Abstract
Background
The frequency of the initial short‐term decline in estimated glomerular filtration rate (eGFR), eGFR dip, following initiation of sodium‐glucose cotransporter‐2 inhibitors (SGLT2i) and its clinical implications in real‐world practice are not clear.
Methods and Results
We built a cohort of 36 638 new users of SGLT2i and 209 025 new users of other antihyperglycemics. Inverse probability weighting was used to estimate the excess rate of eGFR dip, risk of the composite cardiovascular outcome of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or all‐cause mortality, and risk of the composite kidney outcome of eGFR decline >50%, end‐stage kidney disease, or all‐cause mortality. In the first 6 months of therapy, compared with other antihyperglycemics, excess rates of eGFR dip >10% and eGFR dip >30% were 9.86 (95% CI: 8.83–11.00) and 1.15 (0.70–1.62) per 100 SGLT2i users, respectively. In mediation analyses that accounted for eGFR dipping, SGLT2i use was associated with reduced risk of cardiovascular and kidney outcomes (hazard ratio, 0.92 [0.84–0.99] and 0.78 [0.71–0.87], respectively); the magnitude of the association reduced by eGFR dipping was small for both outcomes. SGLT2i was associated with reduced risk of both outcomes in those with higher than average probability of eGFR dip >10% or 30%. Compared with discontinuation, continued use of SGLT2i at 6 months was associated with reduced risk of cardiovascular and kidney outcomes in those with no eGFR dip or eGFR dip ≤10%, in those with eGFR dip >10%, and in those with eGFR dip >30%.
Conclusions
The salutary association of SGLT2i with cardiovascular and kidney outcomes was maintained regardless of eGFR dipping; concerns about eGFR dipping should not preclude use, and occurrence of eGFR dip after SGLT2i initiation may not warrant discontinuation.
Keywords: cardiovascular outcomes, diabetes mellitus, estimated glomerular filtration rate, kidney, kidney function, kidney outcomes, sodium‐glucose cotransporter‐2 inhibitors
Nonstandard Abbreviations and Acronyms
- CDW
Corporate Data Warehouse
- CREDENCE
Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy
- EMPA‐REG OUTCOME
Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients
- SGLT2i
sodium‐glucose cotransporter‐2 inhibitor(s)
- VA
US Department of Veterans Affairs
Clinical Perspective
What Is New?
In the first 6 months of treatment, estimated glomerular filtration rate (eGFR) dipping was more common among sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) users than other antihyperglycemics; however, most eGFR dips were <30%.
eGFR dipping did not abrogate the beneficial association between SGLT2i and cardiovascular and kidney outcomes.
Continued use of SGLT2i (versus discontinuation) was associated with reduced risk of cardiovascular and kidney outcomes regardless of degree of eGFR dipping.
What Are the Clinical Implications?
Real‐world effectiveness of SGLT2i on cardiovascular and kidney outcomes was maintained regardless of eGFR dipping.
Concern about dipping should not preclude initiation of SGLT2i, and occurrence of an eGFR dip following SGLT2i initiation should not, on its own, motivate SGLT2i discontinuation.
As a means of achieving longer‐term reduction in risk of cardiovascular and kidney outcomes, practitioners may consider continuation of treatment with SGLT2i regardless of eGFR dipping.
Several randomized clinical trials provided evidence that sodium‐glucose cotransporter‐2 inhibitors (SGLT2i) reduce the risk of major cardiovascular and kidney outcomes in people with type 2 diabetes mellitus.1, 2, 3, 4, 5, 6, 7 Evidence from recent trials suggests that the salutary properties of SGLT2i may even extend to people without diabetes mellitus.8, 9, 10 However, in several randomized trials, SGLT2i use was associated with an initial estimated glomerular filtration rate (eGFR) decline, eGFR dip, within a few weeks and up to 6 months following initiation of therapy.11 The eGFR dip is generally followed by recovery and stabilization during the subsequent months; in the long‐term, SGLT2i use was associated with relative eGFR preservation compared with placebo.11
However, the frequency and extent of eGFR dip in SGLT2i users relative to other antihyperglycemics, whether eGFR dipping erodes SGLT2i effectiveness on cardiovascular and kidney outcomes, and whether eGFR dip is associated with increased risk of discontinuation of SGLT2i in real‐world practice are not known. Furthermore, whether continued SGLT2i use (versus discontinuation) following an intervening SGLT2i dip is associated with reduced risk of cardiovascular and kidney outcomes is unknown. Addressing this knowledge gap will illuminate our understanding of the clinical ramifications of the eGFR dip in SGLT2i users.
Herein, we leveraged the breadth and depth of the US Department of Veterans Affairs (VA) electronic healthcare databases to build a cohort of 36 638 incident users of SGLT2i, and 209 025 incident users of other antihyperglycemics, and aimed to characterize the rates of eGFR dipping in each antihyperglycemic group, identify characteristics associated with eGFR dipping in SGLT2i users, examine whether and to what extent the effectiveness of SGLT2i on cardiovascular and kidney outcomes was abrogated by an intervening eGFR dip, and finally evaluate the risk of major cardiovascular outcomes and kidney outcomes associated with SGLT2i continuation versus discontinuation according to eGFR dipping category.
Methods
Because of the sensitive nature of the data used in this study, the data sets could only be accessed after obtaining approval from the VA.
Cohort Design
Participants who received antihyperglycemic medication from the VA Health Care System between October 1, 2015, and July 31, 2019, were selected (n=1 293 984). Participants were separated first into an SGLT2i group, which included patients who received an SGLT2i prescription between October 1, 2016, and July 31, 2019 (n=59 133). The other antihyperglycemic group was then selected from participants who did not receive SGLT2i prescription between October 1, 2016, and July 31, 2019. The other antihyperglycemic group included patients who either added on or switched from their existing non‐SGLT2i antihyperglycemic medication to a non‐SGLT2i antihyperglycemic prescription between October 1, 2016, and July 31, 2019 (n=423 193), where the first date of meeting this criterion was considered time of treatment initiation. In each group, participants without a history of SGLT2i exposure within the 1 year before treatment initiation were selected (SGLT2i group, n=55 466; and other antihyperglycemic group, n=420 625). We further excluded participants with history of type 1 diabetes mellitus, those with end‐stage kidney disease, or those who were enrolled in the healthcare system for <1 year at treatment initiation (SGLT2i group, n=51 816; and other antihyperglycemic group, n=387 467). Within participants who remained in the cohort, 46 122 in the SGLT2i group and 312 794 in the other antihyperglycemic group had measurements of glycated hemoglobin (HbA1c), low‐density lipoproteins, blood pressure, height, weight, and had eGFR measurement ≥30 mL/min per 1.73 m2 within the 1 year before treatment initiation. To evaluate the change in eGFR within the first 6 months (180 days) after treatment initiation, we removed participants who had no eGFR measurement or who experienced the composite cardiovascular or kidney outcome in this time period (SGLT2i group, n=36 638; and other antihyperglycemic group, n=209 025). Participants were followed until the occurrence of an outcome or administrative end of follow‐up (January 31, 2020). Informed consent was waived for this study. The cohort flowchart is presented in Figure S1, and study timeline is presented in Figure S2.
