Abstract
Background
High blood pressure (BP) is a well‐known risk factor for atrial fibrillation (AF), but a single BP measurement may provide limited information about AF risk in older adults.
Methods and Results
This study included 1256 MESA (Multi‐Ethnic Study of Atherosclerosis) and 1948 ARIC (Atherosclerosis Risk in Communities) study participants who underwent extended ambulatory electrocardiographic monitoring and who were free of clinically detected cardiovascular disease, including AF. Using BP measurements from 6 examinations (2000–2018 in MESA and 1987–2017 in ARIC study), we calculated individual long‐term mean, trend, and detrended visit‐to‐visit variability in systolic BP and pulse pressure for each participant. Outcomes, assessed at examination 6, included subclinical AF and supraventricular ectopy. Results from each study were combined with inverse variance‐weighted meta‐analysis. At examination 6, the mean age was 73 years in MESA and 79 years in ARIC study, and 4% had subclinical AF. Higher visit‐to‐visit detrended variability in systolic BP was associated with a greater prevalence of subclinical AF (odds ratio [OR], 1.20; 95% CI, 1.02–1.38) and with more premature atrial contractions/hour (geometric mean ratio, 1.08; 95% CI, 1.01–1.15). For pulse pressure as well, higher visit‐to‐visit detrended variability was associated with a greater prevalence of AF (OR, 1.18; 95% CI, 1.00–1.37). In addition, higher long‐term mean pulse pressure was associated with a greater prevalence of subclinical AF (OR, 1.36; 95% CI, 1.08–1.70).
Conclusions
Antecedent visit‐to‐visit variability in systolic BP and pulse pressure, but not current BP, is associated with a higher prevalence of subclinical atrial arrhythmias. Prior longitudinal BP assessment, rather than current BP, may be more helpful in identifying older adults who are at higher risk of atrial arrhythmias.
Keywords: arrhythmia, atrial fibrillation, atrial fibrillation arrhythmia, blood pressure, electrocardiography, older adults
Subject Categories: Arrhythmias, Atrial Fibrillation, Epidemiology, High Blood Pressure
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- DBP
diastolic blood pressure
- MESA
Multi‐Ethnic Study of Atherosclerosis
- PP
pulse pressure
- SBP
systolic blood pressure
- SVE
supraventricular ectopy
- SVT
supraventricular tachycardia
Clinical Perspective
What Is New?
In 2 prospective cohort studies, greater visit‐to‐visit variability in systolic blood pressure and pulse pressure over a period of several years was associated with a higher prevalence of subclinical atrial arrhythmias above and beyond a single cross‐sectional blood pressure measurement.
What Are the Clinical Implications?
These findings suggest that, for older adults, visit‐to‐visit variability in blood pressure over a period of several years is a potentially novel risk factor for atrial arrhythmias.
It is well established that high blood pressure (BP) is an important causative factor for several cardiovascular diseases, including atrial arrhythmias, which are associated with a substantial burden of stroke and other complications.1, 2, 3, 4 Compared with a single BP assessment, BP trajectories over time have been shown to be better predictors of cardiovascular disease mortality.5, 6 Cumulative measures of BP and changes in BP over a period of several years may similarly be more strongly associated with arrhythmia risk than a single cross‐sectional BP measurement. A shift in focus from current elevated BP to a determination of patterns and changes in BP over several years may better inform strategies to more aggressively screen for and treat high BP earlier in life.5, 7, 8 Furthermore, prior studies have found that visit‐to‐visit systolic BP (SBP) variability predicts stroke6 and mortality9, 10 independently of mean SBP. Individual BP variability may represent an individual's inability to maintain homeostasis and is an important marker of cardiovascular outcomes.11 Therefore, BP variability over time may be an important factor in determining atrial arrhythmia risk.12 Visit‐to‐visit variability may be particularly important among older adults because of the increased use of and the potential for nonadherence to BP‐lowering medications,12 a greater burden of comorbid conditions, or aging‐related arterial stiffness.13
In addition to longitudinal aspects of SBP, pulse pressure (PP) is also important, and may more strongly predict cardiovascular risk among older adults.14 Physiologically, aging results in a loss of aortic compliance and increasing SBP. However, diastolic BP (DBP) displays a varying pattern with aging, with DBP increasing until about 60 years of age and then slowly decreasing, largely because of arterial stiffening. These observed patterns in SBP and DBP result in a steep increase in PP with aging.15
Atrial arrhythmias are often asymptomatic. As such, focusing on clinically detected arrhythmias identified from periodic ECGs, diagnosis codes, and death certificates will underestimate the population burden of atrial arrhythmias. The MESA (Multi‐Ethnic Study of Atherosclerosis) and the ARIC (Atherosclerosis Risk in Communities) study recently conducted extended ambulatory cardiac monitoring on study participants. This extended ECG monitoring provides an unbiased, high‐quality assessment of atrial fibrillation (AF) as well as supraventricular ectopy (SVE), an important biomarker of cardiovascular risk.16, 17, 18 In the MESA and ARIC studies, we examined the prevalence of subclinical atrial arrhythmias associated with longitudinal measures of BP, including long‐term mean BP, BP trend (slope), and BP visit‐to‐visit variability in addition to cross‐sectional BP. The objective was to determine whether certain specific ways of assessing BP (long‐term mean, trend, or visit‐to‐visit variability) are associated with subclinical arrhythmias, after adjustment for more conventional BP measures, such as a single cross‐sectional BP value.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of participant privacy issues. However, investigators interested in analyzing MESA data may contact the MESA Coordinating Center at the University of Washington or use the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repositories Information Coordinating Center repository. Investigators interested in analyzing ARIC study data may contact the ARIC Coordinating Center at the University of North Carolina Chapel Hill.
