Abstract
Background
Statin‐mediated efficacy of lowering low‐density lipoprotein (LDL) cholesterol varies in each individual, and its diminished response is associated with worse outcomes. However, there is no established approach to predict hyporesponse to statins. PCSK9 (proprotein convertase subxilisin/kexin type 9) is a serine‐protease associated with LDL metabolism, which circulates as mature and furin‐cleaved PCSK9. Since mature PCSK9 more potently degrades the LDL receptor, its evaluation may enable the identification of statin hyporesponders.
Methods and Results
We analyzed 101 statin‐naive patients with coronary artery disease who commenced a statin. PCSK9 subtypes at baseline and 1 month after statin use were measured by ELISA. Hyporesponse to statins was defined as a percent reduction in LDL cholesterol <15%. The relationship between each PCSK9 subtype level and hyporesponse to statins was investigated. Statins significantly lowered LDL cholesterol level (percent reduction, 40%±21%), whereas 11% of study participants exhibited a hyporeseponse to statins. Multivariable logistic regression analysis demonstrated that baseline mature PCSK9 level was an independent predictor for hyporesponse to statins even after adjusting clinical characteristics (mature PCSK9 per 10‐ng/mL increase: odds ratio [OR], 1.12; 95% CI, 1.01–1.24 [P=0.03]), whereas furin‐cleaved level was not (per 10‐ng/mL increase: OR, 1.37; 95% CI, 0.73–2.58 [P=0.33]). Receiver operating characteristic curve analysis identified mature PCSK9 level of 228 ng/mL as an optimal cutoff to predict hyporesponse to statins (area under the curve, 0.73 [sensitivity, 0.91; specificity, 0.56]).
Conclusions
Baseline mature PCSK9 level >228 ng/mL is associated with hyporesponse to statins. This finding suggests that mature PCSK9 might be a potential determinant of hyporesponse to statins.
Keywords: LDL‐C, proprotein convertase subxilisin/kexin type 9, statin
Subject Categories: Lipids and Cholesterol, Pathophysiology
Nonstandard Abbreviations and Acronyms
- LDLR
low‐density lipoprotein receptor
- PCSK9
proprotein convertase subtilisin/kexin type 9
- rhPCSK9
recombinant human PCSK9
Clinical Perspective
What Is New?
In statin‐naive patients, an elevated mature PCSK9 (proprotein convertase subtilisin/kexin type 9) level, but not a furin‐cleaved level, is significantly associated with hyporesponse to statins.
What Are the Clinical Implications?
Evaluation of mature PCSK9 level might be a useful approach to predict response to a statin.
Achieving a lower level of low‐density lipoprotein (LDL) cholesterol (LDL‐C) with a lipid‐lowering drug has become a cornerstone for the prevention of atherosclerotic cardiovascular disease.1, 2, 3 Statins are a major therapeutic agent used to lower LDL‐C levels by inhibiting 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase, which induces reduced secretion of apolipoprotein B–containing lipoproteins from the liver and the upregulation of LDL receptor (LDLR) activity.4 This agent has been shown to lower LDL‐C level by approximately 30% to 50%.5, 6 However, the extent of statin‐mediated LDL‐C lowering is not consistent among each individual. Recent studies reported that 11% to 20% of patients receiving a statin exhibited its diminished response and an elevated cardiovascular risk.5, 7 In addition, hyporesponse to a statin defined as a percent reduction of LDL‐C <15% was associated with substantial atheroma progression.7 These observations suggest the importance to elucidate factors causing this poor response to a statin, which may enable us to identify hyporesponders to statin therapy and then allocate adequate lipid‐lowering therapy.
LDL metabolism is regulated by PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 is a protease that regulates LDL metabolism by degrading LDLR,8, 9 suggesting that the increased level of PCSK9 may attenuate the response to a statin. However, recent studies did not show any association of PCSK9 level with a change in on‐treatment LDL‐C following statin therapy.10, 11 Pathophysiologically, PCSK9 exists as mature and furin‐cleaved forms in circulation. Several basic studies have demonstrated that mature PCSK9 has the ability to degrade LDLR, whereas furin‐cleaved level has little activity modulating LDLR.12, 13, 14 This observation leads to the hypothesis that the evaluation of these two subtypes may help to predict poor response to a statin. Therefore, the current study aimed to investigate the association of two PCSK9 subtypes with the degree of LDL‐C lowering after treatment with a statin.
