Abstract
Background
Hypertriglyceridemia is associated with increased risk of coronary heart disease but the association is often attributed to concomitant metabolic abnormalities. We investigated the epidemiology of primary isolated hypertriglyceridemia (PIH) and associated cardiovascular risk in a population‐based setting.
Methods and Results
We identified adults with at least one triglyceride level ≥500 mg/dL between 1998 and 2015 in Olmsted County, Minnesota. We also identified age‐ and sex‐matched controls with triglyceride levels <150 mg/dL. There were 3329 individuals with elevated triglyceride levels; after excluding those with concomitant hypercholesterolemia, a secondary cause of high triglycerides, age <18 years or an incomplete record, 517 patients (49.4±14.0 years, 72.0% men) had PIH (triglyceride 627.6±183.6 mg/dL). The age‐ and sex‐adjusted prevalence of PIH in adults was 0.80% (0.72–0.87); the diagnosis was recorded in 60%, 46% were on a lipid‐lowering medication for primary prevention and a triglyceride level <150 mg/dL was achieved in 24.1%. The association of PIH with coronary heart disease was attenuated but remained significant after adjustment for demographic, socioeconomic, and conventional cardiovascular risk factors (hazard ratio [HR], 1.53; 95% CI, 1.06‐2.20; P= 0.022). There was no statistically significant association between PIH and cerebrovascular disease (HR, 1.06; 95% CI, 0.65‐1.73, P= 0.813), peripheral artery disease (HR, 1.27; 95% CI, 0.43‐3.75; P= 0.668), or the composite end point of all 3 (HR, 1.28; 95% CI, 0.92‐1.80; P=0.148) in adjusted models.
Conclusions
PIH was associated with incident coronary heart disease events (although there was attenuation after adjustment for conventional risk factors), supporting a causal role for triglycerides in coronary heart disease. The condition is relatively prevalent but awareness and control are low.
Keywords: coronary heart disease, epidemiology, hypertriglyceridemia
Subject Categories: Epidemiology, Risk Factors, Cardiovascular Disease, Primary Prevention, Secondary Prevention
Nonstandard Abbreviations and Acronyms
- CVD
cerebrovascular disease
- PIH
primary isolated hypertriglyceridemia
- REP
Rochester Epidemiology Project
Clinical Perspective
What Is New?
Primary isolated hypertriglyceridemia (triglyceride level ≥500 mg/dL in the absence of concomitant hypercholesterolemia or a secondary cause of high triglyceride level) is associated with incident coronary heart disease, independent of conventional risk factors.
What Are the Clinical Implications?
Primary isolated hypertiglyceridemia is relatively prevalent 0.80% (0.72–0.87), but only 46% of cases were on a lipid‐lowering medication for primary prevention and a normal triglyceride level was achieved only in 24.1%, highlighting an opportunity for interventions to lower the risk of coronary heart disease in these cases.
Over the years, the concept of elevated triglyceride level as a risk factor for coronary heart disease (CHD) has evolved. Decades ago, hypertriglyceridemia was considered a risk factor for CHD, on par with high cholesterol.1, 2 Subsequently, the focus shifted toward low‐density lipoprotein cholesterol (LDL‐C) as the major risk factor for CHD,3, 4 since epidemiological studies suggested lack of an independent role for triglycerides after adjustment for high‐density lipoprotein cholesterol (HDL‐C).5, 6 More recently, Mendelian randomization studies and clinical trials7, 8, 9, 10, 11 have brought into question a causal role for HDL‐C and renewed interest in triglycerides as a causal risk factor for CHD.
While triglyceride‐rich lipoproteins have been associated with adverse cardiovascular events in multiple studies,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 the extent to which the association is due to concomitant metabolic abnormalities is unclear and little is known about isolated hypertriglyceridemia as a risk factor for CHD.18 Establishing hypertriglyceridemia as an independent risk factor for CHD has been challenging because of bias inherent in non‐population based cohorts,12, 13, 14, 15, 16, 17 the complex nonlinear nature of the association of triglycerides with CHD,23, 24 significant lability in triglyceride levels,12, 25 lack of exclusion of secondary causes of increased triglyceride levels,26, 27, 28 and the co‐occurrence of hypercholesterolemia and additional conventional risk factors with elevated triglyceride levels.29, 30, 31
To address this area of controversy, we investigated whether primary isolated hypertriglyceridemia (PIH)—defined as hypertriglyceridemia in the absence of identifiable secondary causes of triglyceride elevation or concomitant hypercholesterolemia—is an independent risk factor for CHD. To reduce the influence of lability in triglyceride levels, we chose as cases individuals with a high triglyceride level (defined as fasting level ≥500 mg/dL) and controls as those with normal triglyceride level (ie, <150 mg/dL). To reduce referral bias, we conducted our study in the population‐based setting of Olmsted County, Minnesota. Furthermore, we assessed the public health burden of PIH by measuring its prevalence, awareness, and control, using the resources of the Rochester Epidemiology Project (REP).
Methods
Study Design and Data Set
The data of this study are available from the corresponding author upon reasonable request. This study was conducted in Olmsted County, Minnesota, which has an estimated population of 144 248 based on the 2010 census. Given its relative isolation from other metropolitan areas, Olmsted County is ideal for studying disease epidemiology in a population‐based setting. Medical care within this county is provided by Mayo Clinic, Olmsted Medical Center, and their affiliated hospitals, as well as the Rochester Family Medicine Clinics. As part of REP, these healthcare systems are connected by a unique medical records‐linkage system, which covers nearly all residents of Olmsted County. Established more than 50 years ago, REP is supported by the National Institute of Health and has enabled hundreds of epidemiology studies.32, 33 During any given 3‐year period, >90% of residents see a provider in Olmsted County at least once. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards and all methods were performed in accordance to the guidelines. As per institutional policies, participants provided active authorization and informed consent for use of their medical records for research.
