Abstract
Background
This study examines changes in the ideal cardiovascular health (CVH) status and whether these changes are associated with incident cardiovascular disease (CVD) and mortality in the elderly Asian population.
Methods and Results
In the Korea National Health Insurance Service–Senior cohort aged ≥60 years, 208 673 participants without prior CVD, including 109 431 who showed changes in CVH status, were assessed. The association of the changes in cardiovascular risk factors with incident CVD was assessed from 2004 to 2014 in the elderly (aged 60–74 years) and very elderly (≥75 years) groups. During the follow‐up period (7.1 years for CVD and 7.2 years for mortality), 19 429 incident CVD events and 24 225 deaths occurred. In both the elderly and very elderly participants, higher CVH status resulted in a lower risk of CVD and mortality. In the very elderly participants, compared with consistently low CVH, consistently high CVH (subhazard ratio, 0.41; 95% CI, 0.23–0.73) was associated with a lower risk of CVD. This trend was consistently observed in the elderly population. In the very elderly participants, total cholesterol level was not informative enough for the prediction of CVD events. In both the elderly and very elderly groups, body mass index and total cholesterol were not informative enough for the prediction of all‐cause mortality.
Conclusions
In both the elderly and very elderly Asian populations without CVD, a consistent relationship was observed between the improvement of a composite metric of CVH and the reduced risk of CVD. Body mass index and total cholesterol were not informative enough for the prediction of all‐cause mortality in both the elderly and very elderly groups.
Keywords: cardiovascular disease, cardiovascular health, elderly, mortality
Subject Categories: Lifestyle, Primary Prevention, Risk Factors
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- CVH
cardiovascular health
- NHIS
National Health Insurance Service
- sHR
subhazard ratio
Clinical Perspective
What Is New?
In both the elderly (aged 60–74 years) and very elderly (aged ≥75 years) populations, a consistent relationship was observed between the improvement of a composite metric of cardiovascular health and reducing the risk of cardiovascular disease.
However, among the individual health factors, body mass index and total cholesterol were not informative enough for the prediction of all‐cause mortality in both the elderly and very elderly populations.
What Are the Clinical Implications?
Our findings highlight that improving the status of cardiovascular health is beneficial for the elderly and very elderly as well.
The leading cause of death in the elderly is cardiovascular disease (CVD). Prevention of cardiovascular events in elderly participants presents a therapeutic challenge that goes beyond the general underrepresentation of the elderly in clinical trials.1, 2
As a complementary prevention strategy for CVD, primary prevention, which prevents the development of CVD, is increasingly being emphasized.3 The American Heart Association developed a simple tool consisting of 7 metrics (nonsmoking, ideal body weight, physical activity, diet, blood pressure, fasting blood glucose, and total cholesterol level) to promote ideal cardiovascular health (CVH). The substantial benefits of high CVH and ideal metrics for the prevention of incident CVD events and mortality have been reported.4, 5, 6 Among a group of middle‐aged participants without CVD, there was a significant reduction of the CVD risk with the increase of ideal metrics and CVH score.6 However, whether changes in CVH status are related to the development of incident CVD and mortality is unknown in the elderly population.
By using the serial examination data of the Korean National Health Insurance Service (NHIS)–Senior cohort,7 this study examined changes in the ideal CVH status and whether the improvement of CVH status was beneficial for the prevention of incident CVD and mortality in the elderly Asian population.
METHODS
All data and materials have been made publicly available at the NHIS of Korea. The data can be accessed on the National Health Insurance Data Sharing Service homepage of the NHIS (http://nhiss.nhis.or.kr). Applications to use the NHIS data will be reviewed by the inquiry committee of research support and, once approved, raw data will be provided to the authorized researcher for a fee at several permitted sites.
Data were collected from the NHIS–Senior database, which included data for 558 147 individuals selected by a 10% simple random sampling method from a total of 5.5 million subjects aged ≥60 years in the National Health Information Database.8, 9 The NHIS–Senior database covers the following parameters: sociodemographic and socioeconomic information, insurance status, health checkup examinations, and records of participants’ medical and dental histories. These parameters were stratified to cover 13 years (2002–2014) and anonymized to protect the privacy of individuals within the cohort study. This study was approved by the institutional review board of the Yonsei University Health System (4‐2020‐0674). The need for informed consent was waived. The NHIS–Senior database used in this study was established by the NHIS in Korea.
Study Population
From the Korean NHIS–Senior database, 312 736 participants who had a health checkup between 2005 and 2012 were selected, and follow‐up data were reviewed until December 2014. The exclusion criteria were as follows: (1) participants who had an ischemic stroke or transient ischemic attack before enrollment (n=41 993), (2) participants who had a myocardial infarction before enrollment (n=6359), (3) participants who had a hemorrhagic stroke before enrollment (n=1357), (4) participants with vascular disease (n=10 498), (5) participants who had a malignancy before enrollment (n=27 514), (6) participants with a body mass index (BMI) of <18.5 kg/m2 (n=10 139); and (7) those with missing data (n=6203). Finally, we included 208 673 participants, of whom 109 431 had at least 2 health checkups and were without CVD between the first and second examination.
CVH Metrics and Status
To characterize the ideal CVH status, we applied 6 American Heart Association guideline metrics (total cholesterol level, fasting blood glucose level, blood pressure, BMI, cigarette smoking, and exercise) and the cutoff definition for ideal, intermediate, or poor status for each CVH metric (Table S1).3 Information on these 6 metrics was obtained through routine health checks and laboratory measurements. At each test cycle, an interview and physical examination were performed for each patient, and information on medical history and drug use was collected.
We stratified the participants into 3 groups according to the number of ideal metrics as follows: high (5+ ideal metrics), moderate (3 or 4 metrics), and low (≤2 metrics).4, 5 A continuous 12‐point CVH score assigning 0 points for poor metrics, 1 point for intermediate metrics, and 2 points for ideal metrics was also calculated.10 Change in CVH status was examined between the first and second health checkups of the participants with 6 metrics at both time points. All the participants were required to be free of a CVD event between the first and second examinations (Figure S1).
Covariates
The sociodemographic variables included age, sex, economic status, and living in metropolitan cities. The baseline economic status was determined on the basis of the relative economic levels categorized into 10 levels according to their health insurance premiums in the index year. We obtained information on selected comorbid conditions from inpatient and outpatient hospital diagnoses. Baseline comorbidities were defined using the medical claims and prescription drug information before the index date. To ensure diagnostic accuracy, the participants were considered to have comorbidities when their condition was a discharge diagnosis or had been confirmed at least twice in an outpatient setting, in line with previous studies that used data from the NHIS database (Table S2).11, 12
Outcomes
The primary outcome was time to first CVD (combination of coronary heart disease and ischemic stroke or systemic embolism). Coronary heart disease was defined from any discharge diagnoses (International Classification of Diseases , Tenth Revision (ICD‐10) codes for acute myocardial infarction [I21x and I22x], chronic ischemic heart disease [I25.2, I25.5, I25.6, I25.8, and I25.9], or procedure codes for coronary revascularization [M6551, M6552, M6561, M6563, M6562, M6564, M6571, M6572, M6634, O1641, OA641, O1642, OA642, O1647, and OA647]). Ischemic stroke was defined from any discharge diagnoses (ICD‐10 codes I63 and I64) with concomitant brain imaging studies. The accuracy of the diagnosis of an ischemic stroke based on the NHIS claims data was previously validated.9, 12, 13, 14, 15, 16 The definitions of the clinical outcomes are presented in Table S2. The same patient could have >1 study outcome during the study duration, but only the first event of each outcome was considered in the study.