Study Populations
This study used longitudinal data from 2 prospective cohort studies. MESA recruited 6814 Chinese American, Hispanic, White, and Black participants between 45 and 84 years of age who were free of clinically recognized cardiovascular disease from 6 field centers across the United States to undergo baseline examination between 2000 and 2002 (examination 1), with follow‐up examinations through 2016 to 2018 (examination 6).19 At each follow‐up examination, information was collected on demographic factors and several clinical factors, including BP. During examination 6, a subset of MESA participants (n=1557) with and without a history of heart disease or clinically detected AF were enrolled in an ancillary study that conducted extended ambulatory ECG monitoring.20 The ARIC study recruited 15 792 predominantly White and Black study participants aged 45 to 64 years from 4 US communities to undergo baseline examination between 1987 and 1989 (examination 1), with follow‐up examinations through 2018 to 2019 (examination 7).21 At each follow‐up examination, information was collected on demographic factors and clinical factors, including BP. During examination 6 (2016–2017), a subset of ARIC study participants (n=2616) from all 4 sites were enrolled in an ancillary study that included extended ambulatory ECG monitoring.22 Follow‐up examination dates in both cohorts are shown in Figure S1.
Participants from both studies were included in analyses if they had undergone at least 24 continuous hours of ambulatory ECG monitoring at examination 6. Further exclusions were made for those who experienced heart failure, a myocardial infarction, or a stroke, or were diagnosed with clinically detected AF before examination 6 (when ambulatory ECG monitoring occurred). These exclusions were made because the development of these cardiovascular disease events may be potential mediators of possible relationships between BP measures and subsequent atrial arrhythmias. Furthermore, participants were required to have BP measured during at least 4 examinations before examination 6 to be included in analyses. Finally, participants were excluded if they were missing data on baseline covariates (Figure 1). This study was approved by Institutional Review Boards at all participating institutions, with a waiver of consent.
Figure 1. Flowcharts showing inclusion criteria and exclusions for the MESA (Multi‐Ethnic Study of Atherosclerosis) and the ARIC (Atherosclerosis Risk in Communities) study analytic cohorts.
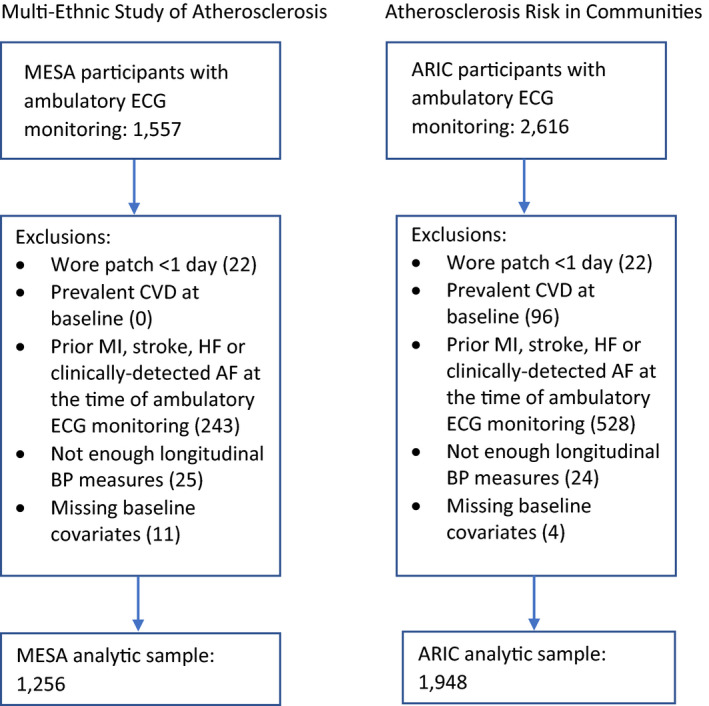
AF indicates atrial fibrillation; BP, blood pressure; CVD, cardiovascular disease; HF, heart failure; and MI, myocardial infarction; and N/A, not applicable.
Exposures
In MESA, resting BP was measured (after a 5‐minute rest period) in a seated position by a certified trained technician using a Dinamap model Pro 100 automated oscillometric sphygmomanometer.23 Each participant contributed 3 BP measurements per visit, and the reported BP was an average of the second and third measurements.19 In the ARIC study, resting BP was measured (after a 5‐minute rest period) via a random zero sphygmomanometer in visits 1 through 4 and an automatic sphygmomanometer (OMRON HEM‐907 XL) in visits 5 and 6 by a certified trained technician. Participants were asked not to smoke, eat, perform physical exertion, or experience cold temperatures 30 minutes before measurements. Each participant contributed 3 BP measurements per visit for visits 1 through 3 and 5 and 6, and the reported BP was an average of the second and third measurements. For visit 4, BP was measured twice and reported as the average.21, 24
We evaluated SBP, DBP, and PP measures. PP is calculated as the difference between SBP and DBP. For SBP, DBP, and PP, we calculated (1) a cross‐sectional measurement, which was assessed at examination 6 (2016–2018 in MESA and 2016–2017 in the ARIC study), corresponding to when extended ambulatory ECG monitoring occurred, (2) a long‐term mean measurement calculated over examinations 1 through 5 (in MESA, this included examinations occurring between 2000 and 2011; and in the ARIC study, this included examinations occurring between 1987 and 2013), (3) a trend measurement, which was calculated as the β coefficient from an individual‐specific linear regression line of the BP values at examinations 1 through 5 with the examination date, in years since baseline, serving as the independent variable, and (4) detrended visit‐to‐visit variability, calculated as the square root of the residual mean square (SD of residuals),25 from the 5 residuals of the individual‐specific linear regression at examinations 1 through 5 (Figure 2). Calculated this way, visit‐to‐visit variability is independent of cross‐sectional BP and long‐term trends in BP.