Case‐Control Selection
We ascertained fasting serum lipid levels including triglycerides, total cholesterol, and HDL‐C. Non‐high‐density lipoprotein cholesterol (non‐HDL‐C) was calculated as the difference between total cholesterol and HDL‐C. We identified all Olmsted county residents with fasting triglyceride ≥500 mg/dL between January 1, 1998 and December 31, 2015. We excluded those with concomitant hypercholesterolemia (defined as non‐HDL‐C ≥190 mg/dL) and patients with secondary causes of hypertriglyceridemia noted within a 1‐year window of the index triglyceride measurement were also excluded. Secondary causes (defined based on electronic health record [EHR] data, as previously described34) included hypothyroidism, significant liver disease (including alcohol abuse), significant kidney disease, uncontrolled diabetes mellitus, morbid obesity, and pregnancy (Table S1). Controls had triglycerides <150 mg/dL and were also selected to not have any of conditions associated with secondary hypertriglyceridemia and were matched based on age (±1 year) and sex to cases (Figure 1). Index date for each matched set was defined as the earliest date of fasting triglyceride level ≥500 mg/dL for the case. The closest triglyceride value on or before the index date was selected for each control. Study variables including demographics and conventional risk factors were extracted from the EHR using the REP resources.
Figure 1. Case‐control selection.
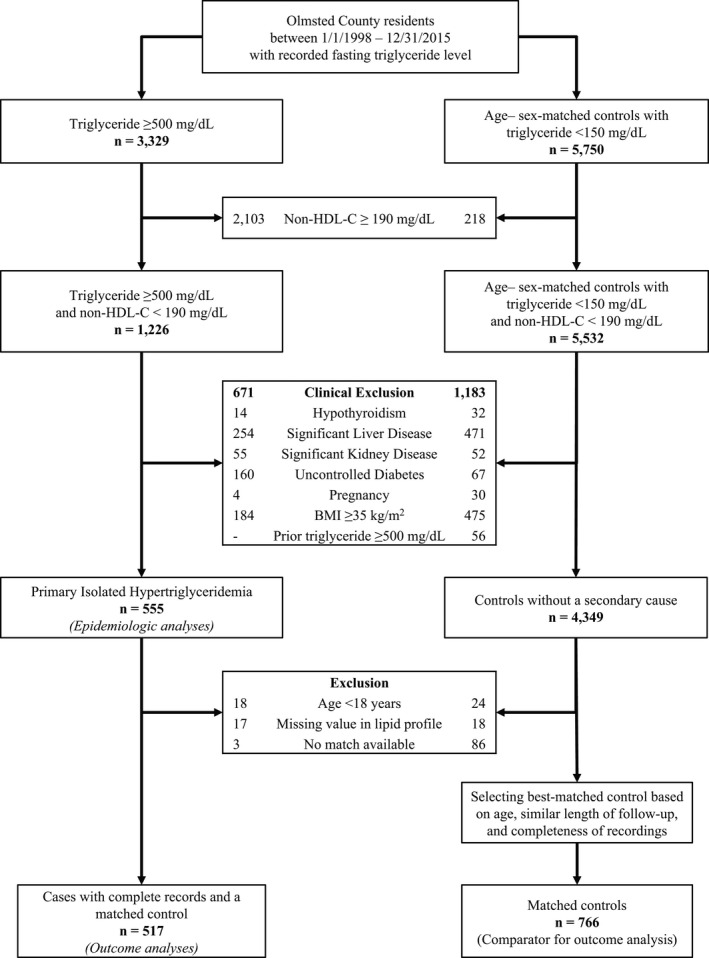
BMI indicates body mass index; and HDL‐C, high‐density lipoprotein cholesterol.
Demographic and Conventional Risk Factors
Age, sex, self‐reported race, and family history of CHD/stroke were obtained from the EHR. To ascertain conventional risk factors we used validated electronic phenotyping algorithms developed by the eMERGE Network and available at www.PheKB.org.35 Body mass index (BMI) was also obtained from the EHR. Self‐reported education level was used as a measure of socioeconomic status.36
Incidence, Prevalence, Awareness, and Control
To estimate the incidence of PIH, incident cases were defined as Olmsted County residents with a triglyceride level ≥500 mg/dL for the first time between 1998 and 2015, without concomitant hypercholesterolemia or an identifiable secondary cause, irrespective of their age and whether or not they had a missing value on the lipid profile or could be matched to a control. Incident cases who were <18 years old, had a missing value in the lipid profile, or did not have a matched control (n=38) were excluded from the outcomes analyses but were included for estimation of incidence. Denominators were based on US Census data for 2000 and 2010 as well as REP recommended interpolations and extrapolations to cover 1998 to 2015.33 Prevalence was estimated using an age‐recursive method, described below, that incorporated estimates of incidence along with relative survival. Awareness was estimated as the proportion of cases in whom the diagnosis of “dyslipidemia,” “hyperlipidemia,” or “hypertriglyceridemia” was noted in the problem list during a 1‐year period following the index date, after manual review of a random set of 250 cases. The most recent triglyceride level in the EHR (and at least 6 months after the index date) was used as an indicator of the degree of control. Three thresholds were used to determine the degree of control: (a) >50% reduction in last triglyceride level compared with initial level, (b) last triglyceride <200 mg/dL, and (c) last triglyceride <150 mg/dL.
