Abstract
Background
Biological sex is an important modifier of cardiovascular disease and women generally have better outcomes compared with men. However, the contribution of cardiac fibroblasts (CFs) to this sexual dimorphism is relatively unexplored.
Methods and Results
Isoproterenol (ISO) was administered to rats as a model for chronic β‐adrenergic receptor (β‐AR)‐mediated cardiovascular disease. ISO‐treated males had higher mortality than females and also developed fibrosis whereas females did not. Gonadectomy did not abrogate this sex difference. To determine the cellular contribution to this phenotype, CFs were studied. CFs from both sexes had increased proliferation in vivo in response to ISO, but CFs from female hearts proliferated more than male cells. In addition, male CFs were significantly more activated to myofibroblasts by ISO. To investigate potential regulatory mechanisms for the sexually dimorphic fibrotic response, β‐AR mRNA and PKA (protein kinase A) activity were measured. In response to ISO treatment, male CFs increased expression of β1‐ and β2‐ARs, whereas expression of both receptors decreased in female CFs. Moreover, ISO‐treated male CFs had higher PKA activity relative to vehicle controls, whereas ISO did not activate PKA in female CFs.
Conclusions
Chronic in vivo β‐AR stimulation causes fibrosis in male but not female rat hearts. Male CFs are more activated than female CFs, consistent with elevated fibrosis in male rat hearts and may be caused by higher β‐AR expression and PKA activation in male CFs. Taken together, our data suggest that CFs play a substantial role in mediating sex differences observed after cardiac injury.
Keywords: cardiac fibroblasts, cardiac fibrosis, isoproterenol, sex differences
Subject Categories: Cardiovascular Disease, Fibrosis, Heart Failure
Nonstandard Abbreviations and Acronyms
- ADRB1
adrenergic receptor β1
- ADRB2
adrenergic receptor β2
- αSMA
αsmooth muscle actin
- β‐AR
β‐adrenergic receptor
- CAS
castrated
- CF
cardiac fibroblast
- Col1a1
collagen 1
- EdU
5‐ethynyl‐2’‐deoxyuridine
- ISO
isoproterenol
- OVX
ovariectomized
- PKA
protein kinase A
- TBP
TATA binding protein
- Tcf21
transcription factor 21
Clinical Perspective
What Is New?
There are significant differences in fibrosis between male and female rats in response to adrenergic stimulation that were not abolished by gonadectomy.
Male and female cardiac fibroblasts make significant contributions to sex differences in the response of the heart to adrenergic stimulation.
What Are the Clinical Implications?
Precision medicine approaches to heart disease need to take cellular sex into consideration during diagnosis and treatment.
Cardiac cell types other than cardiomyocytes need to be considered in therapeutic approaches.
Heart failure (HF) is the clinical outcome of numerous cardiovascular diseases and affects 6.2 million individuals in the United States.1 As cardiac output declines during HF, compensatory neurohormonal mechanisms are engaged in an effort to maintain cardiac function.2 Among these mechanisms, stimulation of β‐ARs by catecholamines plays a major role in regulating cardiovascular performance.3, 4, 5 In the short term, catecholamines preserve heart function, but chronic β‐AR activation results in cardiac dysfunction including elevated apoptosis, hypertrophy, and fibrosis.6
There are sex differences in β‐AR signaling that are associated with improved outcomes of women compared with men. Animal studies show that females have reduced contractile response to β‐AR stimulation compared with males.7, 8 Interestingly, this reduced response in female animals could not be explained by reduced expression levels of β‐ARs in the ventricle but could be abated when females were ovariectomized.7 The sex difference in cardiac contractility upon β‐AR stimulation also transcends to the cellular level. Cardiomyocytes from male rats have enhanced contraction in response to β‐AR stimulation compared with female cells, likely caused by increased β‐adrenergic cAMP signaling.9 In addition to affecting cardiac contractile function, chronic β‐AR stimulation also causes myocardial fibrosis, which is known to be sexually dimorphic.10, 11 In response to β‐AR stimulation, hearts from male mice develop significant fibrosis, which does not occur in female mice.10 The cellular mediators of heart fibrosis, cardiac fibroblasts (CFs), have not yet been investigated for sex differences in response to β‐AR stimulation.
Cardiac injury, including chronic β‐AR stimulation, results in myocardial fibrosis that decreases cardiac compliance and accelerates the progression of HF.4 Fibrosis is caused by overactivation of the remodeling process after injury is sustained, resulting in accumulation of fibrous connective tissue composed of extracellular matrix components, including collagen.12 Hearts from male rats treated with a β‐AR agonist showed elevated levels of collagen I and collagen III deposition compared with control groups.13 Resident fibroblasts are largely responsible for this excessive deposition of extracellular matrix components through their activation to myofibroblasts.14 In response to β‐AR stimulation, CFs proliferate and activate to myofibroblasts to increase contractile forces and express high levels of collagens.15, 16, 17 CFs respond to catecholamines through direct β‐AR activation18 and through paracrine signals from myocytes.16, 19 Interestingly, once CFs activate to myofibroblasts, they can promote cardiac hypertrophy through secretion of paracrine factors,20 suggesting that CFs may play a major role not only in fibrosis but also in cardiac hypertrophy. The specific roles of CFs in sexually dimorphic fibrotic and hypertrophic responses to β‐AR stimulation remain unknown.
Cellular sexual dimorphisms, either intrinsically encoded via sex chromosomes21 or acquired via sex hormones,22, 23 have been linked to numerous disease phenotypes.24, 25 In general, female cells appear to have higher stress tolerance compared with male cells.26, 27, 28 Although limited, data suggest that male and female CFs may respond differently to injury cues.29 Here, we investigate the cellular basis for the sexually dimorphic, fibrotic response to β‐AR activation by ISO. To directly study the effect of ISO on CFs, adult male and female rats were treated with ISO and vehicle and CFs were analyzed in vivo for proliferation. We also analyzed CFs isolated from ISO and vehicle‐treated male and female hearts for myofibroblast activation and gene expression, including β1‐ and β2‐ARs and for a major downstream signaling pathway, PKA (protein kinase A) activity.
Methods
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
All animal work was approved by the Institutional Animal Care and Use Committee at the University of Colorado at Boulder (Protocol #2351) and are in accordance with the National Institutes of Health (NIH) guidelines. Male and Female (250–300 g) Sprague‐Dawley, CD, rats were purchased from Charles River Laboratory and allowed to acclimate in the Jennie Smoly Caruthers Biotechnology Building’s vivarium for 1 week before surgery. Rats were fed ad libitum standard rodent chow and housed in a facility with a 12‐hours light, 12‐hours dark cycle. Female ovariectomized (OVX) rats and male castrated (CAS) rats were purchased from Charles River. Both OVX and CAS animals underwent surgery and recovery at Charles River before delivery. Because there could be a weight match issue because of gonadectomy, rats were age matched to gonad‐intact studies. For these studies, males were 9 weeks of age and females were 13 weeks of age.
β‐adrenergic Stimulation In Vivo
For all studies, ISO was dissolved in 1 μM ascorbic acid. For chronic administration of ISO, subcutaneous administration was achieved by osmotic mini‐pumps implantation. For mini‐pump implantation, rats were anesthetized with isoflurane (5%) via spontaneous inhalation and then placed on a 37°C recirculated heating pad. For the remainder of the procedure, animals were maintained on 2% isoflurane via spontaneous inhalation. All surgical procedures were performed under aseptic conditions with sterile surgical instruments and on a sterile field. Fur was removed from the surgical site by shaving. The operating field was disinfected with Nolvasan surgical scrub and 70% ethanol twice in alternate. Rats received a single injection of sustained‐release buprenorphine (1.0 mg/kg, subcutaneous) before beginning the procedure. An incision was made slightly caudal to the scapulae with scissors. The skin and the muscle layers were separated with curved hemostats. ISO treatment concentration was determined by a titration curve in male rats for 7 days. A treatment of 4 mg/kg/day was found to have the strongest effect on cardiac hypertrophy and heart rate (Figure S1). The sterile mini‐pumps containing either ISO (4 mg/kg/day) or vehicle (1 μM ascorbic acid) were placed under the skin. The incision was closed with a surgical stapler. Animals were observed for any signs of distress and returned to the vivarium where they were observed daily for the duration of the experiment. Any animals showing visible signs of distress were removed from the study and euthanized. PASS2020 software was used to determine group sample sizes for the Gehan‐Wilcoxon 2‐group hazard comparison test via simulation (n=10 000 simulations). Using this method, and assuming a median survival time of 3 days for male rats treated with ISO (4 mg/kg/day) and based upon a 7‐day observation period, sample sizes of 90 male rats and 60 female rats achieved 80% power to detect a hazard ratio of 0.55 at a 5% significance level using a 2‐sided Gehan‐Breslow‐Wilcoxon test. For mini‐pump implantation, the experimenter was unblinded for surgery due to primary end point (death), but subsequent measurements and analyses were performed by a blinded individual. Animals used for the survival studies were also used for other analyses.
Echocardiography
Echocardiography analysis was performed as previously described. Briefly, echocardiographic images were taken, and measurements were made using a Phillips Sonos 5500 system. Anesthetized rats were placed on a recirculating heating pad maintained at 37°C. Hair was removed from the chest using depilatory cream before the image potentiating gel application. M‐mode images were captured for each animal at the level of the papillary muscle (A2 view). Left ventricular dimensions, including functional analysis, were measured using the leading edge convention.