Figure 2. Method of measuring intraindividual components of blood pressure (BP), including cross‐sectional value, long‐term mean, trend, and visit‐to‐visit variability.
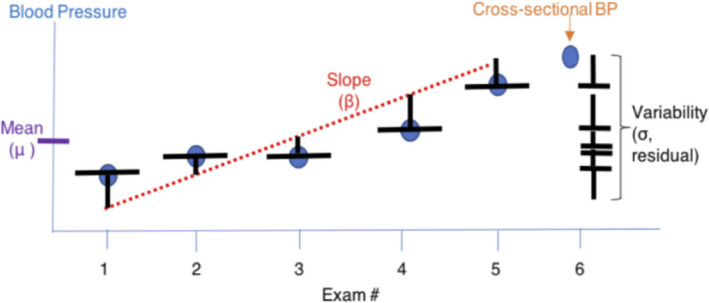
Cross‐sectional BP was assessed at examination 6 (2016–2018 in the MESA [Multi‐Ethnic Study of Atherosclerosis] and 2016–2017 in the ARIC [Atherosclerosis Risk in Communities] study). Long‐term mean BP was calculated over examinations 1 through 5 (in MESA, this included examinations between 2000 and 2011; and in ARIC study, this included examinations between 1987 and 2013). BP trend was calculated as the β coefficient from an individual‐specific linear regression line of the BP values at examinations 1 through 5, with the follow‐up examination date serving as the independent variable. Visit‐to‐visit variability was calculated as the square root of the variance, or the residual mean square, from the 5 residuals of the individual‐specific linear regression.
Outcomes
Outcomes included subclinical AF and SVE. The ECG monitoring device used to measure atrial arrhythmias in both the MESA and ARIC study populations was the Zio Patch XT (iRhythm Technologies, Inc, San Francisco, CA), a US Food and Drug Administration–approved single‐channel ECG patch monitor capable of recording up to 14 days of cardiac rhythm.26 Certified technicians at iRhythm processed and analyzed the ECG data and reported all arrhythmias. The MESA and ARIC study arrhythmia data were verified by the Epidemiological Cardiology Reading Center at Wake Forest University School of Medicine (Winston‐Salem, NC).
Subclinical AF was defined as an irregularly irregular rhythm lasting at least 30 seconds that occurred in a participant without a known diagnosis of AF. SVE measures included (1) frequency of premature atrial contractions (PACs) per hour and (2) frequency of runs of supraventricular tachycardia (SVT) per day, with a run of SVT defined as ≥4 consecutive PACs.
Covariates
The following potential confounders, assessed at baseline (examination 1), were adjusted for in models: age (linear), sex (male/female), race/ethnicity (in MESA, White/Black/Hispanic/Chinese American; in ARIC, White/Black), site (in MESA, Baltimore, MD/Chicago, IL/Los Angeles County, CA/New York, NY/St. Paul, MN/Winston Salem, NC; in ARIC, Forsyth County, NC/Jackson, MS/Washington County, MD/suburbs of Minneapolis–St. Paul, MN), height (linear), weight (linear), diabetes mellitus (yes/no), and use of antihypertensive medications (yes/no). In sensitivity analyses, the following covariates were also included: use of statins (assessed at baseline, yes/no), smoking (assessed at baseline, never/former/current), and alcohol use (assessed at baseline, yes/no).
Statistical Analysis
For the binary outcome of subclinical AF, we used logistic regression to estimate associations with BP exposure variables. For continuous outcomes (PAC frequency and SVT frequency), linear regression was used to estimate associations with BP exposure variables. All models adjusted for baseline characteristics, and in the subclinical AF outcome model, we additionally adjusted for total analyzable monitoring time. Furthermore, models evaluating associations for long‐term mean BP included current BP as a covariate, models evaluating associations for BP trend included current and long‐term mean BP as covariates, and models evaluating visit‐to‐visit BP variability included current BP, long‐term mean BP, and BP trend as covariates.
For variables with a skewed distribution, including the outcome variables of average PACs per hour and average runs of SVT per day, we applied a log transformation. Some participants had no PACs (1% of participants) or runs of SVT (17% of participants), so one PAC and one run of SVT were added for each participant before log transforming these continuous outcomes.
MESA and ARIC study results were combined using a fixed‐effects inverse variance–weighted meta‐analysis.27 SBP and PP exposures were the primary focus in our analyses, because prior research has shown SBP and PP are more powerful independent predictors of cardiovascular risk than DBP among older adults.28 Findings for cross‐sectional BP exposures and long‐term mean BP exposures are presented per 10–mm Hg difference in BP, whereas findings for BP trend exposures are presented per 1–mm Hg difference in trend per year, and findings for visit‐to‐visit variability exposures are presented per 4–mm Hg difference in variability. These units correspond to about one population SD when considering the distributions in MESA and ARIC study.
In secondary analyses, we estimated associations between subclinical atrial arrhythmias and DBP exposure variables and we examined study‐specific differences in associations between BP and subclinical atrial arrhythmias.
Sensitivity Analyses
We conducted 3 sensitivity analyses. First, we repeated analyses in a population restricted to those who did not change their antihypertensive medication use status after baseline (ie, did not start or stop antihypertensive medications during follow‐up). This analysis addressed concerns that the initiation of or discontinuation of antihypertensive medications could alter the longitudinal BP trajectories of participants. Second, we included additional adjustment for statin use, smoking, and alcohol use at baseline because these additional factors may confound the relationship between BP and subclinical atrial arrhythmias. Third, we used an alternative, commonly used method of calculating variability to see if findings differed using this alternative approach. In this approach, we estimated BP variability by taking the SD of BP measurements during examinations 1 through 5.