Associated Cardiovascular Risk
The primary outcome of interest was incident coronary heart disease (CHD). We also evaluated the association of elevated triglyceride level with incident cerebrovascular disease (CVD), peripheral artery disease (PAD), and the composite end point of CHD, CVD, and PAD. CHD was defined as myocardial infarction, surgical or percutaneous coronary revascularization, cardiac angina, high‐grade stenosis on coronary angiography, or an abnormal stress test. CVD was defined as ischemic stroke or transient ischemic attack. PAD was defined as intermittent claudication critical limb ischemia (i.e. rest pain, or gangrene), or other atherosclerosis of the extremities. The details of the EHR‐based phenotyping algorithm and the corresponding International Classification of Diseases ‐ Current Procedural Terminology (ICD‐CPT) codes are summarized in Tables S2 through S4.34, 37, 38
Statistical Analysis
To estimate incidence and prevalence of PIH, we first calculated the age and sex‐specific incidence rates based on numbers of cases and person years in each “age‐sex” bin. Second, the age and sex‐specific “relative survival” (the hazard ratio associated with mortality risk when having the condition compared to the mortality risk in the general population), was estimated by using standard life tables to transform each observed follow‐up time into an observed “cumulative hazard” for each incident case, and combining that with their vital status at the end of follow‐up, in a negative exponential model with the rate ratio as an exponential scale parameter that could depend on age and sex in a loglinear manner. Finally, the age‐sex‐specific incidence and age‐sex‐specific relative hazards were combined using an age‐recursive model that calculates age‐specific prevalence starting at age zero, and increasing a year at a time up to age 95, based on the incidence and differential survival at each successive age (recursive formula is provided in Data S1). The age‐specific prevalence was then used to estimate an overall prevalence adjusted both to the age distribution within the Olmsted population and separately to the US 2010 White population. Standard errors and confidence intervals, both for the age‐specific prevalence estimates and for the overall prevalence estimates were obtained using a bootstrap resampling approach, wherein the incident cases were resampled with replacement in a way that allowed the number of cases in each bootstrap sample to have Poisson variation. In effect, this is equivalent to drawing bootstrap samples from the entire set of incident cases and non‐incident residents in the community.
The risk of incident CHD, CVD, PAD, and their composite end point after the index date were estimated separately by adjusted Cox proportional hazards regression. Unadjusted Kaplan–Meier curves were used to depict time to event in cases and controls. Those noted to have CHD, CVD, or PAD at baseline were excluded from the prospective analyses of incident events but were compared using logistic regression models. The date of the first event was considered as the end point; and for those without events, the last visit or death was considered to be the end of follow‐up. Multivariable Cox regression models that adjusted sequentially for demographic factors (age, sex, race/ethnicity), education level (as a measure of socioeconomic status), and conventional cardiovascular risk factors (family history of CHD/stroke, hypertension, diabetes mellitus, smoking, BMI, and total cholesterol) were used to assess the association of hypertriglyceridemia with incident events. Given the high collinearity between triglyceride level and HDL‐C (Spearman correlation coefficient=−0.68), the latter was also included in an additional separate regression model. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC) and RStudio version 1.2.5033 (RStudio, Inc., Boston, MA). All tests were two‐sided, and P‐values <0.05 were considered statistically significant.
Results
Participant Characteristics
Using the REP resources, 3329 residents of Olmsted County between 1998 and 2015 with a fasting triglyceride level ≥500 mg/dL were identified. We sequentially excluded 2103 people with concomitant hypercholesterolemia (non‐HDL‐C ≥190 mg/dL) and 671 with secondary causes of hypertriglyceridemia, leaving 555 PIH cases for incidence and prevalence analyses. Additionally 38 cases aged <18 years, with missing total cholesterol or HDL‐C, or without a matching control were also excluded, leaving 517 PIH cases for outcomes related analyses. From the remaining population, 766 age‐ (±1 year) and sex‐matched subjects with a triglyceride level <150 mg/dL closest to the index date for cases were identified. The process of selecting cases and controls is summarized in Figure 1.
The mean ages of cases and controls were 49.4±14.0 and 48.6±14.0 years, respectively, and the majority were men, non‐Hispanic, and White. Racial differences were also noted; while 9.1% and 1.5% of cases were Asian and Black, respectively, these percentages in controls were 3.5% and 2.9%. The proportion with hypertension, diabetes mellitus, history of smoking, and obesity was higher in cases than controls (Table 1). The baseline, post diagnosis, and at follow‐up use of lipid‐lowering medications was also greater in cases than controls (Table 1).
Table 1.
Baseline Characteristics of Cases and Controls
| Controls | Cases | P Value | |
|---|---|---|---|
| (n=766) | (n=517) | ||
| Age, y | 48.6±14.0 | 49.4±14.0 | 0.299* |
| Men | 540 (70.5%) | 372 (72.0%) | 0.572† |
| Race | <0.001† | ||
| White | 684 (89.3%) | 427 (82.6%) | |
| Asian | 27 (3.5%) | 47 (9.1%) | |
| Black | 22 (2.9%) | 8 (1.5%) | |
| Other‡ | 33 (4.3%) | 35 (6.8%) | |
| Hispanic ethnicity | 21 (2.7%) | 21 (4.1%) | 0.192† |
| Education§ | <0.001† | ||
| <12 y | 22 (2.9%) | 30 (6.4%) | |
| 12–15 y | 311 (41.5%) | 250 (53.3%) | |
| ≥16 y | 416 (55.5%) | 189 (40.3%) | |
| Medical history | |||
| Hypertension | 345 (45.0%) | 322 (62.3%) | <0.001† |
| Diabetes mellitus | 89 (11.6%) | 173 (33.5%) | <0.001† |
| History of smokingǁ | 334 (43.6%) | 275 (53.2%) | <0.001† |
| BMI, kg/m2 | 26.7±3.8 | 29.4±3.3 | <0.001* |
| BMI categories, kg/m2 | <0.001† | ||
| Overweight (BMI 25–29.9) | 363 (47.4%) | 207 (40.0%) | |
| Obesity (BMI ≥30) | 145 (18.9%) | 223 (43.1%) | |
| Lipid profile (index) | |||
| Triglyceride, mg/dL | 92.8±27.9 | 627.6±183.6 | <0.001* |
| Total cholesterol, mg/dL | 181.4±31.3 | 196.2±24.2 | <0.001* |
| Non‐HDL‐C, mg/dL | 126.4±29.1 | 162.7±20.8 | <0.001* |
| HDL‐C, mg/dL | 55.0±15.3 | 32.9±9.8 | <0.001* |
| Lipid‐lowering medication | |||
| 18 mo before the index date | <0.001† | ||
| Statin only | 102 (13.3%) | 87 (16.8%) | |
| Non‐statin only | 9 (1.2%) | 34 (6.6%) | |
| Both statin and non‐statin | 13 (1.7%) | 24 (4.6%) | |
| 18 mo after the index date | <0.001† | ||
| Statin only | 110 (14.4%) | 91 (17.6%) | |
| Non‐statin only | 18 (2.3%) | 108 (20.9%) | |
| Both statin and non‐statin | 21 (2.7%) | 69 (13.3%) | |
| 18 mo before the last follow‐up¶ | <0.001† | ||
| Statin only | 206 (29.7%) | 173 (37.9%) | |
| Non‐statin only | 23 (3.3%) | 63 (13.8%) | |
| Both statin and non‐statin | 42 (6.1%) | 77 (16.9%) | |
| Triglyceride level at last follow‐up# | |||
| Last measured triglyceride mg/dL§ | 107.0±54.1 | 253.4±153.8 | NA |
| >50% reduction in triglyceride level§ | NA | 337 (72.5%) | NA |
| <200 mg/dL§ | 608 (95.3%) | 211 (45.4%) | NA |
| <150 mg/dL§ | 542 (85.0%) | 112 (24.1%) | NA |
Values are given as mean±SD or n (%).