METHODS
Because of the sensitive nature of the data collected for this study, requests to access the data set from other researchers will require the approval from the corresponding author and the research ethics committee.
Study Design and Protocol
The current investigation is a single‐center prospective observational study designed to evaluate whether two PCSK9 subtypes are associated with the degree of lowering LDL‐C under statin therapy. The inclusion criteria included: (1) patients aged ≥18 years; (2) statin‐naive patients; (3) patients with acute coronary syndrome or stable coronary artery disease (CAD); and (4) patients with a clinical indication to commence statin treatment according to Japanese guidelines.15, 16 This study excluded patients who were previously treated with any lipid‐lowering therapy. During the study period (from February 2018 to November 2019), we screened a total of 127 statin‐naive patients with CAD at the National Cerebral and Cardiovascular Center, Suita, Japan (Figure 1). Of these, the following numbers of patients were excluded from analysis: 10 patients did not agree to enroll in the study; and 4 patients discontinued a statin before the 1‐month visit because of a statin‐related side effect. Ezetimibe was added to the statin therapy before the 1‐month visit in 7 patients. There were no data about lipid parameters and PCSK9 level in 5 patients. As a consequence, the remaining 101 statin‐naive patients with CAD were included into the current analysis (Figure 1).
Figure 1. Study design.
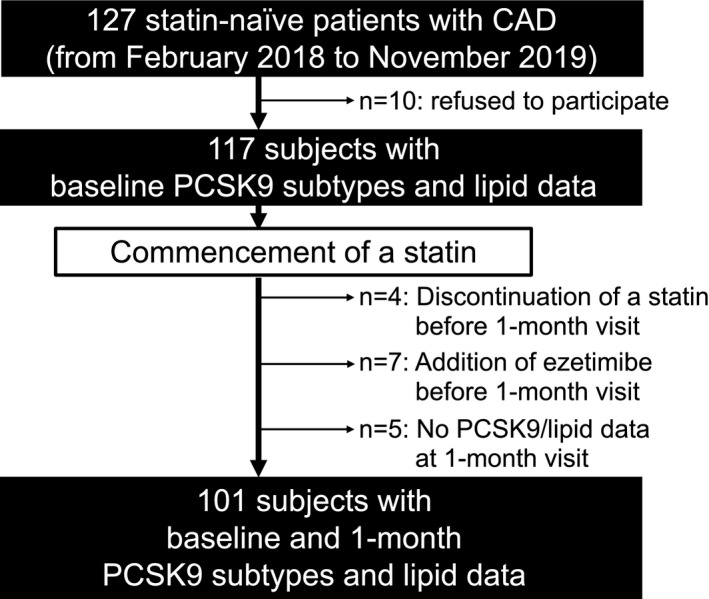
CAD indicates coronary artery disease; and PCSK9, proprotein convertase subtilisin/kexin type 9.
With regard to the study protocol, after informed consent was obtained, a blood sample was collected before the commencement of a statin. The blood sample was drawn from a peripheral vein in the morning after overnight fasting. It was stored at a temperature of −80°C within 2 hours after blood collection. A fixed dose of a statin was commenced and then continued for 1 month. The selection of the statin and its dose was left to each physician's discretion. High‐intensity statin use was defined as rosuvastatin ≥10 mg, pitavastatin ≥4 mg, or atorvastatin ≥20 mg.17 Other medical therapies except nonstatin lipid‐lowering agents were allowed to be concomitantly used during the study period. Clinical follow‐up was performed at 1 month after the therapy, and fasting blood samples were collected again. This study was approved by the institutional review board of the National Cerebral and Cardiovascular Center, Suita, Japan (M28‐119‐4). Each patient gave written informed consent to participate in the study. All clinical investigations were conducted in accordance with the principles of the Declaration of Helsinki.
