Abstract
Background
Anger and extreme stress can trigger potentially fatal cardiovascular events in susceptible people. Political elections, such as the 2016 US presidential election, are significant stressors. Whether they can trigger cardiac arrhythmias is unknown.
Methods and Results
In this retrospective case‐crossover study, we linked cardiac device data, electronic health records, and historic voter registration records from 2436 patients with implanted cardiac devices. The incidence of arrhythmias during the election was compared with a control period with Poisson regression. We also tested for effect modification by demographics, comorbidities, political affiliation, and whether an individual's political affiliation was concordant with county‐level election results. Overall, 2592 arrhythmic events occurred in 655 patients during the hazard period compared with 1533 events in 472 patients during the control period. There was a significant increase in the incidence of composite outcomes for any arrhythmia (incidence rate ratio [IRR], 1.77 [95% CI, 1.42–2.21]), supraventricular arrhythmia (IRR, 1.82 [95% CI, 1.36–2.43]), and ventricular arrhythmia (IRR, 1.60 [95% CI, 1.22–2.10]) during the election relative to the control period. There was also an increase in specific types of arrhythmia, including atrial fibrillation (IRR, 1.50 [95% CI, 1.06–2.11]), supraventricular tachycardia (IRR, 3.7 [95% CI, 2.2–6.2]), nonsustained ventricular tachycardia (IRR, 1.7 [95% CI, 1.3–2.2]), and daily atrial fibrillation burden (P<0.001). No significant interaction was found for sex, race/ethnicity, device type, age ≥65 years, hypertension, coronary artery disease, heart failure, political affiliation, or concordance between individual political affiliation and county‐level election results.
Conclusions
There was a significant increase in cardiac arrhythmias during the 2016 US presidential election. These findings suggest that exposure to stressful sociopolitical events may trigger arrhythmogenesis in susceptible people.
Keywords: arrhythmia, implantable cardioverter‐defibrillator, mental stress, pacemaker, triggers
Subject Categories: Arrhythmias, Mental Health, Risk Factors, Secondary Prevention, Epidemiology
Nonstandard Abbreviations and Acronyms
- ATP
antitachycardia pacing
- GAM
generalized additive model
- IRR
incidence rate ratio
Clinical Perspective
What Is New?
Extreme stress can trigger potentially fatal cardiovascular events.
This study is the first to demonstrate that exposure to a stressful political election, such as the 2016 US presidential election, was associated with a 77% increase in the risk of cardiac arrhythmia in people with underlying cardiovascular disease.
There was also a significant increase in specific types of arrhythmia, including both atrial and ventricular arrhythmias, and daily atrial fibrillation burden.
What Are the Clinical Implications?
Our findings suggest that exposure to stressful sociopolitical events may trigger arrhythmogenesis in susceptible people.
Given that political elections occur every 2 to 4 years in the United States. and at similar frequencies in other countries around the world, the potential impact of recurrent political events on population health is not negligible and warrants further study.
Presidential elections are high‐stakes, stressful political events with far‐reaching implications for individuals and society. For many Americans, the 2016 presidential election between Donald Trump (Republican candidate) and Hillary Clinton (Democratic candidate) stands out as a historic event because of the unprecedented levels of anxiety, animosity, and partisan rhetoric throughout the campaign and the polarized reactions to the unexpected election results.1 There has been considerable speculation that mental stress from political elections may have adverse effects on population health,2, 3 as a higher incidence of acute cardiovascular events, including potentially fatal cardiac arrhythmias, has been reported following natural disasters,4, 5 national tragedies,6, 7, 8 and other large‐scale population stressors.2, 9 People with underlying cardiovascular risk may be especially vulnerable to transient stress‐induced alterations in autonomic, metabolic, inflammatory, and hemodynamic processes that can trigger arrhythmogenesis.10, 11
In this study, we sought to determine whether the stress of a contentious political election increases the risk of arrhythmia in patients with known susceptibility using retrospective data from cardiac devices,7, 8 electronic health records, and historic voter registration records from a large, well‐characterized cohort of patients with cardiac pacemakers and implantable cardioverters‐defibrillators (ICDs) from 2 centers in North Carolina. We further examined whether the risk of arrhythmia differed according to political party affiliation and by the level of concordance between an individual's political affiliation and his/her community's election results.
North Carolina was a key battleground state in the 2016 election, and residents were exposed to a particularly high volume of political advertisements and campaign events leading up to the election.12 Thus, this cohort was uniquely well suited to investigate the association between a stressful political election and the short‐term risk of arrhythmia.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request..
Study Design
As in previous studies,13, 14, 15 we used a case‐crossover design to compare the occurrence of arrhythmia during a prespecified time interval (hazard period) with arrhythmic events during a separate control period.14 We defined the hazard period a priori as a 6‐week time interval (October 25–December 6, 2016) extending from 2 weeks before and 4 weeks after the 2016 presidential election (November 8, 2016) (Figure 1). This hazard period was selected because it ensured sufficient preelection and postelection exposure and because studies have shown an increase in arrhythmias up to 1 month after a stressful event.5, 8
Figure 1. Design of the case‐crossover analysis.
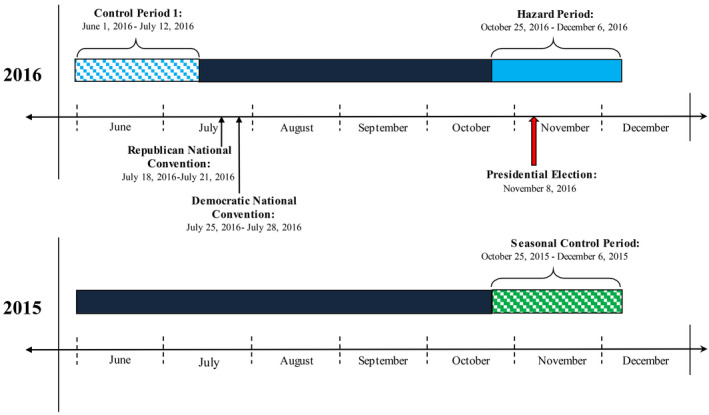
For direct comparison, a 6‐week control period (June 1, 2016–July 12, 2016) was selected a priori because it was recent enough to minimize effects of time‐varying confounders15 and because the seasonal risk of arrhythmia is relatively similar in the proposed hazard and control periods (6.9% in November versus 7.4% in June).16 Alternative control periods closer to the date of the 2016 election were not selected because the monthly incidence of arrhythmia is substantially higher in September (9.9%) than the hazard period (6.9% in November)16 and would introduce carryover effects from the intense media coverage of the national party conventions in late July that persists until the day of the election (Figure 1). Similarly, earlier periods in 2016 were not selected because of the significantly greater incidence of arrhythmia in spring (0.86%) relative to other seasons, whereas the rates of arrhythmia are consistently lower in summer (0.70%) and fall (0.74%).17 In addition, to further control for potential variation in temperature across seasons, a second seasonal control period from the exact same time period in the preceding year (October 25, 2015–December 6, 2015) was included in sensitivity analyses.
