Abstract
Background
In pediatric cardiac surgery, perioperative management has evolved from slow weaning of mechanical ventilation in the intensive care unit to “ultra‐fast‐track” anesthesia with early extubation (EE) in theater to promote a faster recovery. The strategy of EE has not been assessed in adults with congenital heart disease, a growing population of patients who often require surgery.
Methods And Results
Data were collected retrospectively on all patients >16 years of age who underwent adult congenital heart surgery in our tertiary center between December 2012 and January 2020. Coarsened exact matching was performed for relevant baseline variables. Overall, 711 procedures were performed: 133 (18.7%) patients underwent EE and 578 (81.3%) patients received conventional extubation. After matching, patients who received EE required less inotropic or vasopressor support in the early postoperative period (median Vasoactive‐inotropic score 0.5 [0.0–2.0] versus 2.0 [0.0–3.5]; P<0.0001) and had a lower total net fluid balance than patients after conventional extubation (1168±723 versus 847±733 mL; P=0.0002). The overall reintubation rate was low at 0.3%. EE was associated with a significantly shorter postoperative length of stay in higher dependency care units before a “step‐down” to ward‐based care (48 [45–50] versus 50 [47–69] hours; P=0.004). Lower combined intensive care unit and high dependency unit costs were incurred by patients who received EE compared with patients who received conventional extubation (£3949 [3430–4222] versus £4166 [3893–5603]; P<0.0001).
Conclusions
In adult patients undergoing surgery for congenital heart disease, EE is associated with a reduced need for postoperative hemodynamic support, a shorter intensive care unit stay, and lower health‐care‐related costs.
Keywords: adult congenital heart disease, cardiac surgery, coarsened exact matching, early extubation, health economics, health outcomes, propensity score matching
Subject Categories: Cardiovascular Surgery, Health Services, Quality and Outcomes, Cost-Effectiveness, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- ACHS
adult congenital heart surgery
- ACHD
adult congenital heart disease
- CE
conventional extubation
- EE
early extubation
- HDU
high dependency unit
- VIS
vasoactive‐inotropic score
Clinical Perspective
What Is New?
This study assessed the safety, clinical effectiveness, and healthcare cost implications of “ultra‐fast‐track” extubation in a large cohort of adult patients with congenital heart disease undergoing cardiac surgery at a specialist center matched with patients undergoing conventional management.
Early extubation was associated with a reduction in the length of postoperative intensive care and high dependency unit stay, reduced patient costs, and lower requirements for vasoactive, inotropic, and fluid support compared with a conventional extubation strategy.
What Are the Clinical Implications?
Findings from this study support the feasibility and potential safety of an early extubation strategy in adult patients with congenital heart disease with a wide range of congenital heart defects undergoing cardiac surgery.
The population of adults with congenital heart disease (ACHD) has grown steadily during the past several decades, surpassing the number of children with congenital heart disease (CHD) in many developed countries.1 Improvements in surgical and medical management have dramatically improved the survival of patients with CHD, with an increase in the prevalence of ACHD of great complexity.2, 3 This has resulted in an aging group of patients who, far from being cured, have a disease associated with life‐long morbidity. New or residual hemodynamic lesions, failing surgical prostheses, myocardial scarring, and pulmonary vascular disease manifest clinically as heart failure, arrhythmias, and the need for repeated surgical interventions. Optimizing anesthetic and critical care management, with streamlined care in the perioperative period, is essential to improve the outcomes of patients with CHD.
As part of the continuous evolution of anesthetic care for patients with CHD, a paradigm shift has occurred in pediatric cardiac surgery with a move from slow weaning of mechanical ventilation in the intensive care unit (ICU) to an “ultra‐fast‐track” management strategy involving early extubation (EE). This approach of EE in the operating theater is aimed at reducing ICU stay and promotes early ambulation and hospital discharge. Indeed, several studies have demonstrated that EE reduces the duration of ICU and overall hospital stays in the pediatric population without increases in morbidity and mortality, but with evidence of a decrease in postoperative pulmonary complications.4, 5, 6, 7
Careful selection of the pediatric patients suitable for EE is central to this approach, as patients undergoing more complex procedures may benefit from a more conservative, conventional extubation (CE) strategy.8, 9 However, even in children with unusual physiologies, such as patients undergoing total cavopulmonary connection, EE has been associated with improved postoperative hemodynamics, reduced postoperative complications, and earlier hospital discharge, whereas prolonged ventilation was associated with a longer hospital stay.10 EE has also been shown to be beneficial in pediatric patients with right ventricular pressure overload.
In patients with ACHD, little is known about the safety, clinical effectiveness, and healthcare cost implications of EE. We performed clinical and cost analyses of the effects of EE in a large ACHD cohort including a wide range of congenital heart defects and undergoing cardiac surgery at a specialist center.
Methods
The study was approved by the Health Research Authority, England, with a favorable review by a research ethics committee (Integrated Research Application System, IRAS identification 275809). Informed consent was not required given the retrospective nature of the data collection. To minimize the possibility of unintentionally sharing information that can be used to reidentify private information, in line with the conditions of the ethical approval, patient‐level data are not available for use outside of this study.