Necropsy
Total body weight was collected for each rat just before echocardiography and euthanasia. Cardiac tissue, including all major vessels, was removed in fully anesthetized rat. Connective tissue was removed, the heart was cannulated through the aorta with sterile saline, and the cleared heart was blotted. The total heart was weighed, the atria were removed and weighed, the right ventricle was separated from the entire left ventricle, and both ventricles were weighed separately. The tibia was isolated from each rat and measured in order to normalize tissue weights.
Histological Quantification of Collagen Content.
Whole heart tissue embedded in O.C.T. Compound (Sakura Finetek USA, Inc., Torrance, CA) and immediately frozen in liquid nitrogen immediately after necropsy. Tissue was stored at ‐80°C until used and transferred to −20°C an hour before sectioning. For histological analysis, midline sections were cut using a Leica CM1850 cryostat (Leica Biosystems Inc., Buffalo Grove, IL). Three sections from each tissue section were placed on the plus side of a Fisherbrand ColorFrost Plus Microscope Slide (Fisher Scientific, Pittsburgh, PA). Frozen slides were then sent to Premier Laboratories (Longmont, CO) to be stained with Picrosirus red. Images were captured using a Nikon Te‐2000 Widefield Microscope with a 4x objective. The entire cross‐sectioned heart image was generated via image stitching. For all the samples the green channel was used for automated analysis of % fibrotic area using a custom MATLAB script (Data S1). Briefly, the total area of each heart cross‐section was determined using the Image Processing Toolbox. The fibrotic area was determined by identifying and summing the pixel area in which the intensity was above 95% of the total intensity distribution. The % fibrotic area was calculated by dividing the measured fibrotic area by the total area.
Hydroxyproline Measurements for Collagen Content
Hydroxyproline content in the left ventricle of vehicle and ISO‐treated rats was determined by a colormetric Hydroxyproline Assay Kit (Sigma MAK008) according to the manufacturer’s instruction.
Rheology
The shear elastic moduli of rat left ventricles were measured using a rheometer (ARES; TA Instruments, New Castle, DE). Hearts were excised from ISO or vehicle‐treated animals, whereby an 8 mm punch was used to remove a disk‐like portion of the tissue that was approximately 1 mm thick. The left ventricle tissue was characterized within 2 hours of isolation. Tissue was trimmed to yield a sample with a thickness of <1 mm. The shear elastic modulus (G') of the left ventricle tissue was measured using parallel plates modified with sandpaper to prevent tissue slippage.
In Vivo 5‐ethynyl‐2'‐deoxyuridine Labeling of Proliferating Cells
5‐ethynyl‐2'‐deoxyuridine (EdU) labeling is a common, proven, and effective protocol for labeling proliferating cells in vivo.30 In vivo EdU labeling in rats is performed through intraperitoneal injection of 50 µg/g in PBS. Rats were weighed before EdU injection to determine to appropriate dose. EdU injection was performed 24 hours before sacking animals. Vehicle animals were injected with PBS with dimethyl sulfoxide. After sacking, the apex of left ventricle was removed and placed in O.C.T. and frozen in liquid nitrogen until sectioning was performed. Tissue was cryosectioned with 10 µm thick sections. Sections were stained for total nuclei using 4′,6‐diamidino‐2‐phenylindole (Life Technologies D1306, Carlsbad, CA) and cells in the S‐phase were stained for EdU (Thermo Fisher Scientific C10337, Waltham, MA). Sections were imaged on a Nikon spinning disc confocal microscope. The total number of EdU positive and vimentin positive nuclei per image (region of interest) were counted using ImageJ. At least 8 images were quantified per rat. EdU+/vimentin+ nuclei were determined manually by identifying nuclei that overlapped in both EdU and vimentin channels.
Adult Rat Ventricular Fibroblast (CF) Isolation
Rats were injected with Fatal Plus and the heart removed, the left ventricle was isolated, and the remainder of the heart discarded. The left ventricle was then washed with DMEM/F12 (#1103907, Thermo Fisher Scientific) with Pen/Strep (#15070063, Thermo Fisher Scientific) and Fungizone (#15290‐018, Gibco, Gaithersburg, MD) and minced in 1 mg/mL collagenase type II (#LS004177, Worthington Biochemical, Lakewood, NJ) dissolved in Earle’s Balanced Salt Solution (Sigma‐Aldrich E2888‐6X500ML, St. Louis, MO) on a rocker in the 37 degree 5% CO2 incubator for 90 min. This solution was passed through a 100 µm filter to remove large pieces of tissue and the flow through was centrifuged at 600 xg for 5 min. The supernatant was aspirated, and the pellet was resuspended in red blood cell lysis buffer (4.15 g NH4Cl, 0.5 g KHCO3, 0.018 g Na2EDTA, in 500 mL pH to 7.2) for 5 min on ice and centrifuged again for 600 xg for 5 min. The cell pellet was collected and resuspended in growth media (DMEM/F12 with 10% FBS (Thermo Fisher Scientific 16000069), Pen/Strep, Fungizone, and 1 mM L‐Ascorbic acid (Sigma‐Aldrich A4403‐100MG) and plated for 3 hours on tissue culture polystyrene. After 3 hours, nonadherent cells were removed by washing the plate 2 times with warm PBS before adding growth media. CFs were grown on tissue culture polystyrene 24 hours before trypsinizing and plating for imaging or lysing for RNA extraction. Cells tested negative for endothelial marker vascular endothelial‐cadherin and positive for vimentin.
CF Culturing Procedure With α Smooth Muscle Actin Staining
CFs were trypsinized from tissue culture polystyrene and counted using a coulter counter. Cells from each rat were counted and seeded onto gelatin coated coverslips at a density of 10,000 cells/cm2. CFs were cultured on coverslips 24 hours before fixing with 4% PFA in PBS for 30 minutes. Cells were permeabilized with 0.1% TritonX100 for 1 hour and blocked with 5% BSA in PBS for 1 hour. Cells were incubated with primary antibodies alpha smooth muscle actin (αSMA) (AbCam ab7817, Cambridge, UK) and vimentin (Thermo Fisher Scientific PA1‐10003) overnight at 4 C. The next day, cells were washed with PBS and stained with secondary antibody Goat anti Mouse Alexa 488 (AbCam ab150117) 1:500, Goat anti Chicken Alexa 546 (AbCam A‐11040), and 4′,6‐diamidino‐2‐phenylindole (Life Technologies D1306) 1:500. Immunostained samples were imaged with high content confocal microscope using 20x objective (Operetta, PerkinElmer). Myofibroblasts were defined as cells with αSMA staining organized into stress fibers, and the percentage of myofibroblasts was calculated based on (the number of myofibroblasts/total number of cells) × 100%. Quantification of cell area was performed using Harmony Software (PerkinElmer) using vimentin as the cell border indicator.
CF Gene Expression Quantification
Freshly isolated CFs were cultured 24 hours following the isolation, plated CFs were washed with PBS and Trizol reagent was added directly to the plate. The RNA extraction was performed using a phenol chloroform extraction and RNA was quantified using the Nanodrop. The cDNA was synthesized using the Biorad cDNA iScript™ Reverse Transcription Supermix (1708841) with combined oligo(dT) and random primers. Reverse transcriptase–quantitative polymerase chain reaction was performed using Biorad Sybergreen Supermix (1708884). Values were quantified using the ΔΔ Cq method and normalized to TBP (TATA binding protein) or GAPDH. Primers are listed in Table 1.
Table 1.
Primers for RT‐qPCR
| Primer/Gene Name | Sequence |
|---|---|
| TBP Forward | tgcacaggagccaagagtgaa |
| TBP Reverse | cacatcacagctccccacca |
| GAPDH Forward | aggtcggtgtgaacggatttg |
| GAPDH Reverse | tgtagaccatgtagttgaggtca |
| ACTA2 Forward | gatcaccatcgggaatgaacgc |
| ACTA2 Reverse | ctgttataggtggtttcgtggatgc |
| POSTN Forward | tcgtggaaccaaaaattaaagtc |
| POSTN Reverse | cttcgtcattgcaggtcct |
| TCF21 Forward | cattcacccagtcaacctg |
| TCF21 Reverse | ccacttcctttaggtcactctca |
| COL1A1 Forward | cagtcgattcacctacagcacg |
| COL1A1 Reverse | gggatggagggagtttacacg |
| ADRB1 Forward | ctacaacgaccccaagtgct |
| ADRB1 Reverse | acgtagaaggagacgacgga |
| ADRB2 Forward | ccttggcgtgtgctgatcta |
| ADRB2 Reverse | gtccagaactcgcaccagaa |
ACTA2 indicates actin alpha 2; ADRB1, adrenergic receptor β1; ADRB2, adrenergic receptor β2; COL1A1, collagen 1; POSTN, periostin; RT‐qPCR, reverse transcriptase–polymerase chain reaction; TBP, TATA binding protein; and TCF21 transcription factor 21.
CF PKA Activity
CFs from ISO or vehicle‐treated animals were isolated as described. After 24 hours postisolation and culture, cells were trypsinized and resuspended in 100 µL of lysis buffer. A micro BCA assay (Thermo Fisher 23235) was used to quantify protein concentrations in the lysed samples. PKA activity was determined using assay kit (Abcam ab139435). Relative PKA activity was calculated by normalizing PKA activity to total protein concentration.
Statistical Analysis
GraphPad Prism 7.0 was used to perform all statistical analysis. Unless otherwise stated, all data are represented as standard error from the mean (±SEM). Statistical analysis was performed using 2‐tailed Student’s t test, 1‐way, or 2‐way ANOVA with Bonferroni correction post hoc where appropriate. A P value ≤0.05 was considered to be statistically significant. After primary analysis, if outliers were obvious, a robust regression and outlier removal outlier test was performed and then statistical outliers were removed.