Study Population
Adults (aged ≥18 years) who were enrolled in remote monitoring programs at 2 large centers in North Carolina and met the following eligibility criteria were included in the analysis: (1) implantation of an ICD (subcutaneous ICD and single‐ and dual‐chamber devices) or pacemaker (single‐ and dual‐chamber devices) with or without cardiac resynchronization therapy before May 2016; (2) had a device capable of remote diagnostic monitoring that was manufactured by Medtronic (Minneapolis, MN), Boston Scientific (Marlborough, MA), or Abbott (Abbott Park, IL); and (3) had continuous device data during the study period. Patients were excluded if they underwent device reprogramming or replacement with a change of manufacturer during the study period. Institutional Review Boards at each site approved the study protocol and waiver of informed consent. No funding or other research support was provided by the device manufacturers. All authors take responsibility for the integrity of the data and analyses.
Data Sources and Definitions
Details on data sources and linkage procedures are provided in Data S1. Briefly, information on arrhythmia episodes (type, date and time of occurrence, duration, and number of events) and therapy administered (ICD shocks and antitachycardia pacing [ATP]) was obtained from remote monitoring transmissions. Episodes were classified according to established device‐specific algorithms and programmed detection settings. As in prior studies,17, 18, 19 analyses were restricted to events meeting standard diagnostic criteria for atrial fibrillation (AF), supraventricular tachycardia, nonsustained ventricular tachycardia, and ventricular tachycardia/ventricular fibrillation.20, 21 Episodes of AF were reviewed, and only clinically relevant events (eg, AF ≥30 seconds) were included in this analysis. We further examined daily AF burden, defined as the mean percentage of time each day that patients with continuous data recorded by their device experienced AF. Device detection algorithms for AF have demonstrated >95% sensitivity and specificity for detection of atrial arrhythmia episodes and measurement of atrial arrhythmia burden in prior studies.22 Consistent with the approach used in the TRENDS study,23 we applied a minimum threshold of ≥20 seconds of AF burden for analysis and did not distinguish between atrial tachycardias, atrial flutter, or AF. Patients with a prior diagnosis of persistent AF were excluded from analysis, as their AF could not worsen during the period of observation. For device therapies (ATP and ICD shock), only the therapy administered (and not the underlying arrhythmia) was counted to avoid overestimating event rates in analyses of composite outcomes. Multiple events occurring on the same day were counted separately; however, when multiple sustained arrhythmic events occurred with minimal separation (≤60 seconds), only the first rhythm event was included in the analysis. Electrograms for all arrhythmia episodes treated by ICD shock were reviewed and adjudicated by a board‐certified electrophysiologist in a blinded manner.
Automated computer algorithms and standard methods24 were used to abstract demographic information, clinical history, and medications from the electronic health record (Data S1). Clinical data were linked to public voter registration records, which are updated weekly by the North Carolina State Board of Elections. We obtained the November 8, 2016, voter file, which contained personal identifiers and information on voter history and political affiliation (Democrat, Republican, Libertarian, or unaffiliated) from 6.9 million registered voters.
Statistical Analysis
Baseline characteristics are shown as frequencies and percentages for categorical variables, and as means and SDs for continuous variables. Descriptive statistics for patients with and without arrhythmic events during the hazard period were compared using t tests and χ2 tests, as appropriate. Additional descriptive analyses compared patient characteristics according to political party affiliation.
In the primary analysis, the incidence of all arrhythmic events during the hazard period was compared with that of the 2016 control period using Poisson regression with generalized estimating equation, which accounts for correlation between the number of arrhythmia events in the hazard and control periods within a single patient. We also examined the incidence of specific types of arrhythmia (AF, supraventricular tachycardia, nonsustained ventricular tachycardia, and ventricular tachycardia/ventricular fibrillation) as well as composite outcomes for supraventricular arrhythmias (AF and supraventricular tachycardia) and ventricular arrhythmias (nonsustained ventricular tachycardia and ventricular tachycardia/ventricular fibrillation). Inappropriate ICD therapies were excluded from all analyses (n=11 inappropriate ICD shocks occurred in 10 patients). Multivariable analyses were conducted to refine these estimates and control for potential confounders, including demographic characteristics (age, sex, race/ethnicity, time since device implant, and device type), baseline comorbidities (prior diagnoses of congestive heart failure, hypertension, coronary artery disease, renal failure, AF/atrial flutter, diabetes mellitus, left ventricular assist device, and anxiety/depressive disorders), and medications (β blockers and antiarrhythmics). The effects of the hazard period on daily AF burden were fitted nonparametrically using a generalized additive model (GAM) with a cyclic cubic spline function. Unlike linear regression models, GAM models do not assume linearity, allowing for a more flexible fit than models assuming a strict linear association. To avoid overfitting, the optimal smoothing parameters in the GAM were chosen by minimizing the Akaike Information Criterion. The GAM was fitted by using the “mgcv” package in R.25
Prespecified subgroup analyses that included interaction terms were used to ascertain whether the incidence of arrhythmia differed according to sex, age, race/ethnicity, device type, and history of hypertension, coronary artery disease, and congestive heart failure. In addition, because emotional stress from the election may be influenced by political ideology,26 separate analyses were performed in patients with matched voter registration data to assess whether the incidence of arrhythmia differed according to political party affiliation (Democrat versus Republican) and political concordance. “Political concordance” was defined as concordance between individuals' political affiliation (Democrat or Republican) and the election results from their county of residence (Democrat or Republican). An example of political concordance would be a registered Democrat living in a county won by the Democratic presidential candidate.
Sensitivity analyses were performed to assess the robustness of the primary findings. First, we modeled the risk of arrhythmia as a binary (instead of a continuous) outcome using the Mantel‐Haenszel method to determine the relative risk of arrhythmias during the hazard period compared with the control period. Next, to ensure that results were not attributable to seasonal variation in arrhythmia, we repeated the primary analyses using data from a subgroup of patients with complete device data during both the hazard period and the identical 6‐week period in 2015 (October 25, 2015–December 6, 2015).