Study Participants
Data on all patients >16 years of age with ACHD who underwent cardiac surgery at our tertiary center between December 2012 and January 2020 were obtained and studied. Although a wide variety of surgical approaches may be adopted in ACHD surgery and may influence the perioperative course, the population presented consisted of patients presented in a specialist multidisciplinary team meeting where the indications and surgical approach were decided. Demographic and clinical information were extracted from the Royal Brompton and Harefield National Health Service Foundation Trust Clinical Informatics Department, which included consolidated data from the NICOR (National Institute for the Cardiovascular Outcomes Research) Congenital Audit data set. This was enriched with manual data collection from individual hospital records. The complexity of the underlying congenital heart defect was classified in accordance to the Bethesda disease complexity classification.11 The mortality risk related to each surgical procedure was estimated using a validated tool: the adult congenital heart surgery (ACHS) score.12 Survival status and date of death were collected through the health service computer system linked to the national database held by the Office for National Statistics.
Median and maximum daily vasoactive‐inotropic scores (VIS) were calculated for each patient as previously described.13 ICU and high dependency unit (HDU) total patient costs were retrieved by the hospital’s finance department. Length of postoperative high dependency (combined ICU and HDU) stay served as the primary study end point. Secondary outcome variables were the requirement of any postoperative inotropic and vasoactive support, the median and maximum VISs, the net fluid balance in the first 2 postoperative days, transfusion requirements on the day of surgery, complication rates, and ICU and HDU costs.
No differences in the perioperative care protocols were implemented during the studied period.
Statistical Analysis
Analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) and SAS software version 9.4m6 (SAS Institute Inc., Cary, NC). Continuous variables are presented as mean±SD or median and range (with the exception of ventilation time, which is given as median and interquartile range because of a small number of extreme outliers who remained ventilated for a prolonged period), whereas categorical variables are presented as absolute numbers, percentage of total, or both. Matching was achieved using 2 methods, coarsened exact matching followed by propensity score matching, to minimize bias, inefficiency, and model dependence.14 EE was used to define the treatment group with the following clinical variables used in the matching process: age, sex, CHD complexity (Bethesda score), New York Heart Association functional class, ACHS score, pulmonary hypertension, and the presence of active endocarditis. Coarsened exact matching was performed using the R package CEM. Propensity score matching was conducted in a 1:2 fashion where possible without replacement as a sensitivity analysis. A calliper value of 0.2 was used, equal to 0.2 of the SD of the logit of the propensity score. Overall model imbalance was assessed using standardized mean differences for propensity score matching. Multidimensional distribution of the data (L) was assessed for both methods. Propensity matching was performed using the R package MatchIt.
Comparisons between groups were performed using the Wilcoxon rank sum test for continuous variables and the chi‐squared test for categorical variables. For our primary end point, that is, the effect of EE on ICU stay, we used a generalized linear model with log‐gamma distribution, accounting for matched pairs and weighting. A 2‐sided P‐value of <0.05 indicated statistical significance.
Results
A total of 698 patients underwent 711 cardiac surgical procedures during a period of 7.1 years. The vast majority of patients underwent elective surgery (669, 94.1%). Overall, 133 (18.7%) procedures were followed by EE postoperatively. Baseline characteristics of the overall population are shown in Table 1. Mean age was 34.7±14.1 and 384 (54%) were male. The most common CHD diagnoses were tetralogy of Fallot (126, 17.7%) and bicuspid aortic valve disease (85, 12%; Table 2). Patients who were extubated early were more likely to be women (58% versus 43%; P=0.003) and underwent shorter (4.6 [3.8–5.8] versus 5.5 [4.4–7.2] hours; P<0.0001) and less‐complex procedures (ACHS score 0.2 [0.2–0.6] versus 0.6 [0.2–0.7]; P<0.0001) than those who underwent CE. Patients with previous sternotomies were less likely to be extubated earlier (14% versus 24%; P=0.0005) than those undergoing their first sternotomy. However, there was no difference between the EE and CE groups in CHD complexity, functional class, or systemic ventricular function.
Table 1.
