Abstract
Background
Although HIV is associated with increased risk of heart failure (HF), it is not known if people living with HIV develop HF at a younger age compared with individuals without HIV. Crude comparisons of age at diagnosis of HF between individuals with and without HIV does not account for differences in underlying age structures between the populations.
Methods and Results
We used Veterans Health Administration data to compare the age at HF diagnosis between veterans with and without HIV, with adjustment for difference in population age structure. Statistical weights, calculated for each 1‐year strata of veterans with HIV in each calendar year from 2000 to 2018, were applied to the veterans without HIV to standardize the age structure. We identified 5093 veterans with HIV (98% men, 34% White) with first HF episode recorded after HIV diagnosis (median age at incidence of HF, 58 years), and 1 425 987 veterans without HIV (98% men, 78% White) with HF (corresponding age, 72 years), with an absolute difference of 14 years. After accounting for difference in age structure, the adjusted median age at HF diagnosis for veterans without HIV was 63 years, 5 years difference with veterans with HIV (P<0.001). The age differences were consistent across important subgroups such as preserved versus reduced ejection fraction and inpatient versus outpatient index HF.
Conclusions
Veterans with HIV are diagnosed with HF at a significantly younger age compared with veterans without HIV. These findings may have implications for HF prevention in individuals with HIV. Future studies are needed to make the findings more generalizable.
Keywords: cardiovascular disease, heart failure, HIV, incidence, veterans
Subject Categories: Aging, Cardiovascular Disease, Epidemiology, Risk Factors, Heart Failure
Although a number of epidemiological studies have reported that people living with HIV experience premature frailty, early aging of the immune system, and earlier occurrence of certain non–AIDS‐related cancers and atherosclerotic cardiovascular disease,1, 2, 3 others have not replicated these findings.4 On the other hand, despite data showing an increased risk of heart failure (HF) among people living with HIV (PLHIV),5 it is still not known if HF occurs at a younger age in this population compared with individuals without HIV, an important factor to consider for planning HF prevention and detection strategies in PLHIV.5, 6 Studies making direct comparisons of age at diagnosis of HF, and its subtypes, between individuals with and without HIV are lacking. Crude comparison of age at diagnosis of HF is not sufficient to determine if HF occurs at a younger age in individuals with HIV compared with individuals without HIV, because such analyses do not account for differences in the underlying age structures between the 2 populations.3 To address this issue, we used Veterans Health Administration (VHA) data to compare the age at diagnosis of HF, and its subtypes, between veterans with and without HIV, with adjustment for difference in population age structure.
Methods
Data Availability
Data availability is based on restrictions in the data‐use agreements used in this study. The authors are unable to make a data set available. Methodology questions may be directed to the corresponding author.
Data Source and Study Design
This is a retrospective analysis of VHA data on veterans with HF identified through clinical records maintained in the Department of Veterans Affairs (VA) Corporate Data Warehouse and the VA administrative claims data. The study population was veterans who received care in VHA facilities or community hospitals and clinics paid for by the VHA (fee basis), with a record of first diagnosis of HF between 2000 and 2018. Veterans with a first diagnosis of HF were identified through International Classification of Diseases, Ninth Revision (ICD‐9) and International Classification of Diseases , Tenth Revision (ICD‐10) codes. Veterans with HIV were similarly identified on the basis of ICD‐9 (042, V08) or ICD‐10 (B20, Z21) codes. Veterans with HIV with a first episode of HF recorded before the recorded date of HIV ICD code (n=611) were excluded. The study protocol was approved by the Providence VA Medical Center Institutional Review Board. Informed consent requirement was waived.
