Abstract
Background
Comparison of care among centers is currently limited to major end points, such as mortality, length of stay, or complication rates. Creating “care curves” and comparing individual elements of care over time may highlight modifiable differences in intensive care among centers.
Methods and Results
We performed an observational retrospective study at 5 centers in the United States to describe key elements of postoperative care following the stage 1 palliation. A consecutive sample of 502 infants undergoing stage 1 palliation between January 2009 and December 2018 were included. All electronic health record entries relating to mandatory mechanical ventilator rate, opioid administration, and fluid intake/outputs between postoperative days (POD) 0 to 28 were extracted from each institution's data warehouse. During the study period, 502 patients underwent stage 1 palliation among the 5 centers. Patients were weaned to a median mandatory mechanical ventilator rate of 10 breaths/minute by POD 4 at Center 5 but not until POD 7 to 8 at Centers 1 and 2. Opioid administration peaked on POD 2 with extreme variance (median 6.9 versus 1.6 mg/kg per day at Center 3 versus Center 2). Daily fluid balance trends were variable: on POD 3 Center 1 had a median fluid balance of −51 mL/kg per day, ranging between −34 to 19 mL/kg per day among remaining centers. Intercenter differences persist after adjusting for patient and surgical characteristics (P<0.001 for each end point).
Conclusions
It is possible to detail and compare individual elements of care over time that represent modifiable differences among centers, which persist even after adjusting for patient factors. Care curves may be used to guide collaborative quality improvement initiatives.
Keywords: congenital heart disease, intensive care, postoperative care, stage 1 palliation
Nonstandard Abbreviations and Acronyms
- DW
data warehouse
- EMR
electronic medical record
- FB
fluid balance
- MMVR
mandatory mechanical ventilation rate
- POD
postoperative day
- S1P
stage 1 palliation
- TDD
total daily dose
Clinical Perspective
What Is New?
Care curves, similar in concept to growth charts, can be used to describe any element of care in a population undergoing a procedure or with a specific diagnosis.
We created care curves describing ventilatory support, opioid use, and fluid balance in 500 newborns undergoing the stage 1 palliation.
What Are the Clinical Implications?
Care curves can be used to identify the expected trajectory of a patient over time, to identify outlier patients, and to identify outlier practices when used between centers.
An essential component to optimal care is an in‐depth understanding of the structure and patterns of care within a system that determine patient trajectories. Describing the spectrum of interventions and patient responses to a particular situation (eg, following a specific diagnosis or operation) allows healthcare teams to identify patient outliers, question and clarify diagnoses, and to implement quality improvement initiatives for process and outcome metrics that are subpar. In the modern era, transparency in health care is a virtue.1 In nearly every specialty, centers of excellence report patient outcomes and major metrics of quality, including survival, lengths of stay, and major complications.2 While such metrics are undeniably important, they lack specificity as to how a center may improve. Few currently reported metrics are directly actionable, but rather represent the culminated results of measured actions at the bedside. The intensive care unit is a location where such patient outcomes can be significantly influenced, in some cases dramatically so. Patients are sedated, ventilated, administered fluids, and closely monitored in ways that are directly quantifiable, comparable among centers, and modifiable.
In the field of congenital heart disease, few procedures have received more scrutiny than the stage 1 palliation (S1P).3, 4, 5 This procedure is one in which a parallel circulation is surgically established, typically in the newborn period, leaving patients vulnerable to circulatory shock and hypoxemia.6 During recovery, these patients often require prolonged mechanical ventilation, sedation and analgesia, and close fluid management,7 each of which play a role in the rate of recovery. Although the impact of the surgical approach to S1P has been rigorously compared,3 reports describing the details of these elements of postoperative care are lacking and may impact important outcomes such as survival, length of stay, and occurrence of major complications. Accurately characterizing modifiable intensive care elements over time may enhance learning network–sponsored quality improvement projects.8, 9
In 2017, we formed a collaboration of 5 tertiary care centers, organized through the Pediatric Cardiac Intensive Care Society, to compare discrete elements of intensive care following the S1P. In this work, we sought to detail intensive care elements that were extracted from data warehouses (DW) at each site rather than manually retrieved from the clinical electronic medical record (EMR) interface. Specifically, we set out to describe the spectrum of 3 important aspects of postoperative care for the first 28 postoperative days (POD): mandatory mechanical ventilation rate (MMVR), opioid administration, and fluid balance (FB). In addition to statistical modeling, we present a tool for the visualization and comparison of such intensive data, growth chart–like care curves.
Methods
Study Design and Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. The study was approved by the institutional review board at each participating center. Informed consent was not required. Boston Children's Hospital served as the coordinating center (IRB‐P00023338); Ann & Robert H. Lurie Children's Hospital, Children's Hospital Colorado, The Children's Hospital of Philadelphia, and Texas Children's Hospital participated in this study. Consecutive patients undergoing S1P between January 1, 2009, and December 31, 2018 were included; because of logistical challenges, there was variability in the years for which each center contributed data (Table 1). Patients on preoperative extracorporeal membrane oxygenation (ECMO) support, those undergoing a hybrid procedure, and those with 1 or more of the 3 end point variables missing were excluded.
Table 1.