Baseline Characteristics in the Overall Population
| Parameter |
All (n=711) |
Early Extubation (n=133) |
Conventional Extubation (n=578) |
P Value |
|---|---|---|---|---|
| Age, y | 34.7±14.1 | 35±14.7 | 34.6±13.9 | >0.1 |
| Male | 384 (54) | 56 (42.1) | 328 (56.7) | 0.003* |
| BMI, kg/m2 | 25.3±5.4 | 24.5±4.5 | 25.5±5.6 | 0.09 |
| Complexity | ||||
| Simple | 150 (21.1) | 24 (18) | 126 (21.8) | >0.1 |
| Moderate | 451 (63.4) | 90 (67.7) | 361 (62.5) | |
| Great | 110 (15.5) | 19 (14.3) | 91 (15.7) | |
| NYHA class | ||||
| I/II | 595 (83.7) | 121 (91) | 474 (82) | 0.02* |
| III/IV | 116 (16.3) | 12(9) | 104 (18) | |
| Systemic ventricular function | ||||
| Normal | 644 (90.6) | 126 (94.7) | 518 (89.6) | 0.02* |
| Mildly impaired | 10 (1.4) | 4 (3) | 6 (1) | |
| Moderately impaired | 54 (7.6) | 3 (2.3) | 51 (8.8) | |
| Severely impaired | 3 (0.4) | 0 | 3 (0.5) | |
| Pulmonary hypertension† | 155 (21.8) | 28 (21.1) | 127 (22) | >0.1 |
| Hemoglobin, g/L | 140±16 | 139±15 | 140±16 | >0.1 |
| eGFR, mL/min | 100 (21–245) | 99 (49–165) | 100 (21–245) | >0.1 |
| Redo sternotomy | 388 (54.6) | 54 (40.6) | 334 (57.8) | 0.0005* |
| Procedure duration, h | 5.3 (2.3–23.4) | 4.6 (2.4–8.2) | 5.5 (2.3–23.4) | <0.0001* |
| Elective surgery | 669 (94.1) | 129 (97) | 540 (93.4) | 0.54 |
Data are presented as number (percentage), mean±SD, or median (range). BMI indicates body mass index; eGFR, estimated glomerular filtration rate; and NYHA, New York Heart Association.
P<0.05.
Pulmonary hypertension defined as a pulmonary artery systolic pressure ≥31 mmHg on echocardiography.
Table 2.
Congenital Heart Disease Diagnosis and ACHS Scores in the Overall Population
|
All (n=711) |
EE (n=133) |
CE (n=669) |
|
|---|---|---|---|
| Primary diagnosis, % | |||
| ASD | 8.7 | 15.8 | 7.1 |
| VSD | 8.2 | 4.5 | 9.0 |
| AVSD | 6.8 | 6.8 | 6.7 |
| BAV | 12.0 | 5.3 | 13.5 |
| Valvular disease/OTO | 13.4 | 17.3 | 12.5 |
| PAPVC | 9.3 | 18.8 | 7.1 |
| Scimitar | 0.6 | 0.8 | 0.5 |
| Tetralogy of Fallot | 17.7 | 12.8 | 18.9 |
| Coarctation | 4.1 | 2.3 | 4.5 |
| Other aortic disease | 3.5 | 7.5 | 2.6 |
| TGA | 3.8 | 2.3 | 4.2 |
| ccTGA | 0.7 | 0.8 | 0.7 |
| Truncus | 0.7 | 0.8 | 0.7 |
| Ebstein’s anomaly | 3.7 | 0.8 | 4.3 |
| Pulmonary atresia | 3.2 | 1.5 | 3.6 |
| Univentricular/Fontan | 2.7 | 0.8 | 3.1 |
| Coronary artery anomaly | 1.0 | 0.8 | 1.0 |
| Other | 0.1 | 0.8 | 0.0 |
| ACHS score, n (%) | |||
| 0.1 | 58 (8.2) | 19 (14.3) | 39 (6.7) |
| 0.2 | 194 (27.3) | 51 (38.3) | 143 (24.7) |
| 0.3 | 20 (2.8) | 2 (1.5) | 18 (3.1) |
| 0.4 | 57 (8) | 10 (7.5) | 47 (8.1) |
| 0.5 | 27 (3.8) | 5 (3.8) | 22 (3.8) |
| 0.6 | 138 (19.4) | 25 (18.8) | 113 (19.6) |
| 0.7 | 66 (9.3) | 8 (6) | 58 (10) |
| 0.8 | 15 (2.1) | 2 (1.5) | 13 (2.2) |
| 0.9 | 24 (3.4) | 1 (0.8) | 23 (4) |
| 1.0 | 65 (9.1) | 5 (3.8) | 60 (10.4) |
| ≥1.4 | 47 (6.6) | 5 (3.8) | 42 (7.3) |
ACHS indicates adult congenital heart surgery; ASD, atrial septal defect; AVSD, atrioventricular septal defect; BAV, bicuspid aortic valve; ccTGA, congenitally corrected transposition of the great arteries ; CE, conventional extubation; EE, early extubation; OTO, outflow tract obstruction; PAPVC, partial anomalous pulmonary venous connection; TGA, transposition of the great arteries; and VSD, ventricular septal defect.
The complication rate in the overall group was low, with a reintubation rate of 4 (0.6%). In the postoperative period, 25 (3.5%) patients experienced bradyarrhythmia requiring permanent pacemaker implantation, 12 (1.7%) developed hospital‐acquired pneumonia, and 11 (1.5%) required drainage of pericardial or pleural effusions. A return to the operating room for unplanned reintervention was required in 19 (2.7%) patients (11 [57.9%] of these were attributed to uncontrolled bleeding). Neurological complications were rare (6, 0.8%) as was the need for postoperative mechanical circulatory support (6, 0.8%).