The secondary outcome was mortality. Information on death (date and causes of death) was confirmed from the National Population Registry of the Korea National Statistical Office with unique personal identification numbers, in which the central registration of death was conducted on the basis of death certificates.9, 12, 13, 14, 15, 16 The NHIS and National Statistical Office are national agencies serving all Korean residents, so this approach provides a complete event check. Follow‐ups for CVD and mortality were conducted until December 2014. We also analyzed cause‐specific mortality based on causes of death confirmed by Korean National Statistical Office, because health metrics can be related to non–CVD‐related mortality.
Sensitivity Analysis
The analysis was repeated with 4 groups of changes in CVH status, as used in the Framingham Offspring Study.17 The change in the number of ideal metrics between the first and second health checkups as the exposure was evaluated in the Cox analysis by using the unchanged category as reference. We also investigated the associations of time‐varying CVH and changes in the individual CVH metrics with the outcomes.
Statistical Analysis
The 2 steps of the analysis conducted in this study are summarized in Figure S1.
In the Cox proportional hazard regression analysis, CVH status between the first and fifth tests were used as time‐varying variables. Of the 3 versions of CVH status (category CVH status [low, medium, and high], ideal number of metrics [range, 0–6], and 12‐point CVH score), only 1 version of CVH status was used in each Cox proportional hazard regression according to the purpose of each analysis. At each date of an event (CVD or death), the model used the CVH exposure before the event. Coronary heart disease and ischemic stroke/systemic embolism were investigated as a composite CVD end point as well as separate results. In the analysis of the composite CVD end point, the 2025 participants had both the occurrence of coronary heart disease and ischemic stroke/systemic embolism. In this case, on the date of the first event, follow‐up was stopped, and the composite CVD end point was measured. The Fine and Gray method was used to consider death as a competing risk when assessing the composite CVD end point, coronary heart disease, and ischemic stroke or systemic embolism.18 The proportional hazards assumption was tested on the basis of Schoenfeld residuals.19
The changes in CVH categories between the first and second health examination yielded 9 possible combinations of CVH statuses. In these analyses, follow‐up for CVD and mortality began from the second examination. Participants with at least 1 missing CVH metric in the second examination were removed from analysis. The subhazard ratio (sHR) of CVD and hazard ratio of all‐cause mortality for each combination of changes in CVH status were computed in the models using the consistently low CVH as the reference category. All models were adjusted for sex, age, economic status, Hospital Frailty Score, and living in metropolitan cities. The proportional hazard assumption was evaluated by visual inspection of the survival curves and the Kolmogorov test.
The individuals without a second health checkup or those with CVD between the first and second health checkup were excluded from the analysis of the changes in CVH status. To account for attrition between included and excluded subjects, we conducted an additional analysis using inverse probability of attrition weights. The weights for each individual were calculated at the first and second health checkup using the inverse of the estimated probabilities of being without CVD and receiving the next health checkup. The weights were stabilized by the baseline variables of sex, age, economic status, Hospital Frailty Score, living in metropolitan cities, and comorbidities.
Because multiple comparisons were made on the CVH variables, we used the Bonferroni correction, the most conservative approach for declaring significance. Differences were significant if P<0.0083. The statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria; www.R‐project.org).
RESULTS
Baseline Characteristics
The study population included 208 673 participants free of CVD who had data on all 6 CVH metrics at baseline. The mean (SD) age of the study population at baseline was 70.6 years (5.4 years), and 42.5% of the population were men. The comparison of the baseline characteristics between the elderly (aged 60–74 years) and very elderly participants (≥75 years) are presented in Table 1. Compared with the elderly participants, very elderly participants had more comorbidities including hypertension, diabetes mellitus, dyslipidemia, and osteoporosis (Table 1).
Table 1.
Baseline Characteristics
| Characteristics | Elderly, 60–74 y, N=167 317 |
Very Elderly, ≥75 y, N=41 356 |
P Value |
|---|---|---|---|
| Age, mean (SD), y | 68.5 (3.1) | 79.2 (3.8) | <0.001 |
| Men | 74 288 (44.4) | 14 383 (34.8) | <0.001 |
| Economic status, 0–10 | 7.0 (4.0–9.0) | 7.0 (3.0–9.0) | <0.001 |
| Low, 0–4 | 52 329 (31.3) | 13 235 (32.0) | |
| Middle, 5–7 | 39 700 (23.7) | 8404 (20.3) | |
| High, 8–10 | 75 288 (45.0) | 19 717 (47.7) | |
| Living area | |||
| Small city or rural area | 100 798 (60.2) | 26 198 (63.3) | <0.001 |
| Metropolitan city | 66 519 (39.8) | 15 158 (36.7) | |
| Hypertension | 69 373 (41.5) | 21 622 (52.3) | <0.001 |
| Diabetes mellitus | 22 790 (13.6) | 5874 (14.2) | 0.002 |
| Dyslipidemia | 52 086 (31.1) | 11 714 (28.3) | <0.001 |
| Osteoporosis | 46 229 (27.6) | 14 646 (35.4) | <0.001 |
| CVH status no. of ideal metrics | |||
| Low, 0–2 | 69 905 (41.8) | 15 824 (38.3) | <0.001 |
| Moderate, 3–4 | 87 769 (52.5) | 23 279 (56.3) | |
| High, 5–6 | 9643 (5.8) | 2253 (5.4) | |
| No. of ideal metrics, median (IQR)* | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | <0.001 |
| 12‐Point CVH score, median (IQR)† | 7.0 (6.0–9.0) | 8.0 (6.0–9.0) | <0.001 |
Values are reported as number (%) unless otherwise indicated. The relative economic levels categorized into 10 levels according to their health insurance premiums. CVH indicates cardiovascular health; and IQR, interquartile range.
The CVH metrics included nonsmoking, body weight, physical activity, blood pressure, fasting blood glucose, and total cholesterol.
The continuous 12‐point CVH score (range, higher score indicating higher CVH) was calculated by assigning 0 (poor), 1 (intermediate), and 2 (ideal) points to each of the 6 metrics and summing them.
Prevalence of CVH Status During the Total Follow‐Up Period
The baseline characteristics of the study population and the characteristics at each examination are presented in Table S3. The proportion of high CVH status was 5.7% at the first checkup. Those who attended 5 examinations (the maximum) were more likely to be healthy, with the proportion of participants with a high CVH status of 14.1%. Distribution of the number of ideal cardiovascular health metrics at each wave was same in the total study population, and elderly and very elderly population.
Time‐Varying CVH and Risks of CVD and Mortality
The median follow‐up period starting from baseline was 7.1 years (interquartile range [IQR], 4.6–7.9 years) for CVD and 7.2 years (IQR, 4.9–8.0 years) for mortality. During the follow‐up period, 19 429 incident CVD events (9932 coronary heart disease events and 11 522 ischemic stroke or systemic embolism events) and 24 225 deaths occurred. The median times to CVD (2.6 years [IQR, 1.3–4.0 years] versus 2.8 years [IQR, 1.3–4.3 years], P=0.009) and all‐cause death (3.1 years [IQR, 1.7–4.5 years] versus 3.3 years [IQR, 1.9–4.7 years], P=0.001) were significantly short in the very elderly than elderly. The time to CVD and all‐cause mortality according to baseline cardiovascular health is presented in Table S4. After adjustment of clinical variables and competing risk of mortality, in the very elderly participants, compared with low CVH, moderate CVH (sHR, 0.82; 95% CI, 0.77–0.87), and high CVH (sHR, 0.53; 95% CI, 0.46–0.61) were associated with a lower risk of CVD (Table 2). Similarly, CVD risk was significantly reduced for each additional time‐varying ideal metric (sHR, 0.86; 95% CI, 0.84–0.89) and point in the 12‐point CVH score (sHR, 0.87; 95% CI, 0.85–0.88; Table 2). Similar results were observed for all‐cause mortality (Table 2), coronary heart disease, ischemic stroke/systemic embolism (Table S5), and cause‐specific mortality (Table S6). This trend was consistently observed in the elderly population (aged 60–74 years). The incidence rates for CVD and all‐cause mortality according to the CVH category, number of ideal metrics, and level of 12‐point CVH score at baseline are presented in Table 3.