PCSK9 Subtypes' Measurements and Evaluation of Lipid Parameters
Mature and furin‐cleaved PCSK9 concentrations were measured at baseline and 1 month after the commencement of a statin in the stored serum samples using an ELISA (BML, Inc).18, 19, 20, 21 This novel sandwich ELISA has been developed by our coauthors (M.H‐S. and M.H.).18 It enables us to quantitatively measure PCSK9 subtypes by using monoclonal antibodies. The ELISA is characterized by using purified rhPCSK9 (recombinant human PCSK9) or cell lysate of rh⊿218PCSK9 as well as plasma samples.18 Calibration curves in the ELISA for total and mature PCSK9, rhPCSK9 protein, as a primary calibrator and rhPCSK9 culture medium, as a secondary calibrator are obtained.18 The interassay and intra‐assay coefficients of variance to measure each PCSK9 value are as follows: mature PCSK9, 7.7% and 2.2%, and furin‐cleaved PCSK9, 5.6% and 2.1%, respectively. The lower and upper detection limits of mature and furin‐cleaved PCSK9 were 3.9 and 20 000 ng/mL, and 0.7 and 300 ng/mL, respectively.19
LDL‐C level was calculated by its direct measurement as previously reported.22, 23, 24 Hyporesponse to a statin was defined as percent reduction in LDL‐C <15% according to the published article.6 Fasting serum levels of total cholesterol and high‐density lipoprotein cholesterol were measured by enzymatic methods (Sekisui Medical) using an automated analyzer (Hitachi Labospect 008; Hitachi‐Hitec).
Statistical Analysis
Continuous variables were expressed as mean±SD and compared using t test if data were normally distributed. Non‐normally distributed continuous data were summarized as median (interquartile range) and compared using Wilcoxon signed rank test. Spearman correlation coefficient test was used to examine the relationship between the percent reduction of LDL‐C and the percent change in each PCSK9 subtypes' concentration. Logistic regression analysis was conducted to elucidate the association of each PCSK9 subtypes' level with a hyporsponse to a statin. A multivariable logistic regression analysis was performed to evaluate the relationship between each PCSK9 subtypes' level and a hyporeponse to a statin using the following 2 models: (1) adjusted by age and sex; and (2) adjusted by age, sex, hypertension, diabetes mellitus, estimated glomerular filtration rate, types of statin, and high‐intensity statin use. Receiver operating characteristic curve analysis was performed to assess the ability of mature PCSK9 level for the prediction of a hyporesponse to a statin after statin therapy. All P values <0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute).
RESULTS
Clinical Demographics of Study Participants
Clinical characteristics of the study participants are shown in Table 1. Patients had a median age of 72 years, 79% were men, and they had a high prevalence of coronary risk factors (hypertension 68%, type 2 diabetes mellitus 49%, dyslipidemia 50%, and smoking 27%). Furthermore, 70% of the study participants presented with acute coronary syndrome, and multivessel disease was observed in 45% of the study population. Percutaneous coronary intervention was performed in 81% of the study population, and the remaining patients were medically managed.
Table 1.
Baseline Clinical Demographics (N=101)
| Age, y | 72 (61–77) |
| Men, n (%) | 80 (79) |
| BMI, kg/m2 | 23±3 |
| Hypertension, n (%) | 69 (68) |
| Diabetes mellitus, n (%) | 49 (49) |
| Dyslipidemia, n (%) | 51 (50) |
| Chronic kidney disease, n (%) | 32 (32) |
| Smoking, n (%) | 27 (27) |
| History of myocardial infarction, n (%) | 6 (6) |
| History of stroke, n (%) | 5 (5) |
| History of PAD, n (%) | 4 (4) |
| CAD type, n (%) | |
| Acute coronary syndrome | 71 (70) |
| Stable CAD | 30 (30) |
| Multivessel disease | 44 (45) |
| Biochemistry data | |
| Glycated hemoglobin, % | 5.9 (5.6–6.3) |
| eGFR, mL/min per 1.73m2 | 68±21 |
| Systolic BP, mm Hg | 124±18 |
| Diastolic BP, mm Hg | 71±14 |
| Baseline medication use, n (%) | |
| Aspirin | 7 (7) |
| ACEI/ARB | 18 (18) |
| β‐Blocker | 10 (9) |
| Concomitant medications use | |
| Lipid‐lowering therapy, n (%) | |
| Statin | 101 (100) |
| Rosuvastatin | 77 (76) |
| Pitavastatin | 16 (16) |
| Atorvastatin | 8 (8) |
| High‐intensity statin use* | 55 (54) |
| Other established medication, n (%) | |
| Aspirin | 89 (88) |
| Clopidogrel | 27 (27) |
| Prasugrel | 55 (54) |
| ACEI/ARB | 66 (65) |
| β‐Blocker | 55 (54) |
Continuous variables are expressed as mean±SD, and non‐normally distributed continuous data are summarized as median (interquartile range).