All subgroup and sensitivity analyses were performed with composite outcomes for (1) any arrhythmic events, (2) supraventricular arrhythmias, and (3) ventricular arrhythmias to ensure adequate statistical power. Device therapies were not examined as a composite outcome because of the small number of events. Results are shown as relative risks or incidence rate ratios (IRRs) with 95% CIs in forest plots. Less than 1% of patients had missing data; these data were excluded from the analyses. A 2‐sided P<0.05 was considered significant. Analyses were performed with R software, version 3.6.1 (R Core Team, 2019).
Results
Among the 3047 patients who were screened for inclusion, 2449 met eligibility criteria (Figure 2). The final sample included only patients with linked clinical data from the electronic health record (n=2436). Of those patients, 1236 had an ICD (53.2%; 185 single chamber, 579 dual chamber, 517 cardiac resynchronization therapy, and 15 subcutaneous ICD) and 1140 had a pacemaker (46.8%; 73 single chamber, 1011 dual chamber, and 56 cardiac resynchronization therapy).
Figure 2. Cohort selection diagram.
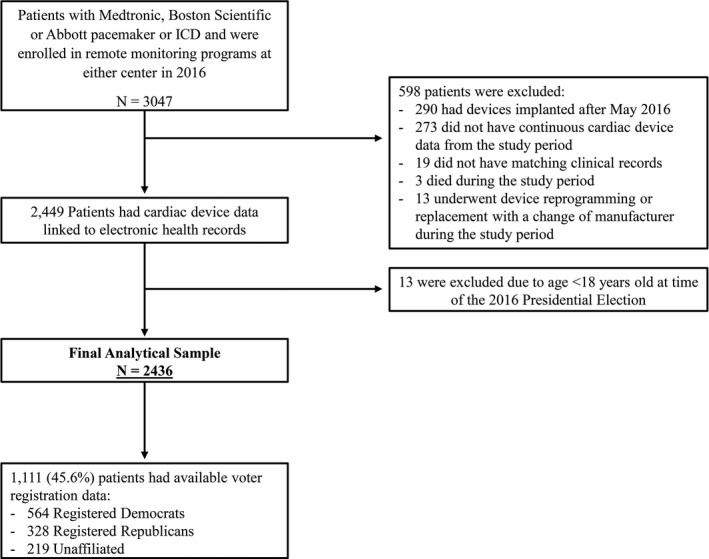
EHR indicates electronic health record; and ICD, implantable cardioverter‐defibrillator.
Baseline characteristics of the study population are shown in Table 1. Most patients were older (mean age, 70.8±12.9 years), White, men with underlying cardiovascular disease (hypertension, congestive heart failure, or AF/atrial flutter), and had prescriptions for β‐blockers, statins, and angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers.
Table 1.
Characteristics of the Patients Who Had an Arrhythmia During the 2016 US Presidential Election and Those Who Did Not
| Characteristics | Overall Sample (N=2436) | Arrhythmia (n=655) | No Arrhythmia (n=1781) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y* | 70.8±12.9 | 69.1±12.9 | 71.4±12.8 | <0.001 |
| Men | 1444 (59.4) | 411 (63.0) | 1033 (58.1) | 0.029 |
| Race/ethnicity† | 0.001 | |||
| White | 1809 (74.5) | 463 (71.0) | 1346 (75.7) | |
| Black | 516 (21.2) | 171 (26.2) | 345 (19.4) | |
| Hispanic | 22 (0.9) | 4 (0.6) | 18 (1.0) | |
| Other | 82 (3.4) | 14 (2.1) | 68 (3.8) | |
| Employment status (retired) | 1555 (68.4) | 406 (66.2) | 1149 (69.1) | 0.187 |
| Device | ||||
| Pacemaker (ICD as referent group) | 1140 (46.8) | 245 (37.4) | 895 (50.3) | <0.001 |
| Left ventricular assist device | 13 (0.6) | 7 (1.1) | 6 (0.4) | 0.054 |
| Time since implant, y* | 3.08±3.08 | 2.85±3.45 | 3.17±2.93 | 0.025 |
| Clinical history | ||||
| Hypertension | 1413 (62.6) | 385 (62.2) | 1028 (62.8) | 0.808 |
| Previous myocardial infarction | 846 (37.5) | 235 (38.0) | 611 (37.3) | 0.770 |
| Congestive heart failure | 1147 (50.8) | 348 (56.2) | 799 (48.8) | 0.002 |
| Coronary artery disease | 867 (38.4) | 239 (38.6) | 628 (38.3) | 0.923 |
| Diabetes mellitus | 341 (15.1) | 78 (12.6) | 263 (16.1) | 0.041 |
| Obstructive sleep apnea | 179 (7.9) | 61 (9.9) | 118 (7.2) | 0.044 |
| Stroke/TIA | 141 (6.2) | 37 (6.0) | 104 (6.3) | 0.845 |
| Lipid disorders | 532 (23.6) | 139 (22.5) | 393 (24.0) | 0.470 |
| Peripheral vascular disease | 179 (7.9) | 40 (6.5) | 139 (8.5) | 0.117 |
| Valvular heart disease | 242 (10.7) | 56 (9.0) | 186 (11.4) | 0.127 |
| Chronic kidney disease | 208 (9.2) | 52 (8.4) | 156 (9.5) | 0.463 |
| COPD | 225 (10.0) | 68 (11.0) | 157 (9.6) | 0.345 |
| Arrhythmias and conduction defects | ||||
| Atrial fibrillation/atrial flutter | 1058 (46.9) | 353 (57.0) | 705 (43.0) | <0.001 |
| Prior sudden cardiac arrest | 53 (2.3) | 18 (2.9) | 35 (2.1) | 0.278 |
| Medications | ||||
| ACE inhibitor or ARB | 1591 (65.5) | 457 (70.1) | 1134 (63.8) | 0.004 |
| β‐Blocker | 1890 (77.8) | 543 (83.3) | 1347 (75.8) | <0.001 |
| Statin | 1554 (64.0) | 421 (64.6) | 1133 (63.7) | 0.739 |
| Calcium channel blockers | 713 (29.3) | 207 (31.7) | 506 (28.5) | 0.119 |
| Antiarrhythmic | 541 (22.3) | 158 (24.2) | 383 (21.5) | 0.169 |
| Anticoagulation | 1192 (49.1) | 342 (52.5) | 850 (47.8) | 0.044 |
| Antiplatelet agent/aspirin | 1677 (69.0) | 473 (72.5) | 1204 (67.7) | 0.023 |
| Antidepressant‡ | 691 (28.4) | 188 (28.8) | 503 (28.3) | 0.800 |
| Lifestyle factors | ||||
| Body mass index, kg/m2 * | 30.06±6.52 | 30.48±6.98 | 29.89±6.33 | 0.053 |
| Alcohol abuse | 7 (0.3) | 1 (0.2) | 6 (0.4) | 0.681 |
| Drug abuse | 23 (1.0) | 8 (1.3) | 15 (0.9) | 0.481 |
| Smoking status | 0.402 | |||
| Current | 176 (7.7) | 50 (7.9) | 126 (7.5) | |
| Former | 1034 (45) | 296 (47) | 738 (44.2) | |
| Never | 1089 (47.4) | 284 (45.1) | 805 (48.2) | |
| Psychiatric comorbidities | ||||
| Major depressive disorder | 131 (5.8) | 30 (4.8) | 101 (6.2) | 0.267 |
| Prior anxiety or depressive disorder§ | 152 (6.7) | 37 (6.0) | 115 (7.0) | 0.399 |
Data are given as number (percentage), unless otherwise indicated. Listed values are for the overall sample, and comparisons are made between those who had an arrhythmia during the hazard period and those who did not. All demographic and clinical data were recorded in the electronic health record before the start of the study period (June 1, 2016). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter‐defibrillator; and TIA, transient ischemic attack.