Table 2.
Time‐Varying Cox Proportional Hazard Model for Incident Cardiovascular Disease and All‐Cause Mortality
| CVH Status, No. of Ideal Metrics | Per Additional Ideal Metric* | Per 1‐Point Increase in the CVH Score* | |||
|---|---|---|---|---|---|
| Low, 0–2 | Moderate, 3–4 | High, 5–6 | |||
| Cardiovascular disease | Adjusted subhazard ratio (95% CI)† | ||||
| Elderly 60~74 y, n/total n=13 761/167 317 | 1 [Reference] | 0.73 (0.71–0.75)‡ | 0.44 (0.41–0.47)‡ | 0.82 (0.81–0.83)‡ | 0.85 (0.85–0.86)‡ |
| Very elderly ≥75 y, n/total n=5668/41 356 | 1 [Reference] | 0.82 (0.77–0.87)‡ | 0.52 (0.46–0.60)‡ | 0.86 (0.84–0.88)‡ | 0.87 (0.85–0.88)‡ |
| All‐cause mortality | Adjusted hazard ratio (95% CI)§ | ||||
| Elderly 60~74 y, n/total n=14 438/99 532 | 1 [Reference] | 0.86 (0.82–0.90)‡ | 0.55 (0.51–0.58)‡ | 0.93 (0.91–0.94)‡ | 0.90 (0.89–0.90)‡ |
| Very elderly ≥75 y, n/total n=9787/41 356 | 1 [Reference] | 0.95 (0.90–1.00) | 0.71 (0.66–0.77)‡ | 0.95 (0.93–0.97)‡ | 0.93 (0.92–0.94)‡ |
CVH, indicates cardiovascular health.
A linear model was used for the analysis, per additional ideal metric and per 1‐point increase in the 12‐point CVH score.
Cardiovascular disease was adjusted for sex, age, economic status, Hospital Frailty Score, and living in metropolitan cities, and competing risk of death.
P<0.0083 for differences reported.
All‐cause mortality was adjusted for sex, age, economic status, Hospital Frailty Score, and living in metropolitan cities.
Table 3.
Incidence Rates for Cardiovascular Disease and All‐Cause Mortality According to Measures of Baseline Cardiovascular Health
| Incidence Rate per 1000 Person‐Years (95% CI) | ||||
|---|---|---|---|---|
| Cardiovascular Disease | All‐Cause Mortality | |||
| Elderly, 60~74 y, N/Total N=14 260/173 109 |
Very Elderly, ≥75 y, N/Total N=6234/45 379 |
Elderly, 60~74 y, N/Total N=15 641/173 109 |
Very Elderly, ≥75 y, N/Total N=11 507/45 379 |
|
| CVH status, no. of ideal metrics | ||||
| Low, 0–2 | 15.5 (15.1–15.9) | 29.3 (28.2–30.5) | 13.8 (13.5–14.2) | 43.2 (41.8–44.6) |
| Moderate, 3–4 | 11.3 (11.0–11.6) | 25.0 (24.1–25.9) | 12.6 (12.3–12.5) | 43.9 (42.8–45.1) |
| High, 5–6 | 6.4 (5.8–7.1) | 18.8 (16.3–21.6) | 9.8 (9.0–10.6) | 40.7 (37.0–44.6) |
| CVH status per no. of ideal metrics | ||||
| 0 | 22.2 (19.8–24.8) | 41.8 (33.2–51.9) | 18.8 (16.7–21.2) | 53.3 (43.9–64.1) |
| 1 | 16.8 (16.1–17.6) | 31.4 (29.1–33.8) | 13.8 (13.2–14.5) | 40.3 (37.8–42.9) |
| 2 | 14.6 (14.2–15.1) | 28.1 (26.8–29.5) | 13.6 (13.2–14.0) | 43.9 (42.3–45.6) |
| 3 | 12.2 (11.8–12.6) | 26.2 (25.1–27.4) | 13.2 (12.8–13.5) | 44.0 (42.5–45.5) |
| 4 | 9.9 (9.5–10.3) | 23 (21.6–24.4) | 11.7 (11.2–12.1) | 43.8 (41.9–45.7) |
| 5 | 6.5 (5.8–7.2) | 19.6 (16.9–22.6) | 9.7 (8.9–10.5) | 42.1 (38.2–46.3) |
| 6 | 5.4 (3.5–7.9) | 9.3 (4.0–18.4) | 11.1 (8.4–13.5) | 24.1 (14.9–36.8) |
| CVH status per points on the CVH score | ||||
| 1 or 2 | 33.6 (27.3–40.9) | 49.5 (31.4–74.4) | 27.1 (21.7–33.4) | 78.9 (56.4–107.4) |
| 3 or 4 | 22.8 (21.5–24.1) | 39.1 (35.3–43.1) | 19.7 (18.5–20.9) | 52.1 (47.9–56.6) |
| 5 or 6 | 16.1 (15.6–16.6) | 31.2 (29.7–32.8) | 15.2 (14.8–15.7) | 46.2 (44.4–48.0) |
| 7 or 8 | 12.3 (12.0–12.6) | 25.4 (24.4–26.5) | 12.6 (12.3–12.9) | 42.7 (41.5–44.0) |
| 9 or 10 | 8.8 (8.4–9.1) | 21.4 (20.2–22.7) | 10.2 (9.8–10.6) | 40.5 (38.8–42.3) |
| ≥11 | 6.4 (5.5–3.5) | 17.3 (14.0–21.2) | 9.0 (8.0–10.1) | 37.3 (32.4–42.7) |
CVH, indicates cardiovascular health.
The time‐varying Cox proportional risk model for the association of individual CVH metrics with the occurrence of CVD event and all‐cause mortality is presented in Table S7. In the very elderly participants, BMI and total cholesterol level were not appropriate for the prediction of CVD events and all‐cause mortality. In the elderly participants, all 6 metrics were appropriate for the prediction of CVD events.
Changes in CVH Status from the First and Second Health Examinations
Changes in CVH status were investigated and calculated in 109 431 individuals. The median interval for participants with all 6 metrics at both time points was 2.0 years (IQR, 1.6–2.8 years). The characteristics of the individuals included in this analysis, as compared with those of the participants, were not examined in the second checkup (n=97 566) and with CVD in the interval (n=1676), and are shown in Table S8.
Figures 1A and 1B and Figure S2 show that 22.1% (n=3740) of the very elderly participants had improved CVH mostly from low to moderate status (15.6%) and from moderate to high status (5.6%), and only 0.8% improved from low to high status. In 15.8% (n=2674) of the very elderly participants with worse cardiovascular health over time, 11.8% went from moderate to low, 3.4% from high to moderate, and 1.5% from high to low cardiovascular health. Stable moderate CVH was more prevalent in the elderly women than in the elderly men (Figures 2A and 2B) but was similarly prevalent regardless of high or low economic status (Figures 2C and 2D). The baseline characteristics of the participants according to the patterns of the changes in CVH status are shown in Table S9.
Figure 1. Heatmap of the unadjusted incidence rates of cardiovascular disease (CVD) (A) and all‐cause mortality (B) according to the patterns of change in cardiovascular health between the first and second examinations in the elderly (60–74 years) and very elderly (≥75 years) populations.