Results
Male Rats Have More Cardiac Hypertrophy, Fibrosis, and Mortality in Response to ISO Treatment
To induce chronic β‐adrenergic signaling in adult male and female rats, the β‐AR agonist, ISO, was delivered for 7 days at 4 mg/kg/day (Figure 1A). ISO treatment caused cardiac hypertrophy in both male (~42%) and female (~28%) rats (Figure 1B); however, ISO‐treated male rat hearts were significantly more hypertrophic than ISO‐treated female rat hearts. Over the course of 7 days of vehicle or ISO treatment, the survival of male rats was significantly lower compared with ISO‐treated female rats, but ISO caused significant mortality in both sexes (Figure S2). Using echocardiography, the hearts of vehicle and ISO‐treated rats were evaluated for functional output after 7 days. ISO treatment caused increased fractional shortening and heart rate in both male (P = 0.000179, P = 0.000147) and female (P = 0.0000343, P = 0.0013) rats compared with vehicle treatment (Table S1).
Figure 1. Male rats increased cardiac hypertrophy and fibrosis in response to ISO treatment compared with female rats.
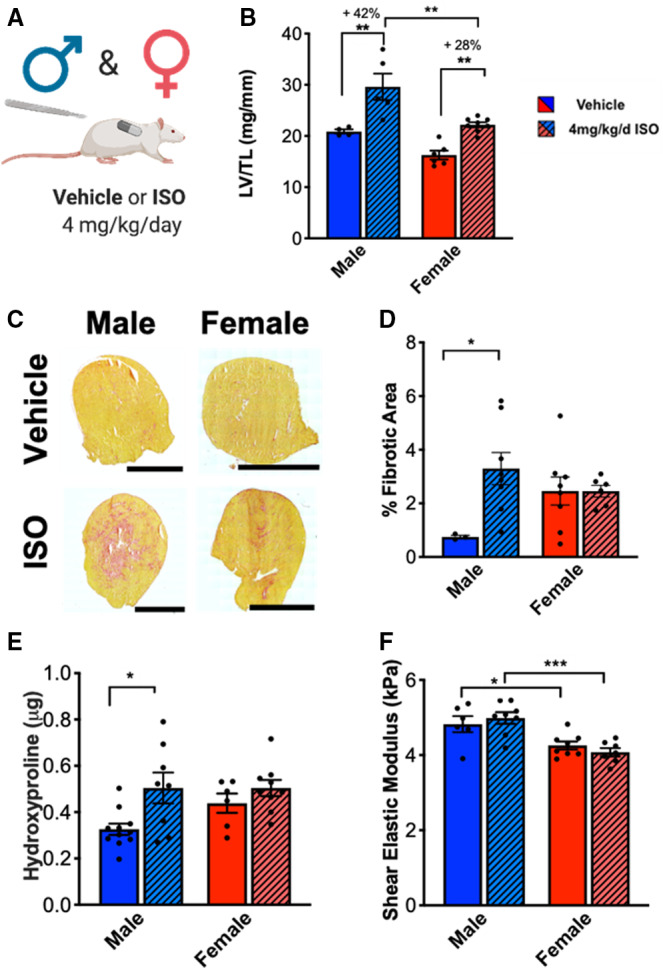
(A) Illustration of experimental design testing ISO treatment on male and female rats. (B) Cardiac hypertrophy measured by left ventricle weight to tibia length (LV/TL). Male vehicle n=4, Male ISO n=5, Female vehicle n=6, Female ISO n=8. (C) Representative images of picrosirius red staining of hearts from male and female rats treated with vehicle or ISO for 7 days. Scale bar = 4 mm. (D) Quantification of picrosirius red staining at 7 days. Male vehicle n=3, Male ISO n=8, Female vehicle n=8, Female ISO n=6. (E) Hydroxyproline biochemical assay on male and female rat hearts treated with vehicle or ISO for 7 days. Male vehicle n=11, Male ISO n=8, Female vehicle n=6, Female ISO n=9. (F) Shear elastic modulus of left ventricle from male and female rat hearts treated with vehicle or ISO for 7 days. Male vehicle n=6, Male ISO n=8, Female vehicle n=8, Female ISO n=7. Two‐way ANOVA with Bonferroni post hoc applied. *P<0.05, **P<0.01, ***P<0.001. All data reported as ±SEM. ISO indicates isoproterenol; and KPa, kilopascal.
To investigate sex differences in the fibrotic response to ISO treatment, hearts from male and female rats were treated with vehicle or ISO for 7 days and evaluated for collagen deposition using picrosirius red staining and hydroxyproline assays. ISO‐treated male rat hearts had significantly more picrosirius red staining compared with vehicle‐treated male rats, whereas staining of ISO‐treated female rats was not different from vehicle‐treated females (Figure 1C and 1D). Hydroxyproline assays revealed similar findings as picrosirius red staining in that ISO‐treated male rat hearts revealed significantly more collagen compared with vehicle‐treated male rat hearts (Figure 1E). Conversely, the collagen content of ISO‐treated female rat hearts was not significantly different from vehicle‐treated female rat hearts. Taken together, these data show that ISO treatment causes a significant fibrotic response only in male rat hearts.
Increased collagen content can lead to increased tissue stiffness,31 so the shear elastic modulus of left ventricular tissue was measured to evaluate whether ISO treatment had an effect on tissue stiffness in male and female hearts. Left ventricles were isolated from animals treated with ISO or vehicle for 7 days and the shear elastic modulus was measured using rheology (Figure 1F). At baseline, male left ventricles were stiffer than female left ventricles, which could potentially affect the development of cardiovascular disease. However, for both male and female tissues, there were no significant differences in the stiffness between vehicle and ISO‐treated samples, which may indicate that the collagen deposited in response to ISO treatment has not been crosslinked into the tissue by 7 days to cause an increase in bulk tissue stiffness.
Gonadectomy Does Not Ameliorate Sex Differences in the Fibrotic Response to ISO
To investigate the role of sex hormones in the sexually dimorphic response to ISO, rats were gonadectomized and treated with vehicle or ISO for 7 days (Figure 2A). ISO treatment caused significant cardiac hypertrophy in both CAS male and OVX females (Figure 2B). Castration did not affect survival in ISO‐treated males, but ovariectomy in ISO‐treated females reduced survival compared with gonadally intact animals (Figure S3A,B). ISO treatment caused an increase in heart rate in OVX females (P = 0.0054) and an increase in fractional shortening in CAS males (P = 0.000454) (Table S2). Similar to gonadally intact animals, ISO treatment caused an increase in collagen content only in CAS males but not in OVX females (Figure 2C). These results suggest that sex hormones alone do not control the fibrotic response to ISO treatment. Further, the decreased survival of OVX females suggests a protective effect of estrogen, consistent with earlier studies.32, 33
Figure 2. ISO‐induced fibrosis is independent of sex hormones.
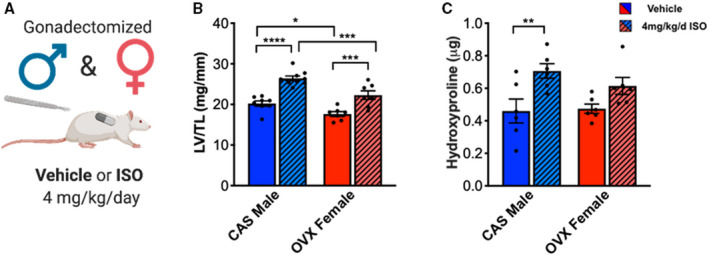
(A) Illustration of experimental design testing ISO treatment on CAS and OVX rats. (B) Cardiac hypertrophy measured by left ventricle weight to tibia length (LV/TL). CAS male vehicle n=8. CAS male ISO n=9. OVX female vehicle n=7. OVX female ISO n=7. (C) Hydroxyproline biochemical assay on CAS male and OVX female rat hearts treated with vehicle or ISO for 7 days. CAS male vehicle n=6. CAS male ISO n=6. OVX female vehicle n=6. OVX female ISO n=6. Two‐way ANOVA with Bonferroni post hoc applied. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. All data reported as ±SEM. CAS indicates castrated; ISO, isoproterenol; and OVX, ovariectomized.
Female CFs are More Proliferative than Male CFs in Response to ISO Treatment
The primary cells responsible for the fibrotic response to cardiac injury are CFs, and their proliferation in the heart contributes to the repair process because an increased population of fibroblasts may lead to increases in collagen deposition. To examine changes in proliferation in the heart in response to ISO treatment, proliferating CFs were labeled with EdU by intraperitoneal injection 24 hours before euthanizing the animals and then counterstaining for vimentin. Proliferation was examined at 2 time points, days 3 and 7, to determine whether ISO treatment caused an early, acute (3 day) or late (7 day) response in CFs (Figure 3A). In both sexes, ISO treatment led to increased proliferation after 3 days; however, females showed higher proliferation rates compared with males (Figure 3B). By 7 days, proliferation rates were decreased in both sexes with ISO treatment but remained higher in ISO‐treated animals compared with vehicle‐treated animals, regardless of sex (Figure 3C). These results point to a short, proliferative phase after ISO treatment that is sexually dimorphic in which female CFs are more proliferative compared with male CFs.
Figure 3. Female CFs are more proliferative than male CFs in response to ISO treatment.