Variables
Data on relevant covariates were obtained from the VHA electronic medical records system. Smoking was dichotomized as current or never/former smoker. For brain natriuretic peptide (BNP), we took the value that was recorded closest to the date of the index HF (ie, the date of the first diagnosis of HF). We took left ventricular ejection fraction (LVEF) values that were obtained from the patients' electronic medical records notes with an automated information extraction application using natural language processing algorithms validated for the VHA; LVEF was mainly assessed using an echocardiogram.7 We selected the LVEF value that was recorded closest to the first HF diagnosis, between 1 year before and 1 year after the index date. LVEF was used to determine HF subtypes, operationally defined as preserved, LVEF ≥45%, and reduced, LVEF <45%. For CD4 count and HIV viral load, we took the value that was recorded closest to the first HF diagnosis, between 1 year before and 1 year after the index date. CD4 count was dichotomized into <200 versus ≥200 cells/µL. Viral load was dichotomized as undetectable (<75 copies/mL) versus detectable (≥75 copies/mL). Antiretroviral therapy (ART) was defined as being on one or more antiretroviral medication within the year preceding the index HF date. Presence of comorbidity was defined by the presence of at least one inpatient or outpatient ICD‐9 or ICD‐10 code for the respective diagnosis recorded prior to the index date of HF.
Statistical Analysis
The overall crude median age of HF diagnosis between veterans with and without HIV was compared using the median test. To account for differences in the underlying age structure between the populations, we calculated the median age of the veterans without HIV with HF after applying statistical weights derived from the age structure of the veteran population with HIV. Data on the age structure of the veteran population were obtained from summary data on VHA users provided by the VHA Support Service Center.8 Statistical weights were calculated by dividing the number of veterans within each 1‐year age strata of veterans with HIV aged ≥20 years for each calendar year from the year 2000 to 2018 by the total number of veterans with HIV for the corresponding calendar year. The weights were applied to the veterans without HIV in each calendar year to standardize the age structure of the population of veterans without HIV to that of the veterans with HIV. The expected number of HF events among the veterans without HIV adjusted to the age structure of the population of veterans with HIV was calculated by multiplying the HF event rate among veterans without HIV within each 1‐year age strata of each calendar year with the standardized population. Additionally, we estimated age‐specific HF incidence in veterans with and without HIV, and calculated standardized incidence ratios (SIRs) across 10‐year age groups (<30, 30–39, 40–49, 50–59, 60–69, 70+ years) to show the absolute and relative risk of HF in veterans with HIV compared with veterans without HIV. Age‐specific HF incidence was calculated for veterans with and without HIV separately by dividing the number diagnosed with a first HF within each 10‐year age group by the total number of veterans at risk for each calendar year from 2000 to 2018. The standard errors of the rates were estimated by dividing the square root of the number of HF events by the total number at risk. SIRs were similarly calculated within each 10‐year age group for each calendar year from 2000 to 2018 by determining the expected number of events in the veterans with HIV using the age‐specific HF incidence rate for the population of veterans without HIV . The standard errors of the SIRs were estimated by dividing the square root of the number of HF events in the veterans with HIV by the expected number of events in the group. The HIV status‐ and age‐specific HF incidence rates and age‐specific SIRs were combined across the years 2000 to 2018 using a fixed‐effect model meta‐analysis. P value for trend of SIRs across age groups was calculated from an inverse variance‐weighted linear regression model.
Secondary analyses comparing age at diagnosis of HF by HIV status was performed within the following important subgroups: (1) inpatient versus outpatient index HF, (2) preserved LVEF versus reduced LVEF, (3) older (aged ≥45 years) versus younger veterans, (4) HF index date 2009 to 2018 versus 2000 to 2008, (5) veterans who received initial HF diagnosis outside the VHA system (ie, received treatment in community hospitals and clinics on a fee basis with VHA paying for the care) versus HF diagnosed within VHA facilities, (6) sex (men versus women), and (7) smoking status (smoker versus nonsmoker). For veterans with HIV, we compared age at diagnosis of HF by CD4 count, viral load, and ART use.
Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Calculation of HF rates and SIRs were performed using Excel Spreadsheets and Stata software (Stata Corp v15, College Station, Texas). A 2‐sided P<0.05 was considered statistically significant.