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blockers; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; and PAD, peripheral arterial disease.
Rosuvastatin ≥10 mg, pitavastatin ≥4 mg, and atorvastatin ≥20 mg.
With regard to statin therapy, rosuvastatin was used in >70% of study patients, whereas the frequency of pitavastatin and atorvastatin use was 16% and 8%, respectively. Fifty‐four percent of patients received a high‐intensity statin. In addition to statin therapy, established medical therapies were concomitantly used at 1 month after baseline as follows: aspirin, 88%; angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, 65%; and β‐blocker, 54%. Since a drug‐eluting stent was implanted in 81% of patients, a P2Y12 inhibitor was used in addition to aspirin (clopidogrel 27%, prasugrel 54%).
LDL‐C Control Following Commencement of a Statin
The serial change in lipid parameters is summarized in Table 2. As expected, a significant reduction of LDL‐C level was observed at 1 month after statin therapy (P<0.001), which corresponded to a percent reduction of 40%±21%. Furthermore, 52% of study participants achieved LDL‐C <1.8 mmol/L at 1 month. By contrast, despite the commencement of a statin, 11% of study patients exhibited a percent reduction of LDL‐C <15%, suggesting a hyporesponse to the therapy. In addition to lowering LDL‐C, statins significantly reduced participants' total cholesterol level and increased their high‐density lipoprotein cholesterol level (P<0.001 and P=0.003, respectively).
Table 2.
Serial Change in Lipid Parameters
|
Baseline (n=101) |
1 mo (n=101) |
P Value | |
|---|---|---|---|
| LDL‐C, mmol/L | 3.1±0.9 | 1.8±0.5 | <0.001 |
| Achieved LDL‐C <1.8 mmol/L, n (%) | … | 53 (52) | … |
| Percent reduction in LDL‐C (%) | … | 40±21 | … |
| Percent reduction in LDL‐C<15%, n (%) | … | 11 (11) | … |
| Total cholesterol, mmol/L | 5.0±1.0 | 3.7±0.6 | <0.001 |
| HDL‐C, mmol/L | 1.2 (1.0–1.5) | 1.2 (1.1–1.6) | 0.003 |
| Mature PCSK9, ng/mL | 225 (187–284) | 312 (265–376) | <0.001 |
| Furin‐cleaved PCSK9, ng/mL | 22 (17–28) | 26 (19–32) | <0.001 |
Continuous variables are expressed as mean±SD, and non‐normally distributed continuous data are summarized as median (interquartile range).
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and PCSK9, proprotein convertase subtilisin/kexin type 9.
Mature and Furin‐Cleaved Levels Under Statin Therapy
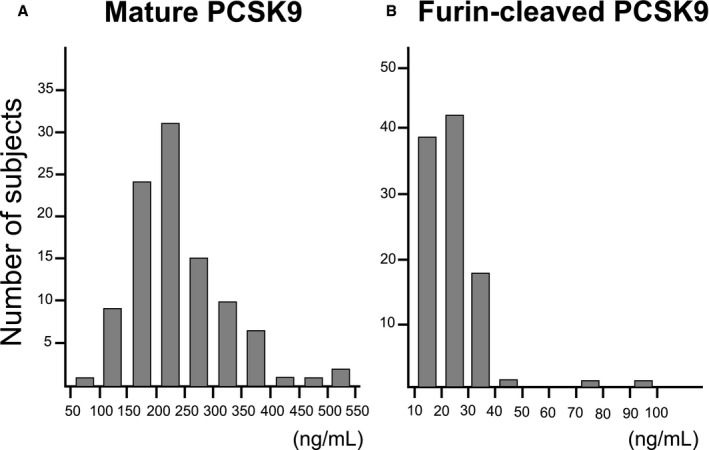
In the current study population, median mature and furin‐cleaved PCSK9 levels at baseline were 225 ng/mL and 22 ng/mL, respectively. The distribution of each PCSK9 subtype concentration is shown in Figure 2. Following the commencement of a statin, a significant increase of mature and furin‐cleaved PCSK9 levels was observed at 1 month (Table 2).