Demographic, Clinical, and Surgical Details and Outcomes of Included Patients
| Variable | Total (n=502) | Center 1 (n=213) | Center 2 (n=40) | Center 3 (n=98) | Center 4 (n=56) | Center 5 (n=95) | P Value |
|---|---|---|---|---|---|---|---|
| Years included, range | 2011–2018 | 2010–2018 | 2009–2018 | 2009–2016 | 2011–2016 | 2011–2016 | … |
| Age (d), median (IQR) | 5 (3–6) | 4 (3–6) | 6 (5–8) | 4 (3–6) | 4 (3–6) | 5 (3–7) | <0.001 |
| Gestational age (wk), median (IQR) | 39 (38–39) | 38 (37–39) | 39 (38–39) | 39 (38–39) | 39 (38–39) | 39 (38–39) | <0.001 |
| Prematurity*, n (%) | 38 (8) | 18 (8) | 1 (3) | 7 (7) | 3 (5) | 9 (8) | 0.624 |
| Weight (kg), median (IQR) | 3.2 (2.9–3.5) | 3.2 (2.9–3.5) | 3.1 (2.8–3.4) | 3.2 (2.8–3.5) | 3.3 (3.0–3.6) | 3.2 (2.8–3.5) | 0.700 |
| Sex (male), n (%) | 330 (66) | 150 (70) | 26 (65) | 65 (66) | 34 (61) | 55 (58) | 0.256 |
| Cardiac diagnosis, n (%) | |||||||
| HLHS | 442 (88) | 173 (81) | 40 (100) | 98 (100) | 48 (86) | 83 (87) | |
| DILV/DIRV | 19 (4) | 12 (6) | 0 (0) | 0 (0) | 5 (9) | 2 (2) | |
| AV canal, unbalanced | 17 (4) | 13 (6) | 0 (0) | 0 (0) | 3 (5) | 1 (1) | <0.001† |
| DORV, hypo LV | 8 (2) | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 5 (5) | |
| TA | 14 (3) | 10 (5) | 0 (0) | 0 (0) | 0 (0) | 4 (4) | |
| Other | 2 (0) | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Surgical characteristics | |||||||
| Type of shunt, n (%) | |||||||
| Blalock‐Taussig shunt | 173 (34) | 46 (22) | 12 (30) | 27 (28) | 30 (54) | 58 (61) | <0.001 |
| Sano shunt | 329 (66) | 167 (78) | 28 (70) | 71 (72) | 26 (46) | 37 (39) | |
| CPB time, min | 166 (140–206) | 176 (142–217) | 181 (142–198) | 164 (147–210) | 85 (79–93) | 185 (162–212) | <0.001 |
| DHCA time, min | 10 (4–26) | 11 (5–25) | 2 (0–4) | 5 (3–9) | 45 (40–49) | 9 (8–13) | <0.001 |
| Aortic clamp time, min | 78 (60–105) | 94 (72–128) | 58 (52–70) | 67 (61–75) | 45 (40–49) | 98 (87–113) | <0.001 |
| ECMO support | |||||||
| ECMO support within 28 d, n (%) | 50 (10) | 29 (14) | 6 (15) | 7 (7) | 1 (2) | 6 (6) | 0.027 |
| Failure to wean from CPB, n (%) | 23 (5) | 12 (6) | 6 (15) | 1 (1) | 0 (0) | 4 (4) | <0.001† |
| Time from surgery to ECMO (d), median (IQR) | 1 (0–9) | 1 (0–8) | 0 (0–0) | 2 (1–10) | 26 | 0 (0–15) | 0.116 |
| ECMO duration (d), median (IQR) | 5 (3–10) | 6 (2–13) | 5 (3–12) | 3 (2–9) | 4 | 4 (3–8) | 0.699 |
| Mandatory ventilation | |||||||
| Length of mandatory ventilation (d), median (IQR) | 8 (5–16) | 12 (8–21) | 12 (8–21) | 6 (4–13) | 3 (2–4) | 6 (5–12) | <0.001 |
| Minimum ventilation rate per day (breaths/min), median (IQR)‡ | 14 (11–17) | 14 (12–18) | 20 (18–26) | 14 (12–16) | 10 (8–10) | 12 (0–14) | <0.001 |
| Opioid use | |||||||
| Firstline opioid infusion, n (%) | |||||||
| Fentanyl | 334 (66) | 140 (66) | 14 (35) | 32 (33) | 56 (100) | 92 (97) | <0.001 |
| Morphine | 168 (34) | 73 (34) | 26 (65) | 66 (67) | 0 (0) | 3 (3) | |
| Length of opioid administration (d), n (%) | 13 (8–25) | 15 (10–27) | 16 (9–27) | 15 (7–24) | 6 (3–10) | 12 (8–26) | <0.001 |
| Opioid TDD (morphine equivalents, mg/kg per d), median (IQR)‡ | 0.57 (0.16–1.65) | 0.79 (0.26–2.1) | 1.17 (0.48–2.41) | 0.42 (0.13–1.59) | 0.15 (0.06–0.73) | 0.34 (0.16–1.14) | <0.001 |
| Fluid management | |||||||
| Daily intake (mL/kg per d), median (IQR)‡ | 136 (124–152) | 135 (121–147) | 144 (131–159) | 147 (134–157) | 130 (118–151) | 132 (111–156) | <0.001 |
| Daily output (mL/kg per d), median (IQR)‡ | 108 (94–122) | 112 (102–131) | 117 (108–128) | 108 (97–120) | 59 (75–53) | 100 (91–122) | <0.001 |
| Daily fluid balance (mL/kg per d), median (IQR)‡ | 25 (15–39) | 18 (7–26) | 25 (19–34) | 35 (25–41) | 71 (48–86) | 26 (18–39) | <0.001 |
| Patients' outcomes | |||||||
| CICU length of stay (d), median (IQR) | 16 (10–28) | 17 (11–30) | 35 (22–56)§ | 9 (7–16) | 11 (9–16) | 23 (16–31) | <0.001 |
| Hospital length of stay (d), median (IQR) | 34 (23–56) | 35 (24–70) | 35 (22–56) | 30 (20–44) | 22 (17–31) | 51 (35–109) | <0.001 |
| Survival at 30 d, n (%) | 473 (94) | 199 (93) | 36 (90) | 92 (94) | 52 (93) | 94 (99) | 0.225 |
| Survival at discharge, n (%) | 444 (88) | 187 (88) | 34 (85) | 89 (91) | 51 (91) | 83 (87) | 0.813 |
Missing data, n: age, 13; gestational age, 92; CBP, DHCA, aortic clamp times: 1; CICU and hospital length of stay, 4. AV indicates atrioventricular; CICU, cardiac intensive care unit; CPB, cardiopulmonary bypass; DHCA, deep hypothermic cardiac arrest; DILV, double inlet left ventricle; DIRV, double inlet right ventricle; DORV, double outlet right ventricle; ECMO, extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; IQR, interquartile range; LV, left ventricle; TA, tricuspid atresia; and TDD, total daily dose.
Prematurity is defined as <37 weeks estimated gestational age.
Fisher exact test. Bonferroni‐corrected pairwise comparisons are reported in Table S1.
Values are medians (IQR) of median ventilation rate per day per patient.
ICU and hospital length of stay identical at Center 2 because of a center‐specific care model dictating location of care.