Data Sources
Data from the VA Corporate Data Warehouse (CDW) were used in this study.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 CDW Outpatient and Inpatient Encounters domains included International Classification of Diseases, Tenth Revision (ICD‐10), diagnosis codes, ICD‐10 procedure codes, and Current Procedural Terminology codes.27 Medication prescriptions were obtained from the CDW Outpatient Pharmacy domain. Laboratory measurements from the CDW Laboratory Results domain were also collected and used.28 The CDW Vital Signs domain provided vital measurements, and the CDW Patient domain and VA Vital Status provided demographic information.29
Exposure and Outcomes
Prescriptions of SGLT2i or other antihyperglycemic medications were identified from outpatient pharmacy records. The distribution of antihyperglycemic medications at treatment initiation is presented in Table S1.15, 30
Differences in rates of eGFR dip >10% and >30% between the SGLT2i and other antihyperglycemic groups were examined. eGFR dip was evaluated on the basis of the difference between baseline eGFR, defined as the average eGFR within 1 year before treatment initiation, and the lowest eGFR value measured within 6 months after the treatment initiation. The difference was transformed into percentage change compared with baseline eGFR. The CKD Epidemiology Collaboration creatinine equation was used to compute eGFR based on serum creatinine, age, race, and sex.31
SGLT2i discontinuation was defined as a >90‐day gap between the last supply date of a prescription and the next prescription, where the date of the last supply was within 6 months after treatment initiation. The associations between discontinuation of SGLT2i and the cardiovascular and kidney outcomes were examined. The composite cardiovascular outcome was defined as nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or all‐cause mortality. The composite kidney outcome was defined as eGFR decline >50% from treatment initiation, end‐stage kidney disease, or all‐cause mortality. Time of end‐stage kidney disease was identified by first occurrence of eGFR <15 mL/min per 1.73 m2, long‐term dialysis, or kidney transplant.
Covariates
Baseline Covariates
Covariate selection was informed by prior knowledge.15, 17, 18, 20, 22, 24, 32 Covariates that may influence antihyperglycemic prescription included age, race (White, Black, and other, where other race included non‐White and non‐Black participants), sex, HbA1c, eGFR, systolic blood pressure, diastolic blood pressure, low‐density lipoproteins, body mass index (computed from height and weight), smoking status (never, former, or current), type of hospital system where the antihyperglycemic was prescribed (outpatient clinic or healthcare system), and the calendar year of enrollment. eGFR was the average eGFR within 1 year before treatment initiation. Clinical comorbidities, such as congestive heart failure, cardiovascular diseases, cancer, alcoholism, hypoglycemia, diabetic ketoacidosis, acute kidney injury, bladder and urinary tract infections, venous thromboembolism, pancreatitis, bone fracture, and albuminuria, were also included.33 Acute kidney injury was defined as an increased serum creatinine of 0.3 mg/dL or 50% within 30 days, and albuminuria status was categorized into no albuminuria (≤30 mg/g), microalbuminuria (>30–≤300 mg/g), and macroalbuminuria (>300 mg/g). History use of glucagon‐like peptide 1 agonists, dipeptidyl peptidase‐4 inhibitors, sulfonylureas, thiazolidinediones, metformin, insulin, α‐glucosidase inhibitors, meglitinides, amylin analogues, statins, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, β‐blockers, loop diuretics, nonloop diuretics, and calcium channel blockers was also used as covariates.33 Covariates were ascertained within the 1 year before the treatment initiation.
Characteristics Within 6 Months After Treatment Initiation
To examine characteristics that may be associated with SGLT2i discontinuation, we additionally evaluated the occurrence of adverse events, including hypoglycemia, diabetic ketoacidosis, amputation, bladder and urinary tract infections, venous thromboembolism, pancreatitis, bone fracture, hospitalizations that were not related to adverse events, and HbA1c change (categorized as increase or lack of increase in HbA1c) within the 6 months after treatment initiation.
Statistical Analysis
Characteristics of the cohort and by treatment group and eGFR dip categories are presented as mean and SD, or number and percentage, as appropriate. A schematic of the analytic approach is presented in Figure S3.
Rates of eGFR Dip and Predictors of the Dip
Differences in rate of eGFR dips, including dip >10% and dip >30%, between the SGLT2i group and other antihyperglycemic group were examined by weighted generalized estimating equations for logistic regression. To balance potential confounders between treatments, inverse probability of treatment weighting method was applied.34 Probability of receiving the assigned treatment at treatment initiation was estimated from logistic regression based on covariates ascertained at treatment initiation. The inverse probability of treatment weighting was then constructed as the inverse probability stabilized by the prevalence of treatments at time of treatment initiation. The inverse probability of treatment weighting was then truncated at the 0.1 and 99.9 percentiles to further stabilize the weighting.35 Absolute rate per 100 patients in each group and the excess rates associated with SGLT2i were estimated on the basis of the predicted probability. We additionally evaluated the excess rates in subgroups based on race, eGFR category, and albuminuria category, and in those with congestive heart failure and those using angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, loop diuretics, and nonloop diuretics. We also investigated the characteristics that may be associated with eGFR dip >10% and dip >30% in SGLT2i users using logistic regressions.
eGFR Dip and the Effectiveness of SGLT2i on Cardiovascular and Kidney Outcomes
We evaluated the mediation effect of eGFR dip on the associations between SGLT2i and composite cardiovascular and kidney outcomes through inverse odds ratio (OR) weighting for causal mediation analysis.36 In mediation analyses, we estimated the direct effect independent of eGFR dip >10% and, in separate analyses, eGFR dip >30%. The total effect of SGLT2i, accounted for eGFR dipping as a mediator was estimated from a Cox model weighted by inverse probability of treatment weighting. Direct effect of SGLT2i was estimated from a similarly weighted Cox model where the model was additionally weighted for the inverse OR of having the mediator in the SGLT2i group. The magnitude of effect abrogated by the mediator was then computed from the difference between hazard ratios (HRs) for the direct effect independent of eGFR dip and the HRs for the total effect. Event rate differences between direct and total effect were estimated on the basis of survival probability, where the difference represented the difference in the rate of the outcome between the SGLT2i and other antihyperglycemic groups that was mediated by differences in the rate of eGFR dipping.
To assess whether the salutary association of SGLT2i on composite cardiovascular and kidney outcomes was abrogated in SGLT2i users with a high probability of experiencing an eGFR dip associated with SGLT2i, we estimated the association between SGLT2i and risk of the composite cardiovascular outcome and the composite kidney outcome in SGLT2i users with a higher or lower than average predicted probability of having an eGFR dip >10%, and separately eGFR dip >30%, associated with SGLT2i (ie, the excess probability of having an eGFR dip associated with SGLT2i compared with other antihyperglycemics after consideration of baseline characteristics). We first estimated the predicted baseline probability of eGFR dip associated with covariates at treatment initiation within the other antihyperglycemic group using logistic regression. Using results from this model, we then computed the baseline probability of dip in the SGLT2i group. Then, the probability of eGFR dip associated with SGLT2i (PSGLT2i dip), conditional on the baseline probability of dipping, was estimated within the SGLT2i group. The SGLT2i users were then separated into high and low risk of experiencing an eGFR dip associated with SGLT2i, based on PSGLT2i dip being above or below the mean predicted probability. The association between SGLT2i and the risk of outcomes in users with predicted probability of SGLT2i‐related eGFR dip higher or lower than the average probability was examined after adjusting for covariates at treatment initiation.
Association Between SGLT2i Discontinuation and Risk of Outcomes
We then examined the association between SGLT2i discontinuation with characteristics occurring between treatment initiation and 6 months after treatment initiation, which included eGFR dip, adverse events, hospitalization, and HbA1c change. Logistic regression was used and adjusted for probability of discontinuation. The probability was estimated from a logistic regression where discontinuation was predicted by the set of baseline covariates.