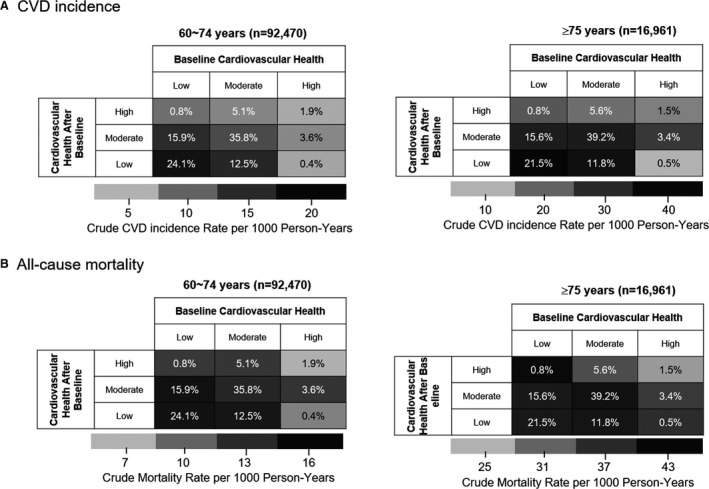
Values indicate the percentage of participants in each category, and colors indicate rate per 1000 person‐years.
Figure 2. Heatmap of the unadjusted incidence rates of cardiovascular disease (CVD) and all‐cause mortality according to the patterns of change in cardiovascular health between the first and second examinations for comparison between women and men (A and B) and between high and low‐to‐moderate economic status (C and D).
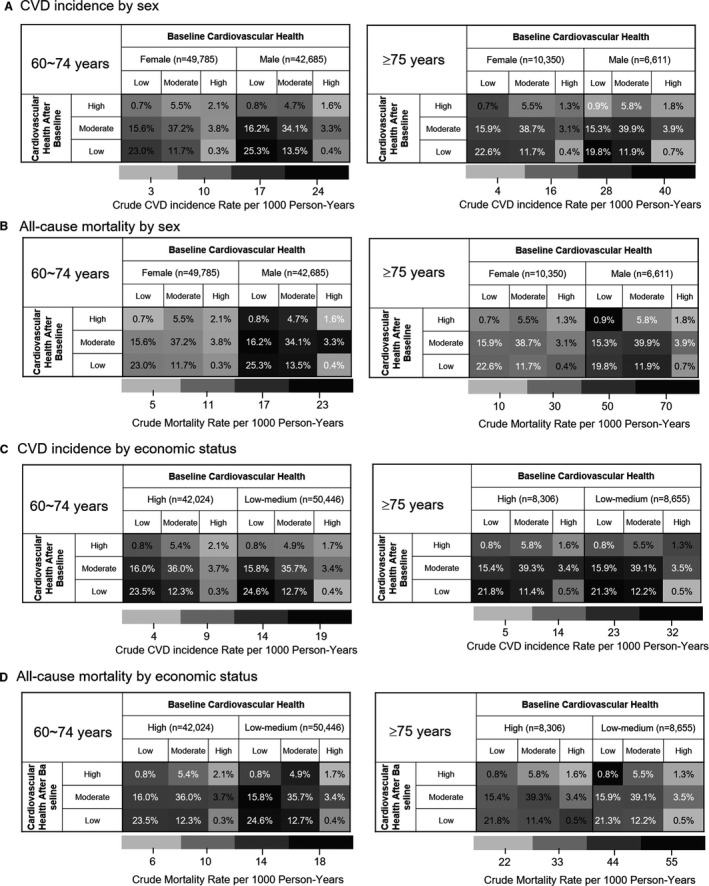
Values indicate the percentage of participants in each category, and black and gray indicate rate per 1000 person‐years.
Changes in CVH (First to Second) and Subsequent Incidence of CVD and Mortality
The median follow‐up of the outcomes starting from the second checkup was 5.1 years (IQR, 3.2–6.0 years) for CVD and 5.2 (IQR, 3.5–6.1 years) for mortality, which resulted in 7745 incident CVD events (elderly, 5980 and very elderly, 1765) and 8930 deaths (elderly, 6099 and very elderly, 2831). Heatmaps (Figures 1 and 2) and Kaplan–Meier curves (Figure S3A and S3B)s were used to depict the crude rates of CVD incidence and all‐cause mortality according to the patterns of change in CVH status in the different age groups. The incidence, absolute difference rates, and adjusted sHRs for CVD and HRs for all‐cause mortality are reported in Table 4 and Figure 3. After weighting to account for attrition, the incidence, absolute difference rates, and adjusted sHRs for CVD and hazard ratios for all‐cause mortality are reported in Table S10.
Table 4.
Change in CVH Status, and Association With Subsequent Incident Cardiovascular Disease and All‐Cause Mortality
| Change in CVH Status | Cardiovascular Disease | All‐Cause Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| N/Total N | Incidence Rate per 1000 Person‐Years (95% CI) | ARD per 1000 Person‐Years (95% CI) | Adjusted sHR (95% CI)* | N/Total N | Incidence Rate per 1000 Person‐Years (95% CI) | ARD per 1000 Person‐Years (95% CI) | Adjusted HR (95% CI)†\ | |
| Elderly, 60–74 y | 5980/92 470 | 6099/92 470 | ||||||
| Consistently low | 1914/22 258 | 18.0 (17.2‐18.8) | Ref | 1 [Reference] | 1607/22 258 | 14.5 (13.8‐15.2) | Ref | 1 [Reference] |
| Low to moderate | 1008/14 680 | 14.8 (13.9‐15.8) | −3.2 (−4.4 to −1.9) | 0.81 (0.75‐0.88)‡ | 1020/14 680 | 14.6 (13.7‐15.5) | ‐0.1 (−1.1 to 1.2) | 1.00 (0.92‐1.08) |
| Low to high | 29/720 | 9.2 (6.1‐13.2) | −8.8 (−13.5 to −4.1) | 0.51 (0.35‐0.73)‡ | 39/720 | 12.1 (8.6‐16.6) | ‐2.4 (−6.6 to 1.8) | 0.86 (0.63‐1.18) |
| Moderate to low | 862/11 556 | 15.3 (14.3‐16.3) | −2.7 (−4.0 to −1.4) | 0.84 (0.77‐0.91)‡ | 830/11 556 | 14.2 (13.‐15.2) | ‐0.3 (−1.5 to 0.9) | 0.95 (0.87‐1.03) |
| Consistently moderate | 1845/33 115 | 11.9 (11.3‐12.4) | −6.1 (−7.0 to −5.2) | 0.66 (0.61‐0.70)‡ | 2089/33 115 | 13.1 (12.6‐13.7) | ‐1.4 (−2.3 to −0.5) | 0.91 (0.85‐0.97)‡ |
| Moderate to high | 160/4755 | 7.7 (6.6‐9.0) | −10.3 (−12.2 to −8.4) | 0.43 (0.37‐0.51)‡ | 256/4755 | 12.2 (10.7‐13.8) | ‐2.3 (−4.1 to −0.6) | 0.88 (0.77‐1.01) |
| High to low | 10/354 | 5.8 (2.8‐10.6) | −12.2 (−18.5 to −5.9) | 0.32 (0.17‐0.59)‡ | 16/354 | 9.1 (5.2‐14.8) | ‐5.4 (−11.1 to 0.2) | 0.59 (0.36‐0.96) |
| High to moderate | 113/3288 | 7.5 (6.2‐9.0) | −10.5 (−12.7 to −8.3) | 0.42 (0.35‐0.51)‡ | 188/3288 | 12.3 (10.6‐14.2) | ‐2.2 (−4.2 to −0.1) | 0.87 (0.75‐1.02) |
| Consistently high | 39/1744 | 5.5 (3.9‐7.6) | −12.5 (−15.6 to −9.3) | 0.32 (0.23‐0.44)‡ | 54/1744 | 7.5 (5.7‐9.8) | ‐6.9 (−9.8 to −4.1) | 0.58 (0.44‐0.76)‡ |
| Very elderly, ≥75 y | 1765/16 961 | 2831/16 961 | ||||||
| Consistently low | 463/3651 | 30.1 (27.4‐33.0) | Ref | 1 [Reference] | 637/3651 | 39.5 (36.5‐42.7) | Ref | 1 [Reference] |