Data are presented as mean±SD.
Data missing: race/ethnicity, n=7 (0.3%).
Antidepressant medications include selective serotonin reuptake inhibitors/serotonin and norepinephrine reuptake inhibitors.
A composite variable was created for any prior diagnoses of anxiety and depressive disorders: generalized anxiety disorder, posttraumatic stress disorder, panic disorder, and major depressive disorder.
Risk of Arrhythmia During the 2016 US Presidential Election
A total of 2592 arrhythmic events occurred in 655 patients during the election period compared with 1533 events in 472 patients during the control period. The incidence of any arrhythmic event was significantly higher during the election relative to the control period (IRR, 1.77 [95% CI, 1.42–2.21]) (Figure 3). As for specific arrhythmias, patients were 1.5 (95% CI, 1.06–2.11) times more likely to experience AF, 3.7 (95% CI, 2.2–6.2) times more likely to experience supraventricular tachycardia, and 1.7 (95% CI, 1.3–2.2) times more likely to experience nonsustained ventricular tachycardia during the election period. They were 11.6 (95% CI, 3.2–42.0) times more likely to receive ATP therapies during the election, whereas associations with ventricular tachycardia/ventricular fibrillation (1.7 [95% CI, 0.9–3.4]) and ICD shock (2.00 [95% CI, 0.50–7.97]) were nonsignificant. Analyses of composite outcomes were consistent with the primary findings and show an elevated risk of supraventricular arrhythmia (1.82 [95% CI, 1.36–2.43]) and ventricular arrhythmia (1.60 [95% CI, 1.22–2.10]) during the election period relative to the control period. In multivariable models, the risk of any arrhythmic event (1.77 [95% CI, 1.42–2.21]; Table 2), supraventricular arrhythmias (1.82 [95% CI, 1.36–2.43]; Table S1), and ventricular arrhythmias (1.60 [95% CI, 1.22–2.10]; Table S2) remained elevated after controlling for all other demographic and clinical confounders.
Figure 3. Incidence rate ratios (IRRs) for arrhythmic events during the 2016 US presidential election.
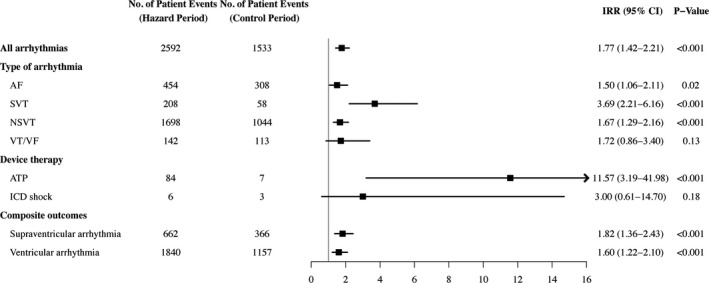
Notes and definitions: Listed values may include multiple arrhythmic events within a single patient and are controlled for by the analysis. IRRs were not adjusted for baseline variables. Composite outcomes: supraventricular arrhythmias (atrial fibrillation [AF] and supraventricular tachycardia [SVT]), ventricular arrhythmias (nonsustained ventricular tachycardia [NSVT] and ventricular tachycardia/ventricular fibrillation [VT/VF]), and device therapy administered (antitachycardia pacing [ATP] and implantable cardioverter‐defibrillator [ICD] shocks). Because Abbott devices do not discriminate between nonsustained events (supraventricular vs ventricular), people with these devices were excluded from analyses of composite outcomes.
Table 2.
IRRs for Any Arrhythmia During the 2016 US Presidential Election
| Variable | IRR (95% CI) | P Value |
|---|---|---|
| Hazard period (control period reference group) | 1.77 (1.42–2.21) | <0.001 |
| Age (in decades) | 0.89 (0.83–0.96) | 0.003 |
| Sex (men reference group) | 0.89 (0.71–1.11) | 0.288 |
| Race/ethnicity (White reference group) | 1.34 (1.04–1.73) | 0.023 |
| Time since diagnosis, y | 1.07 (1.03–1.12) | <0.001 |
| Device type (pacemaker reference group) | 2.17 (1.59–2.95) | <0.001 |
| Congestive heart failure | 0.67 (0.48–0.93) | 0.017 |
| Hypertension | 0.94 (0.73–1.21) | 0.656 |
| Coronary artery disease | 1.04 (0.78–1.40) | 0.783 |
| Chronic kidney disease | 1.25 (0.79–1.98) | 0.334 |
| Diabetes mellitus | 0.75 (0.52–1.08) | 0.125 |
| AF/atrial flutter | 1.62 (1.29–2.04) | <0.001 |
| LVAD | 4.97 (2.18–11.33) | <0.001 |
| Antiarrhythmics medications | 1.21 (0.91–1.60) | 0.191 |
| β‐Blocker medications | 1.05 (0.77–1.44) | 0.744 |
| Prior anxiety or depressive disorder | 0.71 (0.47–1.07) | 0.100 |
Listed values may include multiple arrhythmic events within a single patient. A composite variable was created for any prior diagnoses of anxiety and depressive disorders: generalized anxiety disorder, posttraumatic stress disorder, panic disorder, and major depressive disorder. AF indicates atrial fibrillation; IRR, incidence rate ratio; and LVAD, left ventricular assist device.