Data Extraction
Data collected included demographics (age at surgery, gestational age, weight, and sex) and clinical characteristics (cardiac diagnosis, hypoplastic left heart syndrome versus other anatomies), surgical details (type of shunt [Sano shunt versus modified Blalock‐Taussig shunt], surgical support times), complications (timing and duration of ECMO support), ventilation characteristics (mandatory respiratory rate), opioid administration details (dosages and duration of opioid boluses and continuous infusion), fluid intake and output, and patient‐level outcomes (cardiac intensive care unit and hospital lengths of stay, 30‐day mortality, and survival to hospital discharge). Cardiac diagnosis and surgical details were collected from a surgical database at each center. Demographic data, length of stays, ventilation, and opioid and fluid data were collected from a DW of the EMR as specified below.
To facilitate uniform data collection among centers, we established a common data dictionary as well as structured query language data extraction coding among centers for use within each institution's DW infrastructure. Each center was responsible for understanding their local data infrastructure and altering code as required. Data were queried using SAS version 9.4 (Cary, NC) or Python version 3.7 (Fredericksburg, VA). Following extraction, an investigator at each center manually confirmed a subset of the data (10%) against the source EMR. As has been accomplished by others, we obtained 100% concordance with the EMR after correcting errors in categorization and timing that are part of DW generation.10 Data were then transferred to the coordinating center for transformation into the following variables for each postoperative day 0 through 28.
To represent the degree of mechanical ventilation support per day, we computed the minimum MMVR as the lowest intermittent mandatory ventilator rate between 7 am of the postoperative day and 7 am of the following day. A daily MMVR of 0 may represent a pressure support trial or an extubated patient, whereas an MMVR of 18 would represent a patient who remained fully ventilated for the entire day. We empirically chose this variable as one that is commonly weaned in pediatric patients11 and which in our opinion was a single data element to reflect the degree of respiratory support a patient was receiving over time. To represent opioid use, we computed the total daily dose (TDD) of opioids in morphine equivalents as follows: morphine IV 1 mg=morphine PO 3 mg=fentanyl IV 0.01 mg=methadone IV/PO 1 mg=hydromorphone 0.15 mg=oxycodone 1 mg=hydrocodone 1 mg.12 For each day, the accumulated infusion and intermittent doses of each drug were transformed into morphine equivalents, normalized to dosing weight, and summed for each 24‐hour period. To represent fluid intake and FB, all fluid intake and output entries were summed and normalized to body weight and used to compute a daily FB. A subset (10%) of these transformations was confirmed to reflect the untransformed raw data from each institution at the coordinating center.
Statistical Analysis
Descriptive data are reported as absolute frequencies and percentages for categorical variables, and as mean and SD or median and interquartile range (IQR) for continuous variables, as appropriate. Distributions of continuous variables were tested for normality using the Kolmogorov‐Smirnov test.
Three distinct statistical approaches were used to compare the elements of care among centers. First, intensive care data, as well as demographic, clinical, and surgical details, were compared among centers using a univariate approach. The Pearson χ2 test was used to test categorical data, and the Fisher exact test was used when expected counts were <5. Because of the nonnormal distribution of the continuous variables in the subgroups, a Kruskal‐Wallis test (1‐way ANOVA on ranks) was preferred over ANOVA to compare continuous data among groups. The Bonferroni‐Dunn correction was used to calculate the adjusted P value for pairwise comparisons among centers, in order to specify between‐center differences.
Secondly, we used a Kaplan‐Meier analysis and log‐rank test with overall and pairwise comparison (Bonferroni correction) to compare times to freedom‐from‐MMVR and time to freedom‐from‐opioids among centers. Cox proportional hazard modeling, a semiparametric statistic using time‐related data, was used to estimate a crude and adjusted hazard ratio (HR) for each event.13 Proportional hazard assumptions were checked by comparing the log‐log curve versus log‐time. Variables included in the multivariable model were age at surgery, sex, prematurity, diagnosis (hypoplastic left heart syndrome versus other anatomies), shunt type, surgical times (cardiopulmonary bypass time, aortic clamp time, deep hypothermic circulatory arrest time), and ECMO use in the first 28 PODs. A backward conditional strategy was used for entry and retention of variables in the multivariable model. A candidate variable was retained in the model if the P value was <0.05. Age and weight were tested for correlation using Spearman ρ test according to their nonparametric distribution. Since a significant correlation was proven, only age was included in the model. Results were expressed in terms of HRs and 95% CIs.
Finally, we modeled our 3 main end points over time using generalized mixed‐effects linear models,14 allowing analysis for associations between predictors and changes of continuous outcomes repeatedly measured over time (Data S1).
Development of Care Curves
Daily MMVR, opioid TDD, and daily FB were plotted over time onto growth chart–like curves that display median and percentiles (5, 25, 75, and 95) of each measure over time. A comprehensive care curve for each variable was created to represent the entire cohort.
Results
Study Population
Of the 583 patients undergoing S1P during the study period, 81 were excluded for missing element‐of‐care data such that 502 patients were included. The median age at surgery was 5 days (IQR, 3–6) and 8% of patients were born prematurely. The median weight at surgery was 3.2 (IQR, 2.9–3.5) kg. The vast majority had hypoplastic left heart syndrome as baseline cardiac anatomy (88%), while 12% had other univentricular physiologies (P<0.001 among centers, Table 1; pairwise comparisons between centers by ANOVA‐rank analysis in Table S1). Source of pulmonary blood flow was a Sano shunt in 66% of patients. Ischemic times differed significantly among centers. Specifically, Center 4 exhibited a median cardiopulmonary bypass time of 85 (79–93) minutes, nearly 50% shorter than all other centers, though deep hypothermic circulatory arrest time at this center was significantly longer (Table 1). In the first 28 PODs, 10% of patients required ECMO support (P=0.027 among centers). Overall, the median cardiac intensive care unit length of stay was 16 (IQR, 10–28) days and hospital length of stay was 34 (IQR, 23–56) days (P<0.001 among centers). Thirty‐day survival was 94% (P=0.225 among centers), and survival to hospital discharge was 88% (P=0.813 among centers).
