, May 15 to 17, 2021.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022164
For Sources of Funding and Disclosures, see page 14.
REFERENCES
- 1.Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, American Heart Association Statistics Committee and Stroke Statistics Subcommittee , et al. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. DOI: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. DOI: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Echouffo‐Tcheugui JB, Bishu KG, Fonarow GC, Egede LE. Trends in health care expenditure among US adults with heart failure: the Medical Expenditure Panel Survey 2002–2011. Am Heart J. 2017;186:63–72. DOI: 10.1016/j.ahj.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesyuk W, Kriza C, Kolominsky‐Rabas P. Cost‐of‐illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord. 2018;18:74. DOI: 10.1186/s12872-018-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. DOI: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 6.DeJong C, Kazi DS, Dudley RA, Chen R, Tseng C‐W. Assessment of national coverage and out‐of‐pocket costs for sacubitril/valsartan under Medicare Part D. JAMA Cardiol. 2019;4:828–830. DOI: 10.1001/jamacardio.2019.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J, Feldman R, Rothenberger SD, Hernandez I, Gellad WF. Coverage, formulary restrictions, and out‐of‐pocket costs for sodium‐glucose cotransporter 2 inhibitors and glucagon‐like peptide 1 receptor agonists in the Medicare part D program. JAMA Netw Open. 2020;3:e2020969. DOI: 10.1001/jamanetworkopen.2020.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson LW. Projecting heart failure into bankruptcy in 2012? Am Heart J. 2011;161:1007–1011. DOI: 10.1016/j.ahj.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JW, Cohen SB, Banthin JS. The Medical Expenditure Panel Survey: a national information resource to support healthcare cost research and inform policy and practice. Med Care. 2009;47:S44–S50. DOI: 10.1097/MLR.0b013e3181a23e3a. [DOI] [PubMed] [Google Scholar]
- 10.MEPS‐HC Response Rates by Panel. Medical Expenditure Panel Survey. 2021. Available at: https://meps.ahrq.gov/survey_comp/hc_response_rate.jsp. Accessed March 21, 2021.
- 11.MEPS HC‐207 2018 Medical Conditions. Agency for Healthcare Research and Quality; 2020. Available at: https://meps.ahrq.gov/data_stats/download_data/pufs/h207/h207doc.pdf. Accessed December 19, 2020. [Google Scholar]
- 12.Methodology report #33: sample designs of the Medical Expenditure Panel Survey Household Component, 1996–2006 and 2007–2016. Medical Expenditure Panel Survey. Available at: https://meps.ahrq.gov/data_files/publications/mr33/mr33.pdf. Accessed March 21, 2021.
- 13.Chowdhury S. An assessment of Medical Expenditure Panel Survey sampling and estimation procedures through benchmarking with the National Health Interview Survey. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/workingpapers/wp_13002.pdf. Accessed April 18, 2021. [Google Scholar]
- 14.Emanuel EJ, Glickman A, Johnson D. Measuring the burden of health care costs on US families: the affordability index. JAMA. 2017;318:1863–1864. DOI: 10.1001/jama.2017.15686. [DOI] [PubMed] [Google Scholar]
- 15.Khera R, Valero‐Elizondo J, Okunrintemi V, Saxena A, Das SR, de Lemos JA, Krumholz HM, Nasir K. Association of out‐of‐pocket annual health expenditures with financial hardship in low‐income adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2018;3:729–738. DOI: 10.1001/jamacardio.2018.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MEPS HC‐209 2018 full year consolidated data file. Agency for Healthcare Research and Quality; 2020. Available at: https://meps.ahrq.gov/data_stats/download_data/pufs/h209/h209doc.pdf. Accessed December 20, 2020. [Google Scholar]
- 17.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. DOI: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banthin JS, Selden TM. Income measurement in the Medical Expenditure Panel Survey. Agency for Healthcare Research and Quality Working Paper No. 06005. 2006. Available at: https://meps.ahrq.gov/data_files/publications/workingpapers/wp_06005.pdf. Accessed December 20, 2020.