Results
We identified 5093 veterans with HIV (98% men, 34% White) with a first HF episode within the VA healthcare system recorded after HIV diagnosis and 1 420 894 veterans without HIV (98% men, 78% White) with HF. The baseline characteristics of the participants are shown in Table 1. The prevalence of diabetes mellitus and myocardial infarction were similar between veterans with and without HIV with HF, whereas the prevalence of hypertension and chronic kidney disease was higher among veterans with HIV. On the other hand, the prevalence of depression, smoking, homelessness, and alcohol and substance abuse was significantly higher among veterans with HIV. The median LVEF (47%) and BNP (≈200 pg/mL) were comparable between veterans with and without HIV with HF.
Table 1.
Baseline Characteristics of Veterans With and Without HIV at First HF Record
| Variable* | HF Without HIV | HF With HIV |
|---|---|---|
| n=1 420 894 | n=5093 | |
| Age, y | 72 (63–80) | 58 (52–65) |
| Men | 97% | 98% |
| White | 79% | 35% |
| Black | 14% | 56% |
| Comorbidity | ||
| MI | 18% | 21% |
| DM | 38% | 37% |
| CKD | 13% | 28% |
| Hypertension | 71% | 78% |
| Current smoker | 28% | 54% |
| Homeless | 4% | 27% |
| Depression | 18% | 44% |
| Alcohol | 8% | 33% |
| Substance abuse | 5% | 38% |
| BMI, kg/m2 | 29 (25–34) | 25 (22–30) |
| LVEF, %† | 47 (34–58) | 47 (33–58) |
| BNP, pg/mL | 208 (69–567) | 193 (47–784) |
| Viral load, copies/mL | … | 49 (19–399) |
| CD4 count, cells/µL | … | 392 (204–607) |
| ART | … | 78% |
ART indicates antiretroviral therapy; BMI, body mass index; BNP, brain natriuretic peptide; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; LVEF, left ventricular ejection fraction; and MI, myocardial infraction.
Values are proportions (%) or median (interquartile range).
The mean (SD) interval between LVEF measurement and diagnosis of HF was 41 (78) days.
The median age at index HF was 58 years for veterans with HIV and 72 years for veterans without HIV, with an absolute difference in median age of 14 years between the 2 populations. Comparison of predicted median ages at HF diagnosis for veterans with and without HIV adjusting for sex and race yielded similar results (56 versus 72.0 years, respectively; median difference, 16 years). The predicted median age difference was reduced to 12 years (adjusted median age of 59 versus 71 years for veterans with versus without HIV, respectively) after adjusting for sex, race, and all of the risk factors and comorbidities shown Table 1. The median age at HF diagnosis for veterans without HIV after adjusting to the age structure of the veterans with HIV population was 63 years, leaving an absolute difference of 5 years in median age with veterans with HIV (P<0.001). As shown in the Figure (A), the SIRs of HF for veterans with versus without HIV was highest for the youngest age group and became progressively smaller in the older age groups (P value for trend <0.001). By contrast, as would be expected, the HF incidence rate increased progressively from the younger to the older age groups (Figure [B]).
Figure 1. Standardized incidence ratio (SIR) of heart failure for veterans with HIV vs without HIV (A), and HIV‐specific heart failure incidence (B), by age strata.