Mandatory Ventilation
All patients underwent mechanical ventilation in the postoperative period. Of them, 463 (92%) achieved a freedom‐from‐MMVR by POD 28 in a median time of 8 (IQR, 5–13) days, which differed significantly among centers (P<0.001, Figure 1A, Table S2). In the Cox regression analysis, center affiliation was an independent predictor of time to freedom from MMVR (Table 2). Specifically, when the analysis was adjusted for baseline demographic, clinical, and surgical characteristics, Centers 3, 4, and 5 were associated with a significantly shorter time to freedom from MMVR compared with Center 1 (HRCenter 3 2.10 [95% CI, 1.62–2.71], HRCenter 4 5.08 [3.48–7.41], and HRCenter 5 1.71 [1.31–2.23]). As visualized in Figure S1, MMVR decreases over time in all centers, but center affiliation was independently associated with the trajectory of the mean MMVR over time (Table 3). Modeled trajectories of ventilation rate over time for each center adjusted for baseline patients' characteristics (age, anatomy, shunt type, surgical times, and postoperative ECMO support) are shown in Figure S2A; estimating equations are shown in Data S1.
Figure 1. Kaplan‐Meier curves for the outcomes freedom‐from‐MMVR and freedom‐from‐opioid‐administration by center.
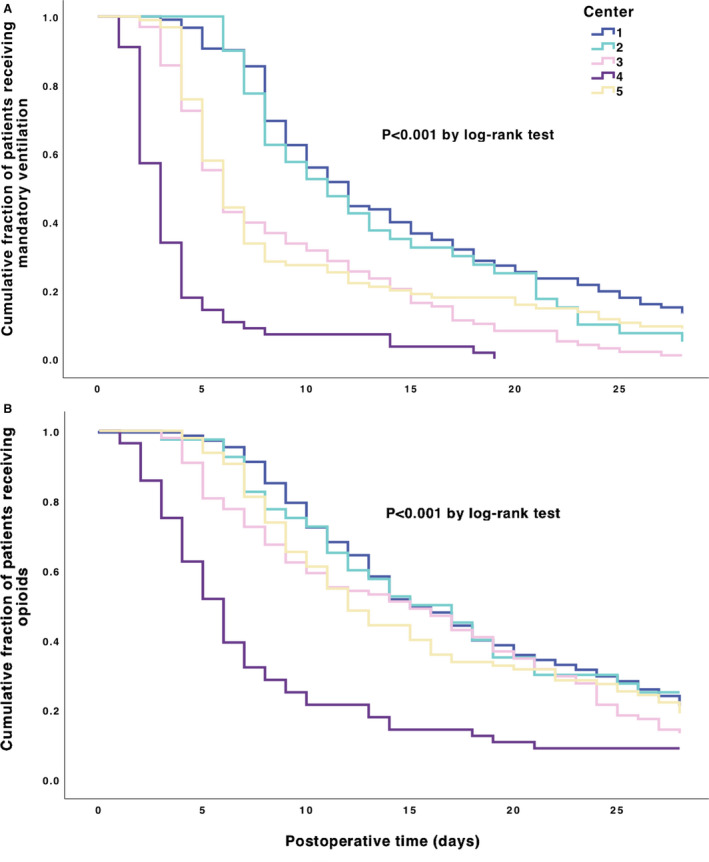
A, Mandatory ventilation: Among 502 patients undergoing Norwood palliation, 463 (92%) achieved a freedom‐from‐mandatory‐ventilation within the first 28 postoperative days, in a median time of 8 days (IQR, 5–13). Overall comparison of distribution by log‐rank test showed a significant difference among centers (P<0.001). Pairwise comparisons by log‐rank test using Bonferroni correction are shown in Table S2 and showed that Center 1 differs significantly from Centers 3, 4, and 5, and Center 4 differs significantly from all other centers (all P<0.001). B, Opioid administration: 411 patients (82%) achieved a freedom‐from‐opioid‐administration within the first 28 postoperative days, in a median time of 11 days (IQR, 7–18). Overall comparison of distribution by log‐rank test showed a significant difference among centers (P<0.001). Pairwise comparisons by log‐rank test using Bonferroni correction are shown in Table S2 and showed that Center 4 differs significantly from all other centers (all P<0.001). IQR indicates interquartile range; and MMVR, mandatory mechanical ventilator rate.
Table 2.
Univariate and Multivariable Cox Regression Analyses for Testing Center Affiliation as Predictor of the Events Freedom‐From‐MMVR and Freedom‐From‐Opioid‐Administration
| End Point and Main Predictor | N Events/N Patients (%) | Hazard Risk, Unadjusted | P Value | Hazard Risk Adjusted for Baseline Factors* | P Value |
|---|---|---|---|---|---|
| Freedom from mandatory ventilator rate | |||||
| Center affiliation | |||||
| Center 1 | 185/203 (87) | Reference | … | Reference | … |
| Center 2 | 38/40 (95) | 1.20 (0.85–1.70) | 0.302 | 0.99 (0.64–1.54) | 0.984 |
| Center 3 | 97/98 (99) | 2.11 (1.65–2.71) | <0.001 | 2.10 (1.62–2.71) | <0.001 |
| Center 4 | 56/56 (100) | 6.93 (5.07–9.46) | <0.001 | 5.08 (3.48–7.41) | <0.001 |
| Center 5 | 87/95 (92) | 1.74 (1.35–2.25) | <0.001 | 1.71 (1.31–2.23) | <0.001 |
| Freedom from opioid administration | |||||
| Center affiliation | |||||
| Center 1 | 168/203 (83) | Reference | Reference | ||
| Center 2 | 30/40 (75) | 0.98 (0.66–1.44) | 0.918 | 0.72 (0.43–1.21) | 0.214 |
| Center 3 | 85/98 (87) | 1.28 (0.99–1.66) | 0.063 | 1.24 (0.94–1.63) | 0.130 |
| Center 4 | 51/56 (91) | 3.00 (2.19–4.11) | <0.001 | 2.92 (2.11–4.04) | <0.001 |
| Center 5 | 77/95 (81) | 1.18 (0.90–1.54) | 0.238 | 1.22 (0.93–1.61) | 0.153 |
Center affiliation was found to be an independent predictor of outcomes. Particularly, being affiliated to Center 3, 4, and 5 predicts a significantly shorter time to freedom‐from‐mandatory‐ventilation compared with Center 1. Being affiliated to Center 4 predicts a significantly shorter time to freedom‐from‐opioid‐administration compared with Center 1. CPB indicates cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; ECMO, extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; MMVR, mandatory mechanical ventilator rate; and POD, postoperative day.