- 19.Dubay L, Kenney GM. Health care access and use among low‐income children: who fares best? Health Aff. 2001;20:112–121. DOI: 10.1377/hlthaff.20.1.112. [DOI] [PubMed] [Google Scholar]
- 20.Distribution of health payments and catastrophic expenditures methodology. World Health Organization; 2005. Available at: https://www.who.int/health_financing/documents/dp_e_05_2‐distribution_of_health_payments.pdf. Accessed December 20, 2020. [Google Scholar]
- 21.Combined expenditure, share, and standard error tables. U.S. Bureau of Labor Statistics. Available at: https://www.bls.gov/cex/csxcombined.htm. Accessed December 20, 2020. [Google Scholar]
- 22.Gotanda H, Jha AK, Kominski GF, Tsugawa Y. Out‐of‐pocket spending and financial burden among low income adults after Medicaid expansions in the United States: quasi‐experimental difference‐in‐difference study. BMJ. 2020;368:m40. DOI: 10.1136/bmj.m40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318:2011–2018. DOI: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CPI inflation calculator. Washington, DC: U.S. Bureau of Labor Statistics. Available at: https://data.bls.gov/cgi‐bin/cpicalc.pl. Accessed December 20, 2020. [Google Scholar]
- 25.Bernard D, Fang Z. Financial burdens and barriers to care among nonelderly adults with heart disease: 2010–2015. J Am Heart Assoc. 2019;8:e008831. DOI: 10.1161/JAHA.118.008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324:488–504. DOI: 10.1001/jama.2020.10262. [DOI] [PubMed] [Google Scholar]
- 27.Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, Writing Committee , et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;77:772–810. DOI: 10.1016/j.jacc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Tan NY, Sangaralingham LR, Sangaralingham SJ, Yao X, Shah ND, Dunlay SM. Comparative effectiveness of sacubitril‐valsartan versus ACE/ARB therapy in heart failure with reduced ejection fraction. JACC Heart Fail. 2020;8:43–54. DOI: 10.1016/j.jchf.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. DOI: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. DOI: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 31.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384:105–116. DOI: 10.1056/NEJMoa2025797. [DOI] [PubMed] [Google Scholar]
- 32.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. DOI: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 33.Olivotto I, Oreziak A, Barriales‐Villa R, Abraham TP, Masri A, Garcia‐Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2020;396:759–769. DOI: 10.1016/S0140-6736(20)31792-X. [DOI] [PubMed] [Google Scholar]
- 34.Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, Yeh RW, Arnold SV, Sperry BW, Maurer MS, et al. Cost‐effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation. 2020;141:1214–1224. DOI: 10.1161/CIRCULATIONAHA.119.045093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, Packer M, Fonarow GC, McMurray JJV, Solomon SD. Estimating lifetime benefits of comprehensive disease‐modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–128. DOI: 10.1016/S0140-6736(20)30748-0. [DOI] [PubMed] [Google Scholar]
- 36.McKillop CN, Waters TM, Kaplan CM, Kaplan EK, Thompson MP, Graetz I. Three years in—changing plan features in the U.S. health insurance marketplace. BMC Health Serv Res. 2018;18:450. DOI: 10.1186/s12913-018-3198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cubanski J, Neuman T, Damico A. How many Medicare Part D enrollees had high out‐of‐pocket drug costs in 2017. Kaiser Family Foundation; 2019. Available at: https://www.kff.org/medicare/issue‐brief/how‐many‐medicare‐part‐d‐enrollees‐had‐high‐out‐of‐pocket‐drug‐costs‐in‐2017/. Accessed January 19, 2021. [Google Scholar]
- 38.Bevan GH, Josephson R, Al‐Kindi SG. Socioeconomic deprivation and heart failure mortality in the United States. J Card Fail. 2020;26:1106–1107. DOI: 10.1016/j.cardfail.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Pool LR, Burgard SA, Needham BL, Elliott MR, Langa KM, Mendes de Leon CF. Association of a negative wealth shock with all‐cause mortality in middle‐aged and older adults in the United States. JAMA. 2018;319:1341–1350. DOI: 10.1001/jama.2018.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akintoye E, Briasoulis A, Egbe A, Dunlay SM, Kushwaha S, Levine D, Afonso L, Mozaffarian D, Weinberger J. National trends in admission and in‐hospital mortality of patients with heart failure in the United States (2001–2014). J Am Heart Assoc. 2017;6:e006955. DOI: 10.1161/JAHA.117.006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angraal S, Zachariah AG, Raaisa R, Khera R, Rao P, Krumholz HM, Spertus JA. Evaluation of internet‐based crowdsourced fundraising to cover health care costs in the United States. JAMA Netw Open. 2021;4:e2033157. DOI: 10.1001/jamanetworkopen.2020.33157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH, Fonarow GC. National differences in trends for heart failure hospitalizations by sex and race/ethnicity. Circ Cardiovasc Qual Outcomes. 2017;10:e003552. DOI: 10.1161/CIRCOUTCOMES.116.003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen LA, Tang F, Jones P, Breeding T, Ponirakis A, Turner SJ. Signs, symptoms, and treatment patterns across serial ambulatory cardiology visits in patients with heart failure: insights from the NCDR PINNACLE® registry. BMC Cardiovasc Disord. 2018;18:80. DOI: 10.1186/s12872-018-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figure S1