BMI indicates body mass index; and HDL‐C, high‐density lipoprotein cholesterol
Test for differences:
t‐test.
Chi‐Square.
Other races include Hawaiian/Pacific islander, American Indian, mixed, refused, or unknown.
Missing data altered percent calculation.
Includes people with unknown values.
In cases with at least 36 months of follow‐up.
At least 6 months after index date.
Incidence and Prevalence
Adjusted to the US White population in 2010, the incidence rate of PIH per 100 000‐years (95% CI) was higher in men 34.84 (31.34–38.34) than women 13.24 (11.15–15.32). The prevalence of PIH in adults indexed to the age of the US White population was 1.20% (1.06–1.34) in men, 0.44% (0.37–0.51) in women, and 0.80% (0.72–0.87) overall (Table 2, Figure 2). Assuming the current adult population of the United States to be ~255 million, there are ~2 000 000 PIH cases in the United States with an annual incidence of ~61 000. The incidence and prevalence of isolated hypertriglyceridemia, regardless of a primary or secondary etiology are shown in Table S5 and Figure S1.
Table 2.
Incidence Rate and Prevalence of Primary Isolated Hypertriglyceridemia
| Incidence Rate* (95% CI) | Prevalence† (95% CI) | |||
|---|---|---|---|---|
| Olmsted County | US‡ | Olmsted County | US‡ | |
| Primary isolated hypertriglyceridemia | ||||
| Men (n=396) | 32.15 (29.06–35.48) | 34.84 (31.34–38.34) | 1.06% (0.94–1.19) | 1.20% (1.06–1.34) |
| Women (n=159) | 11.89 (10.12–13.89) | 13.24 (11.15–15.32) | 0.37% (0.31–0.44) | 0.44% (0.37–0.51) |
| Total (n=555) | 21.61 (19.85–23.48) | 23.98 (21.95–26.01) | 0.69% (0.63–0.77) | 0.80% (0.72–0.87) |
Incidence rates for 100 000 person‐year. Incidence rates were measured by 1 231 727 person‐year of follow‐up in men and 1 336 917 in women.
Prevalence rates are calculated as the mean value of adults (18–95 years), weighted to the population counts of White adults in Olmsted County or the United States from 2010 census estimates. Confidence limits are calculated using the 2.5th and 97.5th percentile of all prevalence rates across 1000 bootstrapped samples (within each sample, the prevalence rate is the mean rate across all ages, as above).
Sex‐specific incidence rates are adjusted to the age distribution of the US White population from 2010, overall incidence rates are adjusted to the age and sex distribution of the US White population from 2010.
Figure 2. Age‐specific prevalence (%) of primary isolated hypertriglyceridemia in adults, adjusted to the US White population.
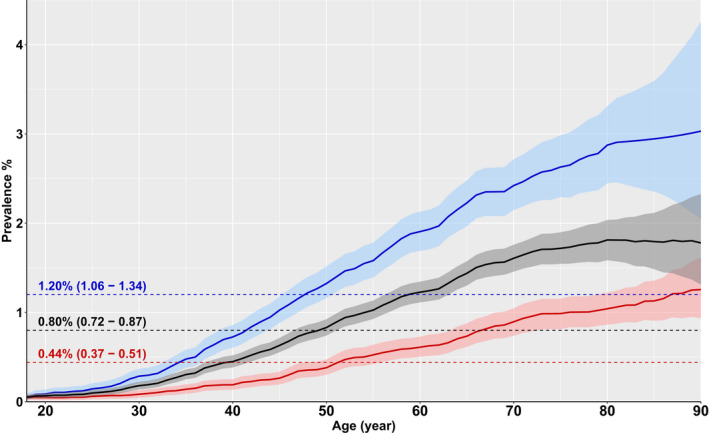
Men are depicted in blue, women in red, and the overall cohort in black. Dashed lines represent mean prevalence values for adults (age 18–95 years).
Awareness, Treatment, and Control
In a 1‐year window after the index date, 60% of cases had a representative diagnosis code or mention of hypertriglyceridemia as a diagnosis in the EHR. In the 18 months before the index date, 28% of cases were on a lipid‐lowering medication, increasing to 52% within 18 months after the index date (Table 1). We also evaluated lipid‐lowering treatment in primary and secondary prevention settings separately (Table S6). The rate of treatment was higher for secondary prevention than for primary prevention (P<0.001) and it was higher in ≥40‐year‐old cases than younger cases (P<0.001). In the primary prevention setting, only 35% of cases <40 years old and 46% of cases 40 to 54 years old were on a lipid‐lowering medication in the 18 months after detection of a high triglyceride level. The rate of lipid‐lowering medication (statin and/or non‐statin) use for primary prevention increased during the study period: from 33% in the first half the study period to 62% in in the second half (P<0.001, Figure S2). The last triglyceride level (at least 6 months after detection), was reduced by >50% or reached <200 mg/dL or <150 mg/dL in 72%, 45%, and 24% of cases, respectively. The degree of control in primary and secondary prevention settings and in different ages categories is illustrated in Figure S3.