Models are adjusted for age, sex, prematurity, diagnosis (HLHS/not HLHS), shunt type, surgical times (CPB time, DHCA time, aortic clamp time), and ECMO support in the 28 PODs (yes/no).
Table 3.
Intergroup Differences in Outcomes Based on Generalized Multivariable Mixed Linear Model
| End Point and Main Predictors | Estimated Change (ln) (95% CI) | P Value | Adjusted Estimated Change (ln) (95% CI) | P Value | |
|---|---|---|---|---|---|
| Ventilation rate, breaths/min | |||||
| Intercept (Center 1) | β0 | 3.04 (3.02–3.06) | REF | 3.09 (3.04–3.16) | REF |
| Center 2 | β1 | 0.36 (0.32–0.40) | <0.001 | 0.31 (0.26–0.37) | <0.001 |
| Center 3 | β2 | 0.16 (0.12–0.20) | <0.001 | 0.11 (0.06–0.15) | <0.001 |
| Center 4 | β3 | −0.27 (−0.30 to −0.13) | <0.001 | −0.35 (−0.44 to −0.25) | <0.001 |
| Center 5 | β4 | 0.07 (0.03–0.12) | 0.001 | 0.07 (0.03–0.12) | 0.002 |
| Time (Center 1 per POD increment) | β5 | −0.09 (−0.09 to ,−0.09) | <0.001 | −0.09 (−0.09 to −0.09) | <0.001 |
| Center 2×POD | β6 | −0.01 (−0.02 to −0.01) | 0.001 | −0.004 (−0.003 to −0.01) | 0.233 |
| Center 3×POD | β7 | −0.11 (−0.12 to −0.09) | <0.001 | −0.11 (−0.12 to −0.09) | <0.001 |
| Center 4×POD | β8 | −0.40 (−0.48 to −0.32) | <0.001 | −0.40 (−0.48 to −0.32) | <0.001 |
| Center 5×POD | β9 | −0.10 (−0.12 to −0.09) | <0.001 | −0.10 (−0.12 to −0.09) | <0.001 |
| Opioid total daily dose, mg/kg per d | |||||
| Intercept (Center 1) | β0 | 1.96 (1.91–2.00) | REF | 0.82 (0.70–0.94) | REF |
| Center 2 | β1 | −0.88 (−1.09 to −0.66) | <0.001 | −1.19 (−1.57 to −0.80) | <0.001 |
| Center 3 | β2 | −0.28 (−0.39 to −0.18) | <0.001 | −0.12 (−0.02 to −0.25) | <0.001 |
| Center 4 | β3 | −2.02 (−2.52 to −1.52) | <0.001 | −1.35 (−1.88 to −0.82) | <0.001 |
| Center 5 | β4 | −1.12 (−1.29 to −0.95) | <0.001 | −0.60 (−0.76 to −0.43) | <0.001 |
| Time (Center 1 per POD increment) | β5 | −0.15 (−0.16 to −0.14) | <0.001 | −0.09 (−0.09 to −0.08) | <0.001 |
| Center 2×POD | β6 | 0.05 (0.03–0.07) | <0.001 | 0.003 (−0.03 to 0.03) | 0.837 |
| Center 3×POD | β7 | −0.02 (−0.04 to −0.01) | 0.009 | −0.06 (−0.08 to −0.05) | <0.001 |
| Center 4×POD | β8 | 0.07 (0.03–0.11) | <0.001 | 0.01 (−0.03 to 0.05) | 0.509 |
| Center 5×POD | β9 | 0.07 (0.05–0.08) | <0.001 | 0.03 (0.02–0.043) | <0.001 |
| End Point and Main Predictors | Estimated Change (95% CI) | P Value | Adjusted Estimated Change (95% CI) | P Value | |
|---|---|---|---|---|---|
| Daily fluid balance | |||||
| Intercept (Center 1) | β0 | −8.66 (−11.21 to −6.10) | REF | −4.68 (−9.17 to −0.19) | REF |
| Center 2 | β1 | 20.62 (14.20–27.05) | <0.001 | 20.40 (13.81–26.99) | <0.001 |
| Center 3 | β2 | 21.40 (16.84–,25.94) | <0.001 | 20.99 (16.26–25.72) | <0.001 |
| Center 4 | β3 | 70.57 (64.97–76.16) | <0.001 | 67.16 (61.02–73.3) | <0.001 |
| Center 5 | β4 | 15.86 (11.26–20.46) | <0.001 | 16.01 (11.28–20.45) | <0.001 |
| Time (Center 1 per POD increment) | β5 | 1.27 (1.14–1.41) | <0.001 | 1.26 (1.13–1.40) | <0.001 |
| Center 2×POD | β6 | −0.68 (−1.02 to −0.34) | <0.001 | −0.68 (−1.01 to −0.33) | <0.001 |
| Center 3×POD | β7 | −0.34 (−0.58 to −0.10) | 0.005 | −0.34 (−0.58 to −0.09) | 0.006 |
| Center 4×POD | β8 | −2.63 (−2.93 to −2.34) | <0.001 | −2.63 (−2.93 to −2.33) | <0.001 |
| Center 5×POD | β9 | −0.12 (−0.36 to −0.13) | 0.335 | −0.11 (−0.36 to −0.13) | 0.367 |
Modeled mean values for POD 0 (ie, y intercept) for Center 1 (the reference center) can be calculated using β0 values for each end point; note that ventilation rate and opioid TDD are presented as the natural log (ln) of the intercept since these models are fitted to a logarithmic curve over time (the modeled mean ventilator rate for center 1 on POD 0=e3.04=20.9 breaths/min). The differences between Center 1 and Centers 2 to 5 are described as β1–4, each with the corresponding P value; the modeled ventilator rate on POD 0 for Center 2=e(3.04+0.36)=29.9. Changes of each end point over time (ie, slope of the modeled line) are computed as β5–9; mean ventilator rate in Center 1 on POD 10=e(3.04−[0.09×10])=8.5 breaths/min. The values are adjusted for age, diagnosis (HLHS/not HLHS), shunt type, surgical times (CBP time, DHCA time, aortic clamp time), and postoperative ECMO support are found in the rightward column. References categories for dichotomous variables not shown in the table are: HLHS, Sano, no ECMO. N observations included in the models: mandatory ventilation N=14 152 (97%), opioid administration N=14 117 (97%), fluid balance N=14 152 (97%). CPB indicates cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; ECMO, extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; POD, postoperative day; and TDD, total daily dose.