RESULTS
The MESA analytic sample included 1256 participants, and the ARIC study analytic sample included 1948 participants. In MESA, 48% of participants were men, whereas in ARIC, the corresponding figure was 40%. At baseline, MESA participants were older (mean age, 57±8 years) than ARIC study participants (mean age, 50±4 years) (Tables 1 and 2). At examination 6, MESA participants were younger (mean age, 73±8 years) than ARIC study participants (mean age, 79±5 years). Antiarrhythmic medications were used by only 1% of MESA participants and <0.5% of ARIC study participants at any time during study follow‐up.
Table 1.
Baseline (2000–2002) Characteristics by Tertiles of SBP Variables: The MESA (n=1256)
| Characteristics | Full Cohort | Cross‐Sectional SBP, mm Hg | Long‐Term Mean SBP, mm Hg | Slope SBP, mm Hg | Visit‐to‐Visit Variability SBP, mm Hg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 78.0 to 116.5 | 117.0 to 134.0 | 134.5 to 275.0 | 79.8 to 111.8 | 111.9 to 125.2 | 125.3 to 170.5 | −26.0 to −1.2 | −1.2 to 1.9 | 1.9 to 17.4 | 0.12 to 4.93 | 0.94 to 8.54 | 8.58 to 33.30 | ||
| (n=1256) | (n=426) | (n=425) | (n=415) | (n=423) | (n=420) | (n=413) | (n=419) | (n=419) | (n=418) | (n=419) | (n=419) | (n=418) | |
| Age, mean (SD), y | 57 (8) | 55 (8) | 57 (8) | 60 (9) | 54 (7) | 57 (8) | 61 (9) | 59 (9) | 56 (8) | 57 (8) | 55 (8) | 57 (8) | 59 (9) |
| Men, % | 48 | 53 | 51 | 40 | 45 | 52 | 46 | 49 | 48 | 46 | 52 | 49 | 42 |
| Race/ethnicity, % | |||||||||||||
| White | 40 | 46 | 40 | 35 | 51 | 39 | 30 | 39 | 43 | 40 | 47 | 40 | 34 |
| Black | 14 | 17 | 13 | 13 | 16 | 15 | 12 | 11 | 16 | 15 | 16 | 15 | 12 |
| Hispanic | 25 | 16 | 25 | 34 | 14 | 25 | 36 | 30 | 21 | 24 | 17 | 25 | 33 |
| Chinese | 20 | 21 | 22 | 18 | 19 | 21 | 22 | 20 | 20 | 21 | 20 | 19 | 22 |
| Height, mean (SD), cm | 167 (10) | 168 (10) | 168 (10) | 166 (10) | 168 (10) | 168 (9) | 166 (10) | 167 (10) | 168 (10) | 167 (10) | 169 (9) | 168 (10) | 166 10) |
| Weight, mean (SD), lb | 172 (37) | 168 (36) | 177 (37) | 173 (37) | 163 (35) | 177 (36) | 178 (38) | 176 (36) | 169 (37) | 172 (37) | 171 (37) | 172 (37) | 174 (36) |
| Antihypertensive use, % | 26 | 18 | 27 | 34 | 13 | 25 | 41 | 37 | 20 | 22 | 15 | 23 | 41 |
| Alcohol, % | 63 | 66 | 61 | 61 | 69 | 61 | 59 | 62 | 65 | 62 | 69 | 65 | 55 |
| Smoking, % | |||||||||||||
| Former | 37 | 32 | 40 | 39 | 37 | 34 | 40 | 40 | 37 | 34 | 36 | 37 | 37 |
| Current | 26 | 11 | 10 | 8 | 13 | 9 | 7 | 9 | 10 | 10 | 26 | 24 | 28 |
| Diabetes mellitus, % | 7 | 5 | 6 | 9 | 4 | 8 | 8 | 8 | 5 | 7 | 3 | 6 | 11 |
| Statin use, % | 13 | 11 | 14 | 13 | 9 | 14 | 15 | 14 | 13 | 10 | 10 | 12 | 16 |
MESA indicates Multi‐Ethnic Study of Atherosclerosis; and SBP, systolic blood pressure.
Table 2.