Cardiovascular Risk
At baseline, CHD (16.2% versus 9.5%; odds ratio [OR], 1.84; 95% CI, 1.32‐2.58; P<0.001) and CVD (6.2% versus 2.9%; OR, 2.23; 95% CI, 1.28‐3.89; P=0.005) were more frequent in cases than controls. During 11.3 years (interquartile range, 6.3–15.6) of follow‐up, the rate of incident CHD events was 18.9% in cases versus 11.8% in controls (hazard ratio [HR], 1.79; 95% CI, 1.32‐2.43; P<0.001), the rate of incident CVD event was 9.3% in cases versus 7.5% in controls (HR, 1.35; 95% CI, 0.91‐1.99; P=0.139) and the rate of incident PAD was 2.0% in cases versus 1.3% in controls (HR, 1.58; 95% CI, 0.66‐3.80; P=0.305) (Table S7). In unadjusted models, PIH cases were at increased risk of both baseline composite end point (20.3% versus 11.6%; OR, 1.94; 95% CI, 1.43‐2.64; P<0.001) and also incident composite end point (21.8% versus 16.1%; HR, 1.54; 95% CI, 1.16‐2.03; P=0.003). A Kaplan–Meier plot for survival free of atherosclerotic cardiovascular disease is presented in Figure 3. Adjustment in Cox proportional hazards regression models was performed incrementally, starting with demographic features and then adding conventional CHD risk factors (Table 3). Cases were at increased risk for CHD after adjustment for age, sex, race and education level (HR, 1.73; 95% CI, 1.26–2.36; P<0.001). The hazard ratio was attenuated but remained significant after additional adjustment for family history of CHD or stroke, hypertension, diabetes mellitus status, smoking, BMI, and total cholesterol level (HR, 1.53; 95% CI, 1.06–2.20; P=0.022). The association was not statistically significant after additional adjustment for HDL‐C. The HRs for other variables in Model 3 are presented in Table S8.
Figure 3. Kaplan–Meier plot for survival free of CHD (red; P<0.001), CVD (blue; P= 0.139), PAD (green; P=0.305), and composite end point (black, P=0.003) as well as number of cases and controls at risk.
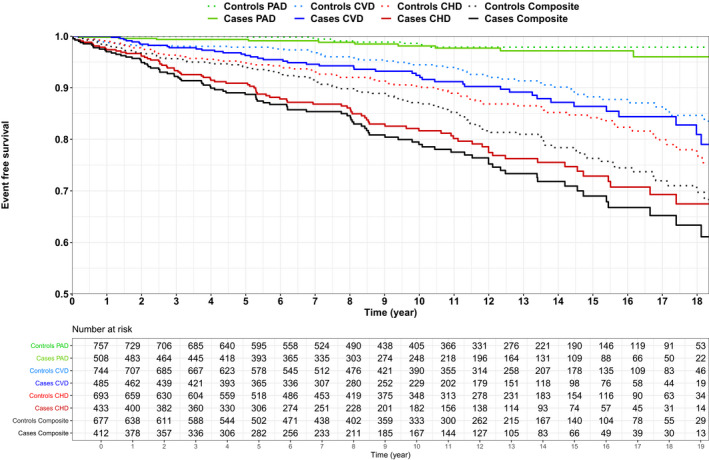
The solid lines represent cases and the dotted lines represent controls. CHD indicates coronary heart disease; CVD, cerebrovascular disease; and PAD, peripheral artery disease.
Table 3.
Multiple Variable Cox Proportional Hazards Regression Models for the Hazard of Incident CHD, CVD, PAD, and Composite End Point During the Follow‐Up Period in PIH Cases in Comparison with Matched Controls
| CHD (Number of Events=164) | CVD (Number of Events=101) | PAD (Number of Events=20) | Composite End Point (Number of Events=199) | |||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Model 1 | 1.79 (1.32–2.43) | <0.001 | 1.35 (0.91–1.99) | 0.139 | 1.58 (0.66–3.80) | 0.305 | 1.54 (1.16–2.03) | 0.003 |
| Model 2 | 1.73 (1.26–2.36) | <0.001 | 1.25 (0.84–1.87) | 0.266 | 1.63 (0.67–3.97) | 0.281 | 1.46 (1.10–1.95) | 0.009 |
| Model 3 | 1.53 (1.06–2.20) | 0.022 | 1.06 (0.65–1.73) | 0.813 | 1.27 (0.43–3.75) | 0.668 | 1.28 (0.92–1.80) | 0.148 |
| Model 4 | 1.21 (0.77–1.91) | 0.403 | 0.74 (0.41–1.36) | 0.334 | 0.71 (0.20–2.57) | 0.606 | 1.00 (0.66–1.52) | 0.992 |
Model 1 is unadjusted.
Model 2 is adjusted for age, sex, race, and education.
Model 3 In addition to covariates listed in Model 2, Model 3 is additionally adjusted for family history of CHD, hypertension, diabetes mellitus, smoking, BMI, and total cholesterol.
HDL‐C is additionally included in the Model 4.
BMI indicates body mass index; CHD, CHD indicates coronary heart disease; CVD, cerebrovascular; HDL‐C, high‐density lipoprotein cholesterol; PAD, peripheral artery disease; and PIH, primary isolated hypertriglyceridemia.