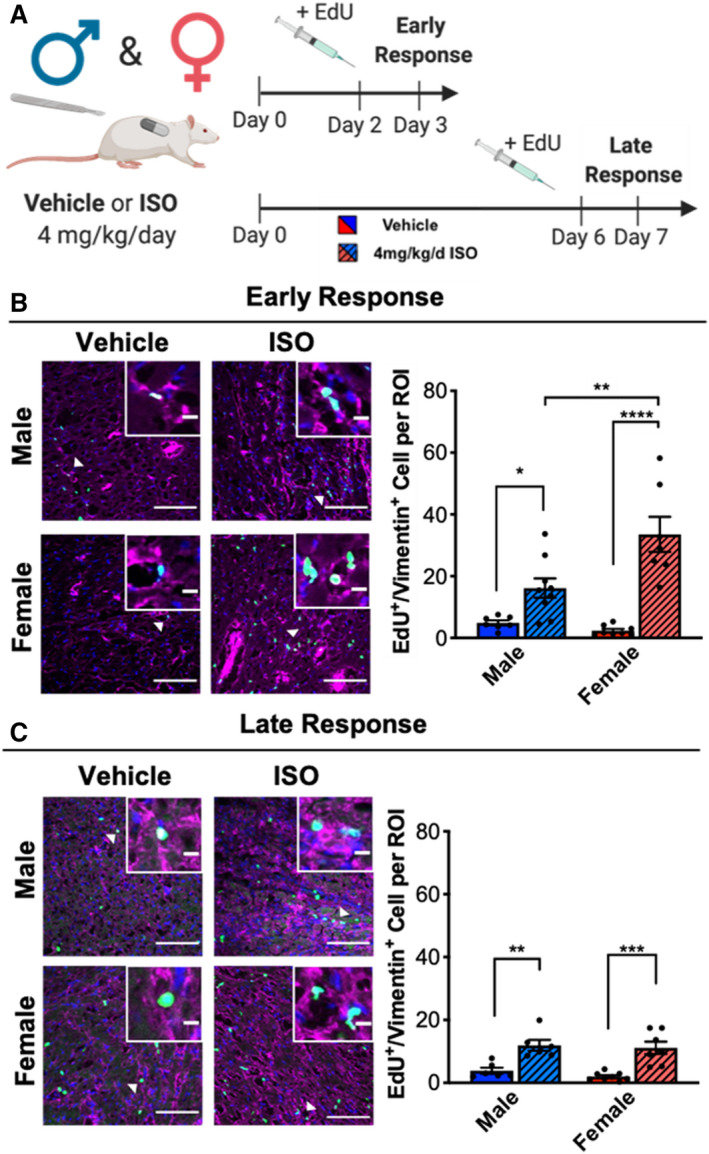
(A) Illustration of experimental design testing ISO treatment on CF proliferation in male and female rats during the early (3 day) or late (7 day) response. (B) Proliferation of CFs after 3 days of ISO treatment measured by EdU staining. Representative images and quantification. White arrows indicate cells shown in insets. DAPI = blue, EdU positive cells = Green, vimentin = pink. Large image scale bar = 100 µm, inset scale bar = 10 µm. Male vehicle n=7, Male ISO n=9, Female vehicle n=8, Female ISO n=7. Two‐way ANOVA with Bonferroni post hoc applied. (C) Proliferation of CFs after 7 days of ISO treatment measured by EdU staining. Representative images and quantification. White arrows indicate cells shown in insets. DAPI = blue, EdU positive cells = Green, vimentin = pink. Large image scale bar = 100 µm, inset scale bar = 10 µm. Male vehicle n=6, Male ISO n=6, Female vehicle n=8, Female ISO n=7. Two‐way ANOVA with Bonferroni post hoc applied. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. All data reported as ±SEM. CF indicates cardiac fibroblasts; DAPI, 4′,6‐diamidino‐2‐phenylindole; EdU, 5‐ethynyl‐2’‐deoxyuridine; ISO, isoproterenol; and ROI, region of interest.
Male CFs Are More Activated to Myofibroblasts at Baseline and Activate Early in Response to ISO Treatment But Deactivate Later
The fibrotic response is associated with CF proliferation but also with myofibroblast activation and the subsequent activation of profibrotic signaling. Because ISO induces CF proliferation at both early and late time points, we isolated CFs at both 3 and 7 days after vehicle or ISO treatment and evaluated myofibroblast activation (Figure 4A). Isolated CFs were cultured for 2 days in vitro and then stained for αSMA, a commonly used myofibroblast marker. Because activated myofibroblasts are larger than quiescent fibroblasts,34 cell area was also measured. Interestingly, vehicle‐treated male CFs were more activated than female CFs, as assessed by αSMA and cell area (Figure 4B). After 3 days of ISO treatment, both male and female CFs expressed more αSMA fibers compared with vehicle controls; similarly, both male and female CFs from ISO‐treated animals were larger than CFs from vehicle‐treated animals.
Figure 4. Male CFs are larger and express more αSMA than females at baseline and with ISO treatment.
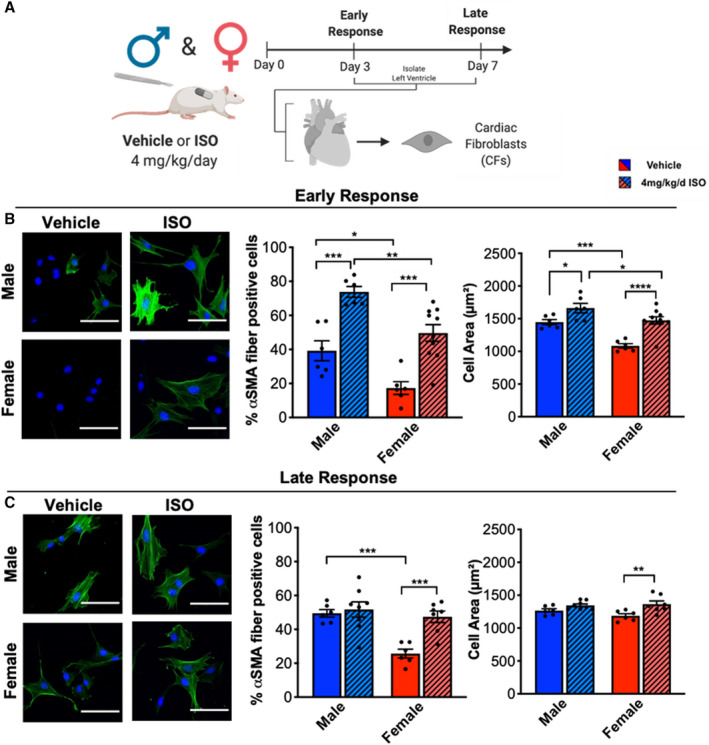
(A) Illustration of experimental design testing ISO treatment on CF activation in male and female rats during the early (3 day) or late (7 day) response. (B) Myofibroblast activation and cell size of CFs after 3 days of ISO treatment measured by αSMA immunostaining. Representative images and quantification. DAPI = blue, αSMA = Green. Scale bar = 100 µm. Male vehicle n=6, Male ISO n=6, Female vehicle n=6, Female ISO n=10. Two‐ANOVA with Bonferroni post hoc applied. (C) Myofibroblast activation and cell size of CFs after 7 days of ISO treatment measured by αSMA immunostaining. Representative images and quantification. DAPI = blue, αSMA = Green. Scale bar = 100 µm. Male vehicle n=6, Male ISO n=8, Female vehicle n=6, Female ISO n=8. Two‐way ANOVA with Bonferroni post hoc applied. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. All data reported as ±SEM. αSMA indicates α smooth muscle actin; CF, cardiac fibroblasts; DAPI, 4′,6‐diamidino‐2‐phenylindole; and ISO, isoproterenol.
By 7 days, only ISO‐treated female CFs were larger and expressed more αSMA fibers compared with vehicle‐treated female CFs, whereas male vehicle‐treated and ISO‐treated CFs were not significantly different (Figure 4C). These data indicate that male CFs maintain higher levels of myofibroblast activation, even without ISO stimulation in vivo. Moreover, male CFs activate to myofibroblasts early, but then deactivate over 7 days of ISO treatment. Conversely, female CFs activate to myofibroblasts early and late with ISO stimulation in vivo.
Male CFs Express Higher Levels of Profibrotic Markers Than Female CFs in Response to ISO Treatment In Vivo
Next, the profibrotic response of CFs from vehicle and ISO‐treated rats was measured by gene expression of αSMA, Col1a1 (collagen 1), Postn (periostin), and Tcf21 (transcription factor 21). In CFs isolated from vehicle‐treated animals, sex differences in expression of Col1a1 and Tcf21 were observed with males expressing higher levels of Col1a1 and females expressing higher levels of Tcf21 (Figure 5A). This result suggests that male CFs may have higher levels of myofibroblast activation than females at baseline, because Col1a1 is a marker of myofibroblast activation and Tcf21 is a marker of quiescent or unactivated fibroblasts. After 3 days of ISO treatment, both sexes increased expression of Postn (a marker of activated myofibroblasts) and decreased expression of Tcf21 compared with vehicle‐treated samples (Figure S4A). However, only male CFs upregulated αSMA expression in response to ISO treatment compared with vehicle. Next, the ISO‐treated samples were normalized to vehicle‐treated controls to evaluate sex differences in response to ISO (Figure 5B). Male CFs expressed significantly more αSMA and Col1a1 and less Tcf21 in response to 3 days of ISO treatment compared with female CFs.
Figure 5. ISO causes myofibroblast activation in both male and female CFs but more dramatic response in males.