Baseline (1987–1989) Characteristics by Tertiles of SBP Variables: The ARIC Study (n=1948)
| Characteristics | Full Cohort | Cross‐Sectional SBP, mm Hg | Long‐Term Mean SBP, mm Hg | Slope SBP, mm Hg | Visit‐to‐Visit Variability SBP, mm Hg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 77.5 to 126.0 | 126.5 to 142 | 142.5 to 209 | 87.4 to 113.9 | 114 to 124.2 | 124.3 to 169.4 | −5.9 to 0.3 | 0.04 to 1.0 | 1.0 to 5.8 | 0.04 to 5.67 | 5.68 to 9.37 | 9.38 to 42.90 | ||
| (n=1948) | (n=653) | (n=639) | (n=656) | (n=648) | (n=652) | (n=648) | (n=649) | (n=650) | (n=649) | (n=649) | (n=650) | (n=649) | |
| Age, mean (SD), y | 50 (4) | 50 (4) | 50 (4) | 51 (5) | 49 (4) | 50 (4) | 51 (5) | 50 (5) | 50 (4) | 51 (4) | 50 (5) | 50 (4) | 51 (4) |
| Men, % | 40 | 44 | 42 | 32 | 36 | 44 | 39 | 47 | 40 | 32 | 44 | 42 | 33 |
| Race/ethnicity, % | |||||||||||||
| White | 74 | 76 | 75 | 72 | 85 | 78 | 60 | 71 | 75 | 77 | 82 | 79 | 62 |
| Black | 26 | 24 | 25 | 28 | 15 | 22 | 40 | 29 | 25 | 23 | 18 | 21 | 38 |
| Hispanic | N/A* | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Chinese | N/A* | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Height, mean (SD), cm | 169 (9) | 169 (9) | 169 (9) | 167 (9) | 168 (9) | 169 (9) | 168 (9) | 169 (10) | 169 (9) | 167 (8) | 169 (10) | 169 (9) | 167 (8) |
| Weight, mean (SD), lb | 167 (34) | 170 (35) | 168 (34) | 164 (33) | 158 (32) | 169 (33) | 175 (35) | 177 (35) | 166 (33) | 159 (31) | 177 (35) | 166 (33) | 159 (31) |
| Antihypertensive use, % | 16 | 14 | 15 | 18 | 8 | 12 | 28 | 22 | 11 | 14 | 10 | 12 | 26 |
| Alcohol, % | 40 | 62 | 58 | 60 | 65 | 63 | 53 | 59 | 60 | 61 | 66 | 63 | 51 |
| Smoking, % | |||||||||||||
| Former | 31 | 32 | 31 | 30 | 29 | 35 | 29 | 29 | 33 | 31 | 32 | 32 | 29 |
| Current | 16 | 17 | 17 | 14 | 21 | 14 | 13 | 5 | 5 | 6 | 16 | 17 | 15 |
| Diabetes mellitus, % | 4 | 4 | 3 | 4 | 1 | 4 | 6 | 4 | 2 | 4 | 3 | 4 | 4 |
| Statin use, % | 0.26 | 0.15 | 0.31 | 0.30 | 0.00 | 0.15 | 0.62 | 0.31 | 0.15 | 0.31 | 0.15 | 0.15 | 0.46 |
ARIC indicates Atherosclerosis Risk in Communities; N/A, not applicable; and SBP, systolic blood pressure.
* ARIC study did not enroll Hispanic or Chinese participants.
The BP exposures in MESA and ARIC study are summarized in Table 3. At examination 6, ARIC study participants had higher SBP and higher PP than MESA participants, whereas DBP was similar in MESA and ARIC study participants. Long‐term mean BP values were similar in both cohorts. BP slope in both cohorts was positive for SBP and PP and was negative for DBP between examinations 1 through 5. The visit‐to‐visit SBP, DBP, and PP detrended variability was larger in ARIC study than in MESA.
Table 3.
Describing BP Exposures
| BP Variable | MESA | ARIC Study | ||||||
|---|---|---|---|---|---|---|---|---|
| Cross‐Sectional Value at Examination 6 | Long‐Term Mean | Slope | Visit‐to‐Visit Variability | Cross‐Sectional Value at Examination 6 | Long‐Term Mean | Slope | Visit‐to‐Visit Variability | |
| SBP, mean (SD), mm Hg | 126.7 (20.1) | 119.5 (15.3) | 0.13 (2.59) | 7.38 (4.35) | 135.1 (18.5) | 119.8 (12.6) | 0.69 (0.89) | 8.43 (5.12) |
| DBP, mean (SD), mm Hg | 66.9 (10.0) | 70.2 (8.6) | −0.58 (1.19) | 3.65 (1.90) | 67.5 (10.5) | 70.9 (7.8) | −0.20 (0.56) | 5.32 (2.89) |
| PP, mean (SD), mm Hg | 57.9 (16.4) | 49.3 (12.3) | 0.72 (2.22) | 5.26 (3.06) | 67.6 (15.7) | 48.9 (9.1) | 0.89 (0.63) | 6.65 (3.89) |
ARIC indicates Atherosclerosis Risk in Communities; BP, blood pressure; DBP, diastolic BP; MESA, Multi‐Ethnic Study of Atherosclerosis; PP, pulse pressure; and SBP, systolic blood pressure.
The median monitor duration was 13.9 days (interquartile interval, 13.2–14.0 days) in MESA and 13.7 days (interquartile interval, 12.8–13.9 days) in ARIC. In MESA, 41 (3.3%) participants had subclinical AF. MESA participants had a median of 3.0 (interquartile interval, 1.0–13.5) PACs/hour and a median of 0.4 (interquartile interval, 0.1–1.2) runs of SVT/day. In ARIC, 73 (3.8%) had subclinical AF. ARIC study participants had a median of 8.3 (interquartile interval, 2.7–32.5) PACs/hour and a median of 0.79 (interquartile interval, 0.36–2.08) runs of SVT/day.
In the primary analyses that combined results from MESA and ARIC study, a 10–mm Hg higher cross‐sectional SBP at examination 6 (2016–2018 in MESA and 2016–2017 in ARIC) was associated with a lower prevalence of subclinical AF (odds ratio [OR], 0.86; 95% CI, 0.76–0.96), but was associated with a greater number of runs SVT/day (geometric mean ratio, 1.04; 95% CI, 1.01–1.07). After adjustment for cross‐sectional BP, long‐term mean BP, and BP slope, a 4–mm Hg higher detrended visit‐to‐visit variability in SBP was associated with a greater prevalence of subclinical AF (OR, 1.20; 95% CI, 1.02–1.38) and with a greater number of PACs/hour (geometric mean ratio, 1.08; 95% CI, 1.01–1.15) (Figure 3). Findings for PP exposures were similar to findings from SBP exposures (Figure 4). For instance, a 10–mm Hg higher cross‐sectional PP was associated with less subclinical AF (OR, 0.73; 95% CI, 0.63–0.84); and a 4–mm Hg higher visit‐to‐visit variability in PP was associated with a greater prevalence of AF (OR, 1.18; 95% CI, 1.00–1.37). In addition, a 10–mm Hg higher long‐term mean PP was associated a greater prevalence of subclinical AF (OR, 1.36; 95% CI, 1.08–1.70).