Opioid Administration
The most frequent first‐line opioid infusion was fentanyl (66%), while morphine was used in the remaining 34%; this finding differed by center (P<0.001). Overall, 411 patients (82%) achieved freedom‐from‐opioid administration by POD 28 in a median time of 11 (IQR, 7–18) days. The peak dosage of opioids took place on POD 2 (3.7 [IQR, 1.5–7.5] mg morphine equivalents/kg per day). Time to freedom‐from‐opioids significantly differed among centers (P<0.001 by log‐rank test, Figure 1B). After adjusting for baseline and surgical variables, Center 4 was associated with a shorter time to freedom‐from‐opioids compared with Center 1 (hazard ratio=HRCenter 4 2.92 [2.11–4.04], Table 2). When trajectories of opioid TDD per kg over time were modeled adjusting for patient's characteristics, we found that the mean opioid TDD significantly decreased over time in all centers, but followed significantly different trajectories among centers (Figure S2B, and all opioid care curves in Figure S3). Centers 1 and 3 decreased TDD of opioids significantly more rapidly than Centers 2, 4, and 5, although these centers also utilized the highest TDDs in the early postoperative period (Table 3).
Fluid Balance
The median daily fluid intake was 136 (IQR, 124–152) mL/kg per day, and median daily output was 108 (IQR, 94–122) mL/kg per day. Median daily FB differed significantly among centers: Center 1 had a median daily FB of 18 (IQR, 7–26) mL/kg per day on POD 0 to 28, Center 4 had a median daily FB of 71 (48–86), while the group median was +25 (IQR, 15–39) mL/kg per day (P<0.001). When data were fitted to a generalized mixed linear model, center affiliation was an independent predictor of the mean daily FB and its changes over time. Moreover, for every POD increase, each center has a significantly less negative FB compared with Center 1 (Table 3). Estimated trajectories of the mean daily FB per kg over time for each center adjusted for baseline patients' characteristics are shown in Figure S2C, and all FB care curves are shown in Figure S4.
Era Effect
There were no significant era‐related differences in survival rates or in lengths of stay (Table S3). In the more contemporary era, more patients received a Sano shunt rather than BT shunt, total cardiopulmonary bypass time was longer, and aortic cross‐clamp time was shorter. Although there were minor statistically significant differences in some of the modifiable end points we collected (eg, duration of opioid administration), the majority of these end points have not changed significantly over time.
Development of Care Curves
Care curves for daily ventilator rate, opioid TDD, and daily FB in the first 28 PODs are shown in Figure 2 and allow for the observation of important differences among groups.
Figure 2. Exemplary care curve plots.
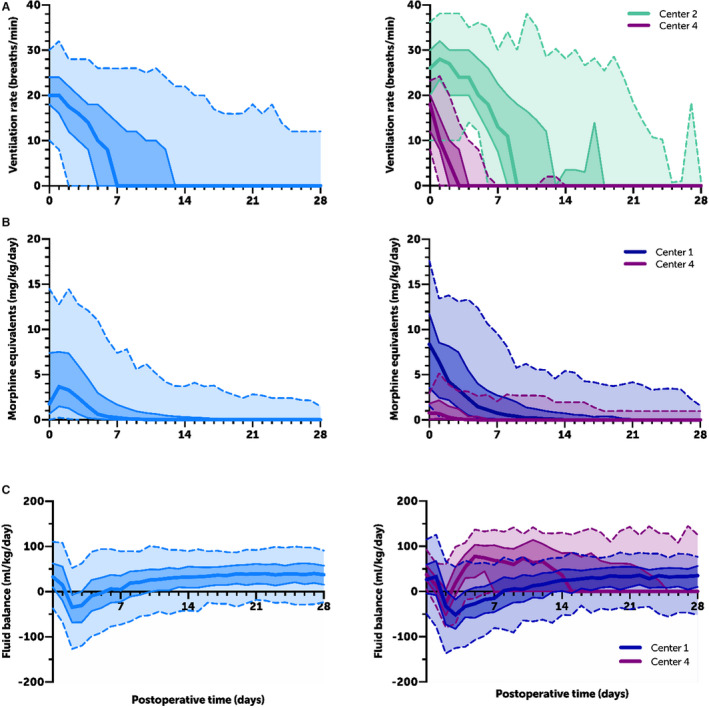
Care curves for (A) postoperative daily ventilation rate, (B) opioid total daily dose, and (C) daily fluid balance for all patients in the cohort (left) and at the 2 centers that differ from each other the most (right). Dark center line=median; dark shaded and solid middle lines=interquartile range; light shaded and dotted outer lines=5% and 95%.
Discussion
We have shown that it is possible to extract, transform, and share data describing daily intensive care elements from DWs housed at different institutions. We demonstrate the depiction of time series data using care curves that are similar in concept to growth charts, enabling contextualization of individual patients in a specific area of care; identifying institutional, disease‐specific practice patterns; and creating targeted quality improvement opportunities. The mortality rate in this cohort was similar to contemporary series following S1P and did not differ among centers. As centers focus on improving morbidity metrics in this setting, it is important to consider that the modifiable elements of intensive care that we characterize here impact typical meaningful outcomes, such as lengths of stay. To be sure, cardiac anatomy, ischemic times, and residual defects play dominant roles not only in mortality but also morbidity end points. However, the factors controlled in the intensive care unit—ventilation, sedation, and fluid management among them—likely play an independent role in determining how a patient responds to their given anatomy, surgical care, and potential residual lesions. Studying and improving these modifiable behaviors may inform and direct intra‐ and interinstitutional quality improvement efforts, reduce hospital‐acquired morbidity, reduce length of stay, optimize outcomes, lower cost per patient, and increase value.