Discussion
The main finding of the present study is that PIH was an independent risk factor for CHD, supporting a causal role for triglycerides in atherosclerosis. The association was attenuated after adjustment for conventional risk factors, suggesting that some of the risk due to elevated triglyceride level is mediated by these factors. Furthermore, the burden of PIH was significant with a prevalence of ~1 in 125 in the US adult White population and an annual incidence of ~61 000 new cases per year. The diagnosis was documented in 60% of cases, 46% of cases were on a lipid‐lowering medication for primary prevention within 18 months after detection of elevated triglyceride level, and control was suboptimal with only 24.1% achieving triglyceride <150 mg/dL level at the last follow up. These findings have public health implications and highlight an opportunity for intervention at the individual and population levels to reduce the burden of CHD.
We estimated prevalence of PIH as 0.80% (~1 in 125), which is double that of familial hypercholesterolemia (~1 in 250).39 The number of cases with any form of hypertriglyceridemia was 6‐fold greater, indicative of the substantial burden of this form of dyslipidemia. The prevalence of PIH increased with age, men were more commonly affected (ratio 2.7:1) and compared with White adults, PIH was more common in Asian adults and less common in Black adults. Awareness of PIH was modest, with only 60% having a diagnosis of hypertriglyceridemia documented in the EHR suggesting that providers, possibly due to time constraints or concern about other co‐morbidities, did not prioritize abnormal triglyceride levels.
The suboptimal control suggests a need to educate providers regarding the causal role of triglyceride in atherosclerosis to encourage consideration of treatment since triglyceride lowering is shown to reduce adverse CHD events.40 The latest American College of Cardiology/American Heart Association (ACC/AHA) guideline on the management of lipid disorders recommends treatment with a statin in those with triglyceride level ≥175 mg/dL and 10‐year CHD risk ≥7.5% (class IIa).41 It is encouraging to note that the rate of lipid‐lowering medication prescriptions after detection of high triglyceride level increased in the second half of the study period in comparison with the first half. Public health interventions that address social determinants of health, encourage a healthy diet, reduce alcohol consumption and processed foods and motivate people to exercise regularly will also help decrease the burden of hypertriglyceridemia and associated CHD risk.
Establishing a causal role of elevated triglycerides in CHD is challenging because of bias inherent in non‐population based cohorts,12, 13, 14, 15, 16, 17 lability in triglyceride levels,12, 25 non‐exclusion of secondary causes26, 27, 28 of increased triglyceride levels and the association of several conventional risk factors with triglyceride levels.29, 30, 31 In this study we attempted to address these limitations of prior reports and demonstrated that PIH was associated with CHD independent of age, sex, race and socioeconomic status. The association remained significant but was attenuated after additional adjustment for conventional risk factors suggesting that some of the attributable risk is mediated by these risk factors.
Our results are consistent with recent studies suggesting LDL‐C and triglycerides are similarly atherogenic since both carry apolipoprotein B particles42, 43 as well as Mendelian randomization studies that indicate triglycerides as a causal risk factor for CHD.7, 8, 9, 10, 11 Evaluating the benefits of isolated triglyceride lowering is not straightforward as many of the medications that decrease triglyceride levels also lower LDL‐C. Furthermore, in trials of triglyceride lowering medications or long‐term cohort studies, few patients with triglyceride level ≥500 mg/dL were included. Assuming triglycerides are causal, the greatest benefit would be expected in these patients. While drugs that increase HDL‐C levels have failed to reduce CHD events,44 the REDUCE‐IT (Reduction of Cardiovascular Events with Icosapent Ethyl‐Intervention) trial revealed a beneficial effect of triglyceride lowering in patients with CHD who were already on a statin.45 A meta‐regression analysis of the fibrate trials demonstrated that a 5% (1%–10%) reduction in CHD events would be achieved by 8.9 mg/dL (0.1 mmol/L) reduction in triglyceride level and the benefit was greater in those with higher baseline triglyceride level.40
We did not find a significant association between elevated triglyceride levels and future CVD or PAD, likely due to the small number of incident CVD and PAD events. In the Copenhagen Heart Study,22 the risk of ischemic stroke increased with increasing triglyceride level. However, the association was attenuated after adjustment for multiple covariates.22 The association of triglyceride levels with PAD has not been studied previously in a population‐based setting. In a multivariable adjusted model, we also did not find a statistically significant association between PIH and the composite end point of CHD, CVD, and PAD.
We limited cases to those with high triglyceride level defined as as fasting triglyceride ≥500 mg/dL. Since triglyceride levels vary significantly within individuals, our goal in doing so was to lessen random measurement bias. Patients with high or very high triglyceride level were often excluded from previous studies12, 13, 18 or composed only a small proportion of the study cohort.14, 15, 19, 20, 21, 22 Given the high collinearity between triglyceride level and HDL‐C, it is difficult to tease out independent effects of the 2 variables. While triglyceride level was not associated with CHD after adjustment for HDL‐C, a growing body of evidence indicates a causal role for triglycerides, not HDL‐C, in atherosclerosis.46, 47 It is also worth noting that a higher rate of CHD in cases was observed despite greater use of lipid‐lowering medication than controls.
Limitations of our study include a relatively low number of non‐White individuals in the cohort. Our findings may not be generalizable to the entire US population. However, given the diversity of the US population, no single cohort is completely representative of the entire country and our study cohort represents a large segment of the US population.32, 48 Strengths include the population‐based setting and exclusion of secondary causes and mixed hyperlipidemia, enabling the study of PIH as a risk factor for CHD. Unlike previous studies, which used the lowest triglyceride tertile or quintile as a referent group,12, 15, 19, 21 we selected controls from the population based on the most accepted definition of a normal triglyceride level. Our study population is a contemporary US cohort, representative of current real‐world practice and care and provides epidemiologic data related to incidence, prevalence, awareness, and control of PIH.