Figure 3. Meta‐analysis results for associations between systolic blood pressure (SBP) exposure variables and atrial arrhythmias.
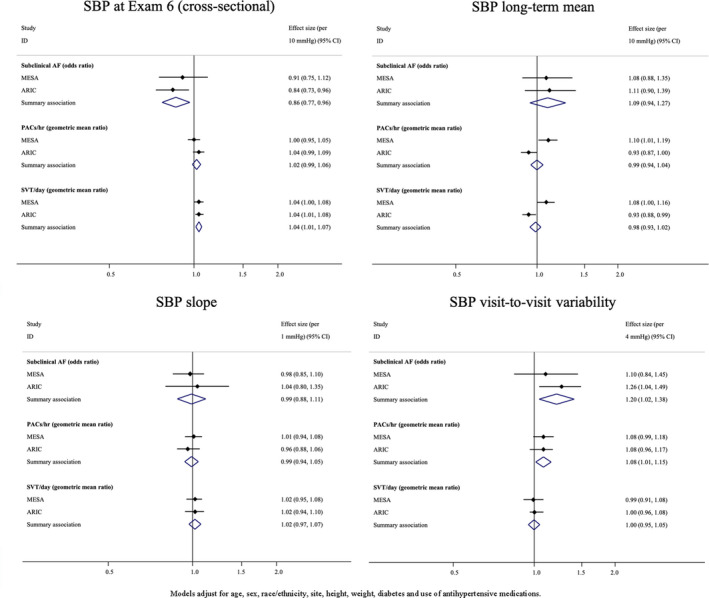
AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; Exam, examination; ID, identifier; MESA, Multi‐Ethnic Study of Atherosclerosis; PAC, premature atrial contraction; and SVT, supraventricular tachycardia.
Figure 4. Meta‐analysis results for associations between pulse pressure (PP) exposure variables and atrial arrhythmias.
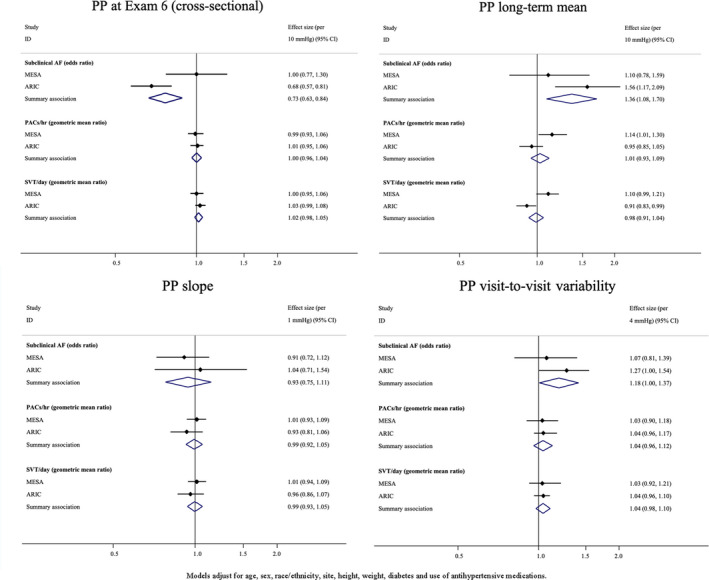
AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; Exam, examination; ID, identifier; MESA, Multi‐Ethnic Study of Atherosclerosis; PAC, premature atrial contraction; and SVT, supraventricular tachycardia.
Secondary analyses examined associations between DBP exposure variables and subclinical AF and SVE outcomes. For DBP, findings were mainly null, with the exception of a greater burden of SVE among participants with a higher cross‐sectional DBP and a negative relationship between long‐term mean and subclinical AF (Figure S2). In secondary analyses considering population‐specific differences, the associations between long‐term mean SBP and SVE differed between MESA and ARIC (P<0.01), and the same was true for associations for runs of SVT/day (P<0.01) (Figure 3). In addition, the meta‐analyzed cross‐sectional findings for PP appeared to be driven by ARIC study–specific estimates. For example, higher cross‐sectional PP in ARIC was associated with less subclinical AF (OR, 0.68; 95% CI, 0.57–0.81), whereas the MESA‐specific estimate was null (OR, 1.00; 95% CI, 0.77–1.30) (P=0.03).
Sensitivity Analyses
Findings from sensitivity analyses restricting to a population who did not change their antihypertensive medication use after baseline and including additional adjustment for statin use, smoking, and alcohol use were consistent with findings from the primary analyses (data not shown). Findings from analyses examining variability, defined as the SD of BP measurements from examinations 1 through 5, produced results consistent with the primary analyses (Table S1).
DISCUSSION
In this study, we found that greater visit‐to‐visit SBP and PP variability were associated with a greater prevalence of subclinical atrial arrhythmias after adjustment for cross‐sectional BP, long‐term mean, and trend in BP. These findings suggest that, for older adults, detrended visit‐to‐visit variability in BP over a period of several years is potentially a novel risk factor of atrial arrhythmias. We also found that higher long‐term mean PP was associated with a greater prevalence of subclinical AF. By contrast, greater cross‐sectional SBP and PP were associated with a lower prevalence of subclinical AF.
