Abstract
Background
The CAVA (Ultrasound‐Accelerated Catheter‐Directed Thrombolysis Versus Anticoagulation for the Prevention of Post‐Thrombotic Syndrome) trial did not show a reduction of post‐thrombotic syndrome (PTS) after additional ultrasound‐accelerated catheter‐directed thrombolysis in patients with acute iliofemoral deep vein thrombosis at 1‐year follow‐up. This prespecified analysis of the CAVA trial aimed to determine the impact of additional thrombolysis on outcomes of PTS at long‐term follow‐up.
Methods and Results
Patients aged 18 to 85 years with a first‐time acute iliofemoral deep vein thrombosis were included and randomly assigned (1:1) to either standard treatment plus ultrasound‐accelerated catheter‐directed thrombolysis or standard treatment alone. The primary outcome was the proportion of PTS (Villalta score ≥5 on 2 occasions ≥3 months apart or venous ulceration) at the final follow‐up visit. Additionally, PTS according to the International Society on Thrombosis and Haemostasis (ISTH) consensus definition was assessed to allow external comparability. Major bleedings were the main safety outcome. At a median follow‐up of 39.0 months (interquartile range, 23.3–63.8), 120 patients (79.8%) participated in the final follow‐up visit: 62 from the intervention group and 58 from the standard treatment group. PTS developed in 19 (30.6%) versus 26 (44.8%) patients, respectively (odds ratio [OR], 0.54; 95% CI, 0.26 to 1.15 [P=0.11]), with an absolute difference between groups of −14.2% (95% CI, −32.0% to 4.8%). Using the ISTH consensus definition, a significant reduction in PTS was observed (29 [46.8%] versus 40 [69.0%]) (OR, 0.40; 95% CI, 0.19–0.84 [P=0.01]) with an absolute difference between groups of −22.2% (95% CI, −39.8% to −2.8%). No new major bleedings occurred following the 12‐month follow‐up.
Conclusions
The impact of additional ultrasound‐accelerated catheter‐directed thrombolysis on the prevention of PTS was found to increase with time. Although this study was limited by its sample size, the overall findings indicate a reduction of mild PTS without impact on quality of life.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00970619.
Keywords: catheter‐directed thrombolysis, Iliofemoral deep vein thrombosis, long‐term follow‐up, post‐thrombotic syndrome, quality of life
Nonstandard Abbreviations and Acronyms
- ATTRACT
Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter‐Directed Thrombolysis
- CAVA
Ultrasound‐Accelerated Catheter‐Directed Thrombolysis Versus Anticoagulation for the Prevention of Post‐Thrombotic Syndrome
- CaVenT
Catheter‐Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis
- EQ5D
EuroQOL‐5D
- ISTH
International Society of Thrombosis and Haemostasis
- PDI
Pain Disability Index
- PTS
post‐thrombotic syndrome
- SF‐36
36‐Item Short Form Health Survey
- VCSS
venous clinical severity score
- VEINES‐QoL/Sym
Venous Insufficiency Epidemiological and Economic Study Quality of Life/Symptoms
Clinical Perspective
What Is New?
This prespecified analysis of the CAVA (Ultrasound‐Accelerated Catheter‐Directed Thrombolysis Versus Anticoagulation for the Prevention of Post‐Thrombotic Syndrome) trial shows that the impact of additional catheter‐directed thrombolysis on the development of post‐thrombotic syndrome increases with time.
Findings indicate a long‐term effect of catheter‐directed thrombolysis on the reduction of mild post‐thrombotic syndrome without associated gain in quality of life.
What Are the Clinical Implications?
Longer clinical follow‐up should be included in future reperfusion studies as the late onset of (mild) post‐thrombotic syndrome might be associated with other factors besides initial patency augmentation.
Post‐thrombotic syndrome (PTS) is a complication of deep vein thrombosis (DVT) occurring in 40% to 60% of affected patients1, 2 when treated according to current guidelines.3, 4 The clinical presentation includes pain, swelling, heaviness, cramps, paresthesia, pruritus, edema, hyperpigmentation of the skin, venous ectasia, and the most serious feature venous ulceration of the post‐thrombotic leg. While it usually occurs within the first year following the acute thrombotic event,1, 5, 6, 7 PTS can also develop many years thereafter.2, 8 It has serious negative implications for the quality of life9 and contributes to rising healthcare costs.10 In the absence of curative treatment options for PTS, emphasis lies on its prevention.