Figure 2. The distribution of two PCSK9 (proprotein convertase subtilisin/kexin type 9) subtypes' concentrations. The histograms of each PCSK9 subtypes' concentration.
Association Between PCSK9 Subtype Levels and Hyporesponse to a Statin
Logistic regression analyses were conducted to evaluate the association of each PCSK9 subtypes' level with a hyporesponse to a statin under the 1‐month statin use (Table 3). In the unadjusted model, the baseline mature PCSK9 level was significantly associated with a hyporesponse to a statin (odds ratio [OR], 1.08; 95% CI, 1.01–1.15 [P=0.02]; per 10‐ng/mL increase), whereas the baseline furin‐cleaved PCSK9 level at baseline was not (OR, 1.37; 95% CI, 0.88–2.11 [P=0.16]; per 10‐ng/mL increase). In the multivariable models, the baseline mature PCSK9 level was still an independent determinant predicting a hyporesponse to a statin, even after adjusting by age and sex (OR, 1.11; 95% CI, 1.03–1.20 [P=0.009]), and adjusted by age, sex, hypertension, diabetes mellitus, estimated glomerular filtration rate, high‐intensity statin use, and its types (OR, 1.12; 95% CI, 1.01–1.24 [P=0.03]).
Table 3.
Logistic Regression Analysis for Predicting Hyporesponse to Statins (Percent Reduction in LDL‐C <15%) by Each PCSK9 Subtypes' Concentration
| Unadjusted Model | Age‐ and Sex‐Adjusted Model | Multivariate‐Adjusted Model* | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Mature PCSK9 (per 10, ng/mL) | 1.08 (1.01–1.15) | 0.02 | 1.11 (1.03–1.20) | 0.009 | 1.12 (1.01–1.24) | 0.03 |
| Furin‐cleaved PCSK9 (per 10, ng/mL) | 1.37 (0.88–2.11) | 0.16 | 1.38 (0.89–2.15) | 0.15 | 1.37 (0.73–2.58) | 0.33 |
LDL‐C indicates low‐density lipoprotein cholesterol; OR, odds ratio; and PCSK9, proprotein convertase subtilisin/kexin type 9.
Adjusted by sex, age, hypertension, diabetes mellitus, estimated glomerular filtration rate, type of statin, and high‐intensity statin use.
Receiver operating characteristics curve analysis elucidated the optimal cutoff value of the baseline mature PCSK9 to predict a hyporesponse to a statin as 228 ng/mL (area under the curve, 0.73 [sensitivity, 91%; specificity, 56%]) (Figure 3). With regard to the association between percent change in each PCSK9 subtype and the degree of statin‐mediated LDL‐C reduction, both the percent change in mature and furin‐cleaved PCSK9 did not exhibit any significant relationships with the percent reduction of LDL‐C (Figure S1).
Figure 3. Receiver operating characteristics analysis for the baseline mature PCSK9 (proprotein convertase subtilisin/kexin) level to predict a hyporesponse to a statin (defined as percent reduction of low‐density lipoprotein cholesterol <15%).
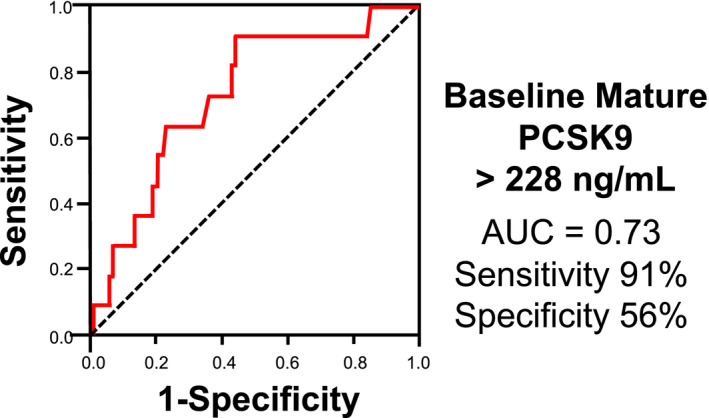
AUC indicates area under the curve.