We focused on reporting findings from our SBP and PP analyses because high SBP and high PP have been shown to be more powerful independent predictors of cardiovascular risk than DBP among older adults.15, 28 Although several studies have examined the relationship of BP trajectories to all‐cause mortality,10, 29, 30 cardiovascular‐specific mortality,5, 30 and other cardiovascular events,10, 30 there is limited prior research on the relationships between long‐term BP trajectories and AF. To the best of our knowledge, our study is the first to evaluate associations between long‐term BP trajectories and subclinical atrial arrhythmias. A prior investigation in ARIC estimated associations between 10‐year BP trajectories and risk of incident clinical AF and found that those with the pattern of long‐term hypertension (defined as SBP ≥140 mm Hg, DBP ≥90 mm Hg, or use of antihypertensives) had a hazard ratio (HR) of 1.31 (95% CI, 1.14–1.51) for clinical AF compared with those without long‐term hypertension.7 Another study found that participants with hypertension whose BP eventually decreased during a 16‐year BP trajectory assessment period, and participants with hypertension whose BP continued to increase, were at greater risk of incident AF compared with normotensive participants (HR, 2.05 [95% CI, 1.24–3.37]; and HR, 1.95 [95% CI, 1.08–3.49], respectively).31 Both of these studies used latent class models to identify a few common BP trajectories. By contrast, we modeled components of long‐term BP trajectories in a more flexible way to investigate how different aspects of longitudinal BP over a period of several years (cross‐sectional, long‐term mean, trend, and detrended visit‐to‐visit variability) were independently related to subclinical atrial arrhythmias.
In contrast to prior studies, which have found that elevated cross‐sectional SBP is associated with a greater prevalence of clinical AF,32, 33 we found that higher cross‐sectional SBP was associated with less subclinical AF. This difference may be explained in part by the older age of MESA and ARIC study participants, or by differences in the underlying demographics of the MESA and ARIC study populations. Another possible explanation is selection bias; healthy participants in MESA and ARIC are more likely to return for follow‐up examinations. In our study, incorporating information on long‐term aspects of BP resulted in important information on subclinical atrial arrhythmia prevalence, whereas the cross‐sectional assessment gave a different and possibly limited view. These findings provide support for the hypothesis that BP from several years in the past may contribute to disease prevalence in older age.
There are important limitations to consider, including the potential for residual confounding attributable to the observational study design. In addition, subclinical arrhythmias measured at examination 6 cannot be treated as truly incident arrhythmias, because extended ambulatory ECG monitoring was not conducted at baseline and many participants likely developed arrhythmias during the period of BP exposure assessment that did not come to clinical attention. Because of this, the temporal relationship between exposure and outcome is not clear. In addition, there are differences in the timing of examinations between the MESA and ARIC study cohorts. In MESA, examinations 1 through 5 occurred over a span of 10 years, while in ARIC study, examinations 1 through 5 occurred over a span of 24 years. Furthermore, it is possible that participants developing more comorbidities over time were less likely to return to follow‐up examinations, resulting in selection bias. Because our results were based on participants surviving to examination 6 and being healthy enough to undergo extended ambulatory ECG monitoring, results may not be fully generalizable to people of the same age group in the general population. It is possible that BP may have been inaccurately measured among participants experiencing AF at the time of their BP assessment, which could explain, in part, the inverse relationship between current SBP at examination 6 and subclinical AF risk. Despite the use of standardized procedures for BP measurement, measurement error, if present, may have attenuated the observed associations between BP exposure variables and arrhythmia risk. Finally, because there are a large number of comparisons made in this study, some significant findings may be attributable to chance alone. Strengths of this study include high‐quality measurements of BP that were conducted over a decade or more at regular study visits rather than sporadically in the course of clinical care, the use of sensitive, unbiased methods to detect AF, and the inclusion of data from 2 large, population‐based cohorts.
In conclusion, among older adults from 2 population‐based cohort studies, we found that information on BP assessed longitudinally for several years, especially visit‐to‐visit BP variability, was associated with the prevalence of subclinical atrial arrhythmias, above and beyond cross‐sectional BP values alone.
Sources of Funding
This MESA (Multi‐Ethnic Study of Atherosclerosis) research was supported by contracts 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 and grant R01HL127659 from the National Heart, Lung, and Blood Institute; and by grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from the National Center for Advancing Translational Sciences. Dr Harding is supported by a National Heart, Lung, and Blood Institute grant T32HL007828. The ARIC (Atherosclerosis Risk in Communities) study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract Nos. HHSN268201700001I, HHSN268201700002I,, HHSN268201700003I,, HHSN268201700005I,, and HHSN268201700004I. This work is additionally supported by R01 HL126637 (Dr Chen).
Disclosures
Dr Psaty serves on the Steering Committee of the Yale Open Data Access Project, funded by Johnson & Johnson. Dr Floyd has consulted for Shionogi Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figure S1–S2
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA (Multi‐Ethnic Study of Atherosclerosis) and ARIC (Atherosclerosis Risk in Communities) study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa‐nhlbi.org. A full list of ARIC study investigators and institutions can be found at https://sites.cscc.unc.edu/aric/PIs_and_Study_Sites.
(J Am Heart Assoc. 2021;10:e020260. DOI: 10.1161/JAHA.120.020260.)
For Sources of Funding and Disclosures, see pages 10 and 11.