Of patients, 35% had ≥20 seconds of AF burden on at least 1 day during the period of observation. Change in mean daily AF burden from the control period to the hazard period is illustrated in Figure 4. Among those people, there was a significant increase in mean daily AF burden during the hazard period relative to the control period (0.6% higher; P<0.001).
Figure 4. Daily mean atrial fibrillation burden (blue dots) during the control period (June 1, 2016 to July 12, 2016) and hazard period (October 25, 2016 to December 6, 2016).
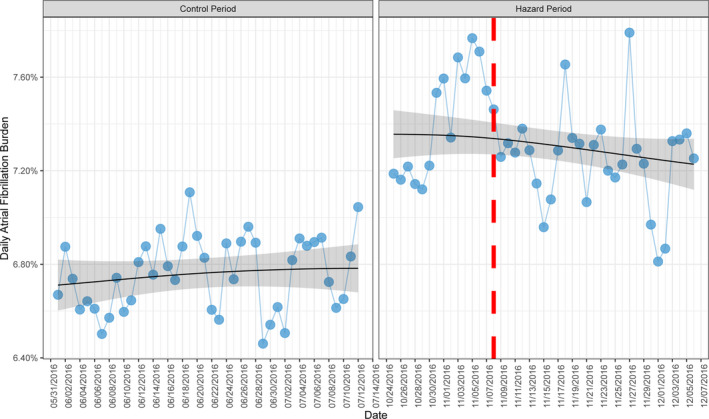
A generalized additive model with a cyclic cubic spline function is fitted to demonstrate trends over these time periods (black line with 95% confidence interval shaded grey). The vertical red line represents the date of the 2016 U.S. presidential election.
Subgroup Analyses
No significant interactions were found for sex, race/ethnicity, device type, age >65 years, hypertension, coronary artery disease, and congestive heart failure for any arrhythmic event, supraventricular arrhythmia, and ventricular arrhythmia (Figure 5).
Figure 5. Subgroup analyses for arrhythmic events during the 2016 US presidential election.
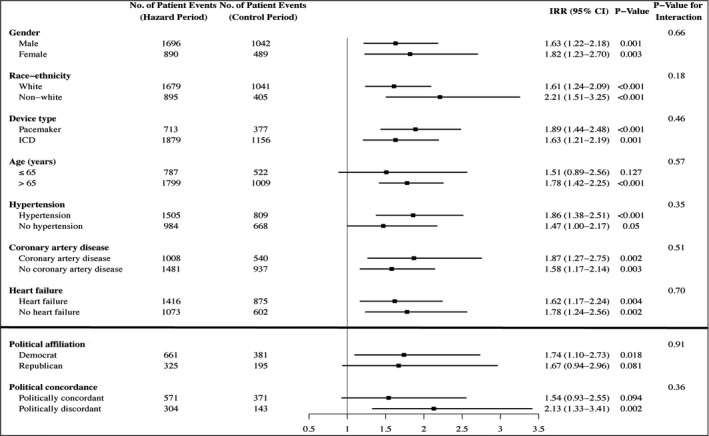
Notes and definitions: Subgroup analyses for sex, race/ethnicity, device type, age, hypertension, coronary artery disease, and congestive heart failure were performed with data from the entire cohort (n=2436). Subgroup analyses for political affiliation and political concordance were limited to people with matched voter registration data (n=1111). Listed values may include multiple arrhythmic events within a single patient and are controlled for by the analysis. Incidence rate ratios (IRRs) were not adjusted for baseline variables. Composite outcomes include: supraventricular arrhythmias (atrial fibrillation and supraventricular tachycardia) and ventricular arrhythmias (nonsustained ventricular tachycardia and ventricular tachycardia/ventricular fibrillation). Because Abbott devices do not discriminate between nonsustained events (supraventricular vs ventricular), people with these devices were excluded from analyses of composite outcomes. ICD indicates implantable cardioverter‐defibrillator.
Voter registration data were available for 1111 patients (45.6%); and of those, 564 were registered Democrats, 328 were Republicans, and 219 were unaffiliated (Table S3). Data from the only registered Libertarian in our sample were excluded from analysis. Overall, Democrats were more likely to be women, to belong to a racial/ethnic minority group, and to have a left ventricular assist device, whereas Republicans were more likely to have a history of myocardial infarction and coronary artery disease. In subgroup analyses, interactions for political affiliation were nonsignificant for any arrhythmia (P=0.91; Figure 4), supraventricular arrhythmias (P=0.07; Figure S1), and ventricular events (P=0.52; Figure S2). Interactions for political concordance were also nonsignificant (P for the interaction: any arrhythmia [P=0.36; Figure 4], supraventricular arrhythmias [P=0.79; Figure S1], and ventricular events [P=0.33; Figure S2]).
Sensitivity Analyses
Results of sensitivity analyses were generally consistent with the main findings of the study. In analyses that modeled the risk of arrhythmia as a binary (instead of a continuous) outcome, the findings showed a significant increase in arrhythmia during the election relative to the 2016 control period (Table S4).
To control for potential seasonal variation in arrhythmia, we repeated the primary analysis with data from a second seasonal cohort using data from a subgroup of patients (n=460) with complete device data during the hazard period and the identical 6‐week period in 2015 (October 25–December 6, 2015). We found a significantly higher risk of arrhythmic events (IRR, 1.29 [95% CI, 1.04–1.59]) and supraventricular arrhythmias (IRR, 1.73 [95% CI, 1.03–2.89]) during the 2016 US presidential election compared with the identical 6‐week period in 2015 (Table S5). Although differences in the occurrence of ventricular arrhythmias were not significant because of the lower number of events in this smaller subgroup of patients (IRR, 1.20 [95% CI, 0.95–1.53]), the point estimate was in the same direction as that of the primary analysis and the total number of ventricular events was higher in the hazard period (n=148) compared with the control period (n=123).
We also repeated the GAM analysis for AF burden with data from the corresponding 6‐week period in 2015. These results were nearly identical to those in the primary analysis (Figure S3), with a significantly higher burden of AF during the 2016 US presidential election compared with the same period 1 year earlier (0.4% higher; P<0.001).
Discussion
In this large cohort of patients with implanted cardiac devices, we found a significant increase in the risk of arrhythmic events, including supraventricular and ventricular arrhythmias, as well as a greater AF burden during the 2016 US presidential election compared with the control period. These associations were independent of known demographic and clinical confounders. Sensitivity analyses further allayed concerns that observed differences might be explained by the analytic approach, or seasonal variation in arrhythmia. These findings reinforce previous observations from studies of other sociopolitical events around the world (eg, the withdrawal of the United Kingdom from the European Union and sociopolitical conflict in Hong Kong),27, 28, 29, 30 suggesting that substantial shifts in political power may negatively affect health outcomes in vulnerable populations.