Conclusion
We report that PIH is associated with the risk of incident CHD in a population‐based setting, consistent with a causal role of triglyceride‐rich lipoproteins in CHD. The association was attenuated after adjustment for conventional risk factors, suggesting that some of the risk due to elevated triglyceride level is mediated by these factors. The burden of PIH was relatively high with a prevalence of ~1 in 125 and the majority did not attain normal triglyceride levels during follow up. Awareness and control were sub‐optimal, highlighting an opportunity for interventions to increase recognition and treatment of PIH, thereby lowering the burden of CHD.
Sources of Funding
This study was funded by grants R01 HL135879 and K24 HL137010 from the National Heart Lung and Blood Institute. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Data S1
Tables S1–S8
Figures S1–S3
Acknowledgments
We thank Ms Luanne Wussow for help with preparation of the manuscript.
(J Am Heart Assoc. 2021;10:e019343. DOI: 10.1161/JAHA.120.019343.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019343
For Sources of Funding and Disclosures, see page 9.
References
- 1.Grundy SM, Gotto A, Bierman E. Recommendations for the treatment of hyperlipidemia in adults. A joint statement of the nutrition committee and the council on arteriosclerosis of the American Heart Association. Arteriosclerosis. 1984;4:445A–468A. [PubMed] [Google Scholar]
- 2.Study Group EAS . Strategies for the prevention of coronary heart disease: a policy statement of the European Atherosclerosis Society. Eur Heart J. 1987;8:77–88. [PubMed] [Google Scholar]
- 3.Steinberg D. Lewis A. Conner Memorial Lecture: oxidative modification of ldl and atherogenesis. Circulation. 1997;95:1062–1071. DOI: 10.1161/01.CIR.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 4.Endo A. The discovery and development of HMG‐CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582. DOI: 10.1016/S0022-2275(20)41379-3. [DOI] [PubMed] [Google Scholar]
- 5.Brown DF, Kinch SH, Doyle JT. Serum triglycerides in health and in ischemic heart disease. N Engl J Med. 1965;273:947–952. DOI: 10.1056/NEJM196510282731802. [DOI] [PubMed] [Google Scholar]
- 6.Hulley SB, Rosenman RH, Bawol RD, Brand RJ. Epidemiology as a guide to clinical decisions: the association between triglyceride and coronary heart disease. N Engl J Med. 1980;302:1383–1389. DOI: 10.1056/NEJM198006193022503. [DOI] [PubMed] [Google Scholar]
- 7.Agerholm‐Larsen B, Nordestgaard BG, Steffensen R, Jensen G, Tybjærg‐Hansen A. Elevated hdl cholesterol is a risk factor for ischemic heart disease in white women when caused by a common mutation in the cholesteryl ester transfer protein gene. Circulation. 2000;101:1907–1912. DOI: 10.1161/01.CIR.101.16.1907. [DOI] [PubMed] [Google Scholar]
- 8.Andersen RV, Wittrup HH, Tybjærg‐Hansen A, Steffensen R, Schnohr P, Nordestgaard BG. Hepatic lipase mutations, elevated high‐density lipoprotein cholesterol, and increased risk of ischemic heart disease: the copenhagen city heart study. J Am Coll Cardiol. 2003;41:1972–1982. DOI: 10.1016/S0735-1097(03)00407-8. [DOI] [PubMed] [Google Scholar]
- 9.Frikke‐Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjærg‐Hansen A. Association of loss‐of‐function mutations in the ABCA1 gene with high‐density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–2532. DOI: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 10.Haase CL, Tybjærg‐Hansen A, Ali Qayyum A, Schou J, Nordestgaard BG, Frikke‐Schmidt R. LCAT, HDL cholesterol and ischemic cardiovascular disease: a mendelian randomization study of hdl cholesterol in 54,500 individuals. J Clin Endocrinol Metab. 2012;97:E248–E256. DOI: 10.1210/jc.2011-1846. [DOI] [PubMed] [Google Scholar]
- 11.Voight BF, Peloso GM, Orho‐Melander M, Frikke‐Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, et al. Plasma hdl cholesterol and risk of myocardial infarction: a mendelian randomisation study. The Lancet. 2012;380:572–580. DOI: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tirosh A, Rudich A, Shochat T, Tekes‐Manova D, Israeli E, Henkin Y, Kochba I, Shai I. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med. 2007;147:377–385. DOI: 10.7326/0003-4819-147-6-200709180-00007. [DOI] [PubMed] [Google Scholar]
- 13.Toth PP, Granowitz C, Hull M, Liassou D, Anderson A, Philip S. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real‐world administrative claims analysis of statin‐treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7:e008740. DOI: 10.1161/JAHA.118.008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klempfner R, Erez A, Sagit B‐Z, Goldenberg I, Fisman E, Kopel E, Shlomo N, Israel A, Tenenbaum A. Elevated triglyceride level is independently associated with increased all‐cause mortality in patients with established coronary heart disease: twenty‐two–year follow‐up of the bezafibrate infarction prevention study and registry. Circ Cardiovasc Qual Outcomes. 2016;9:100–108. DOI: 10.1161/CIRCOUTCOMES.115.002104. [DOI] [PubMed] [Google Scholar]
- 15.Kasai T, Miyauchi K, Yanagisawa N, Kajimoto K, Kubota N, Ogita M, Tsuboi S, Amano A, Daida H. Mortality risk of triglyceride levels in patients with coronary artery disease. Heart. 2013;99:22–29. DOI: 10.1136/heartjnl-2012-302689. [DOI] [PubMed] [Google Scholar]
- 16.Haim M, Benderly M, Brunner D, Behar S, Graff E, Reicher‐Reiss H, Goldbourt U. Elevated serum triglyceride levels and long‐term mortality in patients with coronary heart disease: the bezafibrate infarction prevention (BIP) registry. Circulation. 1999;100:475–482. DOI: 10.1161/01.CIR.100.5.475. [DOI] [PubMed] [Google Scholar]
- 17.Sprecher DL, Pearce GL, Cosgrove DM, Lytle BW, Loop FD, Pashkow FJ. Relation of serum triglyceride levels to survival after coronary artery bypass grafting. Am J Cardiol. 2000;86:285–288. DOI: 10.1016/S0002-9149(00)00915-2. [DOI] [PubMed] [Google Scholar]