Associations between SGLT2i discontinuation and composite cardiovascular or kidney outcomes were examined from Cox survival models weighted by the inverse probability of discontinuation weight, where the weight was constructed on the basis of the probability of discontinuation and updated by additionally including factors that occur between treatment initiation and 6 months after treatment initiation. The effects were estimated in all cohorts, and within those with no eGFR dip or dip ≤10%, eGFR dip >10%, and eGFR dip >30%, separately. The composite cardiovascular and kidney event rates in 1 year by discontinuation and continuation of SGLT2i and their differences were calculated on the basis of the estimated survival probabilities.
Evaluation of Potential Biases
Balance of covariates between the SGLT2i and other antihyperglycemic group, and between SGLT2i continuation and discontinuation, was examined through propensity score distribution and standardized difference of covariates. To test the robustness of our analyses, we tested a negative outcome control as a means to detect the presence of spurious associations.37 Traffic‐related injury was used as the negative outcome control as there is neither biologic plausibility nor a priori evidence suggesting the presence of a relationship with either SGLT2i use or SGLT2i discontinuation.
Other Statistical Considerations
The 95% CIs for rate difference were generated on the basis of 1000 times bootstrapping. A 95% CI for ratio measure that does not cross 1 or for rate that does not cross 0 was considered statistically significant. All analyses were done using SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC). The study was approved by the Institutional Review Board of the VA Saint Louis Health Care System, Saint Louis, MO.
Results
There were 36 638 individuals in the SGLT2i group and 209 025 individuals in the other antihyperglycemic group, corresponding to 447 399 person‐years. Among incident SGLT2i users, there were 20 458 (55.84%) with no eGFR dip, 16 180 (44.16%) with eGFR dip >10%, and 2326 (6.35%) with eGFR dip >30% within the first 6 months of SGLT2i initiation. Baseline demographic and health characteristics in these groups are provided in Table 1.
Table 1.
Baseline Characteristics by Treatment Group and by eGFR Dip in SGLT2i Group
| Baseline Characteristics | Other Antihyperglycemics (n=209 025) | SGLT2i | |||
|---|---|---|---|---|---|
| All SGLT2i (n=36 638) | No eGFR Dip or Dip ≤10% (n=20 458; 55.84%) | eGFR Dip >10% (n=16 180; 44.16%) | eGFR Dip >30% (n=2326; 6.35%) | ||
| Age, mean (SD), y | 65.73 (10.55) | 65.30 (9.11) | 64.71 (9.36) | 66.06 (8.71) | 66.26 (8.12) |
| Race, n (%) | |||||
| White | 145 909 (69.80) | 27 169 (74.16) | 15 479 (75.66) | 11 690 (72.25) | 1623 (69.78) |
| Black | 39 357 (18.83) | 5297 (14.46) | 2685 (13.12) | 2612 (16.14) | 413 (17.76) |
| Other* | 23 759 (11.37) | 4172 (11.39) | 2294 (11.21) | 1878 (11.61) | 290 (12.47) |
| Sex, n (%) | |||||
| Men | 197 727 (94.59) | 35 016 (95.57) | 19 529 (95.46) | 15 487 (95.72) | 2217 (95.31) |
| Women | 11 298 (5.41) | 1622 (4.43) | 929 (4.54) | 693 (4.28) | 109 (4.69) |
| eGFR, mean (SD), mL/min per 1.73 m2 | 76.05 (20.45) | 78.84 (17.51) | 80.77 (17.79) | 75.72 (16.73) | 73.38 (16.62) |
| eGFR category, n (%) | |||||
| eGFR ≥90 mL/min per 1.73 m2 | 57 407 (27.46) | 10 120 (27.62) | 7006 (34.25) | 3114 (19.25) | 342 (14.70) |
| 90 mL/min per 1.73 m2>eGFR≥60 mL/min per 1.73 m2 | 97 261 (46.53) | 19 882 (54.27) | 10 455 (51.10) | 9427 (58.26) | 1261 (54.21) |
| 60 mL/min per 1.73 m2> eGFR≥45 mL/min per 1.73 m2 | 36 044 (17.24) | 5881 (16.05) | 2697 (13.18) | 3184 (19.68) | 627 (26.96) |
| 45 mL/min per 1.73 m2>eGFR ≥30 mL/min per 1.73 m2 | 18 313 (8.76) | 755 (2.06) | 300 (1.47) | 455 (2.81) | 96 (4.13) |
| HbA1c, mean (SD), % | 8.75 (1.93) | 8.71 (1.37) | 8.71 (1.39) | 8.70 (1.34) | 8.76 (1.40) |
| Body mass index, mean (SD), kg/m2 | 32.87 (6.53) | 34.25 (6.41) | 34.17 (6.41) | 34.35 (6.39) | 34.69 (6.58) |
| Low‐density lipoprotein, mean (SD), mg/dL | 89.97 (36.93) | 80.95 (33.77) | 82.10 (34.00) | 79.50 (33.43) | 79.05 (35.05) |
| Systolic blood pressure, mean (SD), mm Hg | 132.92 (17.06) | 132.35 (16.04) | 131.97 (15.67) | 132.82 (16.49) | 133.71 (17.31) |
| Diastolic blood pressure, mean (SD), mm Hg | 76.32 (10.43) | 75.03 (9.81) | 75.32 (9.73) | 74.65 (9.89) | 74.15 (9.89) |
| Congestive heart failure, n (%) | 12 896 (6.17) | 3416 (9.32) | 1496 (7.31) | 1920 (11.87) | 417 (17.93) |
| Alcoholism, n (%) | 12 148 (5.81) | 1479 (4.04) | 817 (3.99) | 662 (4.09) | 111 (4.77) |
| Bone fracture, n (%) | 2656 (1.27) | 423 (1.115) | 208 (1.02) | 215 (1.33) | 51 (2.19) |
| Cancer, n (%) | 42 861 (20.51) | 7599 (20.74) | 3986 (19.48) | 3613 (22.33) | 573 (24.63) |
| Cardiovascular disease, n (%) | 51 284 (24.53) | 14 635 (39.94) | 7671 (37.50) | 6964 (43.04) | 1117 (48.02) |
| Diabetic ketoacidosis, n (%) | 840 (0.40) | 70 (0.19) | 39 (0.19) | 31 (0.19) | 5 (0.21) |
| Hypoglycemia, n (%) | 3855 (1.84) | 1224 (3.34) | 586 (2.86) | 638 (3.94) | 124 (5.33) |
| Pancreatitis, n (%) | 2474 (1.18) | 446 (1.22) | 210 (1.03) | 236 (1.46) | 45 (1.93) |