The potential of catheter‐directed thrombolysis as an additional treatment modality to prevent PTS development has been assessed in 3 randomized controlled trials. The CaVenT (Catheter‐Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis) trial showed a significant preventive effect with an absolute risk reduction of 14.4% (95% CI, 0.2% to 27.9%) in the occurrence of PTS after 2 years, increasing to 28% (95% CI, 14% to 42%) after 5 years of follow‐up. However, the reduction in the occurrence of PTS did not result in a better quality of life.8, 11 Interestingly, the ATTRACT (Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter‐Directed Thrombolysis) trial did not confirm the positive effect of catheter‐directed thrombolysis on the prevention of PTS after 2 years of follow‐up. Although a significantly lower symptom severity was seen, this had no impact on quality of life.12 The limiting effect of additional catheter‐directed thrombolysis on the severity of symptoms was shown in a subanalysis of the ATTRACT trial including only patients with iliofemoral DVT known to have a higher risk of developing PTS.1, 5, 13 Also, an improvement in disease‐specific quality of life was observed in this subgroup.14
Most recently, the CAVA (Ultrasound‐Accelerated Catheter‐Directed Thrombolysis Versus Anticoagulation for the Prevention of Post‐Thrombotic Syndrome) trial, which included only patients with iliofemoral DVT, showed neither a significant preventive effect by the addition of catheter‐directed thrombolysis to standard care on the development of PTS at 1‐year follow‐up, nor a positive impact on quality of life.15 However, a post hoc subanalysis of the CAVA trial data showed that if recanalization was considered successful (ie, an accomplished patency of ≥90% with adequate inflow and outflow in all affected vein segments as established on venous angiogram at the end of the interventional treatment), this was associated with a significantly reduced symptom severity as well as reduced time to regained quality of life.16 However, no difference in the proportion of PTS at 1‐year follow‐up according to the International Society of Thrombosis and Haemostasis (ISTH) consensus definition (ie, a Villalta score of ≥5 or venous ulceration at the 6‐month assessment or later17) was observed.
Since PTS can develop many years after the acute event, this prespecified analysis of the CAVA trial was aimed to evaluate the long‐term effect of additional catheter‐directed thrombolysis on the development of PTS.
Methods
Study Design and Participants
The CAVA trial was a multicenter, randomized, single‐blind, allocation‐concealed, parallel‐group, superiority trial designed to assess the impact of additional ultrasound‐accelerated catheter‐directed thrombolysis compared with standard post‐thrombotic management on the development of PTS after acute iliofemoral DVT. The main outcomes and study protocol have been previously published.15 This prespecified analysis of the long‐term results is part of the protocol as approved by the review boards of all participating centers.
The study was performed in 15 hospitals in the Netherlands, of which 6 were ascertained as interventional centers and therefore responsible for performing all thrombolytic interventions. A full list of participating centers can be found in Data S1. Patients were eligible for participation if aged 18 to 85 years with an objectified first‐time iliofemoral DVT and a maximum symptom duration of 14 days. Increased bleeding risk or limited life expectancy were reasons for exclusion. Table S1 provides a full list of inclusion and exclusion criteria. All participants provided written informed consent before randomization.
Data Sharing
Request for access to the deidentified individual participant data underlying the reported results should be directed to the corresponding author at arina.tencate@maastrichtuniversity.nl.
Randomization and Masking
Patients were randomly assigned (1:1) to standard therapy or to standard therapy with additional ultrasound‐accelerated catheter‐directed thrombolysis (including eventual adjunctive procedures). A web‐based randomization program (TENALEA, ALEA version release 2.2) was used applying a random variable block size (2–12) and stratification for participating center and age (18–50 years, 51–70 years, and 71–85 years). Randomization was performed and communicated to the patient by the study coordinator at the Maastricht University Medical Centre. Patients were asked not to disclose treatment allocation to their treating physician or local study personnel during follow‐up visits. Data analysis was performed by the coordinating researchers who were blinded to treatment allocation.
Procedures
Standard treatment was applied to all included patients and consisted of anticoagulant therapy prescribed according to international guidelines,4 initiation of compression therapy within 24 hours after diagnosis, and early mobilization.
Additionally, patients allocated to the intervention group were admitted to 1 of the 6 interventional centers where thrombolysis had to be initiated no more than 21 days after onset of symptoms. Details on the procedure of ultrasound‐accelerated catheter‐directed thrombolysis have been previously reported.15 Following venography to confirm iliofemoral localization of the thrombus, the catheter of the Ekos Endowave System (Ekos Corporation) was inserted under local anaesthesia and ultrasound guidance and positioned at the level of the thrombosed vein segments. After placement of the catheter, a single bolus dose of 250,000 IU of urokinase diluted in 10 mL NaCl was administered in addition to the continuous infusion of 100,000 IU/h urokinase and 1000 IU/h heparin for the duration of the intervention. Furthermore, during the intervention, standard oral anticoagulant treatment was replaced by therapeutic doses of low‐molecular weight heparin. When thrombolysis was terminated, standard oral anticoagulant therapy was reinstalled 1 hour after removal of the sheath. Thrombolysis was terminated in case of a regained venous patency of ≥90%, 48 hours without improvement of patency as assessed with daily venography, a persisting deviance in coagulation status according to the 6 hourly laboratory tests (activated partial thromboplastin time >80 s, fibrinogen <8 mm in rotational thromboelastometry assay for the fibrin part of the clot, or plasma fibrinogen <1.8 g/L), or when the maximum duration of thrombolysis (96 hours) was exceeded. Adjunctive procedures (eg, thrombosuction, percutaneous transluminal angioplasty, dedicated venous stent placement, endophlebectomy, creating an arteriovenous fistula, or a combination of the former) were recommended in the presence of compression syndromes or a residual venous lumen reduction of ≥50% but performed at the discretion of the operator.