| Low to moderate | 269/2651 | 24.9 (22.1‐28.1) | −5.2 (−9.3 to −1.0) | 0.80 (0.69‐0.93)‡ | 441/2651 | 39.3 (35.7‐43.2) | ‐0.2 (−4.9 to 4.6) | 0.96 (0.84‐1.08) |
| Low to high | 9/136 | 17.0 (7.8‐32.3) | −13.1 (−28.0 to 1.8) | 0.54 (0.28‐1.05) | 23/136 | 42.3 (26.9‐63.6) | 2.9 (−14.1 to 19.9) | 1.04 (0.68‐1.58) |
| Moderate to low | 231/2004 | 27.7 (24.3‐31.5) | −2.4 (−6.9 to 2.2) | 0.89 (0.76‐1.05) | 350/2004 | 40.0 (35.9‐44.4) | 0.5 (−4.7 to 5.7) | 0.95 (0.84‐1.09) |
| Consistently moderate | 680/6645 | 25.3 (23.4‐27.3) | −4.8 (−8.1 to −1.6) | 0.81 (0.72‐0.91)‡ | 1141/6645 | 40.8 (38.5‐43.3) | 1.4 (−2.5 to 5.3) | 0.97 (0.88‐1.07) |
| Moderate to high | 53/953 | 14.6 (10.9‐19.1) | −15.5 (−21.5 to −9.6) | 0.47 (0.35‐0.62)‡ | 123/953 | 33.1 (27.5‐39.5) | ‐6.3 (−13.3 to 0.6) | 0.81 (0.66‐0.98) |
| High to low | 3/86 | 8.5 (1.8‐24.9) | −21.6 (−39.8 to −3.4) | 0.27 (0.09‐0.81) | 13/86 | 36.6 (19.5‐62.6) | ‐2.8 (−23.7 to 18.0) | 0.79 (0.46‐1.37) |
| High to moderate | 45/584 | 20.8 (15.2‐27.9) | −9.3 (−17.0 to −1.6) | 0.66 (0.49‐0.91)‡ | 78/584 | 35.1 (27.8‐43.9) | ‐4.3 (−13.1 to 4.4) | 0.81 (0.64‐1.02) |
| Consistently high | 12/251 | 13.0 (6.7‐22.7) | −17.1 (−28.5 to −5.8) | 0.41 (0.23‐0.73)‡ | 25/251 | 26.7 (17.3‐39.5) | ‐12.7 (−25.7 to 0.3) | 0.60 (0.40‐0.90) |
ARD indicates absolute rate difference; CVH, cardiovascular health; HR, hazard ratio; Ref, reference; and sHR, subhazard ratio.
Cardiovascular disease was adjusted for sex, age, economic status, Hospital Frailty Score, living in metropolitan cities, and competing risk of death.
All‐cause mortality was adjusted for sex, age, economic status, Hospital Frailty Score, and living in metropolitan cities.
P<0.0083 for differences reported.
Figure 3. Hazard ratios of cardiovascular disease (green dots) and all‐cause mortality (red dots) according to the pattern of change in the cardiovascular health status between the first and second health examinations in the elderly (60–74 years) (A) and very elderly (≥75 years) (B) populations.
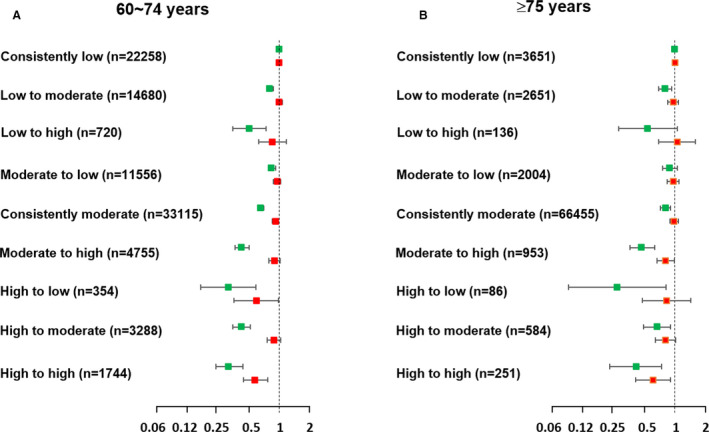
For the very elderly population (aged ≥75 years), the multivariable analysis with adjustment of competing risk of death showed that compared with the individuals with consistently low CVH status (CVD incident rate per 1000 person‐years, 30.1; 95% CI, 27.4–33.0), the low‐to‐moderate (sHR, 0.80; 95% CI, 0.69–0.93), consistently moderate (sHR, 0.80; 95% CI, 0.72–0.91), moderate‐to‐high (sHR, 0.47; 95% CI, 0.35–0.62), high‐to‐low (sHR, 0.27; 95% CI, 0.09–0.80), high‐to‐moderate (sHR, 0.66; 95% CI, 0.49–0.90), and consistently high CVH status groups (sHR, 0.42; 95% CI, 0.23–0.74) showed lower CVD risks (Table 4). In the elderly groups, compared with the individuals with consistently low CVH status, a lower CVD risk was observed in all of the other groups with the improvement of CVH status. The results were similar for all‐cause mortality.
Number of participants according to changes in the levels of individual CVH metrics between the first and second examinations are presented in Table S11. The associations of the changes in the individual CVH metrics with the subsequent occurrence of incident CVD events and all‐cause mortality are presented in Table 5 and Table S12. Compared with the low‐low group, the high‐high group of total cholesterol level was associated lower risk of CVD event in elderly but not in the very elderly population (P interaction=0.005). In both the elderly and very elderly groups, compared with the low‐low group, the high‐high group of BMI and total cholesterol were not informative enough for prediction of all‐cause mortality.
Table 5.