The clinical utility of this effort takes several forms. On a patient level, a care curve is useful in the same way that a specialized growth chart is for an individual patient. For example, specialized growth charts for patients with cystic fibrosis are useful to track a patient's growth over time and to identify deviations in trajectory that might prompt further evaluation. In the same way, a patient who “falls off” of a mechanical ventilation care curve might be evaluated for a paralyzed diaphragm, a residual anatomic lesion, or an infection, for example. Here we note the importance of matching patients based on diagnosis and procedure, as well as potentially other variables known to affect ventilator performance (eg, gestational age or presence of restrictive atrial septum). In future work, this may best be done using an interactive electronic tool rather than a static care curve rendering.
On an institution level, a care curve is useful to identify patterns and variance among centers. In this work, we noted many differences between centers that may represent clinically actionable findings, highlighted as follows.
Mechanical Ventilation
Center 4, where the S1P was performed with median cardiopulmonary bypass time of 85 minutes (primarily under deep hypothermic circulatory arrest), experienced dramatically less intense ventilation and less opioid use than the other centers, highlighting the dominant impact that operative factors have on the postoperative course. Still, important differences remain between the centers whose ischemic times were similar. We quantified these differences using mixed modeling, and these differences can also be visually appreciated by comparing care curves. For example, at Center 5, 50% of patients were weaned to a ventilator rate of 10 (and thus doing the majority of their own work of breathing) by POD 4, a milestone that was not reached until POD 7 to 8 at Centers 1 and 2. The median time between reaching a rate of 10 and a rate of 0 (pressure support ventilation) also differed among centers, and was as high as 4 days at Center 1 and as low as 1 day at Center 2. These findings may lead to the following quality improvement efforts. First, this variance may diminish simply by quantifying mechanical ventilation care in this way. Instead of only showing a target date of extubation, displaying a target weaning trajectory may be much more actionable. Second, our findings highlight the benefits of comparing the details of our care that may explain such variance, such as who weans the ventilator and according to what protocol. Care curves provide an infrastructure for comparison of the details between centers by quantifying variance.
Opioid Use
There were also major differences in the doses of opioid used. For example, the median narcotic dose on POD 2 at Center 2 was 1.6 mg/kg per day morphine equivalents and at Center 3 was 6.9 mg/kg per day. The rate of opioid weaning following the peak (most often on POD 2) also varied from a daily wean of 1.4 mg/kg per day (Center 3) to 0.5 mg/kg per day (Center 5). These findings may enable quality improvement by (1) raising awareness among centers, (2) creating real‐time benchmarking for use at the bedside, and (3) informing updates to sedation protocols, including the choice of initial opioid and how medications are titrated at each center.
Fluid Balance
With respect to FB, Center 1 had a median FB of −51 mL/kg per day on POD 3, a time when median FB ranged between −34 and 19 mL/kg per day at the remaining 4 centers. The quality improvement action items including a detailed examination of each center's practices surrounding diuretic administration and nutrition, as well as the effects of these practices on renal function, would be important next steps.
We believe that these observations will add dimension to nationwide efforts to improve the management of patients following S1P. Several highly successful such initiatives already exist, including the Pediatric Cardiac Critical Care Consortium with a focus on postoperative cardiac care, and the National Pediatric Cardiology QI Collaborative with a focus on interstage morbidity and mortality following S1P.15, 16, 17
In this effort, we identified several important lessons for the use of automatically extracted data. The first is the absolute importance of data integrity. While typographical errors are minimized in automated data extraction, it is vital to identify and correct systematic errors in data collection and transformation, a process that requires a nuanced understanding of each institution's database infrastructure (ie, what is stored where, and how are entries recorded). For example, in several instances we encountered errant chest tube output values because the fluid level of the chest drainage system (eg, Pleur‐evac) was recorded in place of the fluid out per hour, requiring an additional data transformation step. Each of the participating institutions benefited from the expertise of a dedicated team who extracted and validated the primary data against the clinical EMR interface. Eventually, it would be ideal for such raw data elements to be transformed into a standard structure and maintained alongside current quality end points.18 The second was the establishment of a centralized data coordinating center, which performed the data transformations and statistical analyses. This provided a second, external layer of quality checking that we found valuable. Finally, it is vital to create visualization tools optimized for granular data. Here, we plotted data in growth chart–like care curves that illustrate the median, variability, and trends over time of each variable. It is possible that the use of such (likely institution‐specific) curves would be useful to identify outlier patients or to identify otherwise inconspicuous practice patterns. Future efforts should incorporate multiple related data elements into single parameters to further enhance data depth, such as the incorporation of all ventilatory parameters into a single numerical score.19
We note several limitations to our work. First, there were many variables that contribute to outcomes that we did not quantify, because our purpose was not to create a comprehensive model of outcome but rather to describe differences in modifiable elements of care. The most important of these was the presence of major anatomic risk factors (eg, severe ventricular dysfunction, severe atrioventricular valve regurgitation, or ventriculo‐coronary fistula) and technical performance scores.20 Collecting these data, along with other ventilator parameters and noninvasive ventilatory support, other classes of medications (eg, sedatives, diuretics, inotropes), and blood product use may allow for more comprehensive modeling of patient outcomes. Eventually, this may permit statistical weighting of each of the contributions that each of these factors make on outcomes. Second, there were several potential sources of bias in our study. Our observations span a 10‐year period of time (with some intercenter variability) during which practice may have varied, including the implementation of new clinical guidelines for intensive care unit management.21 We also excluded 81 patients for whom data capture in the DW was incomplete. Finally, not all centers reported data for nonhypoplastic left heart syndrome S1P procedures.
Conclusions
It is possible to create “care curves” and compare individual elements of care over time that highlight important modifiable differences in intensive care. MMVR, opioid TDD, and FB following S1P differ in clinically and statistically significant ways among centers even after adjusting for patient and operative factors. In the context of already excellent clinical outcomes, these differences suggest that care can be further optimized to improve alternative end points, such as length of stay, cost, and comfort.
Sources of Funding
This work was funded by a multicenter grant from the Gerber Foundation (Kheir).
Disclosures
None.
Supporting information
Data S1
Tables S1–S3
Figures S1–S4
Acknowledgments
The authors thank the following for assistance with data extraction and transformation: Sarah van den Bosch, Manasee Godsay, and Julie Ferullo, Boston Children's Hospital; Marisa Payan, Children's Hospital Colorado; Kathleen Van't Hof, Ann & Robert H. Lurie Children's Hospital of Chicago; and Rakesh Kriplani and Warren Boudreau, Texas Children's Hospital.