| Bladder and urinary tract infections, n (%) | 7328 (3.51) | 746 (2.04) | 377 (1.84) | 369 (2.28) | 76 (3.27) |
| Venous thromboembolism, n (%) | 1359 (0.65) | 214 (0.58) | 113 (0.55) | 101 (0.62) | 14 (0.60) |
| Acute kidney injury, n (%) | 18 821 (9.00) | 3101 (8.46) | 1388 (6.78) | 1713 (10.59) | 406 (17.45) |
| Albuminuria, n (%) | |||||
| None (≤30 mg/g) | 88 410 (42.30) | 14 414 (39.34) | 8871 (43.36) | 5543 (34.26) | 595 (25.58) |
| Microalbuminuria (>30–≤300 mg/g) | 102 139 (48.86) | 18 608 (50.79) | 10 053 (49.14) | 8555 (52.87) | 1306 (56.15) |
| Macroalbuminuria (>300 mg/g) | 18 476 (8.84) | 3616 (9.87) | 1534 (7.50) | 2082 (12.87) | 425 (18.27) |
| Metformin, n (%) | 109 958 (52.61) | 29 760 (81.23) | 16 616 (81.22) | 13 144 (81.24) | 1876 (80.65) |
| Insulin, n (%) | 56 150 (26.86) | 20 705 (56.51) | 10 892 (53.24) | 9813 (60.65) | 1543 (66.34) |
| Sulfonylureas, n (%) | 61 094 (29.23) | 17 734 (48.40) | 9999 (48.88) | 7735 (47.81) | 1048 (45.06) |
| DPP4, n (%) | 9683 (4.63) | 9362 (25.56) | 5298 (25.90) | 4064 (25.12) | 509 (21.88) |
| GLP1, n (%) | 2921 (1.40) | 4798 (13.10) | 2616 (12.79) | 2182 (13.49) | 333 (14.32) |
| Thiazolidinediones, n (%) | 3918 (1.87) | 3081 (8.41) | 1666 (8.14) | 1415 (8.75) | 211 (9.07) |
| Total No. of diabetes mellitus medications used, mean (SD) | 1.18 (0.81) | 2.35 (0.93) | 2.32 (0.94) | 2.39 (0.92) | 2.39 (0.92) |
| ACEI/ARB, n (%) | 118 277 (56.59) | 25 686 (70.11) | 13 906 (67.97) | 11 780 (72.80) | 1801 (77.43) |
| Calcium channel blockers, n (%) | 56 994 (27.27) | 11 133 (30.39) | 5716 (27.94) | 5417 (33.48) | 877 (37.70) |
| β‐Blockers, n (%) | 79 898 (38.22) | 18 992 (51.84) | 9859 (48.19) | 9133 (56.45) | 1478 (63.54) |
| Diuretics, n (%) | |||||
| Loop diuretics | 25 216 (12.06) | 5970 (16.29) | 2690 (13.15) | 3280 (20.27) | 662 (28.46) |
| Nonloop diuretics | 51 521 (24.65) | 9771 (26.67) | 5030 (24.59) | 4741 (29.30) | 759 (32.63) |
| Statins, n (%) | 144 393 (69.08) | 31 222 (85.22) | 17 179 (83.97) | 14 043 (86.79) | 2066 (88.82) |
| Type of hospital system, n (%) | |||||
| Outpatient clinic | 126 384 (60.46) | 19 387 (52.92) | 10 959 (53.57) | 8428 (52.09) | 1174 (50.47) |
| Healthcare system | 82 641 (39.54) | 17 251 (47.08) | 9499 (46.43) | 7752 (47.91) | 1152 (49.53) |
| Year of treatment initial, n (%) | |||||
| 2016 | 18 247 (8.73) | 746 (2.04) | 412 (2.01) | 334 (2.06) | 35 (1.50) |
| 2017 | 76 758 (36.72) | 6747 (18.42) | 3868 (18.91) | 2879 (17.79) | 388 (16.68) |
| 2018 | 72 696 (34.78) | 13 478 (36.79) | 7608 (37.19) | 5870 (36.28) | 812 (34.91) |
| 2019 | 41 324 (19.77) | 15 667 (42.76) | 8570 (41.89) | 7097 (43.86) | 1091 (46.90) |
| Smoking status, n (%) | |||||
| Never | 95 093 (45.49) | 17 062 (46.57) | 9555 (46.71) | 7507 (46.40) | 1093 (46.99) |
| Former | 65 260 (31.22) | 12 092 (33.00) | 6685 (32.68) | 5407 (33.42) | 753 (32.37) |
| Current | 48 672 (23.29) | 7484 (20.43) | 4218 (20.62) | 3266 (20.19) | 480 (20.64) |
Data are presented as mean (SD) or number (percentage). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; DPP4, dipeptidyl peptidase‐4 inhibitor; eGFR, estimated glomerular filtration rate; GLP1, glucagon‐like peptide‐1 agonist; HbA1c, glycated hemoglobin; and SGLT2i, sodium‐glucose co‐transporter‐2 inhibitor.
*Other race includes non‐White and non‐Black participants.
Rates of eGFR Dip in the First 6 Months Among SGLT2i Users and Users of Other Antihyperglycemics
Unadjusted rates of eGFR dip >10% within the first 6 months were 44.16 (95% CI: 43.66–44.67) and 30.37 (30.17–30.56) per 100 people in the SGLT2i and other antihyperglycemic group, respectively (Table S2). In adjusted analyses, compared with other antihyperglycemics, excess rate of eGFR dip >10% was 9.86 (8.83–11.0) per 100 users of SGLT2i (Figure S4). Results showed consistently higher rates of eGFR dip >10% in the SGLT2i group than other antihyperglycemics in prespecified subgroups (Table S3).
Unadjusted rates of eGFR dip >30% within the first 6 months were 6.35 (95% CI: 6.10–6.60) and 4.12 (4.03–4.20) in the SGLT2i and other antihyperglycemic groups, respectively. In adjusted analyses, the excess rate of eGFR dip >30% attributable to SGLT2i was small (1.15 [0.70–1.62] per 100 users of SGLT2i) (Figure 1), and it was nonsignificant in several prespecified subgroups (Table S3).
Figure 1. Adjusted rates of estimated glomerular filtration rate (eGFR) dip in the first 6 months among users of sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) and other antihyperglycemics.
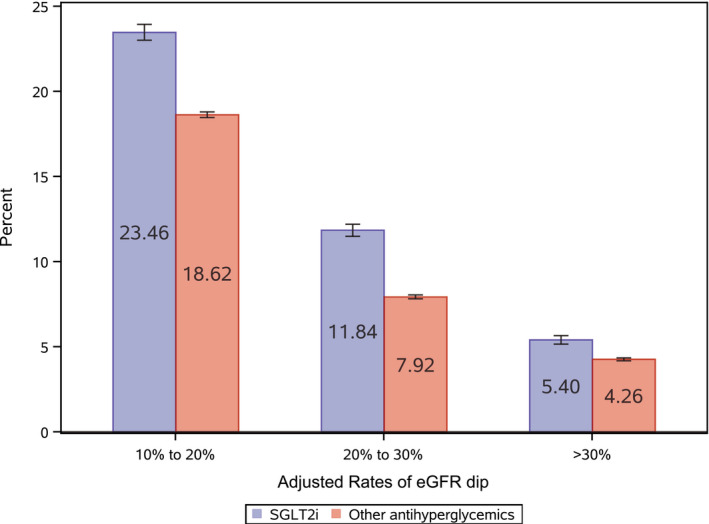
Adjusted rates of eGFR dip >10% to 20%, >20% to 30%, and >30% in the SGLT2i group (blue) and the other antihyperglycemic group (red); model was adjusted for covariates at treatment initiation. Error bars represent 95% CIs.
Characteristics Associated With eGFR Dip Among SGLT2i Users
Black race, lower eGFR category, congestive heart failure, history of acute kidney injury, microalbuminuria, macroalbuminuria, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker use, loop diuretic use, and nonloop diuretic use were associated with higher risk of eGFR dip >10% and eGFR dip >30% (Table 2).
Table 2.