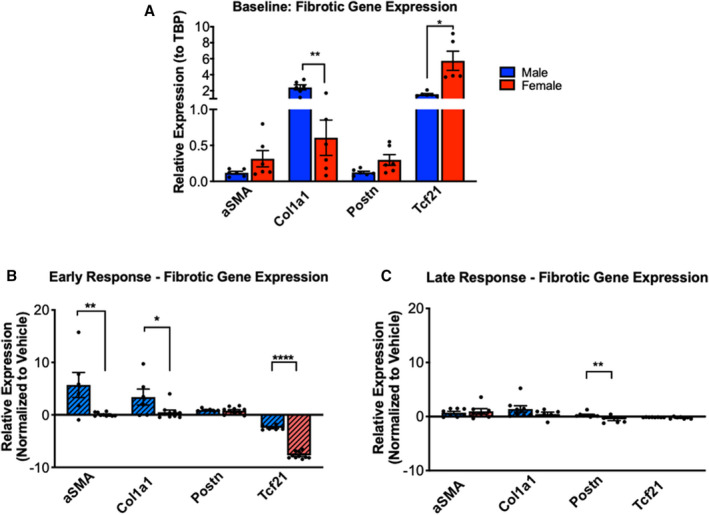
(A) mRNA expression levels of myofibroblast and fibroblast genes in male and female CFs from vehicle‐treated rats. αSMA, Col1a1, and Postn, and Tcf21 gene expression (normalized to TBP) was measured by RT‐qPCR. Male n=6, Female n=6. Unpaired student’s t‐test applied. (B) mRNA expression levels of myofibroblast and fibroblast genes in male and female CFs from 3‐day ISO‐treated rats. αSMA, Col1a1, and Postn, and Tcf21 gene expression (normalized to TBP) was measured by RT‐qPCR. Values were normalized to vehicle‐treated samples. ISO Male n=6, ISO Female n=10. Unpaired Student’s t test applied. (C) mRNA expression levels of myofibroblast and fibroblast genes in male and female CFs from 7‐day ISO‐treated rats. αSMA, Col1a1, and Postn, and Tcf21 gene expression (normalized to TBP) was measured by RT‐qPCR. Values were normalized to vehicle‐treated samples. ISO Male n=8, ISO Female n=7 (expect Col1a1 n=5). Unpaired Student’s t test applied. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. All data reported as ±SEM. αSMA indicates α smooth muscle actin; CF, cardiac fibroblasts; Col1a1, collagen 1; ISO, isoproterenol; Postn, periostin; RT‐qPCR, reverse transcriptase–polymerase chain reaction; TBP, TATA binding protein; and Tcf21 transcription factor 21.
Although these changes in gene expression of fibrotic markers were observed with 3 days of ISO treatment, by 7 days of treatment, the differences between the vehicle and ISO‐treated rats were dampened in both sexes (Figure S4B). After 7 days, only male CFs had an increase in expression of αSMA and a decrease in expression of Tcf21 with ISO treatment compared with vehicle‐treated CFs. There were no significant differences at 7 days between female ISO‐treated and vehicle‐treated samples. Moreover, in response to 7 days of ISO treatment, male CFs expressed significantly more Postn compared with female CFs (Figure 5C).
Taken together, these data show that there is an acute, early phase of myofibroblast activation in response to ISO that occurs within 3 days and is diminished by 7 days. In the early phase, CFs from male ISO hearts express increased levels of αSMA and Col1a and decreased levels of Tcf21 compared with CFs from female ISO hearts, indicating higher myofibroblast activation of male CFs. These data correlate with αSMA staining, where male CFs are more activated than female CFs. Interestingly, CFs from hearts of female ISO treated animals show higher proliferation rates, whereas the CFs from male ISO treated animals show higher expression of myofibroblast markers.
CFs Regulate β‐AR mRNAs and Activate PKA in Response to ISO Treatment
ISO‐mediated cardiac fibrosis is linked to increased β‐AR signaling in CFs,20, 35 so we hypothesized that the sexually dimorphic CF response to ISO was regulated by the β‐AR expression profile. To determine if the prominent fibrotic response in male CFs was caused by increased β‐AR signaling, gene expression of the adrenergic receptor β1 (ADRB1) and adrenergic receptor β2 (ADRB2) was measured. While radioligand binding assays are a more direct measure of receptor functionality, gene expression has been shown to correlate with binding and downstream β‐AR signaling.36, 37, 38 At baseline, sex differences in expression of ADRB1 and ADRB2 were observed, with male CFs expressing higher levels of both ADRB1 and ADRB2 compared with female CFs (Figure 6A). This result suggested to us that males may be more responsive to ISO treatment than females, and we found that after 3 days of ISO treatment, male CFs expressed significantly more ADRB1 and ADRB2 than female CFs (Figure 6B). Moreover, only in male CFs was ADRB2 expression upregulated with ISO treatment compared with vehicle, whereas female CFs downregulated expression with ISO treatment compared with vehicle. Similarly, with 7 days of ISO treatment, male CFs from ISO‐treated rats expressed more ADRB1 and ADRB2 than female CFs (Figure 6C). Male CFs from ISO‐treated rats also downregulated ADRB1 compared with vehicle‐treated CFs, whereas females did not. Collectively, these results highlight the role of sex in the regulation of β‐AR signaling in response to stimulation in CFs.
Figure 6. Male CFs express more adrenergic receptor β1 and β2 at baseline and with ISO treatment than females.
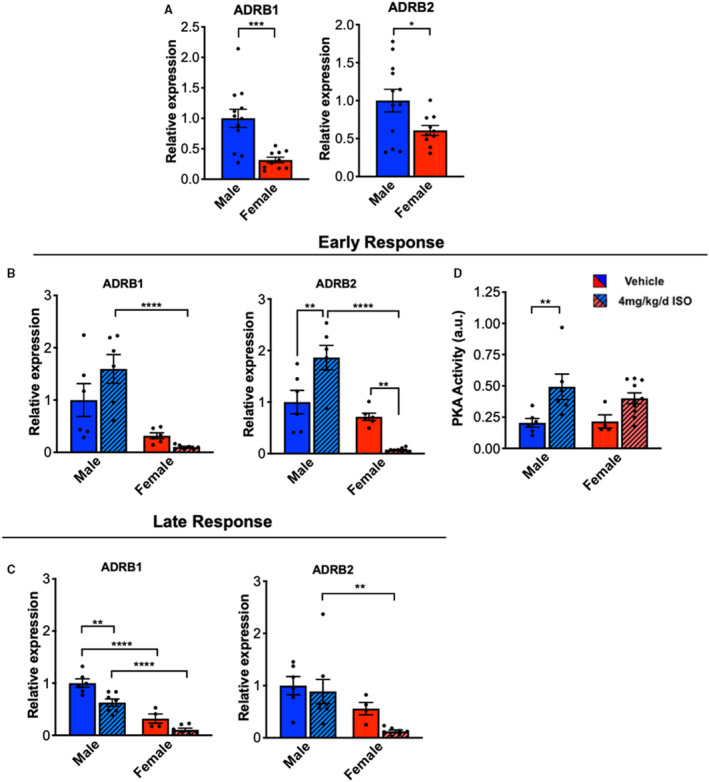
(A) mRNA expression levels of adrenergic receptor β1 (ADRB1) and adrenergic receptor β2 (ADRB2) in male and female CFs from vehicle‐treated rats. ADRB1 and ADRB2 gene expression (normalized to GAPDH) was measured by RT‐qPCR. Male n=12, Female n=10. Unpaired Student’s t test applied. (B & C) mRNA expression levels of ADRB1 and ADRB2 in male and female CFs from vehicle and ISO‐treated rats at early (B) or late (C) time points. ADRB1 and ADRB2 gene expression (normalized to GAPDH) was measured by RT‐qPCR. ADRB1 Early: Male vehicle n=6, Male ISO n=6, Female vehicle n=6, Female ISO n=9. Two statistical outliers were detected but were not removed as this did not affect statistical significance. ADRB2 Early: Male vehicle n=6, Male ISO n=6, Female vehicle n=6, Female ISO n=9. ADRB1 Late: Male vehicle n=6, Male ISO n=7, Female vehicle n=4, Female ISO n=7. A statistical outlier was removed in the ADRB1 late data set because it was far outside of expected values and we suspect due to error (Male ISO: 3.326). If the outlier is not removed, the results indicate that male ISO and male vehicle are significantly different. ADRB2 Late: Male vehicle n=6, Male ISO n=8, Female vehicle n=4, Female ISO n=7. Two‐way ANOVA with Bonferroni post hoc applied. (D) PKA activity in CFs from vehicle or ISO‐treated male and female rats after 3 days of treatment. Male vehicle n=6, Male ISO n=6, Female vehicle n=4, Female ISO n=10. Two‐way ANOVA with Bonferroni post hoc applied. **P<0.01, ****P<0.0001. All data reported as ± SEM. CF indicates cardiac fibroblasts; ISO, isoproterenol; PKA, protein kinase A; and RT‐qPCR, reverse transcriptase–polymerase chain reaction.
β‐AR signaling results in activation of PKA in cardiac myofibroblasts and plays a substantial role in ISO‐induced cardiac hypertrophy.20 To determine if PKA activity is sexually dimorphic in CFs in response to ISO treatment, PKA activity was measured in isolated CFs from ISO‐ or vehicle‐treated rats. ISO‐treated male CFs had significantly higher PKA activity compared with vehicle‐treated male CFs (Figure 6D). Conversely, PKA activity in female ISO‐ and vehicle‐treated CFs was not significantly different. These results suggest that the elevated profibrotic genetic programming observed in male CFs could be a result of elevated PKA activity.