(J Am Heart Assoc. 2021;10:e019396. DOI: 10.1161/JAHA.120.019396.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019396
For Sources of Funding and Disclosures, see page 11.
References
- 1.Wasfy JH, Borden WB, Secemsky EA, McCabe JM, Yeh RW. Public reporting in cardiovascular medicine: accountability, unintended consequences, and promise for improvement. Circulation. 2015;131:1518–1527. DOI: 10.1161/CIRCULATIONAHA.114.014118. [DOI] [PubMed] [Google Scholar]
- 2.Jha AK. Public reporting of surgical outcomes: surgeons, hospitals, or both? JAMA. 2017;318:1429–1430. DOI: 10.1001/jama.2017.13815. [DOI] [PubMed] [Google Scholar]
- 3.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, et al. Comparison of shunt types in the Norwood procedure for single‐ventricle lesions. N Engl J Med. 2010;362:1980–1992. DOI: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan M, Sleeper LA, Ohye RG, Frommelt PC, Caldarone CA, Tweddell JS, Lu M, Pearson GD, Gaynor JW, Pizarro C, et al. Technical performance score is associated with outcomes after the Norwood procedure. J Thorac Cardiovasc Surg. 2014;148:2208–2214.e6. DOI: 10.1016/j.jtcvs.2014.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newburger JW, Sleeper LA, Gaynor JW, Hollenbeck‐Pringle D, Frommelt PC, Li JS, Mahle WT, Williams IA, Atz AM, Burns KM, et al. Transplant‐free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137:2246–2253. DOI: 10.1161/CIRCULATIONAHA.117.029375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia‐hypoplastic left heart syndrome. N Engl J Med. 1983;308:23–26. DOI: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 7.Hornik CP, He X, Jacobs JP, Li JS, Jaquiss RDB, Jacobs ML, O’Brien SM, Peterson ED, Pasquali SK. Complications after the Norwood operation: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2011;92:1734–1740. DOI: 10.1016/j.athoracsur.2011.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lannon CM, Peterson LE. Pediatric collaborative networks for quality improvement and research. Acad Pediatr. 2013;13:S69–S74. DOI: 10.1016/j.acap.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JB, Iyer SB, Beekman RH, Jenkins KJ, Klitzner TS, Kugler JD, Martin GR, Neish SR, Rosenthal GL, Lannon CM. National pediatric cardiology quality improvement collaborative: lessons from development and early years. Prog Pediatr Cardiol. 2011;32:103–109. DOI: 10.1016/j.ppedcard.2011.10.008. [DOI] [Google Scholar]
- 10.Denney MJ, Long DM, Armistead MG, Anderson JL, Conway BN. Validating the extract, transform, load process used to populate a large clinical research database. Int J Med Inform. 2016;94:271–274. DOI: 10.1016/j.ijmedinf.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newth CJL, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, Pollack M, Zimmerman J, Anand KJS, Carcillo JA, et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. 2009;10:1–11. DOI: 10.1097/PCC.0b013e318193724d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudchadkar SR, Easley RB, Brady KM, Yaster M. Pain and sedation management. In: Nichols DG, Shaffner DH, eds. Rogers’ Textbook of Pediatric Intensive Care. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 13.Cox D. Regression models and life‐tables. J R Stat Soc B. 1972;34:187–220. DOI: 10.1111/j.2517-6161.1972.tb00899.x. [DOI] [Google Scholar]
- 14.Laird N, Ware J. Random‐effects models for longitudinal data. Biometrics. 1982;38:963–974. DOI: 10.2307/2529876. [DOI] [PubMed] [Google Scholar]
- 15.Gaies M, Pasquali SK, Banerjee M, Dimick JB, Birkmeyer JD, Zhang W, Alten JA, Chanani N, Cooper DS, Costello JM, et al. Improvement in pediatric cardiac surgical outcomes through interhospital collaboration. J Am Coll Cardiol. 2019;74:2786–2795. DOI: 10.1016/j.jacc.2019.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaies M, Tabbutt S, Schwartz SM, Bird GL, Alten JA, Shekerdemian LS, Klugman D, Thiagarajan RR, Gaynor JW, Jacobs JP, et al. Clinical epidemiology of extubation failure in the pediatric cardiac ICU: a report from the pediatric cardiac critical care consortium. Pediatr Crit Care Med. 2015;16:837–845. DOI: 10.1097/PCC.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schidlow DN, Gauvreau K, Patel M, Uzark K, Brown DW. Site of interstage care, resource utilization, and interstage mortality: a report from the NPC‐QIC registry. Pediatr Cardiol. 2015;36:126–131. DOI: 10.1007/s00246-014-0974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandel JC, Kreda DA, Mandl KD, Kohane IS, Ramoni RB. SMART on FHIR: a standards‐based, interoperable apps platform for electronic health records. J Am Med Inform Assoc. 2016;23:899–908. DOI: 10.1093/jamia/ocv189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha AM, van den Bosch SJ, Pozerski K, Zhou L, Kheir JN. Development of a respiratory support score as a visualization tool in intensive care. Respir Care. 2020;65:1268–1275. DOI: 10.4187/respcare.07341. [DOI] [PubMed] [Google Scholar]
- 20.Nathan M, Gauvreau K, Samnaliev M, Ozonoff AL, Jenkins K, Bergersen L, Connor J, Pigula FA, Colan SD, Mayer JE, et al. Technical performance score predicts resource utilization in congenital cardiac procedures. J Am Coll Cardiol. 2016;67:2696–2698. DOI: 10.1016/j.jacc.2016.03.545. [DOI] [PubMed] [Google Scholar]
- 21.Mills KI, Kaza AK, Walsh BK, Bond HC, Ford M, Wypij D, Thiagarajan RR, Almodovar MC, Quinonez LG, Baird CW, et al. Phosphodiesterase inhibitor‐based vasodilation improves oxygen delivery and clinical outcomes following stage 1 palliation. J Am Heart Assoc. 2016;5:e003554. DOI: 10.1161/JAHA.116.003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1–S4