Characteristics Associated With eGFR Dip Among SGLT2i Users
| Characteristics | Adjusted Odds Ratio (95% CI) | |
|---|---|---|
| eGFR Dip >10% | eGFR Dip >30% | |
| Age | 1.00 (0.99–1.00) | 0.99 (0.98–0.99) |
| Race (reference=White) | ||
| Black | 1.40 (1.31–1.49) | 1.29 (1.15–1.46) |
| Other* | 1.02 (0.95–1.09) | 1.01 (0.88–1.15) |
| Women | 1.09 (0.98–1.21) | 1.20 (0.97–1.48) |
| eGFR category (reference=eGFR ≥90 mL/min per 1.73 m2) | ||
| 90 mL/min per 1.73 m2>eGFR≥60 mL/min per 1.73 m2 | 2.02 (1.91–2.14) | 1.90 (1.66–2.16) |
| 60 mL/min per 1.73 m2>eGFR≥45 mL/min per 1.73 m2 | 2.44 (2.26–2.63) | 2.89 (2.48–3.38) |
| 45 mL/min per 1.73 m2>eGFR≥30 mL/min per 1.73 m2 | 2.90 (2.46–3.41) | 3.01 (2.31–3.92) |
| Albuminuria (reference=no albuminuria) | ||
| Microalbuminuria (>30–≤300 mg/g) | 1.22 (1.16–1.27) | 1.46 (1.32–1.61) |
| Macroalbuminuria (>300 mg/g) | 1.63 (1.50–1.76) | 1.90 (1.65–2.18) |
| HbA1c | 1.00 (0.99–1.02) | 1.03 (1.00–1.06) |
| Low‐density lipoprotein | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Systolic blood pressure | 1.00 (1.00–1.00) | 1.00 (1.00–1.01) |
| Diastolic blood pressure | 1.00 (0.99–1.00) | 0.99 (0.99–1.00) |
| Congestive heart failure | 1.19 (1.09–1.29) | 1.30 (1.13–1.49) |
| Cardiovascular disease | 1.04 (0.99–1.09) | 1.06 (0.97–1.17) |
| Acute kidney injury | 1.12 (1.03–1.21) | 1.45 (1.28–1.65) |
| ACEI/ARB | 1.10 (1.04–1.15) | 1.22 (1.10–1.35) |
| Diuretics (reference=no diuretic use) | ||
| Loop diuretics | 1.38 (1.29–1.48) | 1.70 (1.50–1.93) |
| Nonloop diuretics | 1.29 (1.23–1.36) | 1.59 (1.44–1.77) |
| Statins | 0.98 (0.92–1.04) | 0.99 (0.86–1.14) |
Models additionally adjusted for sex, body mass index, smoking status, type of hospital system, cancer, alcoholism, hypoglycemia, diabetic ketoacidosis, bladder and urinary tract infections, venous thromboembolism, pancreatitis, bone fracture, and history use of glucagon‐like peptide‐1 agonist, dipeptidyl peptidase‐4 inhibitor, sulfonylureas, thiazolidinediones, metformin, insulin, α‐glucosidase inhibitors, meglitinides, amylin analogues, β‐blockers, and calcium channel blockers. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; and SGLT2i, sodium‐glucose co‐transporter‐2 inhibitor.
*Other race includes non‐White and non‐Black participants.
eGFR Dip and the Effectiveness of SGLT2i on Cardiovascular and Kidney Outcomes
To estimate whether and to what extent the protective association between SGLT2i and cardiovascular and kidney outcomes may be abrogated by an intervening eGFR dip (conditioned on the probability of eGFR dip), we first developed mediation analyses where eGFR dip was considered a mediator. In analyses for the composite cardiovascular outcome, the total effect size that accounted for eGFR dipping as a mediator was 0.92 (95% CI: 0.84–0.99); estimates of the association independent of eGFR dip >10% and eGFR dip >30% yielded an HR of 0.88 (0.81–0.92) and 0.90 (0.82–0.98), respectively, suggesting that the magnitude of risk reduction abrogated by eGFR dipping was 3.78% (2.22%–5.44%) and 1.18% (0.62%–2.22%) for eGFR dip >10% and eGFR dip >30%, respectively (Figure 2 and Table S4).
Figure 2. Risk of composite cardiovascular and kidney outcomes associated with sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) (vs other antihyperglycemics) based on mediation analyses.
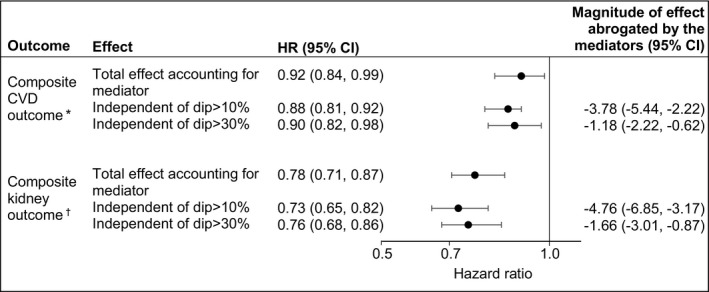
Mediation analyses based on inverse odds ratio weighting and adjusted for covariates measured at treatment initiation. The total effect accounted for estimated glomerular filtration rate (eGFR) dipping as the mediator. The estimates independent of eGFR dip represent the effect that was not mediated by eGFR dip. The magnitude of effect abrogated by the mediator was estimated from the difference between hazard ratios (HRs) independent of eGFR dip and the HRs for the total effect and presented as a percentage. CVD indicates cardiovascular disease.
*Composite CVD outcome of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or all‐cause mortality. †Composite kidney outcome of eGFR decline >50%, end‐stage kidney disease, or all‐cause mortality.
In analyses, for the composite kidney outcome, the total effect size that accounted for eGFR dipping as a mediator was 0.78 (95% CI: 0.71–0.87); estimates of the association independent of eGFR dip >10% and eGFR dip >30% yielded an HR of 0.73 (0.65–0.82) and 0.76 (0.68–1.86), suggesting that the magnitude of risk reduction abrogated by eGFR dipping was 4.76% (3.17%–6.85%) and 1.66% (0.87%–3.01%) for eGFR dip >10% and eGFR dip >30%, respectively (Figure 2 and Table S4).
To determine whether the association between SGLT2i and cardiovascular and kidney outcomes remained significant in people with higher than average probability of experiencing an eGFR dip following SGLT2i initiation, we tested the association in groups based on the predicted probability of eGFR dipping associated with SGLT2i exposure (categorized as above and below average; where average probability for eGFR dip >10% and eGFR dip >30% was 11.64% and 1.92%, respectively). The results suggest that the association between SGLT2i and cardiovascular and kidney outcomes remained significant even in those with higher than average probability of eGFR dip >10% and eGFR dip >30% (Figure 3 and Table S5). The association was also significant in those with below average probability of eGFR dipping (Figure 3 and Table S5).
Figure 3. Risk of composite cardiovascular and kidney outcomes associated with sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) (vs other antihyperglycemics) in groups based on predicted probability of SGLT2i‐related estimated glomerular filtration rate (eGFR) dip.
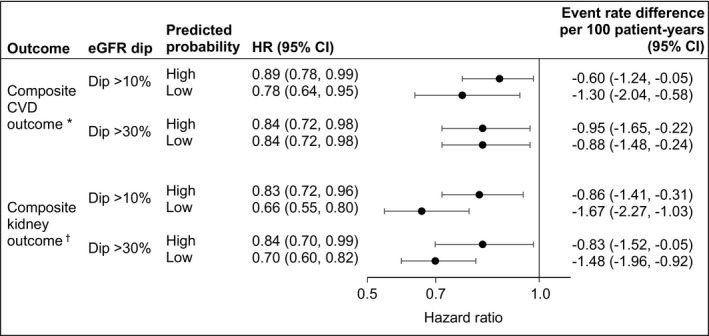
High and low probability of eGFR dip, categorized as above and below average predicted probability of eGFR dip associated with SGLT2i. Average predicted probability was 11.64% and 1.92% for eGFR dip >10% and eGFR dip >30%, respectively. Model was adjusted for covariates measured at treatment initiation. CVD indicates cardiovascular disease; and HR, hazard ratio.