Discussion
Cardiovascular disease is the largest cause of mortality worldwide and biological sex is an important risk factor.39, 40 Many cardiovascular diseases involve elevated β‐adrenergic signaling by catecholamines that lead to HF and are associated with pathological cardiac hypertrophy and myocardial fibrosis, but the influence of sex is not well understood and is understudied.41, 42 Here, we investigated sex differences in the fibrotic response of rats upon ISO treatment (Figure 7). We found that male rats experienced increased cardiac hypertrophy, fibrosis, and death in response to ISO treatment compared with female animals. In addition, when the response of CFs, the primary cellular mediators of fibrosis, was measured, we found that male CFs were less proliferative but more activated than their female counterparts. A recent report suggested that in response to ISO treatment, CFs may secrete factors that promote cardiac myocyte hypertrophy by activation of PKA.20 Interestingly, our data suggest that differential expression and regulation of β‐ARs and activation of PKA in male and female CFs might be responsible for this sexually dimorphic fibrotic response. These results point to the fact that CFs modulate their response to ISO differently in males and females, and this may contribute to the higher survival in younger women with cardiovascular disease compared with men.
Figure 7. Summary of study results and implications.
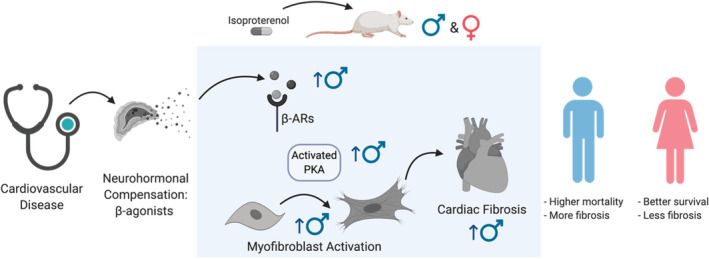
Here, ISO was used as a mimic for chronic stimulation of β‐adrenergic receptors (β‐ARs) in male and female rats. Results indicate a cellular sexually dimorphic response of cardiac fibroblasts to ISO. Male cardiac fibroblasts express more β‐ARs than females, leading to increased activation of PKA, and positively correlates with increased myofibroblast activation. We posit that increased myofibroblast activation of male cardiac fibroblasts from ISO treatment leads to increased collagen deposition and fibrosis in male hearts, while females are protected. Cardiac fibrosis negatively correlates with survival in patients with cardiovascular disease. ISO indicates isoproterenol; and PKA, protein kinase A.
Sex differences in β‐adrenergic responsiveness in the heart have been associated with cardio‐protection in females. For example, cardiomyocytes respond in a sexually dimorphic manner in response to ISO treatment. Cardiomyocytes from male hearts show enhanced contractile responsiveness to β‐adrenergic stimulation compared with female hearts,9, 43, 44 which can lead to maladaptive cardiac hypertrophy. Besides hypertrophy, treatment with ISO also causes interstitial fibrosis. Interestingly, women show less inflammation, fibrosis, and apoptosis in HF compared with men.45 Similarly, results herein show that ISO treatment causes less fibrosis in female rats compared with male rats, suggesting rats recapitulate some of the findings in humans. Others have observed a similar response in mice, where males, but not females, develop significant fibrosis in response to ISO treatment.10 It remains unclear why this sexually dimorphic fibrotic response to β‐adrenergic stimulation exists, and whether it may be a result of differential response by CFs. Excessive β‐adrenergic stimulation in the heart has been linked to increased CF proliferation and collagen synthesis;18, 46, 47 however, this current study is the first to investigate sex as a modifier of these phenotypes.
Sex hormones, in particular estrogen, have been investigated as a potential mediator of sexually dimorphic responses to cardiac injury. Reports show that premenopausal women have a lower cardiovascular risk compared with age‐matched men, whereas postmenopausal women do not retain this protection.48 Moreover, cardiac pathologies are exacerbated in OVX animals in experimental studies. For instance, infarct size is significantly larger during ischemic insult in OVX animals compared with either sham or OVX animals treated with estrogen.49 The data presented here show that removal of sex hormone signaling via gonadectomy eliminated the sex differences in survival in response to ISO treatment, suggesting that estrogen likely plays a protective role in the survival of animals with ISO treatment. However, removal of testosterone did not improve survival of the males. Interestingly, our data show that gonadectomy does not ameliorate the differences in the fibrotic response in male and female hearts to ISO, which may indicate that the sexually dimorphic fibrotic response does not depend on estrogen or androgen signaling. Although estrogen treatment has been shown to influence CF proliferation,50 collagen expression,51 and matrix metalloprotease expression,52 estrogen does not appear to affect myofibroblast activation.52 Taken together, these data suggest that although sex hormones play a role in the immediate survival response to β‐adrenergic stimulation, they do not appear to influence the resulting fibrotic response. These findings may explain, in part, why even after menopause, women still retain protection to developing cardiac fibrosis compared with men.53, 54 Interestingly, sex differences in cellular phenotypes may reflect intrinsic differences between male and female cells driven by chromosomes.21 For example, proteomic analysis of endothelial cells revealed enrichment of X‐chromosome encoded proteins in female cells compared with male cells after wounding, which suggests that cellular sexual dimorphisms could be a result of expression of genes that escape X‐chromosome inactivation.55 Perhaps the sex differences in CF response to ISO are driven by X‐ or Y‐linked genes.
CFs promote wound healing after cardiac injury (eg, as caused by chronic β‐adrenergic stimulation) by proliferating and activating to a myofibroblast phenotype.56 It is becoming increasingly evident that these 2 common outputs, activation and proliferation, are not always interconnected responses that contribute to fibrosis. For instance, a recent study analyzed CFs from several genetic mouse lines treated with ISO and found that each mouse line demonstrated varying levels of fibrosis, and when CFs were isolated, they demonstrated different levels of myofibroblast activation.15 Interestingly, these CFs isolated from ISO‐treated mice all showed similar levels of proliferation regardless of the genetic background. This finding suggests that myofibroblast activation, rather than proliferation, correlates with the severity of cardiac fibrosis whereas proliferation may be less correlated. Indeed, we show that male rats treated with ISO display increased markers of myofibroblast activation that correlates with increased cardiac fibrosis, whereas conversely, ISO‐treated females showed increased levels of proliferation but no cardiac fibrosis. This sex difference in the CF activation versus proliferation response could be potentiating the differences in cardiac fibrosis and perhaps indicates differential intracellular signaling between the sexes. Further investigations are needed to decipher the interconnection between fibrosis severity, myofibroblast activation, and proliferation.
β‐ARs mediate the response to ISO in the heart. Although the mammalian heart generally expresses predominantly β1‐AR (75%–85%), a substantial amount of β2‐AR is detected as well.57 CFs, on the other hand, express mostly β2‐AR.58 In vitro stimulation of these receptors by agonists promotes fibroblast proliferation,18, 59 collagen synthesis,16 and autophagy.46 Although reports are controversial, β2‐AR appears to be the functional receptor controlling the agonist‐induced fibroblast response.17, 46, 60, 61 Here, we demonstrate that male CFs express more ADRB2 than females, which suggests that male CFs are more sensitive to ISO treatment than females. Of note, sex differences have been observed in β‐AR expression and function in male and female blood vessels.62 Sex differences in drug metabolism often lead to different drug efficacy in men and women,63 which implies that higher doses of ISO could be used to achieve the same levels of hypertrophy and fibrosis in females as compared with males. In designing these experiments, we were very reluctant to give different doses to male and female rats. We made the decision to use the maximum level in males and females that did not result in 100% lethality in males. The goal of the experiment was to determine the difference in response with the same stimuli, not to try to replicate the same injury. Pharmacokinetic studies also suggest that β1‐AR binding affinity is not different between males and females,64 so the differential effects of ISO on males and females likely is caused by other factors, like receptor expression levels. Moreover, it is well accepted that in response to chronic β‐AR stimulation, the heart downregulates β‐AR expression in an attempt to limit detrimental effects.65 Interestingly, in response to ISO treatment, males increase their expression of ADRB2 whereas females decrease their expression, which may explain why male CFs have a more exaggerated myofibroblast response to ISO than females. Perhaps females are capable of reducing the impact of ISO on the CFs by downregulating expression of the key receptors for its effect, and consequentially show reduced myofibroblast activation and subsequent fibrosis. These findings support clinical findings that suggest that women are generally less sensitive to β‐receptor agonists66 and less predisposed to fibrosis after cardiac injury.67
Besides affecting collagen deposition and fibrosis, CFs can influence cardiac hypertrophy.68 The fibroblast secretome can be amplified by cardiomyocyte‐fibroblast crosstalk.68 Interestingly, ISO can further influence this crosstalk, in which β2‐AR stimulation via ISO activates PKA signaling in CFs, which influences secretion of prohypertrophic factors by CFs that can ultimately induce cardiac hypertrophy by signaling to cardiomyocytes.20 A recent study from our group showed that activated myofibroblasts can secrete insulinlike growth factor 1 to promote cardiomyocyte hypertrophy.69 Here, we show that CFs from male rats increase PKA activation in response to ISO treatment, whereas CFs from females do not, which suggests that ISO treatment in male rats may results in cardiac hypertrophy, in part, through increased PKA signaling in CFs.