Although our study is the first to investigate the role of a stressful political election in triggering arrhythmic events, prior studies have reported a marked increase in acute cardiovascular events following natural disasters,4, 5 industrial accidents,31 terrorist attacks,6 and other large‐scale population stressors.2, 9 A higher incidence of ventricular tachyarrhythmias has also been reported in patients with ICDs following national tragedies, such as the attacks on the World Trade Center on September 11, 2001.7, 8 Our findings extend this work by demonstrating a 77% increase in clinical arrhythmia in people with underlying cardiovascular disease exposed to a highly polarized political election. In addition, AF was detected in approximately one third of individuals during the study, and we observed a significantly higher burden of AF in those people during the election relative to the control periods in 2015 and 2016. These findings raise the possibility that acute mental stress from a political election may have more long‐term consequences on cardiovascular health, as increases in the frequency and duration of tachyarrhythmias have been strongly associated with hemodynamic instability, worsening heart failure, hospitalization, and death in patients with ICDs.32 Transient increases in daily AF burden have also been associated with a higher short‐term risk of stroke and worse quality of life.33 This study, however, was not designed to examine long‐term clinical outcomes. Whether arrhythmic events trigged by sociopolitical events are associated with long‐term morbidity requires further investigation.
A novel feature of this study was the examination of individual risk factors for arrhythmia as well as the social and political conditions that may influence cardiovascular health. Although negative emotions, social isolation, and loneliness have been associated with cardiovascular morbidity and mortality in previous studies,11, 34 we did not observe a higher incidence of arrhythmia among people who voted for the losing candidate (Democrats in the 2016 election) or among those who may have felt socially or ideologically disconnected from their community (politically discordant people). We also found no variation in risk according to demographic characteristics and comorbidities. Instead, our study showed that the increased risk of arrhythmia associated with the 2016 election was similar among individuals of all demographic, clinical, and ideological backgrounds. Although we may expect to see a higher incidence of emotion‐triggered arrhythmia in people with underlying heart disease, previous studies have also failed to show a relationship between the type and severity of structural heart disease and anger‐triggered arrhythmias.35, 36 The absence of effect modification by demographic, clinical, and political characteristics in the current analysis could also be attributed to the small number of events that occurred in this cohort during the 6‐week study periods, particularly among those with matched voter registration data. It is worth noting that the total number of arrhythmic events was higher in the hazard period relative to the control period in most of the subgroup analyses. Larger, fully powered studies are needed to clarify the influence of social and political factors on arrhythmia burden.
Although mechanisms were not directly assessed in this study, acute mental stress and negative emotions are associated with increases in adrenergic activity, sympathetic activation, and reduced vagal tone, which can produce dynamic changes in cardiac electrophysiology that trigger arrhythmogenesis and maintain arrhythmogenic substrate.10, 11 Anger and severe emotional distress have also been shown to precipitate ischemia and abrupt plaque rupture, which are potent triggers of arrhythmia, especially in the context of coronary heart disease.9 Other studies have suggested that alterations in the hypothalamus‐pituitary‐adrenal axis may lead to proinflammatory responses that accelerate structural remodeling secondary to an underlying disease process (eg, hypertension, renal dysfunction, or heart failure), thereby increasing susceptibility to cardiac conduction and repolarization abnormalities.10, 37 Sustained increases in stress hormones, such as cortisol, may activate biological processes that facilitate arrhymia.9, 11 Mental stress and salivary cortisol have also been shown to increase during a stressful political election,38 suggesting that a biological link between election‐related stress and arrhythmia is possible.37 Additional metabolic pathways,10 endothelial dysfunction,37 underlying psychiatric disorders,39 and unhealthy behaviors (sleep disturbance, poor diet, smoking, medication nonadherence, substance abuse, excessive caffeine consumption, and decreased physical activity)37, 40 may also contribute to electrical instability in the heart and arrhythmogenesis.
Implications of Findings
Although the absolute risk of acute cardiovascular events is generally low for infrequent events and uncommon triggers (eg, earthquakes),13 national political elections occur every 2 to 4 years in the United States (midterms and general election) and at similar frequencies in other countries around the world. The pathophysiologic consequences of stressful sociopolitical events can accumulate and may amplify the effects of other long‐term stressors (eg, caregiving and marital or work‐related stress) and behavioral risk factors for cardiovascular disease (eg, substance abuse). Therefore, the potential population‐level health impact of recurrent political events is not negligible and warrants further study to help inform future public health strategies.
Findings from this investigation also raise important questions about whether appropriate therapeutic strategies can mitigate the risk of emotion‐triggered arrhythmia in susceptible people during periods of heightened social and political stress. Cognitive‐behavioral therapy, yoga, and other stress management techniques have been shown to reduce mental stress and physiological arousal, and improve health outcomes in patients with established cardiovascular disease.41 Preliminary data suggest that β‐blockers may also be effective at reducing the effect of negative emotions on arrhythmia reoccurrence.42 Further research and randomized trials are needed to determine the clinical benefits of these tools in populations prone to emotionally triggered cardiac events.
Strengths and Limitations
A major strength of our study was its case‐crossover design. Because each patient served as his or her own control, confounding by non–time‐varying patient factors was eliminated.14 Furthermore, unlike previous studies of short‐term triggers of arrhythmia, which were limited to small samples of predominantly male patients and focused on a single device or outcome (eg, ICD shock), this investigation included a large sample, 40% of whom were women, and assessed multiple arrhythmia outcomes in a wide range of devices from several manufacturers.
Several limitations should be noted. First, because this was a retrospective observational study, no causal relationship should be inferred. Second, this study did not collect information on important time‐varying confounders (eg, subjective emotional distress, changes in medications, New York Heart Association class, hospitalizations, and environmental factors) that may have influenced study findings. Although information on time‐varying covariates is often not available in retrospective studies,2, 5, 6, 7, 8 time‐varying clinical factors should be considered in future prospective studies. A third limitation is that although ICD shocks were adjudicated, arrhythmias were assessed using validated device detection algorithms.43 Although this may have resulted in occasional misclassification of events, programmed detection settings were consistent throughout the hazard and control periods for all patients; thus, it is unlikely to have affected risk estimates. However, because electrograms of arrhythmic events treated with ATP are not consistently stored in the device, inappropriate ATP therapies could not be removed from analyses. It is possible that the risk estimates for ATP were partly driven by a high number of inappropriate ATP therapies for atrial arrhythmias. Fourth, we cannot discount the possibility that longer or shorter periods of assessment may have led to different results. A fifth limitation was that political affiliation was ascertained from voter registration records, which may not reflect actual voter behavior. We note, however, that 92% of affiliated Republicans and 94% of Democrats in North Carolina voted with their party in the 2016 presidential election.44
In conclusion, exposure to a stressful political election was associated with an increased risk of both supraventricular and ventricular arrhythmias, and a higher burden of AF in people with underlying cardiovascular disease. These findings suggest that exposure to stressful sociopolitical events may promote arrhythmogenesis in susceptible people.