DISCUSSION
While numerous large‐scale clinical trials have consistently demonstrated the clinical benefit of a statin to reduce the frequency of atherosclerotic cardiovascular disease,1, 5, 6 its efficacy to lower LDL‐C differs in each individual, and some patients do not fully respond to the therapy.5, 25 In the present study, each of two PCSK9 subtypes exhibited different properties in the degree of lowering LDL‐C under statin use. In particular, an elevated mature PCSK9 level was associated with an attenuated efficacy for the statin to lower LDL‐C level, whereas an elevated furin‐cleaved level was not. These findings indicate the association of mature PCSK9 with LDL metabolism under statin therapy.
In the current analysis, the degree of LDL‐C lowering with a statin was associated with an elevation of mature PCSK9 level but not furin‐cleaved level. This finding further supports the different properties of each PCSK9 subtype in vivo. PCSK9 is initially produced as an ≈75 kDa proprotein. Mature PCSK9 is formed by removal of the signal peptide and secreted consisting of a prodomain, catalytic domain, and C‐terminal domain.25 The prodomain is associated with the C‐terminal domain after autocatalytic cleavage, and PCSK9 has degradation activity of LDLR not by its proteolytic active site but by binding of its catalytic domain and prosegment with the epidermal growth factor A of LDLR.26 Given that statins lower LDL‐C level through the overexpression of LDLR at the surface of a hepatocyte,27 an increased level of mature PCSK9 could attenuate the efficacy of a statin, resulting in hyporesponse. With regard to furin‐cleaved PCSK9, furin cleaves some of the mature PCSK9 at the 218 RFHR position of the catalytic domain, resulting in prosegment detachment and loss of binding ability to LDLR.12 This property could account for the absence of an association between furin‐cleaved PCSK9 and the extent of LDL‐C lowering.
The current study provides insights into the association of a statin‐mediated PCSK9 increase with its response. Statins increase the activity and nuclear translocation of sterol regulatory element‐binding protein 2, which activates PCSK9 genes.26, 27 In our analysis, following commencement of a statin, a significant elevation of both mature and furin‐cleaved PCSK9 levels was observed. In particular, mature PCSK9 was still a dominant subtype in circulation under statin therapy. However, these increases in PCSK9 subtypes' concentrations did not necessarily affect the magnitude of LDL‐C lowering. Circulating LDL‐C level generally depends on the balance between cholesterol synthesis and its clearance. The commencement of a statin triggers reduced cholesterol synthesis, LDLR overexpression, and the secretion of PCSK9 subtypes.10, 28, 29 Since some circulating PCSK9, especially the mature form, can bind to LDLR and then both removes from the circulation, the increased amount of PCSK9 subtypes under the therapy may not invariably reflect the true degree of its statin‐mediated secretion. Therefore, evaluating the PCSK9 subtypes' levels before statin use may be a better clinically applicable tool to estimate the extent of LDL‐C reduction following statin use.
Predicting a poor response to a statin by evaluating the PCSK9 subtypes may help to customize a lipid‐lowering therapy for each individual. Recently, an injectional agent for PCSK9 modulation has been used to substantially lower LDL‐C levels. While its favorable efficacy on atherosclerotic cardiovascular disease has been demonstrated,30, 31 its high medication cost has been a barrier to patients requiring more potent agents. Our observation suggests that measuring the mature PCSK9 level might guide physicians to select potent lipid‐lowering agent. In addition, this approach may have great potential for statin hyporesponders to better understand the expected effect of a statin, its related‐future cardiovascular risks, and the need for additional lipid‐lowering drugs.
Several caveats should be noted. First, this investigation was a single‐center study, and the number of poor responses to a statin was relatively small. Second, the type and dose of a statin was selected by each physician, which may be a potential bias. Third, the approved maximum dose of statins (atorvastatin 20 mg, rosuvastatin 10 mg, and pitavastatin 4 mg) in Japan is lower than those in Europe and the United States. Therefore, the current study does not evaluate the association of PCSK9 with LDL‐C level under the guideline‐recommended high‐intensity statin regimen (atorvastatin 40 mg to 80 mg and rosuvastatin 40 mg) in other countries. However, all of the study population exhibited CAD at baseline, and therefore physicians commenced an adequate statin dose and intensity following the cholesterol guideline from the Japan Atherosclerosis Society.16 Fourth, adherence to statin therapy was assessed by patients' self‐reports and not by research nurses. Hence, there is a possibility that poor adherence might have existed in some patients, which may have affected their response to a statin.