Matched Cohorts
We matched 100 patients in the EE group to 225 patients in the CE group through progressive coarsening and coarsened exact matching (Table 3). For patients undergoing more than 1 surgery during the study period, the most recent procedure was used for matching. After matching, a minority of patients in each group were patients deemed to be at higher perioperative risk (presence of active endocarditis, nonelective surgery, and/or an ACHS score >0.5), with no significant difference in the proportion of high‐risk patients between the EE and CE groups (31% versus 32.4%; P>0.1). In the matched cohort, patients who received CE were ventilated for a median (interquartile range) of 12 (5–19) hours following surgery, and 56.9% were ventilated for >6 hours postoperatively. In the postoperative period, only 47 (47%) patients in the EE group required vasoactive agents compared with 158 (70.2%) patients in the CE group (P=0.004). In the early postoperative period (days 0 and 1), patients with EE required less inotropic or vasopressor support, as demonstrated by a lower VIS, compared with patients following CE (median VIS 0.5 [range, 0.0–2.0] versus 2.0 [range, 0.0–3.5]; P<0.0001). Prolonged inotrope/vasopressor support, defined as a VIS>0 after day 1, was required in 4 (4%) patients following EE versus 29 (12.9%) patients following CE (P=0.02). The total net fluid balance by the third postoperative day was more positive after CE than EE (1168±723 mL versus 847±733 mL; P=0.0002; Figure). Indeed, 64% of patients after EE achieved a net fluid balance <1 L positive within 48 hours of surgery compared with 101 (44.9%) patients in the CE group (P=0.001). Coagulation factor requirements, but not transfusion requirements, were lower in the EE compared with the CE group after the day of surgery (P=0.003 and P>0.1, respectively).
Table 3.
Baseline Characteristics in CEM‐Matched and PSM‐Matched Cohorts
| Parameter | CEM | PSM, 1:2 Matching | ||||
|---|---|---|---|---|---|---|
|
EE (n=100) |
CE (n=225) |
P Value |
EE (n=132) |
CE (n=259) |
P Value | |
| Age, y | 35±14.5 | 34.5±13.5 | >0.1 | 35±14.8 | 35.1±14.2 | >0.1 |
| Male | 41 (41) | 110 (48.9) | >0.1 | 55 (41.7) | 113 (43.6) | >0.1 |
| BMI, kg/m2 | 24.3±4.3 | 25.6±6.2 | >0.1 | 24.5±4.5 | 25.4±5.7 | >0.1 |
| Complexity | ||||||
| Simple | 13 (13) | 20 (8.9) | >0.1 | 23 (17.4) | 49 (18.9) | >0.1 |
| Moderate | 82 (82) | 198 (88) | 90 (68.2) | 175 (67.6) | ||
| Great | 5 (5) | 7 (3.1) | 19 (14.4) | 35 (13.5) | ||
| NYHA class | ||||||
| I/II | 94 (94) | 216 (96) | >0.1 | 121 (91.7) | 237 (91.5) | >0.1 |
| III/IV | 6 (6) | 9 (4) | 11 (8.3) | 22 (8.5) | ||
| Systemic ventricular function | ||||||
| Normal | 96 (96) | 214 (95.1) | >0.1 | 125 (94.7) | 250 (96.5) | >0.1 |
| Mildly impaired | 2 (2) | 1 (0.4) | 4 (3) | 5 (1.9) | ||
| Moderately impaired impaired | 2 (2) | 10 (4.4) | 3 (2.3) | 4 (1.5) | ||
| Severely impaired | 0 | 0 | 0 | 0 | ||
| Pulmonary hypertension, n (%) | 23 (23) | 38 (16.9) | >0.1 | 28 (21.2) | 52 (20.1) | >0.1 |
| Hemoglobin, g/L | 138±14 | 142±15 | 0.09 | 139±14.4 | 140±13.5 | >0.1 |
| eGFR, mL/min | 98 (61–164) | 102 (41–175) | >0.1 | 99 (49–165) | 103 (21–228) | 0.08 |
| Redo sternotomy | 38 (38) | 110 (48.9) | 0.09 | 54 (40.9) | 120 (46.3) | >0.1 |
| Procedure duration, h | 4.6 (2.4–8.2) | 4.7 (2.3–7.9) | >0.1 | 4.6 (2.4–8.2) | 4.6 (2.3–10.8) | >0.1 |
| Elective surgery | 97 (97) | 223 (99.1) | >0.1 | 128 (97) | 253 (97.7) | >0.1 |
Data are presented as number (percentage), mean±SD, or median (range). BMI indicates body mass index; CE, conventional extubation; CEM, coarsened exact matching; EE, early extubation; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; and PSM, propensity score matching.
Figure 1. Lower average (A) and (B) maximum VIS and in net‐positive fluid balance (C) in the EE versus the conventional extubation group in the first 48 hours postoperatively.