*Composite CVD outcome of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or all‐cause mortality. †Composite kidney outcome of eGFR decline >50%, end‐stage kidney disease, or all‐cause mortality.
eGFR Dip and Characteristics Associated With SGLT2i Discontinuation at 6 Months
Rates of SGLT2i discontinuation at 6 months were 21.83% in the overall SGLT2i group, and 22.10%, 20.12%, and 29.58% in those with no eGFR dip, 10%<eGFR dip≤30%, and eGFR dip >30%, respectively.
In analyses that balanced demographic and health characteristics at baseline, we considered a battery of putative characteristics that may be associated with SGLT2i discontinuation and occurred following SGLT2i initiation and before 6 months. eGFR dip >30%, but not eGFR dip of 10% to 30%, was associated with increased risk of SGLT2i discontinuation (Table S6). The analyses also identified adverse events, including amputation, pancreatitis, bladder and urinary tract infections, hospitalization, and HbA1c increase, as characteristics associated with increased odds of SGLT2i discontinuation (Table S6).
Risk of Cardiovascular and Kidney Outcomes Associated With SGLT2i Continuation or Discontinuation at 6 Months by eGFR Dipping Category
We examined the risk of a composite cardiovascular outcome (of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or all‐cause mortality) associated with continued use of SGLT2i versus discontinuation at 6 months in each eGFR dipping category. The results suggested that compared with discontinuation, continued use of SGLT2i was associated with reduced risk of cardiovascular outcomes in nondippers, in those with eGFR dip >10%, and in those with eGFR dip >30% (Figure 4 and Table S7).
Figure 4. Risk of composite cardiovascular and kidney outcomes associated with sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) continuation vs discontinuation by estimated glomerular filtration rate (eGFR) dipping category.
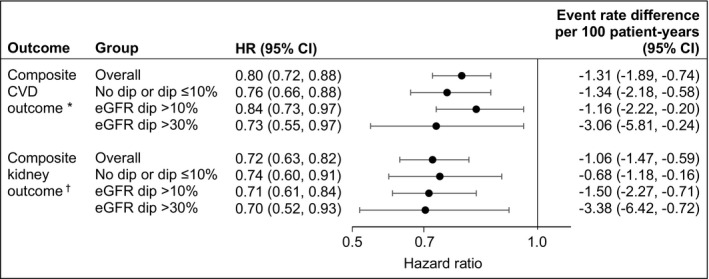
Model adjusted for both covariates measured at treatment initiation and characteristics evaluated within 6 months after treatment initiation. CVD indicates cardiovascular disease; and HR, hazard ratio.
*Composite CVD outcome of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or all‐cause mortality. †Composite kidney outcome of eGFR decline >50%, end‐stage kidney disease, or all‐cause mortality.
We then examined the risk of a composite kidney outcome (of eGFR decline >50%, end‐stage kidney disease, or all‐cause mortality) associated with continued use of SGLT2i versus discontinuation in each eGFR dipping category. The results suggested that compared with discontinuation, continued use of SGLT2i was associated with reduced risk of composite kidney outcome in nondippers, in those with eGFR dip >10%, and in those with eGFR dip >30% (Figure 4 and Table S7).
Evaluation of Potential Biases
Propensity score distribution and standardized difference of covariates across treatment groups and across discontinuation status are presented in Figures S5 through S7. Plots suggested good overlap of the propensity score across groups, and all covariates are well balanced after weighting. A negative outcome control was applied following the same analytic algorithm to examine if the associations observed were attributable to possible spurious biases. Traffic‐related injury, which should not causally exhibit an association with SGLT2i use or SGLT2i continuation, was used as negative outcome control. There was no significant association between SGLT2i and traffic‐related injury (OR: 0.96 [95% CI: 0.83–1.10]) or between SGLT2i continuation and traffic‐related injury (HR: 0.93 [95% CI: 0.63–1.36]).
Discussion
In this cohort study of 36 638 incident users of SGLT2i and 209 025 incident users of other antihyperglycemics, our results suggest that eGFR dip was more frequent following initiation of SGLT2i than other antihyperglycemics; however, most eGFR dips were <30%. Mediation analyses suggested that eGFR dip following initiation of SGLT2i does not substantially abrogate the effectiveness of SGLT2i on cardiovascular and kidney outcomes. Analyses based on the predicted probability of eGFR dipping suggested that even in those with higher than average probability of eGFR dipping, SGLT2i use was still associated with reduced risk of cardiovascular and kidney outcomes. Although eGFR dip <30% was not associated with SGLT2i discontinuation, eGFR dip >30% (a relatively infrequent event) was associated with SGLT2i discontinuation. Compared with those who discontinue SGLT2i in the first 6 months of therapy, and after accounting for characteristics that were associated with discontinuation, SGLT2i continuation was associated with reduced risk of cardiovascular and kidney outcomes regardless of eGFR dipping.
Our findings suggest that eGFR dipping is not uncommon following SGLT2i initiation in real‐world setting (rate of eGFR dip >10% was 9.86 [95% CI: 8.83–11.0] per 100 users of SGLT2i); however, most eGFR dips were <30% as rate of eGFR dip >30% was relatively infrequent (1.15 [0.70–1.62] per 100 users of SGLT2i). In the EMPA‐REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients), 28.3% of empagliflozin users experienced an eGFR dip >10% and 1.4% experienced an eGFR dip of >30%.38 In the CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) trial, rates of eGFR dip >10% and >30% were 21% and 4%, respectively.4 The relatively higher rates of eGFR dipping in these randomized trials compared with the real‐world data provided in this report are likely a reflection of the higher underlying risk of eGFR dip among trial participants.
Our mediation analyses, which considered the contribution of an intervening eGFR dip following initiation of SGLT2i, suggested that dipping does not abrogate the salutary association between SGLT2i and cardiovascular and kidney outcomes. Furthermore, our analyses suggest that even in those with higher than average probability of eGFR dipping, the association between SGLT2i and cardiovascular and kidney outcomes remained protective. Last, the results suggested that even after accounting for characteristics associated with discontinuation at 6 months, continuation of SGLT2i use was associated with reduced risk of the composite cardiovascular and kidney outcomes. Taken together, the constellation of findings suggests that, although eGFR dipping may be more common in SGLT2i users (than other antihyperglycemics), concern about dipping should not preclude initiation of SGLT2i. Another key message from our analyses is that regardless of eGFR dip in the first 6 months following initiation of SGLT2i, continued use of SGLT2i may be more beneficial to long‐term kidney and cardiovascular outcomes than discontinuation; practitioners may consider continuation of treatment with SGLT2i as a means of achieving longer‐term reduction in risk of cardiovascular and kidney outcomes.