CONCLUSIONS
The baseline mature PCSK9 level predicted a hyporesponse to statin therapy in patients with CAD, whereas the furin‐cleaved level did not. As expected, all of these PCSK9 levels significantly increased at 1 month after the therapy commenced. However, this statin‐mediated increase in PCSK9 did not help to estimate response to a statin. Our findings reveal that mature PCSK9 level before statin treatment might be an important marker to identify statin hyporesponders who require more potent lipid‐lowering therapy.
Sources of Funding
This study was supported by the Japan Research Foundation for Clinical Pharmacology (grant number: H29).
Disclosures
Yu Kataoka has received research support from Nipro and Abbott, and honoraria from Nipro, Abbott, Kowa, Amgen, Sanofi, Astellas, Takeda, and Daiichi‐Sankyo. The remaining authors have no disclosures to report.
Supporting information
Figure S1
Acknowledgments
We thank the Biobank at the National Cerebral and Cardiovascular Center for their assistance with blood sample storage.
(J Am Heart Assoc. 2021;10:e019525. DOI: 10.1161/JAHA.120.019525.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019525.
For Sources of Funding and Disclosures, see page 7.
References
- 1.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. DOI: 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 2.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart J‐C, Gotto AM, Greten H, Kastelein JJP, Shepherd J, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. DOI: 10.1056/NEJMoa050461 [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies C, Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta‐analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370:1829–1839. DOI: 10.1016/S0140-6736(07)61778-4 [DOI] [PubMed] [Google Scholar]
- 4.Endo A. The discovery and development of HMG‐CoA reductase inhibitors. J Lipid Res. 1992;5:67–80. [PubMed] [Google Scholar]
- 5.Ridker PM, Mora S, Rose L, Group JTS . Percent reduction in LDL cholesterol following high‐intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid‐lowering agents. Eur Heart J. 2016;37:1373–1379. DOI: 10.1093/eurheartj/ehw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. DOI: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kataoka Y, St John J, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen SE, Nicholls SJ. Atheroma progression in hyporesponders to statin therapy. Arterioscler Thromb Vasc Biol. 2015;35:990–995. DOI: 10.1161/ATVBAHA.114.304477 [DOI] [PubMed] [Google Scholar]
- 8.Bergeron N, Phan BA, Ding Y, Fong A, Krauss RM. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation. 2015;132:1648–1666. DOI: 10.1161/CIRCULATIONAHA.115.016080 [DOI] [PubMed] [Google Scholar]
- 9.Seidah NG, Awan Z, Chretien M, Mbikay M. Pcsk9: a key modulator of cardiovascular health. Circ Res. 2014;114:1022–1036. DOI: 10.1161/CIRCRESAHA.114.301621 [DOI] [PubMed] [Google Scholar]
- 10.Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. High‐dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res. 2010;51:2714–2721. DOI: 10.1194/jlr.M008144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakoski SG, Xu F, Vega GL, Grundy SM, Chandalia M, Lam C, Lowe RS, Stepanavage ME, Musliner TA, Cohen JC, et al. Indices of cholesterol metabolism and relative responsiveness to ezetimibe and simvastatin. J Clin Endocrinol Metab. 2010;95:800–809. DOI: 10.1210/jc.2009-1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Essalmani R, Susan‐Resiga D, Chamberland A, Abifadel M, Creemers JW, Boileau C, Seidah NG, Prat A. In vivo evidence that furin from hepatocytes inactivates PCSK9. J Biol Chem. 2011;286:4257–4263. DOI: 10.1074/jbc.M110.192104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjannet S, Rhainds D, Hamelin J, Nassoury N, Seidah NG. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post‐translational modifications. J Biol Chem. 2006;281:30561–30572. DOI: 10.1074/jbc.M606495200 [DOI] [PubMed] [Google Scholar]