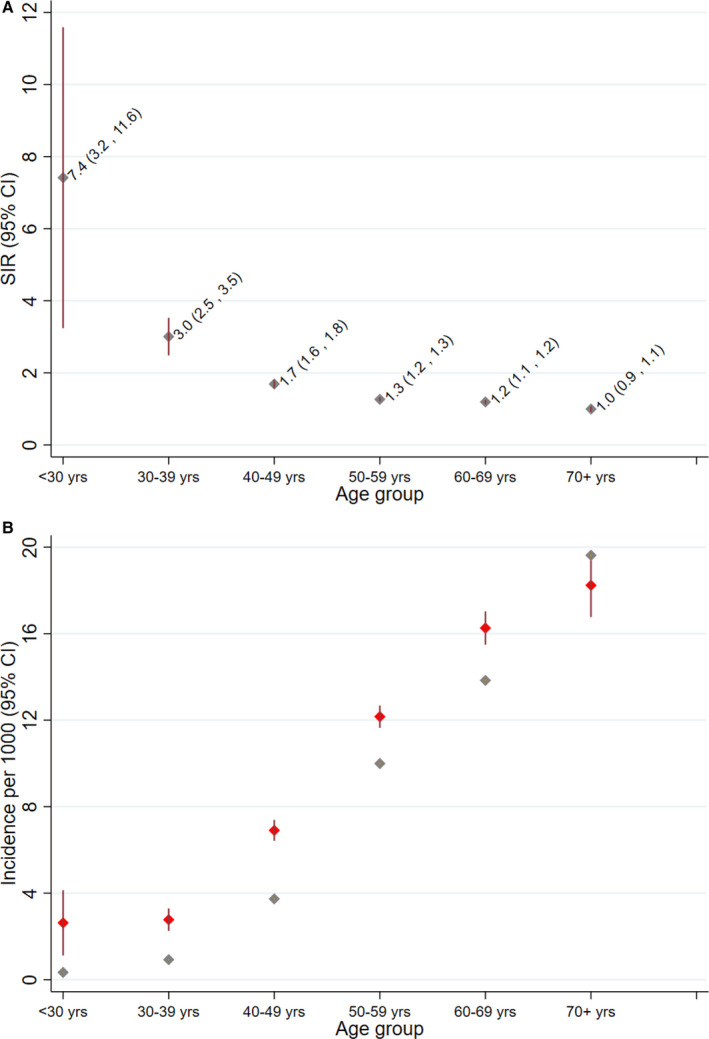
Vertical error bars represent 95% CIs. B, Red diamonds represent incidence in veterans with HIV, and light‐gray diamonds represent incidence in veterans without HIV. The vertical error bars are small for veterans without HIV because of the large underlying population and the narrow confidence intervals.
In subgroup analyses comparing median age of veterans with versus without HIV: (1) inpatient versus outpatient index HF, (2) preserved versus reduced LVEF (3) age ≥45 years versus <45 years, (4) time period 2009 to 2018 versus 2000 to 2008, (5) index HF diagnosed in community versus VHA facilities, (6) men versus women, and (7) smoker versus nonsmoker, yielded similar results to the main analyses (Table 2). The median age difference in the subgroups after adjusting for population age structure ranged from 2 to 6 years. The adjusted median age differences were similar for inpatient versus outpatient index HF (5 years each), time period 2009 to 2018 versus 2000 to 2008 (3 versus 4 years), community versus VHA facilities (5 years each), and men versus women (5 versus 4 years).The median age differences were comparable for HF with preserved LVEF versus reduced LVEF (3 versus 5 years), veterans aged >=45 years versus 20 to 45 years (4 versus 2 years), and smokers versus nonsmokers (4 versus 6 years) (Table 2).
Table 2.