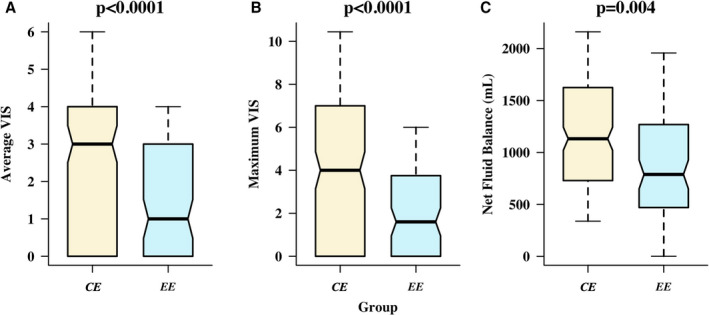
The boxplots present the 10th, 25th, 50th, 75th, and 90th quantiles. Comparisons between groups performed using the Wilcoxon signed rank test. EE indicates early extubation; CE, conventional extubation; and VIS, vasoactive‐inotropic score.
There was no evidence of increased risk in the rate of major complications in the EE compared with the CE group (7.0% versus 15.6%; P=0.05). The reintubation rate was very low in the matched cohort as a whole (0.3%), precluding statistical comparisons between groups: none in the EE group and 1 (0.4%) in the CE group.
EE was associated with a significantly shorter postoperative length of stay (LOS) in higher dependency care units (ICU or HDU) before the “step‐down” to ward‐based care (48 [range, 45–50] versus 50 [range, 47–69] hours; P=0.004). This was driven by a shorter LOS in the ICU in the EE versus the CE group (P<0.0001). In turn, lower combined ICU and HDU costs were incurred by patients with EE compared with patients with CE (£3949 [range, 3430–4222] versus £4166 [range, 3893–5603]; P<0.0001).
Sensitivity Analysis Using Propensity Score Matching
As a sensitivity analysis, we also performed propensity score matching. Using this method, 132 patients who received EE were matched to 259 patients with CE. “Adequate” balance was achieved with an absolute standardized mean difference of <0.1 for each variable (Table 4, Figures S1 and S2). However, multivariable imbalance was higher following propensity score matching (L1=0.992) than following coarsened exact matching, where there was a moderate 12% imbalance reduction from L1=0.996 to L1=0.88. Our findings were similar using propensity score matching: EE was still associated with a significantly shorter postoperative stay in higher dependency care units before a “step‐down” to ward‐based care (48 [range, 46–51] versus 51 [range, 47–69] hours; P=0.0004). Lower combined ICU and HDU costs were incurred by patients with EE compared with patients with CE (P<0.0001). Patients with EE had lower median VISs (P<0.0001) and lower total net fluid balance than the patients in the CE group (P=0.0003) during the early postoperative period.
Table 4.
Early Postoperative Outcomes in the CEM‐Matched Cohort
| Parameter |
Early Extubation (n=100) |
Conventional Extubation (n=225) | P Value |
|---|---|---|---|
| No. catecholamines required on theater exit | |||
| 0 | 47 (47) | 67 (30) | 0.001* |
| 1 | 40 (40) | 81 (36) | |
| 2 | 13 (13) | 71 (32) | |
| 3 | 0 (0) | 5 (2) | |
| Average VIS, units | |||
| Day 0 | 1 (0–20) | 3 (0–28) | <0.0001* |
| Day 1 | 0 (0–9) | 0 (0–38) | <0.0001* |
| Maximum VIS, units | |||
| Day 0 | 2 (0–31) | 5 (0–54) | <0.0001* |
| Day 1 | 0 (0–12) | 0 (0–90) | <0.0001* |
| Any catecholamine required >48 h | 4 (4) | 29 (12.9) | 0.02* |
| Net fluid balance, mL | |||
| Day 0 | 1446±907 | 2097±1069 | <0.0001* |
| Day 1 | 191±1010 | 239±949 | >0.1 |
| Overall postoperative ward | 1592±1543 | 2226±1692 | 0.001* |
| Cellsave during operation, mL | 464±208 | 421±235 | >0.1 |
| Blood transfusion requirement | 13 (13) | 45 (20) | >0.1 |
| Coagulation factor requirement | 9 (9) | 51 (22.7) | 0.006* |
| Postoperative length of stay | |||
| ICU, h | 21 (1–51) | 23 (5–1032) | 0.0001* |
| HDU, h | 26.5 (3–169) | 27 (5–196) | >0.1 |
| Higher level care (ICU plus HDU), h | 48 (23–191) | 50 (26–1228) | 0.0002* |
| Total postoperative, d | 6 (3–32) | 7 (3–194) | 0.09 |
| ICU cost, £ | 2449 (117–5948) | 2682 (583–120354) | <0.0001* |
| HDU cost, £ | 1405 (159–8957) | 1431 (265–10389) | >0.1 |
| Higher level care cost (ICU plus HDU), £ | 3949 (1283–11523) | 4166 (1951–130743) | <0.0001* |
| Major complications | 7 (7) | 35 (15.6) | 0.05 |
Data are presented as number (percentage), mean±SD, or median (range). CEM indicates coarsened exact matching; HDU, high dependency unit; ICU, intensive care unit; and VIS, vasoactive‐inotropic score.