Our analyses of factors associated with eGFR discontinuation suggest that the occurrence of adverse events, hospitalization, or increase in HbA1c (a marker of worsening glycemic control) was associated with discontinuation of SGLT2i. Furthermore, eGFR dip >30% was also associated with increased risk of discontinuation. These results may be useful in guiding efforts to examine whether some of these patient groups may benefit from resumption of treatment with SGLT2i.39
The mechanism underpinning the eGFR dip in SGLT2i is not entirely clear.11, 40, 41 It has been suggested that this initial dip is reminiscent of the mild decline in eGFR observed in some patients following initiation of angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, which is generally attributed to postglomerular (efferent) vasodilatation and reduced hyperfiltration.11, 42 Several other hypotheses are being tested, including potential contribution of enhanced proximal tubular natriuresis leading to activation of tubuloglomerular feedback and resultant preglomerular (afferent) vasoconstriction.11, 40, 41, 43 The constellation of evidence from randomized controlled trials and real‐world studies suggests that the initial eGFR dip is likely a functional (and reversible) dip that does not reflect kidney injury and is then followed by eGFR stabilization, and ultimately reduced risk of adverse cardiovascular and kidney outcomes.11, 17, 18, 41 The results of our analyses are congruent with this understanding and support the assessment that eGFR dip does not substantially erode the effectiveness of SGLT2i on cardiovascular and kidney outcomes, and that even in those with eGFR dip >30%, continued therapy with SGLT2i was more beneficial for longer‐term cardiovascular and kidney outcomes than discontinued therapy.
This study has several limitations. We used observational real‐world data from the VA to build our cohort, which was mostly composed of older, White, and male participants, which may limit the generalizability of study findings. Although our analytic approach evaluated SGLT2i versus other active non‐SGLT2i antihyperglycemics, considered known confounders, and applied inverse probability weighting to generate balance in characteristics between the 2 treatment groups, we cannot completely rule out the possibility of residual confounding. Although we used validated definitions to define covariates, exposures, and outcomes based on diagnostic codes, procedure codes, laboratory data, and pharmacy data, we cannot completely rule out misclassification. Because empagliflozin represents >97% of SGLT2i use at the VA, we restricted our analyses to empagliflozin, and we did not examine within SGLT2i class differences. We defined discontinuation based on pharmacy records; hence, the exact discontinuation date may not be accurate. Although we estimated the probability of discontinuation by leveraging a priori knowledge through inclusion of a comprehensive set of covariates, the direct clinical reason (or indication) of medication discontinuation may not have been accounted for in our analyses. The estimation of absolute rate difference was based on the baseline risk in our cohort, which may vary in other populations with different baseline risks.
The study has several strengths. We used large‐scale real‐world data from the VA, which operates the largest integrated healthcare system in the United States; VA data are captured during routine clinical care, which might more closely recapitulate real‐world experiences.17, 18, 44 The selection of antihyperglycemics or discontinuation in VA was less likely driven by financial considerations. We developed our research aims, study design, and execution to specifically address the knowledge gap of real‐world clinical implications of eGFR dip and the effect of discontinuation versus continuation on clinical outcomes, which may not be addressed in randomized controlled trials.45 In addition to reporting relative risk, we reported absolute risk differences that may be meaningful in informing clinical decision making.45 We used a new user design (new SGLT2i users) with active comparator (new other antihyperglycemic users) and applied advanced statistical methods, including inverse probability of treatment weighting, and additionally accounted for events in the first 6 months of therapy that may be associated with discontinuation to evaluate the risk of outcomes in users who continue versus discontinue SGLT2i. We additionally evaluated both risk of major cardiovascular and kidney outcomes to address questions relevant to the clinical community. Finally, the successful testing of negative controls, generally used to detect spurious associations, lessens concern that the observed associations of interest may be attributable to biases.
In sum, our results suggest that eGFR dipping is not uncommon following initiation of SGLT2i; however, most eGFR dips were <30%. The salutary association between SGLT2i and cardiovascular and kidney outcomes was not abrogated by eGFR dipping. Continuation of SGLT2i (versus discontinuation) was associated with reduced risk of cardiovascular and kidney outcomes in those with no eGFR dip, and in those with eGFR dip >10% and eGFR dip >30%.
Sources of Funding
This research was funded by the US Department of Veterans Affairs and the Institute for Public Health at Washington University in Saint Louis, MO (for Dr. Al‐Aly) and American Society of Nephrology (grants to Y. Xie and B. Bowe). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Disclosures
None.
Supporting information
Tables S1–S7
Figures S1–S7
Acknowledgments
Author contributions: Research area and study design: Y. Xie, B. Bowe, and Dr. Al‐Aly; data acquisition: Y. Xie and A.K. Gibson; data analysis and interpretation: Y. Xie, B. Bowe, A.K. Gibson, and Dr. Al‐Aly; statistical analysis: Y. Xie; drafting the manuscript: Y. Xie and Dr Al‐Aly; critical revision of the manuscript: Y. Xie, B. Bowe, A.K. Gibson, Dr. McGill, Dr. Maddukuri, and Dr. Al‐Aly; administrative, technical, or material support: Dr. Al‐Aly; supervision and mentorship: Dr. Al‐Aly. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. Dr. Al‐Aly takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
(J Am Heart Assoc. 2021;10:e020237. DOI: 10.1161/JAHA.120.020237.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020237
For Sources of Funding and Disclosures, see page 12.
References
- 1.Neal B,Perkovic V,Mahaffey KW,de Zeeuw D,Fulcher G,Erondu N,Shaw W,Law G,Desai M,Matthews DR, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. DOI: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 2.Wiviott SD,Raz I,Bonaca MP,Mosenzon O,Kato ET,Cahn A,Silverman MG,Zelniker TA,Kuder JF,Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. DOI: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 3.Zinman B,Wanner C,Lachin JM,Fitchett D,Bluhmki E,Hantel S,Mattheus M,Devins T,Johansen OE,Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. DOI: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V,Jardine MJ,Neal B,Bompoint S,Heerspink HJL,Charytan DM,Edwards R,Agarwal R,Bakris G,Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. DOI: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 5.Mosenzon O,Wiviott SD,Cahn A,Rozenberg A,Yanuv I,Goodrich EL,Murphy SA,Heerspink HJL,Zelniker TA,Dwyer JP, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–617. DOI: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 6.Wanner C,Inzucchi SE,Lachin JM,Fitchett D,von Eynatten M,Mattheus M,Johansen OE,Woerle HJ,Broedl UC,Zinman B, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. DOI: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 7.Heerspink HJ,Desai M,Jardine M,Balis D,Meininger G,Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368–375. DOI: 10.1681/ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMurray JJV,Solomon SD,Inzucchi SE,Køber L,Kosiborod MN,Martinez FA,Ponikowski P,Sabatine MS,Anand IS,Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. DOI: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 9.Packer M,Anker SD,Butler J,Filippatos G,Pocock SJ,Carson P,Januzzi J,Verma S,Tsutsui H,Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. DOI: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 10.Petrie MC,Verma S,Docherty KF,Inzucchi SE,Anand I,Belohlávek J,Böhm M,Chiang C‐E,Chopra VK,de Boer RA, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. DOI: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Nicola L,Gabbai FB,Garofalo C,Conte G,Minutolo R. Nephroprotection by SGLT2 inhibition: back to the future? J Clin Med. 2020;9:2243. DOI: 10.3390/jcm9072243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y,Bowe B,Li T,Xian H,Balasubramanian S,Al‐Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27:3153–3163. DOI: 10.1681/ASN.2015121377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y,Bowe B,Li T,Xian H,Yan Y,Al‐Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open. 2017;7:e015735. DOI: 10.1136/bmjopen-2016-015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y,Bowe B,Li T,Xian H,Yan Y,Al‐Aly Z. Long‐term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91:1482–1494. DOI: 10.1016/j.kint.