Age at First Record of Heart Failure by Various Subgroups With and Without Adjustment for Interpopulation Difference in Age Structure
| Subgroup Analyses | Veterans Without HIV | Veterans With HIV | |||
|---|---|---|---|---|---|
| No. | Crude Age, y, Median (Mean) | Adjusted Age, y, Median (Mean) | No. | Crude Age, y Median (Mean) | |
| Inpatient vs outpatient | |||||
| Inpatient | 209 828 | 70 (70.3) | 63 (62.9) | 1794 | 58 (58.6) |
| Outpatient | 1 085 978 | 72 (71.5) | 63 (63.6) | 2726 | 58 (58.8) |
| HFPEF vs HFREF | |||||
| EF ≥45% | 507 176 | 70 (70.0) | 63 (63.5) | 2290 | 60 (59.9) |
| EF <45% | 429 123 | 69 (69.1) | 62 (61.5) | 1921 | 57 (57.7) |
| Older vs younger | |||||
| Age ≥45 y | 1 400 585 | 72 (71.4) | 63 (63.3) | 4769 | 59 (59.9) |
| Age 20–45 y | 19 248 | 41 (38.9) | 43 (42.2) | 324 | 41 (39.5) |
| Time trends | |||||
| 2009–2018 | 692 321 | 71 (71.6) | 64 (64.6) | 3049 | 61 (60.8) |
| 2000–2008 | 727 510 | 72 (70.5) | 59 (61.1) | 2044 | 55 (55.4) |
| Community vs VHA care | |||||
| Community, fee basis | 124 025 | 66 (67.4) | 63 (62.5) | 573 | 58 (58.1) |
| VHA, non–fee basis | 1 295 806 | 72 (71.3) | 63 (63.4) | 4520 | 58 (58.7) |
| Men vs women | |||||
| Men | 1 384 011 | 72 (71.1) | 63 (63.5) | 4988 | 58 (58.7) |
| Women | 35 596 | 64 (66.0) | 57 (57.4) | 105 | 53 (53.3) |
| Smoker vs nonsmoker | |||||
| Smoker | 300 362 | 67 (67.2) | 63 (62.6) | 2775 | 59 (58.6) |
| Nonsmoker | 1 021 513 | 74 (72.5) | 64 (64.0) | 2318 | 58 (58.7) |
Crude age: unadjusted age at first record of heart failure. Adjusted age: age at first record of heart failure after accounting for difference in population age structure between veteran populations with and without HIV. EF indicates ejection fraction; HFPEF, heart failure with preserved ejection fraction; HFREF, heart failure with reduced ejection fraction; and VHA, Veterans Health Administration.
In analyses of HIV‐specific variables, the median age at HF diagnosis was lower in those with CD4 count ≤200 versus >200 cell/µL (56 versus 60 years), those with HIV viral load >75 versus ≤75 copies/mL (55 versus 61 years), and those not on ART versus those on ART (56 versus 59 years). Comparisons of mean age, instead of median age, yielded similar results (Table 2).
Discussion
In the analyses of data involving up to 1 420 894 veterans without HIV and 5093 veterans with HIV with first diagnosis of HF in the VA healthcare system, we found that veterans with HIV were on average 14 years younger than veterans without HIV at the time of diagnosis of HF, which is partially a reflection of the difference in the underlying population age structure of veterans with and without HIV. However, veterans with HIV were still on average 5 years younger at their first diagnosis of HF compared with veterans without HIV after accounting for differences in population structure. These conclusions are further supported by our finding of higher SIRs at younger age groups. Epidemiological studies have shown that HIV infection is associated with increased risk of HF independent of cardiovascular risk factors or coronary artery disease.5 Our recent meta‐analysis of extant observational studies on HIV and cardiac dysfunction showed that PLHIV have a higher prevalence of cardiac dysfunction and HF.9 The current findings indicate that HF may develop at a younger age in veterans living with HIV, extending the prior works showing association between HIV and risk of incident HF.
The age differences were broadly consistent across the following important subgroups: inpatient versus outpatient index HF, HF with preserved versus reduced LVEF, community versus VHA diagnosed index HF, men versus women, time period 2009 to 2018 versus 2000 to 2008, and smokers versus nonsmokers. The age difference was somewhat smaller for younger age groups (2 years versus 4 years for those aged <45 years versus ≥45 years) despite higher SIRs of HF in the younger age group, likely because of the low number of participants aged <45 years (≈1.4% of the total number). That the age difference was broadly similar comparing (preserved versus reduced LVEF versus 5 years, respectively) implies that the earlier occurrence of HF is relevant to both HF subtypes. This may be particularly important given suggestions from epidemiological studies that HIV‐associated HF may be shifting toward HF with preserved LVEF.10, 11
In analyses restricted to individuals with HIV, we found that the median age at HF diagnosis was lower in individuals with lower CD4 count, higher HIV viral load, or those not on ART. These finding are consistent with a prior study reporting more advancement in biological age in individuals with lower CD4 count,12 whereas the reported association of ART with biological aging has been variable,12, 13, 14 and suggest that HIV‐associated immunological and inflammatory changes may play a role in earlier onset of end‐organ diseases.15 Whether interventions to mitigate these pathophysiological processes result in delay in onset of HF in PLHIV remains to be determined.