Indicates statistical significance (P<0.05).
Discussion
In this study, we demonstrate that on‐table extubation is feasible and safe in patients with ACHD with a wide spectrum of anatomic complexity undergoing cardiac surgery. In the population studied, an EE strategy was associated with a reduction in the length of postoperative ICU and HDU stays and was associated with a significant reduction in ICU and HDU patient costs compared with CE. Furthermore, EE had postoperative hemodynamic benefits, with patients in the EE group requiring less vasoactive, inotropic, and fluid support and lower transfusion rates than those who received CE.
Despite evidence supporting EE in pediatric populations undergoing cardiac surgery, especially when a total cavopulmonary connection is performed, the evidence has been much weaker for patients with ACHD.4, 5, 7, 8, 9, 15 A single, small retrospective study of 67 patients reported that EE was feasible and safe in patients with ACHD undergoing noncomplex surgery.16 Ours is the first study, to our knowledge, examining a large population of adult patients undergoing congenital cardiac surgery including complex surgical procedures. The ACHD population is growing with a disproportionate increase in diseases of higher complexity, and these patients face a higher risk of perioperative complications compared with patients with acquired cardiac conditions.17, 18 Reassuringly, EE was not associated with an increase in postoperative complications in our cohort. EE appears to be safe and feasible for the majority of patients with ACHD in routine practice, when EE is appropriate. An optimal approach for extubation timing will mitigate unnecessary risks and increased costs.
In our population, EE was associated with reduced vasoactive drug use. This is likely related to the limited intake of hypnotic drugs, such as propofol and benzodiazepines, which cause vasodilation. A clear correlation between vasoactive medication and unfavorable outcomes in adults undergoing cardiac surgery has been reported.19 Moreover, vasodilation is likely to result in excessive fluid administration. Indeed, the first day fluid balance was significantly higher in the CE compared with the EE group, but was not different on day 2 when most of the patients with CE had been extubated and off vasoactive support, thus reaching a similar physiological state to patients with EE. Fluid overload and a significantly positive fluid balance perioperatively is a predictor of poor outcome in patients with CHD.20, 21 Cardiopulmonary bypass predisposes to this by causing hemodilution and increased capillary permeability, with an accumulation of fluid in the extracellular compartment that is enhanced by fluid administration and blood transfusions in the early postoperative period. Oedema and ascites raise the intra‐abdominal pressure, causing renal venous hypertension and a drop in renal perfusion pressure. The resultant acute kidney injury worsens fluid overload and is associated with a 3‐fold increased risk of postoperative mortality. Patients with complex CHD and/or cyanosis are at particularly increased risk of renal dysfunction, which can be exacerbated around surgery.22, 23 By extubating patients with ACHD on the operating table, anesthetists may avoid a vicious circle of sedation, vasoactive drugs, and fluid replacement, protecting patients from end‐organ damage and improving outcomes.
The reduced need for fluid support in patients extubated early may also explain the reduced need for blood products and coagulation factors in the EE group in our study. Observational studies suggest that transfusion is harmful after cardiac surgery, associated with postoperative infection, low cardiac output, acute kidney injury, and death.24, 25, 26 However, transfusion requirements during surgery may influence the decision to extubate early, as concerns about bleeding usually lead to a longer period of ventilation, allowing for a return to the operating room and/or keeping the chest stented open. Furthermore, the greater requirement for coagulation factors in the CE group could be an indicator of a sicker patient group postoperatively, which might impact the decision to prolong ventilation. Indeed, patient selection for EE is key. EE is likely to be beneficial for patients with pulmonary arterial hypertension and/or significant right ventricular impairment, both of which are prevalent in the ACHD cohort. Intermittent positive pressure ventilation increases right ventricular afterload and is poorly tolerated by these patients. Moreover, most sedative and anesthetic agents required to maintain ventilation depress myocardial contractility and cause nonselective vasodilation, increasing the risk of systemic hypotension and hemodynamic collapse.27, 28, 29 Further studies might look at this particular group of patients and the effect of ultra‐fast‐track extubation in this unique physiological setting.