- 14.Han B, Eacho PI, Knierman MD, Troutt JS, Konrad RJ, Yu X, Schroeder KM. Isolation and characterization of the circulating truncated form of PCSK9. J Lipid Res. 2014;55:1505–1514. DOI: 10.1194/jlr.M049346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, Sakamoto T, Tsujita K, Hagiwara N, Miyazaki S, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J. 2019;83:1085–1196. DOI: 10.1253/circj.CJ-19-0133 [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25:846–984. DOI: 10.5551/jat.GL2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura M, Uno K, Hirayama A, Ako J, Nohara A, Arai H, Harada‐Shiba M. Exploration into lipid management and persistent risk in patients hospitalised for acute coronary syndrome in Japan (EXPLORE‐J): protocol for a prospective observational study. BMJ Open. 2017;7:e014427. DOI: 10.1136/bmjopen-2016-014427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori M, Ishihara M, Yuasa Y, Makino H, Yanagi K, Tamanaha T, Kishimoto I, Kujiraoka T, Hattori H, Harada‐Shiba M. Removal of plasma mature and furin‐cleaved proprotein convertase subtilisin/kexin 9 by low‐density lipoprotein‐apheresis in familial hypercholesterolemia: development and application of a new assay for PCSK9. J Clin Endocrinol Metab. 2015;100:E41–49. DOI: 10.1210/jc.2014-3066 [DOI] [PubMed] [Google Scholar]
- 19.Kataoka YU, Harada‐Shiba M, Nakao K, Nakashima T, Kawakami S, Fujino M, Kanaya T, Nagai T, Tahara Y, Asaumi Y, et al. Mature proprotein convertase subtilisin/kexin type 9, coronary atheroma burden, and vessel remodeling in heterozygous familial hypercholesterolemia. J Clin Lipidol. 2017;11:e413. DOI: 10.1016/j.jacl.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 20.Nakamura A, Kanazawa M, Kagaya Y, Kondo M, Sato K, Endo H, Nozaki E. Plasma kinetics of mature PCSK9, furin‐cleaved PCSK9, and Lp(a) with or without administration of PCSK9 inhibitors in acute myocardial infarction. J Cardiol. 2020;76:395–401. DOI: 10.1016/j.jjcc.2020.04.006 [DOI] [PubMed] [Google Scholar]
- 21.Nozue T, Hattori H, Ogawa K, Kujiraoka T, Iwasaki T, Hirano T, Michishita I. Correlation between serum levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) and atherogenic lipoproteins in patients with coronary artery disease. Lipids Health Dis. 2016;15:165. DOI: 10.1186/s12944-016-0339-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the Friedewald equation for estimating low‐density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–2068. DOI: 10.1001/jama.2013.280532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Jang S, Jeong H, Ryu OH. Validation of the Friedewald formula for estimating low density lipoprotein cholesterol: the Korea National Health and Nutrition Examination Survey, 2009 to 2011. Korean J Intern Med. 2020;35:150–159. DOI: 10.3904/kjim.2017.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer MK, Barter PJ, Lundman P, Nicholls SJ, Toth PP, Karlson BW. Comparing a novel equation for calculating low‐density lipoprotein cholesterol with the Friedewald equation: a voyager analysis. Clin Biochem. 2019;64:24–29. DOI: 10.1016/j.clinbiochem.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 25.Akyea RK, Kai J, Qureshi N, Iyen B, Weng SF. Sub‐optimal cholesterol response to initiation of statins and future risk of cardiovascular disease. Heart. 2019;105:975–981. DOI: 10.1136/heartjnl-2018-314253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown MS, Goldstein JL. A receptor‐mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. DOI: 10.1126/science.3513311 [DOI] [PubMed] [Google Scholar]
- 27.Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–398. DOI: 10.1194/jlr.M700437-JLR200 [DOI] [PubMed] [Google Scholar]
- 28.Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, Prat A. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis‐regulated convertase‐1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004;24:1454–1459. DOI: 10.1161/01.ATV.0000134621.14315.43 [DOI] [PubMed] [Google Scholar]
- 29.Dong B, Wu M, Li H, Kraemer FB, Adeli K, Seidah NG, Park SW, Liu J. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL‐cholesterol lowering effect of statins in dyslipidemic hamsters. J Lipid Res. 2010;51:1486–1495. DOI: 10.1194/jlr.M003566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. DOI: 10.1056/NEJMoa1501031 [DOI] [PubMed] [Google Scholar]
- 31.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. DOI: 10.1056/NEJMoa1500858 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