REFERENCES
- 1.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82:2N–9N. DOI: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham heart study. Circulation. 2003;107:2920–2925. DOI: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 3.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. DOI: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. DOI: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tielemans SM, Geleijnse JM, Menotti A, Boshuizen HC, Soedamah‐Muthu SS, Jacobs DR Jr, Blackburn H, Kromhout D. Ten‐year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: the Minnesota business and professional men study and the Zutphen study. J Am Heart Assoc. 2015;4:e001378. DOI: 10.1161/JAHA.114.001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. DOI: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 7.Norby FL, Soliman EZ, Chen LY, Bengtson LG, Loehr LR, Agarwal SK, Alonso A. Trajectories of cardiovascular risk factors and incidence of atrial fibrillation over a 25‐year follow‐up: the ARIC study (atherosclerosis risk in communities). Circulation. 2016;134:599–610. DOI: 10.1161/CIRCULATIONAHA.115.020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maddox TM, Ross C, Tavel HM, Lyons EE, Tillquist M, Ho PM, Rumsfeld JS, Margolis KL, O'Connor PJ, Selby JV, et al. Blood pressure trajectories and associations with treatment intensification, medication adherence, and outcomes among newly diagnosed coronary artery disease patients. Circ Cardiovasc Qual Outcomes. 2010;3:347–357. DOI: 10.1161/CIRCOUTCOMES.110.957308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit‐to‐visit variability in systolic blood pressure and all‐cause mortality in the general population: findings from NHANES iii, 1988 to 1994. Hypertension. 2011;57:160–166. DOI: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 10.Suchy‐Dicey AM, Wallace ER, Mitchell SV, Aguilar M, Gottesman RF, Rice K, Kronmal R, Psaty BM, Longstreth WT Jr. Blood pressure variability and the risk of all‐cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens. 2013;26:1210–1217. DOI: 10.1093/ajh/hpt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Brown MD. Relationship of visit‐to‐visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 2012;35:55–61. DOI: 10.1038/hr.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Shlipak MG, Stawski RS, Peralta CA, Psaty BM, Harris TB, Satterfield S, Shiroma EJ, Newman AB, Odden MC, et al. Visit‐to‐visit blood pressure variability and mortality and cardiovascular outcomes among older adults: the health, aging, and body composition study. Am J Hypertens. 2017;30:151–158. DOI: 10.1093/ajh/hpw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–256. DOI: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glynn RJ, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Arch Intern Med. 2000;160:2765–2772. DOI: 10.1001/archinte.160.18.2765. [DOI] [PubMed] [Google Scholar]
- 15.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? Circulation. 1999;100:354–360. DOI: 10.1161/01.CIR.100.4.354. [DOI] [PubMed] [Google Scholar]
- 16.Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–728. DOI: 10.7326/0003-4819-159-11-201312030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engstrom G, Hedblad B, Juul‐Moller S, Tyden P, Janzon L. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke. 2000;31:2925–2929. DOI: 10.1161/01.STR.31.12.2925. [DOI] [PubMed] [Google Scholar]
- 18.Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. DOI: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. DOI: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Heckbert SR, Austin TR, Jensen PN, Floyd JS, Psaty BM, Soliman EZ, Kronmal RA. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: the multi‐ethnic study of atherosclerosis. J Electrocardiol. 2018;51:997–1002. DOI: 10.1016/j.jelectrocard.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The ARIC investigators . The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. DOI: 10.1093/oxfordjournals.aje.a115184. [DOI] [PubMed] [Google Scholar]
- 22.Rooney MR, Soliman EZ, Lutsey PL, Norby FL, Loehr LR, Mosley TH, Zhang M, Gottesman RF, Coresh J, Folsom AR, et al. Prevalence and characteristics of subclinical atrial fibrillation in a community‐dwelling elderly population: the ARIC study. Circ Arrhythm Electrophysiol. 2019;12:e007390. DOI: 10.1161/CIRCEP.119.007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey M 3rd. Blood pressure monitoring: automated oscillometric devices. J Clin Monit. 1991;7:56–67. DOI: 10.1007/BF01617900. [DOI] [PubMed] [Google Scholar]
- 24.Balakrishnan P, Beaty T, Young JH, Colantuoni E, Matsushita K. Methods to estimate underlying blood pressure: the atherosclerosis risk in communities (ARIC) study. PLoS One. 2017;12:e0179234.– 10.1371/journal.pone.0179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao S, Hendrie HC, Wang C, Stump TE, Stewart JC, Kesterson J, Clark DO, Callahan CM. Redefined blood pressure variability measure and its association with mortality in elderly primary care patients. Hypertension. 2014;64:45–52. DOI: 10.1161/HYPERTENSIONAHA.114.03576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang MJ, Roetker NS, Folsom AR, Alonso A, Heckbert SR, Chen LY. Feasibility of using a leadless patch monitor in community cohort studies: the multi‐ethnic study of atherosclerosis. Pacing Clin Electrophysiol. 2018;41:1389–1390. DOI: 10.1111/pace.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlesselman JJ, Collins JA. Evaluating systematic reviews and meta‐analyses. Semin Reprod Med. 2003;21:95–105. DOI: 10.1055/s-2003-39999. [DOI] [PubMed] [Google Scholar]
- 28.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. DOI: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 29.Ravindrarajah R, Hazra NC, Hamada S, Charlton J, Jackson SHD, Dregan A, Gulliford MC. Systolic blood pressure trajectory, frailty, and all‐cause mortality >80 years of age: cohort study using electronic health records. Circulation. 2017;135:2357–2368. DOI: 10.1161/CIRCULATIONAHA.116.026687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smitson CC, Scherzer R, Shlipak MG, Psaty BM, Newman AB, Sarnak MJ, Odden MC, Peralta CA. Association of blood pressure trajectory with mortality, incident cardiovascular disease, and heart failure in the cardiovascular health study. Am J Hypertens. 2017;30:587–593. DOI: 10.1093/ajh/hpx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman F, Yin X, Larson MG, Ellinor PT, Lubitz SA, Vasan RS, McManus DD, Magnani JW, Benjamin EJ. Trajectories of risk factors and risk of new‐onset atrial fibrillation in the Framingham heart study. Hypertension. 2016;68:597–605. DOI: 10.1161/HYPERTENSIONAHA.116.07683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D, Yang P‐S, Kim T‐H, Jang E, Shin H, Kim HY, Yu HT, Uhm J‐S, Kim J‐Y, Pak H‐N, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72:1233–1245. DOI: 10.1016/j.jacc.2018.05.076. [DOI] [PubMed] [Google Scholar]
- 33.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. JAMA. 1994;271:840–844. DOI: 10.1001/jama.1994.03510350050036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1–S2