Sources of Funding
This study was supported by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health to Dr Rosman (K23HL141644). Dr Salmoirago‐Blotcher is funded by National Institute of Health (grants R01HL149672 and R21HL140492). Support for the data linkage and database management was provided by the Translational and Clinical Sciences Institute (UL1TR002489 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences, National Institutes of Health).
Disclosures
Dr Bumgarner receives consulting fees from Medtronic as well as support from Boston Scientific, Biotronik, and Abbott. Dr Gehi receives research support from Bristol Myers Squib Foundation, consulting fees from Biosense‐Webster, and speaker's honoraria from Abbott, Biotronik, and Zoll Medical. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S5
Figures S1–S3
Acknowledgments
We thank George L. Adams, MD, MHS, and Jennifer Hanks, RN, for their contributions to this study.
(J Am Heart Assoc. 2021;10:e020559. DOI: 10.1161/JAHA.120.020559.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020559
For Sources of Funding and Disclosures, see page 11.
References
- 1.Fleming PJ, Lopez WD, Mesa H, Rion R, Rabinowitz E, Bryce R, Doshi M. A qualitative study on the impact of the 2016 US election on the health of immigrant families in Southeast Michigan. BMC Public Health. 2019;19:947. DOI: 10.1186/s12889-019-7290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilbert‐Lampen U, Leistner D, Greven S, Pohl T, Sper S, Völker C, Güthlin D, Plasse A, Knez A, Küchenhoff H, et al. Cardiovascular events during World Cup soccer. N Engl J Med. 2008;358:475–483. DOI: 10.1056/NEJMoa0707427. [DOI] [PubMed] [Google Scholar]
- 3.Montoya‐Williams D, Fuentes‐Afflick E. Political determinants of population health. JAMA Netw Open. 2019;2:e197063. DOI: 10.1001/jamanetworkopen.2019.7063. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Ohira T, Takeishi Y, Hosoya M, Yasumura S, Satoh H, Kawasaki Y, Takahashi A, Sakai A, Ohtsuru A, et al. Increased prevalence of atrial fibrillation after the Great East Japan Earthquake: results from the Fukushima Health Management Survey. Int J Cardiol. 2015;198:102–105. DOI: 10.1016/j.ijcard.2015.06.151. [DOI] [PubMed] [Google Scholar]
- 5.Nakano M, Kondo M, Wakayama Y, Kawana A, Hasebe Y, Shafee MA, Fukuda K, Shimokawa H. Increased incidence of tachyarrhythmias and heart failure hospitalization in patients with implanted cardiac devices after the great East Japan earthquake disaster. Circ J. 2012;76(5):1283–1285. DOI: 10.1253/circj.CJ-12-0261. [DOI] [PubMed] [Google Scholar]
- 6.Meisel SR, Kutz I, Dayan KI, Pauzner H, Chetboun I, Arbel Y, David D. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet. 1991;338:660–661. DOI: 10.1016/0140-6736(91)91234-L. [DOI] [PubMed] [Google Scholar]
- 7.Shedd OL, Sears SF, Harvill JL, Arshad A, Conti JB, Steinberg JS, Curtis AB. The World Trade Center attack: increased frequency of defibrillator shocks for ventricular arrhythmias in patients living remotely from New York City. J Am Coll Cardiol. 2004;44:1265–1267. DOI: 10.1016/j.jacc.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg JS, Arshad A, Kowalski M, Kukar A, Suma V, Vloka M, Ehlert F, Herweg B, Donnelly J, Philip J, et al. Increased incidence of life‐threatening ventricular arrhythmias in implantable defibrillator patients after the World Trade Center attack. J Am Coll Cardiol. 2004;44:1261–1264. DOI: 10.1016/j.jacc.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863–1872. DOI: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- 10.Lampert R. Behavioral influences on cardiac arrhythmias. Trends Cardiovasc Med. 2016;26:68–77. DOI: 10.1016/j.tcm.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106:1800–1805. DOI: 10.1161/01.CIR.0000031733.51374.C1. [DOI] [PubMed] [Google Scholar]
- 12.Fowler EF, Ridout TN, Franz MM. Political advertising in 2016: the presidential election as outlier? Forum. 2016;14:445. DOI: 10.1515/for-2016-0040. [DOI] [Google Scholar]
- 13.Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation. 2011;124:346–354. DOI: 10.1161/CIRCULATIONAHA.110.968776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mostofsky E, Coull BA, Mittleman MA. Analysis of observational self‐matched data to examine acute triggers of outcome events with abrupt onset. Epidemiology. 2018;29:804–816. DOI: 10.1097/EDE.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mostofsky E, Maclure M, Sherwood JB, Tofler GH, Muller JE, Mittleman MA. Risk of acute myocardial infarction after the death of a significant person in one's life: the determinants of myocardial infarction onset study. Circulation. 2012;125:491–496. DOI: 10.1161/CIRCULATIONAHA.111.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra F, Bonelli P, Flori M, Cipolletta L, Carbucicchio C, Izquierdo M, Kozluk E, Shivkumar K, Vaseghi M, Patani F, et al. Temporal trends and temperature‐related incidence of electrical storm: the TEMPEST study (temperature‐related incidence of electrical storm). Circ Arrhythm Electrophysiol. 2017;10:e004634. DOI: 10.1161/CIRCEP.116.004634. [DOI] [PubMed] [Google Scholar]
- 17.Maan A, Sherfesee L, Lexcen D, Heist EK, Cheng A. Diurnal, seasonal, and monthly variations in ventricular arrhythmias in patients with implantable cardioverter‐defibrillators. JACC Clin Electrophysiol. 2019;5:979–986. DOI: 10.1016/j.jacep.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Jędrzejczyk‐Patej E, Lenarczyk R, Mazurek M, Liberska A, Przybylska‐Siedlecka K, Podolecki T, Kowalczyk J, Sokal A, Leopold‐Jadczyk A, Kowalski O, et al. Can we rely on machines? Device‐detected atrial high rates correspond well with atrial arrhythmias in cardiac resynchronization recipients. Europace. 2015;18:436–444. DOI: 10.1093/europace/euv095. [DOI] [PubMed] [Google Scholar]
- 19.Link MS, Luttmann‐Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, Dockery DW, Laden F. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol. 2013;62:816–825. DOI: 10.1016/j.jacc.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calkins H, Hindricks G, Cappato R, Kim Y‐H, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace. 2017;20:157–208. DOI: 10.1093/europace/eux275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stiles MK, Fauchier L, Morillo CA, Wilkoff BL; ESC Scientific Document Group . 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter‐defibrillator programming and testing. Europace. 2019;21:1442–1443. DOI: 10.1093/europace/euz065. [DOI] [PubMed] [Google Scholar]
- 22.Purerfellner H, Gillis AM, Holbrook R, Hettrick DA. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol. 2004;27:983–992. DOI: 10.1111/j.1540-8159.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 23.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, Gasparini M, Lewalter T, Camm JA, Singer DE. Device‐detected atrial fibrillation and risk for stroke: an analysis of >10 000 patients from the SOS AF project (stroke prevention strategies based on atrial fibrillation information from implanted devices). Eur Heart J. 2013;35:508–516. DOI: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dusetzina SB, Tyree S, Meyer AM, Meyer A, Green L, Carpenter WR. Linking Data for Health Services Research: A Framework and Instructional Guide. Rockville, MD; 2014. [PubMed] [Google Scholar]
- 25.Wood S. Generalized Additive Models. New York, NY: Chapman and Hall/CRC; 2017. [Google Scholar]
- 26.Association AP . Stress in America: coping with change. February 15, 2017. Available at: http://www.Apa.Org/news/press/releases/stress/2016/coping‐with‐change.Pdf. Accessed October 15, 2019.