Conclusions
In conclusion, this research reveals sex differences in the establishment of cardiac fibrosis owing to chronic β‐adrenergic stimulation. Upon ISO treatment, male rats are more susceptible to cardiac fibrosis compared with female rats, likely because of the higher activation of male CFs to myofibroblasts. Our data also indicate that sex hormones do not influence this sexually dimorphic difference in cardiac fibrosis. Ultimately, our findings highlight that CFs play a major role in mediating the sexually dimorphic response to cardiac injury with β‐adrenergic stimulation.
Sources of Funding
CJW was funded by the NIH Ruth L. Kirschstein National Research Service Award (NRSA) Individual Predoctoral Fellowship (NIH FHL142223). TLC was funded by The American Heart Association Predoctoral Fellowship (17PRE33661129) and the NIH T32 Biophysics Training grant (T32GM065103). CT was funded by the American Heart Association (AHA 14PRE20380468). KRL was funded by Beckman Scholars Award from the Arnold and Mabel Beckman Foundation and CU Boulder’s Biological Science Initiative (BSI). NIH R01 grants (NIH R01 HL132353 for KSA and NIH R01 GM29090 for LAL).
Disclosures
None.
Supporting information
Data S1
Tables S1–S2
Figures S1–S4
Acknowledgments
Some imaging work was performed at the BioFrontiers Institute Advanced Light Microscopy Core. Spinning disc confocal microscopy was performed on Nikon Ti‐E microscope supported by the BioFrontiers Institute and the Howard Hughes Medical Institute. Widefield microscopy was performed on a Nikon Te‐2000 microscope supported by NIH grant R01CA107098S1.
(J Am Heart Assoc. 2021;10:e06256. DOI: 10.1161/JAHA.120.018876.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018876
For Sources of Funding and Disclosures, see page 14.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. DOI: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. Physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol. 2009;54:1747–1762. DOI: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ Res. 2013;113:739–753. DOI: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lucia C, Eguchi A, Koch WJ. New insights in cardiac β‐Adrenergic signaling during heart failure and aging. Front. Pharmacol. 2018;9:904. DOI: 10.3389/fphar.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodde OE, Bruck H, Leineweber K. Cardiac adrenoceptors: Physiological and pathophysiological relevance. J. Pharmacol. Sci. 2006;100:323–337. DOI: 10.1254/jphs.crj06001x. [DOI] [PubMed] [Google Scholar]
- 6.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of β‐adrenergic signaling in heart failure? Circ. Res. 2003;93:896–906. DOI: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh VJ, Chandrasekera PC, Lasley RD. Sex differences and the effects of ovariectomy on the β‐adrenergic contractile response. Am J Physiol Circ Physiol. 2011;301:H1127–H1134. DOI: 10.1152/ajpheart.00711.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeker GS, Hood AR, Katra RP, Poelzing S, Pogwizd SM. Sex differences in β‐adrenergic responsiveness of action potentials and intracellular calcium handling in isolated rabbit hearts. PLoS One. 2014;9. DOI: 10.1371/journal.pone.0111411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT, Schwertz DW. Mechanisms of sex differences in rat cardiac myocyte response to β‐adrenergic stimulation. Am J Physiol ‐ Hear Circ Physiol. 2002;282. DOI: 10.1152/ajpheart.2002.282.1.H256. [DOI] [PubMed] [Google Scholar]
- 10.Zhu B, Liu K, Yang C, Qiao Y, Li Z. Gender‐related differences in β‐adrenergic receptor‐mediated cardiac remodeling. Can J Physiol Pharmacol. 2016;94:1349–1355. DOI: 10.1139/cjpp-2016-0103. [DOI] [PubMed] [Google Scholar]
- 11.Medzikovic L, Aryan L, Eghbali M. Connecting sex differences, estrogen signaling, and microRNAs in cardiac fibrosis. J. Mol. Med. 2019;97:1385–1398. [DOI] [PubMed] [Google Scholar]
- 12.Samuel JL, Delcayre C. Cardiac fibrosis. Bull Acad Natl Med. 2017;201:775–784. [Google Scholar]
- 13.Wu X, Li M, Chen SQ, Li S, Guo F. Pin1 facilitates isoproterenol‐induced cardiac fibrosis and collagen deposition by promoting oxidative stress and activating the MEK1/2‐ERK1/2 signal transduction pathway in rats. Int J Mol Med. 2018;41:1573–1583. DOI: 10.3892/ijmm.2017.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res. 2016;365(3):1–19. DOI: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Ranjbarvaziri S, Lay FD, Zhao P, Miller MJ, Dhaliwal JS, Huertas‐Vazquez A, Wu X, Qiao R, Soffer JM, et al. Genetic regulation of fibroblast activation and proliferation in cardiac fibrosis. Circulation. 2018;138:1224–1235. DOI: 10.1161/CIRCULATIONAHA.118.035420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuamnaichati N, Sato VH, Moongkarndi P, Parichatikanond W, Mangmool S. Sustained β‐AR stimulation induces synthesis and secretion of growth factors in cardiac myocytes that affect on cardiac fibroblast activation. Life Sci. 2018;193:257–269. DOI: 10.1016/j.lfs.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Turner NA, Porter KE, Smith WHT, White HL, Ball SG, Balmforth AJ. Chronic β2‐adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res. 2003;57:784–792. DOI: 10.1016/S0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 18.Sun M, Yu H, Zhang Y, Li Z, Gao W. MicroRNA‐214 mediates isoproterenol‐induced proliferation and collagen synthesis in cardiac fibroblasts. Sci Rep. 2015;5:1–10. DOI: 10.1038/srep18351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamin IJ, Jalil JE, Tan LB, Cho K, Weber KT, Clark WA. Isoproterenol‐induced myocardial fibrosis in relation to myocyte necrosis. Circ Res. 1989;65:657–670. DOI: 10.1161/01.RES.65.3.657. [DOI] [PubMed] [Google Scholar]
- 20.Imaeda A, Tanaka S, Tonegawa K, Fuchigami S, Obana M, Maeda M, Kihara M, Kiyonari H, Conway SJ, Fujio Y, et al. Myofibroblast β2 adrenergic signaling amplifies cardiac hypertrophy in mice. Biochem Biophys Res Commun. 2019;510:149–155. DOI: 10.1016/j.bbrc.2019.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snell DM, Turner JMA. Sex chromosome effects on male‐female differences in mammals. Curr. Biol. 2018;28:R1313–R1324. DOI: 10.1016/j.cub.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadel S, Kovats S. Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Front. Immunol. 2018;91653: 10.3389/fimmu.2018.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold AP. A general theory of sexual differentiation. J. Neurosci. Res. 2017;95:291–300. DOI: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman RJG, Kapteijn DMC, Haitjema S, Bekker MN, Mokry M, Pasterkamp G, Civelek M, den Ruijter HM. Intrinsic transcriptomic sex differences in human endothelial cells at birth and in adults are associated with coronary artery disease targets. Sci Rep. 2020;10: DOI: 10.1038/s41598-020-69451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Addis R, Campesi I, Fois M, Capobianco G, Dessole S, Fenu G, Montella A, Cattaneo MG, Vicentini LM, Franconi F. Human umbilical endothelial cells (HUVECs) have a sex: characterisation of the phenotype of male and female cells. Biol Sex Differ. 2014;5: DOI: 10.1186/s13293-014-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz M, Blaschke B, Benn A, Hammer E, Witt E, Kirwan J, Fritsche‐Guenther R, Gloaguen Y, Bartsch C, Vietzke A, et al. Sex‐specific metabolic and functional differences in human umbilical vein endothelial cells from twin pairs. Atherosclerosis. 2019;291:99–106. DOI: 10.1016/j.atherosclerosis.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Balaji S, Dong X, Li H, Zhang Y, Steen E, Lingappan K. Sex‐specific differences in primary neonatal murine lung fibroblasts exposed to hyperoxia in vitro: Implications for bronchopulmonary dysplasia. Physiol Genomics. 2018;50:940–946. DOI: 10.1152/physiolgenomics.00075.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguado BA, Schuetze KB, Grim JC, Walker CJ, Cox AC, Ceccato TL, Tan A‐C, Sucharov CC, Leinwand LA, Taylor MRG, et al. Transcatheter aortic valve replacements alter circulating serum factors to mediate myofibroblast deactivation. Sci Transl Med. 2019;11. DOI: 10.1126/scitranslmed.aav3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin M, Lee HW, Zhao L, Eghblai‐Webb M. Gender‐related differences in proliferative response of cardiac fibroblasts to hypoxia: Effects of estrogen. Mol Cell Biochem. 2000;215:21–30. DOI: 10.1023/a:1026585420021. [DOI] [PubMed] [Google Scholar]
- 30.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl. Acad Sci USA. 2008;105:2415–2420. DOI: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta. 2013;1832:884–890. DOI: 10.1016/j.bbadis.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang S, Liu Y, Sun D, Zhou C, Liu A, Xu C, Hao Y, Li D, Yan C, Sun H. Chronic activation of the G protein‐coupled receptor 30 with Agonist G‐1 attenuates heart failure. PLoS One. 2012;7(10):e48185. DOI: 10.1371/journal.pone.0048185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhuiyan MS, Shioda N, Shibuya M, Iwabuchi Y, Fukunaga K. Activation of endothelial nitric oxide synthase by a vanadium compound ameliorates pressure overload‐induced cardiac injury in ovariectomized rats. Hypertension. 2009;53:57–63. DOI: 10.1161/HYPERTENSIONAHA.108.118356. [DOI] [PubMed] [Google Scholar]