The strength of the study is use of the largest data available to date that directly addresses the question of earlier age of onset of HF in patients with HIV. Although our previous article using VHA data reported on age‐specific incidence rate of HF,4 that study was based on a smaller number of incident HF cases in veterans with HIV (n=941) with age‐matched controls, precluding direct comparison of age of HF onset between the groups. Also, various subgroup analyses indicate the robustness of the findings, suggesting HF to be among the age‐related diseases that develop early in PLHIV.15
We acknowledge a number of important limitations. First, this was retrospective observational study; therefore, causal inference is limited because of residual confounding and reverse causation. To limit the effect of these sources of error, we restricted analyses to first‐diagnosed HF only, and for veterans with HIV, to those where HIV diagnosis preceded the diagnosis of HF. Second, the identification of first HF episodes using VHA data (not complemented with Medicare and Medicaid data) is limited, because some veterans may be diagnosed with their first HF episode outside the VHA system. However, we found similar results when restricting our analyses to HF cases first diagnosed in community facilities (on a fee basis), indicating that any effect of such bias is likely small. Third, our results are mostly applicable to men with HIV given the predominantly male composition of the veteran population. However, the similar results obtained in the small subset of female participants in this study suggest that the findings may be relevant to female patients with HIV. Finally, it is not clear if the findings are necessarily generalizable to patients with HIV outside of the United States, or even outside of the VA, because factors related to risk‐factor burden and HIV treatment tend to differ from place to place.
Conclusions
In a retrospective analysis of large record‐linkage data from the VA healthcare system, we found that veterans with HIV are diagnosed with HF at significantly younger age compared with veterans without HIV after accounting for differences in age structure between the 2 populations. The results were consistent across several important subgroups. These findings imply that preventive measures such as screening for HF and treatment of risk factors (eg, hypertension, diabetes mellitus) may have to occur at an earlier age for individuals with HIV. Future studies using non‐VHA data and with larger representation of women should help to make the findings more broadly generalizable.
Sources of Funding
The research reported/outlined here was supported by the Department of Veterans Affairs, Veterans Health Administration, VISN 1 Career Development Award to Dr Erqou. Dr Erqou is also funded by the Center for Aids Research (P30 AI042853), Rhode Island Foundation, and Lifespan Cardiovascular Institute. This work is partially supported (investigator's time effort and publication cost) by the VA Health Service Research and Development Merit Review grant IRP 20‐003 (Dr Wu). Drs Rudolph and Wu are funded by the VA Health Services Research and Development Center of Innovation in Long Term Services and Supports (CIN 13‐4193; C19‐20‐213). Drs Erqou, Choudhary, Lally, Rudolph, and Wu, and Ms Jiang are employees of the VHA. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Disclosures
None.
(J Am Heart Assoc. 2021;10:e018983. DOI: 10.1161/JAHA.120.018983.)
For Sources of Funding and Disclosures, see page 6.