- 18.Andersson C, Lyass A, Vasan RS, Massaro JM, D'Agostino RB Sr, Robins SJ. Long‐term risk of cardiovascular events across a spectrum of adverse major plasma lipid combinations in the Framingham Heart Study. Am Heart J. 2014;168:878–883.e1. DOI: 10.1016/j.ahj.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw K‐T, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 western prospective studies. Circulation. 2007;115:450–458. DOI: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 20.Nordestgaard BG, Benn M, Schnohr P, Tybjærg‐Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. DOI: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 21.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. DOI: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 22.Freiberg JJ, Tybjærg‐Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142–2152. DOI: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 23.Brunzell JD, Deeb SS.Familial Lipoprotein Lipase Deficiency, Apo C‐II Deficiency, and Hepatic Lipase Deficiency. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA. eds. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw‐Hill; Accessed May 02, 2021. Available at: https://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225539482. Accessed May 3, 2021. [Google Scholar]
- 24.Nordestgaard B, Zilversmit D. Large lipoproteins are excluded from the arterial wall in diabetic cholesterol‐fed rabbits. J Lipid Res. 1988;29:1491–1500. DOI: 10.1016/S0022-2275(20)38428-5. [DOI] [PubMed] [Google Scholar]
- 25.Varbo A, Benn M, Tybjærg‐Hansen A, Jørgensen AB, Frikke‐Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. DOI: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler DC, Bernard DB. Lipid abnormalities in the nephrotic syndrome: causes, consequences, and treatment. Am J Kidney Dis. 1994;23:331–346. DOI: 10.1016/S0272-6386(12)80994-2. [DOI] [PubMed] [Google Scholar]
- 27.O'brien T, Dinneen SF, O'brien PC, Palumbo PJ. Hyperlipidemia in patients with primary and secondary hypothyroidism. Mayo Clin Proc. 1993;68:860–866. DOI: 10.1016/S0025-6196(12)60694-6. [DOI] [PubMed] [Google Scholar]
- 28.Crook D, Cust MP, Gangar KF, Worthington M, Hillard TC, Stevenson JC, Whitehead MI, Wynn V. Comparison of transdermal and oral estrogen‐progestin replacement therapy: effects on serum lipids and lipoproteins. Am J Obstet Gynecol. 1992;166:950–955. DOI: 10.1016/0002-9378(92)91370-P. [DOI] [PubMed] [Google Scholar]
- 29.Smellie WSA. Hypertriglyceridaemia in diabetes. BMJ. 2006;333:1257–1260. DOI: 10.1136/bmj.39043.398738.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appel G. Lipid abnormalities in renal disease. Kidney Int. 1991;39:169–183. DOI: 10.1038/ki.1991.22. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian S, Chait A. Hypertriglyceridemia secondary to obesity and diabetes. Biochim et Biophys Acta (BBA) ‐ Mol Cell Biol Lipids. 2012;1821:819–825. DOI: 10.1016/j.bbalip.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Rocca WA, Yawn BP, Sauver JLS, Grossardt BR, Melton LJ. History of the rochester epidemiology project: half a century of medical records linkage in a us population. Mayo Clin Proc. 2012;87:1202–1213. DOI: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. DOI: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safarova MS, Liu H, Kullo IJ. Rapid identification of familial hypercholesterolemia from electronic health records: the search study. J Clin Lipidol. 2016;10:1230–1239. DOI: 10.1016/j.jacl.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirby JC, Speltz P, Rasmussen LV, Basford M, Gottesman O, Peissig PL, Pacheco JA, Tromp G, Pathak J, Carrell DS, et al. PheKB: a catalog and workflow for creating electronic phenotype algorithms for transportability. Journal of the American Medical Informatics Association. 2016;23:1046–1052. DOI: 10.1093/jamia/ocv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Smith GD. Indicators of socioeconomic position (part 1). J Epidemiol Commun Health. 2006;60:7–12. DOI: 10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kullo IJ, Fan J, Pathak J, Savova GK, Ali Z, Chute CG. Leveraging informatics for genetic studies: use of the electronic medical record to enable a genome‐wide association study of peripheral arterial disease. J Am Med Inform Assoc. 2010;17:568–574. DOI: 10.1136/jamia.2010.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Bielinski SJ, Sohn S, Murphy S, Wagholikar KB, Jonnalagadda SR, Ravikumar K, Wu ST, Kullo IJ, Chute CG. An information extraction framework for cohort identification using electronic health records. AMIA Summits Transl Sci Proc. 2013;2013:149. [PMC free article] [PubMed] [Google Scholar]
- 39.Bucholz EM, Rodday AM, Kolor K, Khoury MJ, de Ferranti SD. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among us adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999–2014). Circulation. 2018;137:2218–2230. DOI: 10.1161/CIRCULATIONAHA.117.032321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic VJTL. Effects of fibrates on cardiovascular outcomes: a systematic review and meta‐analysis. Lancet. 2010;375:1875–1884. DOI: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 41.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–e350. DOI: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Ference BA, Kastelein JJ, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver‐Williams C, Wood AM, Butterworth ASJJ. Association of triglyceride‐lowering LPL variants and LDL‐C–lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. DOI: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA. Apolipoprotein B particles and cardiovascular disease. JAMA Cardiol. 2019;4:1287–1295. DOI: 10.1001/jamacardio.2019.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eyvazian VA, Frishman WH. Evacetrapib: another CETP inhibitor for dyslipidemia with no clinical benefit. Cardiol Rev. 2017;25:43–52. DOI: 10.1097/CRD.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 45.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. DOI: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 46.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. DOI: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 47.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. DOI: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauver JLS, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the rochester epidemiology project. Mayo Clin Proc. 2012;87:151–160. DOI: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S8
Figures S1–S3