- 34.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–379. DOI: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv T, Du Y, Cao N, Zhang S, Gong Y, Bai Y, Wang W, Liu H. Proliferation in cardiac fibroblasts induced by β1‐adrenoceptor autoantibody and the underlying mechanisms. Sci Rep. 2016;6:32430. DOI: 10.1038/srep32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myagmar B‐E, Flynn JM, Cowley PM, Swigart PM, Montgomery MD, Thai K, Nair D, Gupta R, Deng DX, Hosoda C, et al. Adrenergic receptors in individual ventricular myocytes: the beta‐1 and alpha‐1B are in all cells, the alpha‐1A is in a subpopulation, and the beta‐2 and beta‐3 are mostly absent. Circ Res. 2017;120:1103–1115. DOI: 10.1161/CIRCRESAHA.117.310520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al‐Gburi S, Deussen A, Zatschler B, Weber S, Künzel S, El‐Armouche A, Lorenz K, Cybularz M, Morawietz H, Kopaliani I. Sex‐difference in expression and function of beta‐adrenoceptors in macrovessels: role of the endothelium. Basic Res Cardiol. 2017;112: DOI: 10.1007/s00395-017-0617-2. [DOI] [PubMed] [Google Scholar]
- 38.Dang S, Zhang Z‐Y, Li K‐L, Zheng J, Qian L‐L, Liu X‐Y, Wu Y, Zhang C‐Y, Zhao X‐X, Yu Z‐M, et al. Blockade of β‐adrenergic signaling suppresses inflammasome and alleviates cardiac fibrosis. Ann Transl Med. 2020;8:127. DOI: 10.21037/atm.2020.02.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosca L, Barrett‐Connor E, Kass WN. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. DOI: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regitz‐Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97:1–37. DOI: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 41.Lindenfeld JoAnn, Cleveland JC, Kao DP, White M, Wichman S, Bristow JC, Peterson V, Rodegheri‐Brito J, Korst A, Blain‐Nelson P, et al. Sex‐related differences in age‐associated downregulation of human ventricular myocardial β1‐adrenergic receptors. J Hear Lung Transplant. 2016;35:352–361. DOI: 10.1016/j.healun.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Madamanchi A. β‐adrenergic receptor signaling in cardiac function and heart failure. McGill J. Med. 2007;10:99–104. DOI: 10.26443/mjm.v10i2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curl CL, Wendt IR, Kotsanas G. Effects of gender on intracellular [Ca2+] in rat cardiac myocytes. Pflugers Arch Eur J Physiol. 2001;441:709–716. DOI: 10.1007/s004240000473. [DOI] [PubMed] [Google Scholar]
- 44.Schwertz DW, Vizgirda V, Solaro RJ, Piano MR, Ryjewski C. Sexual dimorphism in rat left atrial function and response to adrenergic stimulation. Mol Cell Biochem. 1999;200:143–153. DOI: 10.1023/a:1007011807383. [DOI] [PubMed] [Google Scholar]
- 45.Kessler EL, Rivaud MR, Vos MA, Van Veen TAB. Sex‐specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol. Sex Differ. 2019;10:7. DOI: 10.1186/s13293-019-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aránguiz‐Urroz P, Canales J, Copaja M, Troncoso R, Vicencio JM, Carrillo C, Lara H, Lavandero S, Díaz‐Araya G. Beta2‐adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim Biophys Acta. 2011;1812:23–31. DOI: 10.1016/j.bbadis.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Colombo F, Gosselin H, El‐Helou V, Calderone A. β‐Adrenergic receptor‐mediated DNA synthesis in neonatal rat cardiac fibroblasts proceeds via a phosphatidylinositol 3‐kinase dependent pathway refractory to the antiproliferative action of cyclic AMP. J Cell Physiol. 2003;195:322–330. DOI: 10.1002/jcp.10251. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, De Ferranti S, Després JP, Fullerton HJ, et al. Executive summary: Heart disease and stroke statistics‐2016 update: A Report from the American Heart Association. Circulation. 2016;133:447–454. DOI: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 49.Kam KWL, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of β 1‐Adrenoceptor upon ischemic insult in the rat heart. J Pharmacol Exp Ther. 2004;309:8–15. DOI: 10.1124/jpet.103.058339. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe T, Akishita M, He H, Miyahara Y, Nagano K, Nakaoka T, Yamashita N, Kozaki K, Ouchi Y. 17β‐estradiol inhibits cardiac fibroblast growth through both subtypes of estrogen receptor. Biochem Biophys Res Commun. 2003;311:454–459. DOI: 10.1016/j.bbrc.2003.09.232. [DOI] [PubMed] [Google Scholar]
- 51.Dworatzek E, Mahmoodzadeh S, Schriever C, Kusumoto K, Kramer L, Santos G, Fliegner D, Leung Y‐K, Ho S‐M, Zimmermann W‐H, et al. Sex‐specific regulation of collagen i and III expression by 17β‐Estradiol in cardiac fibroblasts: Role of estrogen receptors. Cardiovasc Res. 2019;115:315–327. DOI: 10.1093/cvr/cvy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz‐Zagrosek V. 17b‐Estradiol inhibits matrix metalloproteinase‐2 transcription via MAP kinase in fibroblasts. Cardiovasc Res. 2010;85:719–728. DOI: 10.1093/cvr/cvp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Treibel TA, Kozor R, Fontana M, Torlasco C, Reant P, Badiani S, Espinoza M, Yap J, Diez J, Hughes AD, et al. Sex dimorphism in the myocardial response to aortic stenosis. JACC Cardiovasc Imaging. 2018;11:962–973. DOI: 10.1016/j.jcmg.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ambale Venkatesh B, Volpe GJ, Donekal S, Mewton N, Liu C‐Y, Shea S, Liu K, Burke G, Wu C, Bluemke DA, et al. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: The Multi‐Ethnic study of Atherosclerosis study. Hypertension. 2014;64:508–515. DOI: 10.1161/HYPERTENSIONAHA.114.03697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witt E, Lorenz M, Völker U, Stangl K, Hammer E, Stangl V. Sex‐specific differences in the intracellular proteome of human endothelial cells from dizygotic twins. J Proteomics. 2019;201:48–56. DOI: 10.1016/j.jprot.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Tallquist MD, Molkentin JD. Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 2017;14:484–491. DOI: 10.1038/nrcardio.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. β1‐ and β2‐adrenergic‐receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective β1‐receptor down‐regulation in heart failure. Circ Res. 1986;59:297–309. DOI: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 58.Meszaros JG, Gonzalez AM, Endo‐Mochizuki Y, Villegas S, Villarreal F, Brunton LL. Identification of G protein‐coupled signaling pathways in cardiac fibroblasts: Cross talk between G(q) and G(s). Am J Physiol ‐ Cell Physiol. 2000;278:C154–C162. DOI: 10.1152/ajpcell.2000.278.1.C154. [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Eckhart AD, Eguchi S, Koch WJ. β‐adrenergic receptor‐mediated DNA synthesis in cardiac fibroblasts is dependent on transactivation of the epidermal growth factor receptor and subsequent activation of extracellular signal‐regulated kinases. J Biol Chem. 2002;277:32116–32123. DOI: 10.1074/jbc.M204895200. [DOI] [PubMed] [Google Scholar]
- 60.Lv T, Du Y, Cao N, Zhang S, Gong Y, Bai Y, Wang W, Liu H. Proliferation in cardiac fibroblasts induced by β1‐adrenoceptor autoantibody and the underlying mechanisms. Sci Rep. 2016;6:1–15. DOI: 10.1038/srep32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pullar CE, Isseroff RR. The β2‐adrenergic receptor activates pro‐migratory and pro‐proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci. 2006;119:592–602. DOI: 10.1242/jcs.02772. [DOI] [PubMed] [Google Scholar]
- 62.Al‐Gburi S, Deussen A, Zatschler B, Weber S, Künzel S, El‐Armouche A, Lorenz K, Cybularz M, Morawietz H, Kopaliani I. Sex‐difference in expression and function of beta‐adrenoceptors in macrovessels: role of the endothelium. Basic Res Cardiol. 2017;112:1–16. DOI: 10.1007/s00395-017-0617-2. [DOI] [PubMed] [Google Scholar]
- 63.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009;48:143–157. DOI: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dent MR, Tappia PS, Dhalla NS. Gender differences in β‐adrenoceptor system in cardiac hypertrophy due to arteriovenous fistula. J Cell Physiol. 2011;226:181–186. DOI: 10.1002/jcp.22321. [DOI] [PubMed] [Google Scholar]
- 65.Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of β‐Adrenergic Receptor Desensitization and Resensitization. Adv Pharmacol. 1997;42:416–420. [DOI] [PubMed] [Google Scholar]
- 66.Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski JC, Ceconi C, Drexel H, Kjeldsen K, Savarese G, et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3:163–182. DOI: 10.1093/ehjcvp/pvw042. [DOI] [PubMed] [Google Scholar]
- 67.Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, Knosalla C, Golz S, Hetzer R, Regitz‐Zagrosek V. Sex‐dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014;16:1160–1167. DOI: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 68.Fujiu K, Nagai R. Fibroblast‐mediated pathways in cardiac hypertrophy. J. Mol. Cell. Cardiol. 2014;70:64–73. DOI: 10.1016/j.yjmcc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Ceccato TL, Starbuck RB, Hall JK, Walker CJ, Brown TE, Killgore JP, Anseth KS, Leinwand LA. Defining the cardiac fibroblast secretome in a fibrotic microenvironment. J Am Heart Assoc. 2020;9(19): DOI: 10.1161/JAHA.120.017025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2
Figures S1–S4