Association of Change in the Individual Cardiovascular Health Metrics With the Risk of Subsequent Incident Cardiovascular Disease Events and All‐Cause Mortality
| Pattern of Change | Low‐Low | Low‐Moderate | Low‐High | Moderate‐Low | Moderate‐Moderate | Moderate‐High | High‐Low | High‐Moderate | High‐High |
|---|---|---|---|---|---|---|---|---|---|
| Incident cardiovascular disease, adjusted sHR (95% CI)* | |||||||||
| Elderly 60–74, y | |||||||||
| Smoking | 1 [Reference] | 0.84 (0.69–1.02) | 0.84 (0.74–0.96) | 0.87 (0.70–1.09) | 0.77 (0.65–0.91)† | 0.74 (0.64–0.85)† | 0.81 (0.71–0.93)† | 0.76 (0.67–0.87)† | 0.68 (0.63–0.74)† |
| Body mass index | 1 [Reference] | 0.80 (0.58–1.09) | 1.67 (0.78–3.57) | 0.82 (0.60–1.12) | 0.82 (0.69–0.98) | 0.80 (0.66–0.97) | 1.66 (0.87–3.15) | 0.74 (0.61–0.91)† | 0.74 (0.63–0.88)† |
| Physical activity | 1 [Reference] | 0.87 (0.80–0.94)† | 0.87 (0.79–0.95)† | 0.91 (0.82–1.01) | 0.68 (0.62–0.76)† | 0.78 (0.70–0.87)† | 0.87 (0.76–1.00) | 0.84 (0.74–0.96)† | 0.75 (0.68–0.83)† |
| Blood pressure | 1 [Reference] | 0.89 (0.82–0.96)† | 0.70 (0.58–0.86)† | 0.87 (0.80–0.95)† | 0.79 (0.73–0.85)† | 0.55 (0.48–0.63)† | 0.81 (0.66–0.99) | 0.58 (0.51–0.66)† | 0.44 (0.37–0.51)† |
| Total cholesterol | 1 [Reference] | 0.73 (0.64–0.84)† | 0.81 (0.67–0.98)† | 0.70 (0.61–0.80)† | 0.66 (0.59–0.74)† | 0.65 (0.58–0.73)† | 0.88 (0.72–1.08) | 0.67 (0.59–0.75)† | 0.56 (0.51–0.62)† ‡ |
| Fasting glucose | 1 [Reference] | 0.84 (0.74–0.97) | 0.63 (0.50–0.79)† | 0.84 (0.73–0.97) | 0.66 (0.59–0.73)† | 0.52 (0.46–0.59)† | 0.76 (0.61–0.94)† | 0.54 (0.48–0.60)† | 0.50 (0.45–0.55)† |
| Very elderly, ≥75 y | |||||||||
| Smoking | 1 [Reference] | 0.97 (0.67–1.41) | 0.80 (0.62–1.03) | 0.67 (0.39–1.15) | 0.67 (0.46–0.96) | 0.76 (0.58–0.99) | 0.66 (0.50–0.89)† | 0.69 (0.53–0.90)† | 0.67 (0.57–0.80)† |
| Body mass index | 1 [Reference] | 0.79 (0.42–1.49) | 1.30 (0.40–4.25) | 1.06 (0.59–1.93) | 0.67 (0.46–0.98) | 0.91 (0.62–1.36) | 1.05 (0.37–2.98) | 0.85 (0.57–1.28) | 0.78 (0.54–1.12) |
| Physical activity | 1 [Reference] | 0.92 (0.80–1.06) | 0.81 (0.69–0.95) | 0.86 (0.69–1.06) | 0.80 (0.66–0.98) | 0.76 (0.61–0.95) | 1.09 (0.87–1.38) | 0.80 (0.62–1.03) | 0.75 (0.62–0.92)† |
| Blood pressure | 1 [Reference] | 0.98 (0.85–1.13) | 0.69 (0.48–1.01) | 0.97 (0.83–1.13) | 0.82 (0.71–0.93)† | 0.71 (0.54–0.92) | 0.95 (0.67–1.35) | 0.61 (0.46–0.82)† | 0.51 (0.37–0.72)† |
| Total cholesterol | 1 [Reference] | 1.01 (0.77–1.32) | 0.94 (0.66–1.35) | 1.03 (0.79–1.35) | 0.90 (0.73–1.12) | 0.80 (0.63–1.02) | 1.06 (0.68–1.64) | 0.92 (0.72–1.18) | 0.81 (0.66–1.01)† |
| Fasting glucose | 1 [Reference] | 0.75 (0.57–0.99) | 0.83 (0.59–1.17) | 0.82 (0.63–1.08) | 0.65 (0.52–0.81)† | 0.56 (0.45–0.71)† | 0.65 (0.44–0.95) | 0.60 (0.48–0.75)† | 0.56 (0.46–0.69)† |
| All‐cause mortality, adjusted HR (95% CI)§ | |||||||||
| Elderly, 60–74 y | |||||||||
| Smoking | 1 [Reference] | 0.86 (0.73–1.02) | 0.89 (0.80–1.00) | 0.70 (0.57–0.87)† | 0.60 (0.50–0.71)† | 0.58 (0.50–0.66)† | 0.78 (0.69–0.89)† | 0.61 (0.53–0.69)† | 0.59 (0.55–0.64)† |
| Body mass index | 1 [Reference] | 0.89 (0.63–1.26) | 1.16 (0.43–3.14) | 1.11 (0.80–1.54) | 0.77 (0.63–0.95) | 1.01 (0.81–1.25) | 0.55 (0.18–1.74) | 0.82 (0.66–1.03) | 1.03 (0.84–1.25) |
| Physical activity | 1 [Reference] | 0.92 (0.85–1.00) | 0.87 (0.80–0.96)† | 0.86 (0.77–0.95)† | 0.67 (0.60–0.74)† | 0.75 (0.67–0.84)† | 0.84 (0.74–0.96) | 0.79 (0.70–0.90) | 0.75 (0.68–0.83)† |
| Blood pressure | 1 [Reference] | 1.00 (0.92–1.09) | 0.99 (0.83–1.19) | 0.97 (0.89–1.07) | 0.98 (0.88–1.02) | 0.86 (0.76–0.97) | 0.98 (0.80–1.19) | 0.86 (0.76–0.97) | 0.91 (0.80–1.03) |
| Total cholesterol | 1 [Reference] | 0.92 (0.78–1.07) | 0.99 (0.81–1.22) | 0.91 (0.77–1.06) | 0.79 (0.69–0.90)† | 0.95 (0.83–1.09) | 1.28 (1.04–1.59) | 0.88 (0.76–1.01) | 1.00 (0.89–1.13) |
| Fasting glucose | 1 [Reference] | 0.86 (0.75–1.00) | 0.88 (0.71–1.08) | 0.79 (0.69–0.92)† | 0.68 (0.61–0.76)† | 0.62 (0.55–0.70)† | 0.85 (0.70–1.05) | 0.59 (0.52–0.66)† | 0.56 (0.51–0.62)† |
| Very elderly, ≥75 y | |||||||||
| Smoking | 1 [Reference] | 0.83 (0.62–1.12) | 0.85 (0.70–1.01) | 1.04 (0.75–1.44) | 0.70 (0.53–0.93) | 0.65 (0.53–0.80)† | 0.75 (0.61–0.93)† | 0.73 (0.60–0.89)† | 0.65 (0.58–0.74)† |
| Body mass index | 1 [Reference] | 1.22 (0.63–2.38) | 2.40 (0.82–7.00) | 1.51 (0.81–2.84) | 1.16 (0.75–1.80) | 1.91 (1.22–3.00)† | 1.91 (0.72–4.40) | 1.56 (0.99–2.46) | 1.68 (1.09–2.59) |
| Physical activity | 1 [Reference] | 0.94 (0.84–1.05) | 0.80 (0.70–0.91)† | 0.83 (0.70–0.97) | 0.83 (0.71–0.96) | 0.75 (0.62–0.90)† | 0.93 (0.77–1.13) | 0.78 (0.63–0.95) | 0.67 (0.57–0.79)† |
| Blood pressure | 1 [Reference] | 1.10 (0.97–1.24) | 1.23 (0.96–1.58) | 1.06 (0.93–1.20) | 1.00 (0.89–1.11) | 1.04 (0.86–1.26) | 1.06 (0.80–1.40) | 1.03 (0.84–1.25) | 0.94 (0.75–1.16) |
| Total cholesterol | 1 [Reference] | 0.92 (0.73–1.17) | 1.05 (0.79–1.41) | 1.00 (0.79–1.26) | 0.80 (0.66–0.97) | 0.94 (0.77–1.15) | 1.49 (1.07–2.07) | 0.98 (0.80–1.20) | 1.04 (0.87–1.24) |
| Fasting glucose | 1 [Reference] | 0.72 (0.58–0.89)† | 0.66 (0.49–0.88)† | 0.84 (0.68–1.04) | 0.63 (0.53–0.75)† | 0.58 (0.49–0.70)† | 0.74 (0.56–0.99) | 0.58 (0.49–0.70)† | 0.57 (0.48–0.66)† |
HR indicates hazard ratio; and sHR, subhazard ratio.
Cardiovascular disease was adjusted for sex, age, economic status, Hospital Frailty Score, living in metropolitan cities, and competing risk of death.
P<0.0083 for differences reported.
Compared with low‐low group, high‐high group of total cholesterol level was associated lower risk of CVD event in elderly but not in the very elderly population (p interaction=0.005).
All‐cause mortality was adjusted for sex, age, economic status, Hospital Frailty Score, and living in metropolitan cities.
Sensitivity Analysis
The results for 4 groups of changes in the CVH score showed that the high (score of ≥8)‐to‐low (score of <8), low‐to‐high, and consistently high CVH status groups had lower risks of CVD and mortality compared with the consistently low group (Table S13).
DISCUSSION
Principal Findings
In this study, time‐varying moderate and high measures of CVH were associated with lower risks of CVD and mortality than low CVH status in both the elderly and very elderly populations. In both the elderly and very elderly populations, consistent relationships were found between the improvement of the composite metric of CVH and the reduced risk of CVD. However, among the individual health factors, total cholesterol level was not appropriate for the prediction of CVD events in the very elderly participants. BMI and total cholesterol level were not appropriate for the prediction of all‐cause mortality in both the elderly and very elderly groups. This study is showing that improving CVH status is beneficial for both the elderly and very elderly populations.