References
- 1.Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, Fiocchi F, Lattanzi A, Rossi R, Modena M, et al. Coronary aging in HIV‐infected patients. Clin Infect Dis. 2009;49:1756–1762. DOI: 10.1086/648080. [DOI] [PubMed] [Google Scholar]
- 2.Gross A, Jaeger P, Kreisberg J, Licon K, Jepsen K, Khosroheidari M, Morsey B, Swindells S, Shen H, Ng C, et al. Methylome‐wide analysis of chronic HIV infection reveals five‐year increase in biological age and epigenetic targeting of HLA. Mol Cell. 2016;62:157–168. DOI: 10.1016/j.molcel.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiels MS, Althoff KN, Pfeiffer RM, Achenbach CJ, Abraham AG, Castilho J, Cescon A, D'Souza G, Dubrow R, Eron JJ, et al. HIV infection, immunosuppression, and age at diagnosis of non‐AIDS‐defining cancers. Clin Infect Dis. 2017;64:468–475. DOI: 10.1093/cid/ciw764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert CG, Oursler KK, Rimland D, Rodriguez‐Barradas MC, Dubrow R, Park LS, et al. Comparison of risk and age at diagnosis of myocardial infarction, end‐stage renal disease, and non‐AIDS‐defining cancer in HIV‐infected versus uninfected adults. Clin Infect Dis. 2015;60:627–638. DOI: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang C‐C, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So‐Armah KA, Vasan RS, Oursler KA, Gottdiener J, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. DOI: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erqou S, Jiang L, Choudhary G, Lally M, Bloomfield GS, Zullo AR, Shireman TI, Freiberg M, Justice AC, Rudolph J, et al. Heart failure outcomes and associated factors among veterans with human immunodeficiency virus infection. JACC Heart Fail. 2020;8:501–511. DOI: 10.1016/j.jchf.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garvin JH, DuVall SL, South BR, Bray BE, Bolton D, Heavirland J, Pickard S, Heidenreich P, Shen S, Weir C, et al. Automated extraction of ejection fraction for quality measurement using regular expressions in unstructured information management architecture (UIMA) for heart failure. J Am Med Inform Assoc. 2012;19:859–866. DOI: 10.1136/amiajnl-2011-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veterans Health Administration (VHA) Support Service Center. HIV Cube. Available at: https://vssc.med.va.gov/VSSCMainApp/products.aspx?PgmArea=72. Accessed September 5, 2019. (Access is restricted by the U.S. Veteran's Affairs Department).
- 9.Erqou S, Lodebo BT, Masri A, Altibi AM, Echouffo‐Tcheugui JB, Dzudie A, Ataklte F, Choudhary G, Bloomfield GS, Wu W‐C, et al. Cardiac dysfunction among people living with HIV: a systematic review and meta‐analysis. JACC Heart Fail. 2019;7:98–108. DOI: 10.1016/j.jchf.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J. 2013;34:3538–3546. DOI: 10.1093/eurheartj/eht388. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, Grinspon SK, Levin J, Longenecker CT, Post WS. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140:e98–e124. DOI: 10.1161/CIR.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Francesco D, Wit FW, Bürkle A, Oehlke S, Kootstra NA, Winston A, Franceschi C, Garagnani P, Pirazzini C, Libert C, et al. Do people living with HIV experience greater age advancement than their HIV‐negative counterparts? AIDS. 2019;33:259–268. DOI: 10.1097/QAD.0000000000002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rickabaugh TM, Baxter RM, Sehl M, Sinsheimer JS, Hultin PM, Hultin LE, Quach A, Martínez‐Maza O, Horvath S, Vilain E, et al. Acceleration of age‐associated methylation patterns in HIV‐1‐infected adults. PLoS One. 2015;10:e0119201. DOI: 10.1371/journal.pone.0119201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson KN, Hui Q, Rimland D, Xu K, Freiberg MS, Justic AC, Marconi VC, Sun YV. Identification of HIV infection‐related DNA methylation sites and advanced epigenetic aging in HIV‐positive, treatment‐naive U.S. veterans. AIDS. 2017;31:571–575. DOI: 10.1097/QAD.0000000000001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. DOI: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is based on restrictions in the data‐use agreements used in this study. The authors are unable to make a data set available. Methodology questions may be directed to the corresponding author.