Although total hospital LOS was not significantly longer in patients with CE, EE was associated with a more rapid discharge from ICU, promoting early recovery with a small but statistically significant reduction in ICU costs. Costs related to congenital cardiac surgery contribute significantly to the resource‐intensive nature of ACHD care. Widespread adoption of measures, such as EE, which reduce costs but improve the quality of care, should be encouraged given the financial constraints of the public healthcare system. This might be particularly relevant during the COVID‐19 pandemic. Moreover, ICU beds have become an even more valuable resource to safeguard. Reduction in ICU LOS can speed up patient turnover. Our results confirm the conclusion of a wide Cochrane meta‐analysis concerning fast‐track protocols in adult cardiac surgery. Fast‐track interventions shortened the LOS in the ICU, but did not reduce the total hospital LOS.30This was a nonrandomized, retrospective single‐center study and thus suffers from potential selection and ascertainment bias despite robust matching using both coarsened exact and propensity score matching techniques. This cohort included patients undergoing elective and nonelective surgery as well as patients who required prolonged ventilation, reflecting modern ACHD practice and the heterogeneity and complexity of the population managed in our center. The matching process ensured that patients with similar baseline and surgical characteristics were matched between treatment groups without a priori exclusion of specific risk cohorts, thus including as wide a population as possible. This allowed better matching of the populations and makes our conclusions applicable to a wider ACHD population. Furthermore, large‐scale, prospective studies are required to establish the best timing for EE (on‐table extubation versus timely extubation in recovery or ICU).
In conclusion, we showed that EE is feasible and potentially safe in patients with ACHD undergoing a wide spectrum of congenital cardiac surgical procedures, even though further data are required to confirm these findings, especially in patients with ACHD at higher perioperative risk. This management strategy reduces the need for vasoactive drugs and fluid resuscitation while achieving a low complication rate. Intensive care stay is also shortened, leading to a reduction in costs.
Sources of Funding
Support was provided solely from Institutional and/or Departmental sources.
Disclosures
Dr Constantine has received a personal education grant from Actelion Pharmaceuticals (a Janssen Pharmaceutical company of Johnson & Johnson). Professor Dimopoulos has received unrestricted educational support from Actelion Pharmaceuticals and has been a consultant to and received grants and personal fees from Actelion Pharmaceuticals, Pfizer, GlaxoSmithKline, and Bayer/Merck Sharp & Dohme. No support is related with the aim of this study. The remaining authors have no disclosures to report.
Supporting information
Figures S1–S2
(J Am Heart Assoc. 2021;10:e020201. DOI: 10.1161/JAHA.120.020201.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020201
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1.Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle‐Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101–109. DOI: 10.1161/CIRCULATIONAHA.115.019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marelli AJ, Raluca I‐I, Mackie AS, Liming G, Nandini D, Mohammed K, Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2000;130:749–756. DOI: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 3.Khan A, Gurvitz M. Epidemiology of ACHD: what has changed and what is changing? Prog Cardiovasc Dis. 2018;61:275–281. DOI: 10.1016/j.pcad.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Marianeschi SM, Seddio F, McElhinney DB, Colagrande L, Abella RF, de la Torre T, Meli M, Iorio FS, Marcelletti CF. Fast‐track congenital heart operations: a less invasive technique and early extubation. Ann Thorac Surg. 2000;69:872–876. DOI: 10.1016/S0003-4975(99)01330-2. [DOI] [PubMed] [Google Scholar]
- 5.Kim KM, Kwak JG, Shin BC‐H, Kim ER, Lee J‐H, Kim EH, Kim JT, Kim W‐H. Early experiences with ultra‐fast‐track extubation after surgery for congenital heart disease at a single center. Korean J Thorac Cardiovasc Surg. 2018;51:247–253. DOI: 10.5090/kjtcs.2018.51.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kin N, Weismann C, Srivastava S, Chakravarti S, Bodian C, Hossain S, Krol M, Hollinger I, Nguyen K, Mittnacht AJC. Factors affecting the decision to defer endotracheal extubation after surgery for congenital heart disease: a prospective observational study. Anesth Analg. 2011;113:329–335. DOI: 10.1213/ANE.0b013e31821cd236. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton BCS, Honjo O, Alghamdi AA, Caldarone CA, Schwartz SM, Van Arsdell GS, Holtby H. Efficacy of evolving early‐extubation strategy on early postoperative functional recovery in pediatric open‐heart surgery: a matched case‐control study. Semin Cardiothorac Vasc Anesth. 2014;18:290–296. DOI: 10.1177/1089253213519291. [DOI] [PubMed] [Google Scholar]
- 8.Miller JW, Vu D, Chai PJ, Kreutzer J, Hossain MM, Jacobs JP, Loepke AW. Patient and procedural characteristics for successful and failed immediate tracheal extubation in the operating room following cardiac surgery in infancy. Paediatr Anaesth. 2014;24:830–839. DOI: 10.1111/pan.12413. [DOI] [PubMed] [Google Scholar]