CVH Metrics in the Very Elderly Population
The recently published European Society of Cardiology and European Atherosclerosis Society guidelines extended the age of the risk assessment system from 65 years to 70 years and recommended statin use for primary prevention according to the individual level of risk until aged 75 years.20 With this in mind, we have defined very elderly as referring to individuals aged >75 years herein.
The present study found that in the overall Asian very elderly population, 61.8%, 21.8%, and 16.3% of the participants showed stable, improved, and deteriorated CVH, respectively. This trend is consistently observed in both the very elderly and elderly populations. This study had a higher number of participants with improved health metrics than other studies.6, 21, 22 This can be related with the high proportion of participants with intermediate or ideal BMI and physical activity in the Asian elderly cohort. Worldwide, 31.1% (95% CI, 30.9–31.2) of adults are physically inactive, with proportions ranging from 17.0% (95% CI, 16.8–17.2) in Southeast Asia to about 43% in the Americas and Eastern Mediterranean regions. Adults aged 60 years or older from Southeast Asia are much more active than are individuals of the same age from all other regions, and more active than are young adults (aged 15–29 years) from the Americas, the Eastern Mediterranean, Europe, and the western Pacific regions.23
The National Health and Nutrition Examination Surveys21 of the United States found that the prevalence of smoking, hypercholesterolemia, and hypertension decreased significantly, but the prevalence of obesity and diabetes mellitus increased significantly. However, dietary and physical activity levels were unchanged from 1988/1994 to 2008. In the elderly Asian cohort of this study, the proportion of participants with ideal body weights was >60%. Moreover, those of the participants with intermediate or ideal physical activity levels were 28.5% and 64.2% in the first and second health examinations, respectively. This can be explained by the fact that this study included only individuals aged >60 years. In the ARIC (Atherosclerosis Risk in Communities) study,22 the general trend was a decrease in the number of ideal metrics, with only 7% of the participants showing improved CVH over 26 years from 1987 to 2013. In a UK general community (Whitehall II), 13% of the participants showed improved CVH. The baseline mean ages of the participants in the ARIC and Whitehall II studies were 52.1 and 44.8 years, respectively.6 Moreover, the ideal other individual CVH metrics, including smoking habit, were high in this study.
CVH Change and CVD Disease
The relationship between change of CVH and CVD event is still controversial. In the ARIC study,22 the improvement in CVH through follow‐up over 26 years was associated with a lower prevalence of CVD (no data on incident CVD) and better cardiac structure and function. In addition, in the Framingham Heart Study,10 loss of ideal CVH metrics over 6 years was not statistically significantly associated with coronary artery calcification progression after adjustment for the number of baseline ideal metrics. In the Whitehall II study,6 time‐varying moderate and high measures of CVH were associated with a lower CVD risk than low CVH. However, no consistent relationship was observed between the direction of change in the category of a composite metric of CVH and the risk of CVD.
In this study, the time‐varying moderate and high measures of CVH were associated with lower CVD and mortality risks than low CVH status in both the elderly and very elderly populations. In the elderly group, compared with the persistently low CVH group, the CVD risk was decreased in all of the other groups. By contrast, compared with the very elderly individuals with persistently low CVH status (consistently low group), all of the other groups except the low‐to‐moderate and low‐to‐high groups had a decreased CVD risk. Because the very elderly population with low baseline CVH status did not show decreased CVD risk even after improvement of their health metrics, the effect of baseline health condition might be more important in the very elderly population.
Individual CVH Metrics and CVD Risk in the Very Elderly Population
Because of the lack of data in the very elderly participants, there are no clinical support tools specifically designed to assess cardiovascular risk in this population. Statin therapy, blood pressure control, smoking cessation, physical activity, and maintaining a normal body weight are interventions that have been shown to reduce the incidence of primary cardiovascular events in the very elderly. The present study showed that maintaining optimal BMI and cholesterol level were not related with reduced mortality in both the elderly and very elderly population. No significant association of BMI with outcomes might be attributable to the obesity paradox.24, 25 For example, obesity is associated with mortality at a younger age, but not at an older age. Thus, the association might be driven by this age difference.
The association between weight loss and cardiovascular mortality is less clear in the very elderly population. Ghaem Maralani et al reported that obesity increased risk of CVD‐related death only in those aged <70 years, but not in those aged ≥70 years.26 Takata et al observed a similar outcome that cardiovascular mortality was 78% less in overweight octogenarians than in those who were underweight.27 Thus, although dietary recommendations can be made for the very elderly participants, weight loss recommendations for this population are not well supported by the literature.
The best available evidence for the primary prevention of cardiovascular events in older adults supports the use of statin therapy and blood pressure control. This study showed ideal total cholesterol was positively associated with CVD events in elderly participants. It can be a benefit from lipid‐lowering therapy. Statin therapy reduces the risk of myocardial infarction and stroke, although close monitoring for adverse events is warranted. It was the same in the Asian very elderly (≥75 years) population.28 However, in participants aged >75 years because of the lack of evidence, the American College of Cardiology/American Heart Association guidelines on the treatment of blood cholesterol to reduce cardiovascular risk in adults do not recommend using their atherosclerotic risk calculator. It is suggested that therapy could be considered on the basis of a discussion of benefits and risks, adverse effects, drug interactions, and patient preference.29
LIMITATIONS
This study has several limitations. First, studies that use administrative databases might be susceptible to errors arising from coding inaccuracies. To minimize this problem, we applied the definition that has been previously validated in previous studies that used the Korean NHIS sample cohort.9, 12, 13, 14, 15, 16 Second, the individuals excluded from the analysis of the changes in CVH had a less favorable cardiovascular risk profile, which could lead to an underestimation of the reported associations. To check this problem, we did an analysis with weighting to account for attrition. The weighted result to account for attrition was consistent with the main result. Third, the changes in the distribution of CVH across repeated examinations might have partly been related to aging, temporal trends, and cohort attrition. Fourth, some categories of CVH change were small, which might explain why some associations are not statistically significant.
CONCLUSIONS
In both the elderly and very elderly populations, a consistent relationship was observed between the improvement of a composite metric of CVH and reducing the risk of CVD. However, among the individual health factors, total cholesterol level was not appropriate for the prediction of CVD events in the very elderly participants. BMI and total cholesterol level were not appropriate for the prediction of all‐cause mortality in both the elderly and very elderly groups.
Sources of Funding
This study was supported by a research grant from the Korean Healthcare Technology R&D project funded by the Ministry of Health and Welfare (HI15C1200, HC19C0130), and a CMB‐Yuhan research grant from Yonsei University College of Medicine (6‐2019‐0124).
Disclosures
Dr Joung has served as a speaker for Bayer, BMS/Pfizer, Medtronic, and Daiichi‐Sankyo; and received research funds from Medtronic and Abbott. No fees were directly or personally received. The remaining authors have no disclosures to report.
Supporting information
Acknowledgments
Access to the National Health Information Database was provided by the NHIS of Korea. The authors thank the NHIS for their cooperation.
(J Am Heart Assoc. 2021;10:e019482. DOI: 10.1161/JAHA.120.019482.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019482.
For Sources of Funding and Disclosures, see page 12.