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Xie Y,Bowe B,Li T,Xian H,Yan Y,Al‐Aly Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018;93:741–752. DOI: 10.1016/j.kint.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Xie Y,Bowe B,Yan Y,Xian H,Li T,Al‐Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ. 2019;365:l1580. DOI: 10.1136/bmj.l1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y,Bowe B,Gibson A,McGill J,Maddukuri G,Yan Y,Al‐Aly Z. Comparative effectiveness of SGLT2 inhibitors, GLP1 receptor agonists, DPP4 inhibitors and sulfonylureas on risk of kidney outcomes: emulation of a target trial using healthcare databases. Diabetes Care. 2020;43:2859–2869. DOI: 10.2337/dc20-1890. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y,Bowe B,Gibson A,McGill J,Yan Y,Maddukuri G,Al‐Aly Z. Comparative effectiveness of the sodium‐glucose co‐transporter‐2 inhibitor empagliflozin vs. other antihyperglycemics on risk of major adverse kidney events. Diabetes Care. 2020;43:2785–2795. DOI: 10.2337/dc20-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowe B,Cai M,Xie Y,Gibson AK,Maddukuri G,Al‐Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID‐19. Clin J Am Soc Nephrol. 2020;16:14–25. DOI: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowe B,Xie Y,Li T,Yan Y,Xian H,Al‐Aly Z. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: a cohort study. Lancet Planet Health. 2017;1:e267–e276. DOI: 10.1016/S2542-5196(17)30117-1. [DOI] [PubMed] [Google Scholar]
- 21.Bowe B,Xie Y,Li T,Yan Y,Xian H,Al‐Aly Z. The 2016 global and national burden of diabetes mellitus attributable to PM2.5 air pollution. Lancet Planet Health. 2018;2:e301–e312. DOI: 10.1016/S2542-5196(18)30140-2. [DOI] [PubMed] [Google Scholar]
- 22.Bowe B,Xie Y,Li T,Mokdad AH,Xian H,Yan Y,Maddukuri G,Al‐Aly Z. Changes in the US burden of chronic kidney disease from 2002 to 2016: an analysis of the global burden of disease study. JAMA Netw Open. 2018;1:e184412. DOI: 10.1001/jamanetworkopen.2018.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowe B,Xie Y,Li T,Yan Y,Xian H,Al‐Aly Z. Estimates of the 2016 global burden of kidney disease attributable to ambient fine particulate matter air pollution. BMJ Open. 2019;9:e022450. DOI: 10.1136/bmjopen-2018-022450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowe B,Xie Y,Yan Y,Al‐Aly Z. Burden of cause‐specific mortality associated with PM2.5 air pollution in the United States. JAMA Netw Open. 2019;2:e1915834. DOI: 10.1001/jamanetworkopen.2019.15834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowe B,Artimovich E,Xie Y,Yan Y,Cai M,Al‐Aly Z. The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: a modelling study. BMJ Glob Health. 2020;5:e002063. DOI: 10.1136/bmjgh-2019-002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowe B,Xie Y,Yan Y,Xian H,Al‐Aly Z. Diabetes minimally mediated the association between PM2.5 air pollution and kidney outcomes. Sci Rep. 2020;10:4586. DOI: 10.1038/s41598-020-61115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent BM,Wiitala WL,Burns JA,Iwashyna TJ,Prescott HC. Using Veterans Affairs corporate data warehouse to identify 30‐day hospital readmissions. Health Serv Outcomes Res Methodol. 2018;18:143–154. DOI: 10.1007/s10742-018-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VIReC Research User Guide: Veterans Health Administration Decision Support System Clinical National Data Extracts H. Menlo Park, CA: US Department of Veterans Affairs; VA Information Resource Center; 2009. [Google Scholar]
- 29.Maynard C. Ascertaining veterans' vital status: VA data sources for mortality ascertainment and cause of death. Database & Methods Cyberseminar Series. Washington, DC: US Department of Veterans Affairs; 2017. [Google Scholar]
- 30.Xie Y,Bowe B,Li T,Xian H,Al‐Aly Z. Blood urea nitrogen and risk of insulin use among people with diabetes. Diab Vasc Dis Res. 2018;15:409–416. DOI: 10.1177/1479164118785050. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS,Stevens LA,Schmid CH,Zhang YL,Castro AF III,Feldman HI,Kusek JW,Eggers P,Van Lente F,Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Y,Bowe B,Mokdad AH,Xian H,Yan Y,Li T,Maddukuri G,Tsai CY,Floyd T,Al‐Aly Z. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. DOI: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Hernán M. Antihyperglycemic therapy and cardiovascular risk: design and emulation of a target trial using healthcare databases. Washington, DC: Patient‐Centered Outcomes Research Institute; 2019. [Google Scholar]
- 34.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. DOI: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole SR,Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. DOI: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tchetgen Tchetgen EJ. Inverse odds ratio‐weighted estimation for causal mediation analysis. Stat Med. 2013;32:4567–4580. DOI: 10.1002/sim.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipsitch M,Tchetgen Tchetgen E,Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–388. DOI: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraus BJ,Weir MR,Bakris GL,Mattheus M,Cherney DZI,Sattar N,Heerspink HJL,Ritter I,von Eynatten M,Zinman B, et al. Characterization and implications of the initial estimated glomerular filtration rate “dip” upon sodium‐glucose co‐transporter‐2 inhibition with empagliflozin in the EMPA‐REG OUTCOME trial. Kidney Int. 2021;99:750–762. DOI: 10.1016/j.kint.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 39.Zhu NA,Harris SB. Therapeutic inertia in people with type 2 diabetes in primary care: a challenge that just won't go away. Diabetes Spectr. 2020;33:44–49. DOI: 10.2337/ds19-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarafidis P,Ferro CJ,Morales E,Ortiz A,Malyszko J,Hojs R,Khazim K,Ekart R,Valdivielso J,Fouque D, et al. SGLT‐2 inhibitors and GLP‐1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease: a consensus statement by the EURECA‐m and the DIABESITY working groups of the ERA‐EDTA. Nephrol Dial Transplant. 2019;34:208–230. DOI: 10.1093/ndt/gfy407. [DOI] [PubMed] [Google Scholar]
- 41.Nespoux J,Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond). 2018;132:1329–1339. DOI: 10.1042/CS20171298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Bommel EJM,Muskiet MHA,van Baar MJB,Tonneijck L,Smits MM,Emanuel AL,Bozovic A,Danser AHJ,Geurts F,Hoorn EJ, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post‐glomerular vasodilatation rather than pre‐glomerular vasoconstriction in metformin‐treated patients with type 2 diabetes in the randomized, double‐blind RED trial. Kidney Int. 2020;97:202–212. DOI: 10.1016/j.kint.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Kidokoro K,Cherney DZI,Bozovic A,Nagasu H,Satoh M,Kanda E,Sasaki T,Kashihara N. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140:303–315. DOI: 10.1161/CIRCULATIONAHA.118.037418. [DOI] [PubMed] [Google Scholar]
- 44.Xie Y,Bowe B,Maddukuri G,Al‐Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid‐19 and seasonal influenza: cohort study. BMJ. 2020;371:m4677. DOI: 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cefalu WT,Kaul S,Gerstein HC,Holman RR,Zinman B,Skyler JS,Green JB,Buse JB,Inzucchi SE,Leiter LA, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors' expert forum. Diabetes Care. 2018;41:14–31. DOI: 10.2337/dci17-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figures S1–S7