- 27.Jofre‐Bonet M, Serra‐Sastre V, Vandoros S. The impact of the great recession on health‐related risk factors, behaviour and outcomes in England. Soc Sci Med. 2018;197:213–225. DOI: 10.1016/j.socscimed.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Vandoros S, Avendano M, Kawachi I. The EU referendum and mental health in the short term: a natural experiment using antidepressant prescriptions in England. J Epidemiol Community Health. 2019;73:168–175. DOI: 10.1136/jech-2018-210637. [DOI] [PubMed] [Google Scholar]
- 29.Ni MY, Kim Y, McDowell I, Wong S, Qiu H, Wong IO, Galea S, Leung GM. Mental health during and after protests, riots and revolutions: a systematic review. Aust N Z J Psychiatry. 2020;54:232–243. DOI: 10.1177/0004867419899165. [DOI] [PubMed] [Google Scholar]
- 30.Ni MY, Yao XI, Leung KSM, Yau C, Leung CMC, Lun P, Flores FP, Chang WC, Cowling BJ, Leung GM. Depression and post‐traumatic stress during major social unrest in Hong Kong: a 10‐year prospective cohort study. Lancet. 2020;395:273–284. DOI: 10.1016/S0140-6736(19)33160-5. [DOI] [PubMed] [Google Scholar]
- 31.Ruidavets J‐B, Paterniti S, Bongard V, Giroux M, Cassadou S, Ferrières J. Triggering of acute coronary syndromes after a chemical plant explosion. Heart. 2006;92:257–258. DOI: 10.1136/hrt.2005.061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson WG, John RM. Ventricular arrhythmias in patients with implanted defibrillators. Circulation. 2011;124:e411–e414. DOI: 10.1161/CIRCULATIONAHA.111.064816. [DOI] [PubMed] [Google Scholar]
- 33.Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137:e623–e644. DOI: 10.1161/CIR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta‐analysis of longitudinal observational studies. Heart. 2016;102:1009–1016. DOI: 10.1136/heartjnl-2015-308790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stopper M, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D, Lampert R. Electrophysiologic characteristics of anger‐triggered arrhythmias. Heart Rhythm. 2007;4:268–273. DOI: 10.1016/j.hrthm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Taylor E, Berger R, Hummel JD, Dinerman JL, Kenknight B, Arria AM, Tomaselli G, Calkins H. Analysis of the pattern of initiation of sustained ventricular arrhythmias in patients with implantable defibrillators. J Cardiovasc Electrophysiol. 2000;11:719–726. DOI: 10.1111/j.1540-8167.2000.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 37.Kivimaki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15:215–229. DOI: 10.1038/nrcardio.2017.189. [DOI] [PubMed] [Google Scholar]
- 38.Stanton SJ, LaBar KS, Saini EK, Kuhn CM, Beehner JC. Stressful politics: voters’ cortisol responses to the outcome of the 2008 United States presidential election. Psychoneuroendocrinology. 2010;35:768–774. DOI: 10.1016/j.psyneuen.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Rosman L, Lampert R, Ramsey CM, Dziura J, Chui PW, Brandt C, Haskell S, Burg MM. Posttraumatic stress disorder and risk for early incident atrial fibrillation: a prospective cohort study of 1.1 million young adults. J Am Heart Assoc. 2019;8:e013741. DOI: 10.1161/JAHA.119.013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voskoboinik A, Kalman JM, Kistler PM. Caffeine and arrhythmias: time to grind the data. JACC Clin Electrophysiol. 2018;4:425–432. DOI: 10.1016/j.jacep.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Khatib MN, Kirubakaran R, Gaidhane S, Shankar AH, Quazi SZ. Yoga for improving functional capacity, quality of life and cardiovascular outcomes in people with heart failure. Cochrane Database Syst Rev. 2017;2017:CD012015. DOI: 10.1002/14651858.CD012015.pub2. [DOI] [Google Scholar]
- 42.Lampert R, Burg MM, Jamner LD, Dziura J, Brandt C, Li F, Donovan T, Soufer R. Effect of beta‐blockers on triggering of symptomatic atrial fibrillation by anger or stress. Heart Rhythm. 2019;16:1167–1173. DOI: 10.1016/j.hrthm.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman ES, Israel CW, Nair GM, Armaganijan L, Divakaramenon S, Mairesse GH, Brandes A, Crystal E, Costantini O, Sandhu RK, et al. Positive predictive value of device‐detected atrial high‐rate episodes at different rates and durations: an analysis from ASSERT. Heart Rhythm. 2012;9:1241–1246. DOI: 10.1016/j.hrthm.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Pew Research Center . August, 2018. For most trump voters, “very warm” feelings for him endured. Available at: https://www.pewresearch.org/politics/2018/08/09/for‐most‐trump‐voters‐very‐warm‐feelings‐for‐him‐endured/. Accessed August 1, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
Figures S1–S3