References
- 1.Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med. 2014;174:1868–1870. DOI: 10.1001/jamainternmed.2014.4758. [DOI] [PubMed] [Google Scholar]
- 2.Konrat C, Boutron I, Trinquart L, Auleley GR, Ricordeau P, Ravaud P. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. PLoS One. 2012;7:e33559. DOI: 10.1371/journal.pone.0033559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. DOI: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 4.Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta‐analysis of prospective studies. Clin Cardiol. 2017;40:1339–1346. DOI: 10.1002/clc.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaye B, Canonico M, Perier MC, Samieri C, Berr C, Dartigues JF, Tzourio C, Elbaz A, Empana JP. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the three‐city study. J Am Coll Cardiol. 2017;69:3015–3026. DOI: 10.1016/j.jacc.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 6.van Sloten TT, Tafflet M, Perier MC, Dugravot A, Climie RED, Singh‐Manoux A, Empana JP. Association of Change in Cardiovascular Risk Factors With Incident Cardiovascular Events. JAMA. 2018;320:1793–1804. DOI: 10.1001/jama.2018.16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmot MG, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A, Marmot MG, Smith GDavey. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. DOI: 10.1016/0140-6736(91)93068-K. [DOI] [PubMed] [Google Scholar]
- 8.Kim YI, Kim Y‐Y, Yoon JL, Won CW, Ha S, Cho K‐D, Park BR, Bae S, Lee E‐J, Park SY, et al. Cohort profile: national health insurance service‐senior (NHIS‐senior) cohort in Korea. BMJ Open. 2019;9:e024344. DOI: 10.1136/bmjopen-2018-024344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Yang P‐S, Yu HT, Kim T‐H, Jang E, Sung J‐H, Pak H‐N, Lee M‐Y, Lee M‐H, Lip GYH, et al. Risk of dementia in stroke‐free patients diagnosed with atrial fibrillation: data from a population‐based cohort. Eur Heart J. 2019;40:2313–2323. DOI: 10.1093/eurheartj/ehz386. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SJ, Onuma O, Massaro JM, Zhang X, Fu YP, Hoffmann U, Fox CS, O'Donnell CJ. Maintenance of ideal cardiovascular health and coronary artery calcium progression in low‐risk men and women in the Framingham heart study. Circ Cardiovasc Imaging. 2018;11:e006209. DOI: 10.1161/CIRCIMAGING.117.006209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SS, Ae Kong K, Kim D, Lim Y‐M, Yang P‐S, Yi J‐E, Kim M, Kwon K, Bum Pyun W, Joung B, et al. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J. 2017;38:2599–2607. DOI: 10.1093/eurheartj/ehx316. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Yang P‐S, Jang E, Yu HT, Kim T‐H, Uhm J‐S, Kim JY, Pak H‐N, Lee M‐H, Joung B, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018;104:2010–2017. DOI: 10.1136/heartjnl-2017-312930. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Yang P‐S, Jang E, Yu HT, Kim T‐H, Uhm J‐S, Kim J‐Y, Pak H‐N, Lee M‐H, Joung B, et al. 10‐year nationwide trends of the incidence, prevalence, and adverse outcomes of non‐valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–26. DOI: 10.1016/j.ahj.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Kim TH, Baek YS, Uhm JS, Pak HN, Lee MH, Joung B. The trends of atrial fibrillation‐related hospital visit and cost, treatment pattern and mortality in Korea: 10‐year nationwide sample cohort data. Korean Circ J. 2017;47:56–64. DOI: 10.4070/kcj.2016.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Yang P‐S, Kim T‐H, Jang E, Shin H, Kim HY, Yu HT, Uhm J‐S, Kim J‐Y, Pak H‐N, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72:1233–1245. DOI: 10.1016/j.jacc.2018.05.076. [DOI] [PubMed] [Google Scholar]
- 16.Kim T‐H, Yang P‐S, Yu HT, Jang E, Shin H, Kim HY, Uhm J‐S, Kim J‐Y, Sung J‐H, Pak H‐N, et al. Effect of hypertension duration and blood pressure level on ischaemic stroke risk in atrial fibrillation: nationwide data covering the entire Korean population. Eur Heart J. 2019;40:809–819. DOI: 10.1093/eurheartj/ehy877. [DOI] [PubMed] [Google Scholar]
- 17.Enserro DM, Vasan RS, Xanthakis V. Twenty‐year trends in the American Heart Association Cardiovascular Health Score and impact on subclinical and clinical cardiovascular disease: the Framingham offspring study. J Am Heart Assoc. 2018;7:e008741. DOI: 10.1161/JAHA.118.008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. DOI: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 19.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. DOI: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 20.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. DOI: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 21.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd‐Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the national health and nutrition examination surveys. Circulation. 2012;125:2595–2602. DOI: 10.1161/CIRCULATIONAHA.111.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation. 2015;132:1979–1989. DOI: 10.1161/CIRCULATIONAHA.115.017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, Lancet Physical Activity Series Working Group . Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–257. DOI: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 24.Romero‐Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez‐Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. DOI: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 25.Lavie CJ, De Schutter A, Patel D, Artham SM, Milani RV. Body composition and coronary heart disease mortality–an obesity or a lean paradox? Mayo Clin Proc. 2011;86:857–864. DOI: 10.4065/mcp.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghaem Maralani H, Tai BC, Wong TY, Tai ES, Li J, Wang JJ, Mitchell P. The prognostic role of body mass index on mortality amongst the middle‐aged and elderly: a competing risk analysis. Diabetes Res Clin Pract. 2014;103:42–50. DOI: 10.1016/j.diabres.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Takata Y, Ansai T, Soh I, Akifusa S, Sonoki K, Fujisawa K, Awano S, Kagiyama S, Hamasaki T, Nakamichi I, et al. Association between body mass index and mortality in an 80‐year‐old population. J Am Geriatr Soc. 2007;55:913–917. DOI: 10.1111/j.1532-5415.2007.01170.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim K, Lee CJ, Shim CY, Kim JS, Kim BK, Park S, Chang HJ, Hong GR, Ko YG, Kang SM, et al. Statin and clinical outcomes of primary prevention in individuals aged >75years: the SCOPE‐75 study. Atherosclerosis. 2019;284:31–36. DOI: 10.1016/j.atherosclerosis.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. DOI: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Yang PS, Kim D, Yu HT, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B, Lip GYH. CHA2DS2‐VASc score for identifying truly low‐risk atrial fibrillation for stroke: a Korean nationwide cohort study. Stroke. 2017;48:2984–2990. DOI: 10.1161/STROKEAHA.117.018551. [DOI] [PubMed] [Google Scholar]
- 31.Lee HY, Yang PS, Kim TH, Uhm JS, Pak HN, Lee MH, Joung B. Atrial fibrillation and the risk of myocardial infarction: a nation‐wide propensity‐matched study. Sci Rep. 2017;7:12716. DOI: 10.1038/s41598-017-13061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seong SC, Kim Y‐Y, Park SK, Khang YH, Kim HC, Park JH, Kang H‐J, Do C‐H, Song J‐S, Lee E‐J, et al. Cohort profile: the National Health Insurance Service‐National Health Screening Cohort (NHIS‐HEALS) in Korea. BMJ Open. 2017;7:e016640. DOI: 10.1136/bmjopen-2017-016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung H, Yang P‐S, Jang E, Yu HT, Kim T‐H, Uhm J‐S, Kim J‐Y, Pak H‐N, Lee M‐H, Joung B, et al. Effectiveness and safety of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation with hypertrophic cardiomyopathy: a nationwide cohort study. Chest. 2019;155:354–363. DOI: 10.1016/j.chest.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Kim D, Yang PS, Kim TH, Uhm JS, Park J, Pak HN, Lee MH, Joung B. Effect of atrial fibrillation on the incidence and outcome of osteoporotic fracture‐ A nationwide population‐based study. Circ J. 2018;82:1999–2006. DOI: 10.1253/circj.CJ-17-1179. [DOI] [PubMed] [Google Scholar]
- 35.Song S, Yang PS, Kim TH, Uhm JS, Pak HN, Lee MH, Joung B. Relation of chronic obstructive pulmonary disease to cardiovascular disease in the general population. Am J Cardiol. 2017;120:1399–1404. DOI: 10.1016/j.amjcard.2017.07.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