- 9.Mittnacht AJC, Thanjan M, Srivastava S, Joashi U, Bodian C, Hossain S, Kin N, Hollinger I, Nguyen K. Extubation in the operating room after congenital heart surgery in children. J Thorac Cardiovasc Surg. 2008;136:88–93. DOI: 10.1016/j.jtcvs.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Ovroutski S, Kramer P, Nordmeyer S, Cho M‐Y, Redlin M, Miera O, Photiadis J, Berger F. Early extubation is associated with improved early outcome after extracardiac total cavopulmonary connection independently of duration of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2018;54:953–958. DOI: 10.1093/ejcts/ezy179. [DOI] [PubMed] [Google Scholar]
- 11.Webb GD, Williams RG. 32nd Bethesda Conference: “care of the adult with congenital heart disease”. J Am Coll Cardiol. 2001;37:1162–1165. [DOI] [PubMed] [Google Scholar]
- 12.Fuller SM, He X, Jacobs JP, Pasquali SK, Gaynor JW, Mascio CE, Hill KD, Jacobs ML, Kim YY. Estimating mortality risk for adult congenital heart surgery: an analysis of the society of thoracic surgeons congenital heart surgery database. Ann Thorac Surg. 2015;100(5):1728–1736.discussion 1735–1736. DOI: 10.1016/j.athoracsur.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC. Vasoactive‐inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–238. DOI: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 14.King G, Nielsen R. Why propensity scores should not be used for matching. Polit Anal. 2019;27:435–454. DOI: 10.1017/pan.2019.11. [DOI] [Google Scholar]
- 15.Nawrocki P, Wisniewski K, Schmidt C, Bruenen A, Debus V, Malec E, Januszewska K. Extubation on the operating table in patients with right ventricular pressure overload undergoing biventricular repair†. Eur J Cardiothorac Surg. 2019;56:904–910. DOI: 10.1093/ejcts/ezz139. [DOI] [PubMed] [Google Scholar]
- 16.Weismann CG, Yang SF, Bodian C, Hollinger I, Nguyen K, Mittnacht AJC. Early extubation in adults undergoing surgery for congenital heart disease. J Cardiothorac Vasc Anesth. 2012;26:773–776. DOI: 10.1053/j.jvca.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Nasr VG, Faraoni D, Valente AM, DiNardo JA. Outcomes and costs of cardiac surgery in adults with congenital heart disease. Pediatr Cardiol. 2017;38:1359–1364. DOI: 10.1007/s00246-017-1669-7. [DOI] [PubMed] [Google Scholar]
- 18.Karangelis D, Mazine A, Narsupalli S, Mendis S, Veldtman G, Nikolaidis N. Morbidity after cardiac surgery in patients with adult congenital heart disease in comparison with acquired disease. Heart Lung Circ. 2018;27:739–744. DOI: 10.1016/j.hlc.2017.05.133. [DOI] [PubMed] [Google Scholar]
- 19.Koponen T, Karttunen J, Musialowicz T, Pietiläinen L, Uusaro A, Lahtinen P. Vasoactive‐inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122:428–436. DOI: 10.1016/j.bja.2018.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grist G, Whittaker C, Merrigan K, Fenton J, Worrall E, O’Brien J, Lofland G. The correlation of fluid balance changes during cardiopulmonary bypass to mortality in pediatric and congenital heart surgery patients. J Extra Corpor Technol. 2011;43:215–226. [PMC free article] [PubMed] [Google Scholar]
- 21.Hazle MA, Gajarski RJ, Yu S, Donohue J, Blatt NB. Fluid overload in infants following congenital heart surgery. Pediatr Crit Care Med. 2013;14:44–49. DOI: 10.1097/PCC.0b013e3182712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimopoulos K, Diller G‐P, Koltsida E, Pijuan‐Domenech A, Papadopoulou SA, Babu‐Narayan SV, Salukhe TV, Piepoli MF, Poole‐Wilson PA, Best N, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320–2328. DOI: 10.1161/CIRCULATIONAHA.107.734921. [DOI] [PubMed] [Google Scholar]
- 23.Price S, Jaggar SI, Jordan S, Trenfield S, Khan M, Sethia B, Shore D, Evans TW. Adult congenital heart disease: intensive care management and outcome prediction. Intensive Care Med. 2007;33:652–659. DOI: 10.1007/s00134-007-0544-z. [DOI] [PubMed] [Google Scholar]
- 24.Murphy GJ, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. DOI: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 25.Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH. Morbidity and mortality risk associated with red blood cell and blood‐component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616. DOI: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 26.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Chan CT, Wong P‐Y, Beattie WS. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology. 2011;115:523–530. DOI: 10.1097/ALN.0b013e318229a7e8. [DOI] [PubMed] [Google Scholar]
- 27.Viitanen A, Salmenperä M, Heinonen J, Hynynen M. Pulmonary vascular resistance before and after cardiopulmonary bypass. The effect of PaCO2. Chest. 1989;95:773–778. DOI: 10.1378/chest.95.4.773. [DOI] [PubMed] [Google Scholar]
- 28.Viitanen A, Salmenperä M, Heinonen J. Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology. 1990;73:393–400. DOI: 10.1097/00000542-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Gordon C, Collard CD, Pan W. Intraoperative management of pulmonary hypertension and associated right heart failure. Current Opinion in Anesthesiology. 2010;23:49–56. DOI: 10.1097/ACO.0b013e3283346c51. [DOI] [PubMed] [Google Scholar]
- 30.Wong W‐T, Lai VK, Chee YE, Lee A. Fast‐track cardiac care for adult cardiac surgical patients. Cochrane Database of Systematic Reviews. 2016;9:CD003587. DOI: 10.1002/14651858.CD003587.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