Regular follow‐up study visits were performed at the outpatient clinic at 3, 6, and 12 months after inclusion and annually thereafter if preferred by the patient. During the study’s long‐term follow‐up phase (ie, follow‐up assessments of more than 12 months performed after inclusion of the last patient) visits were clustered. The closing study visit included assessment of clinical scores, health‐related quality of life, and a standardized extensive duplex ultrasound. A detailed overview of the follow‐up schedule and performed assessments can be found in Table S2.
Outcomes
Outcomes of this prespecified analysis of long‐term results of the CAVA trial conform with the outcomes previously reported.15 The primary outcome was the proportion of patients with PTS during follow‐up later than 12 months assessed according to the original definition: the development of venous ulceration or a Villalta score ≥5 on 2 separate occasions at least 3 months apart with the first assessment at least 3 months after the acute event.18 In addition, the proportion of patients with PTS according to the ISTH consensus scoring method (venous ulceration or a Villalta score ≥5 after 6 months of follow‐up or later)17 was reported, as well as the severity of PTS assessed using both the Villalta scale (0–33: differentiating into none [<5], mild [5–9], moderate [10–14], or severe [≥15 or venous ulceration] PTS)17, 18 and the venous clinical severity score (VCSS) (0–30; with higher scores indicating more complaints).19 The occurrence of major bleedings20 was recorded as the main safety outcome. Other adverse events such as recurrent (nonstent) DVT, in‐stent thrombosis, pulmonary embolism, or death were secondary outcomes.
Another secondary outcome was health‐related quality of life assessed using the generic 36‐Item Short Form Health Survey (SF‐36, version 2),21 EuroQOL‐5D (EQ5D),22 and Pain Disability Index (PDI),23 as well as the disease‐specific VEINES‐QoL/Sym (Venous Insufficiency Epidemiological and Economic Study Quality of Life/Symptoms) questionnaire calculated using its original relative summary score24, 25 and the intrinsic score method.26 The present report addresses the results of the aforementioned predefined primary and secondary outcomes at follow‐up later than 12 months.
Statistical Analysis
Sample size calculation for the main CAVA trial was based on the assumption that the addition of catheter‐directed thrombolysis to standard treatment would result in a 17% absolute risk reduction in the proportion of PTS compared with standard treatment alone. At a 2‐sided significance level of 5% and a statistical power of 80%, with compensation for potential loss of patients during follow‐up, a total of 180 patients were to be included.15 The analysis included all randomized patients from the modified intention‐to‐treat population who were still available and agreed to partake in the study follow‐up closing visit. The primary analysis compared the proportion of patients with PTS at long‐term follow‐up using a univariate analysis of proportions (χ2) analysis. Subsequently, the associated odds ratios (ORs) and their corresponding 95% CIs were calculated using StatPages and Open Source Epidemiologic Statistics for Public Health (OpenEpi). Hazard ratios (HRs) and their corresponding 95% CIs were calculated using Cox proportional hazard models stratified for center and adjusted for age, sex, clinical presentation of the thrombotic event, and extent of the index thrombosis at duplex ultrasound using the lower extremity thrombosis classification.13, 27
Details on patient characteristics and risk factors, treatment characteristics, symptom severity, and adverse events were assessed using descriptive statistics and reported as appropriate. A mixed design ANOVA was performed to test for differences between groups and to assess changes over time (comparing scores at long‐term with scores at 12‐month follow‐up). In case of a significant difference, clinical relevance was determined using the validated minimal clinically important differences (for the EQ5D28 and VEINES‐QoL25) or as calculated according to Norman et al.29
A significance level of ≤0.05 (2‐sided) was considered significant. In case of multiple testing adjusted significance levels based on the Bonferroni correction were used. Analyses were performed using SPSS (version 25, IBM), StatPages, or Open Source Epidemiologic Statistics for Public Health (OpenEpi). A data safety monitoring committee oversaw the conduct of the study. The study is registered at ClinicalTrials.gov, number NCT00970619.
Role of the Funding Source
The CAVA trial was funded by a grant from ZonMw (The Netherlands Organisation for Health Research and Development, project number 171101001) and additional funding was provided by the board of the Maastricht University Medical Centre. The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The authors involved in analyzing the data (P.N. and A.t.C.H.) had full access to all of the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
Between May 28, 2010, and September 18, 2017, a total of 184 patients were included and randomly assigned, of which 152 (82.6%) were included in the original modified intention‐to‐treat analysis (Figure). Of these patients, 120 patients (78.9%) participated in the long‐term follow‐up: 62 (81%) patients allocated standard post‐thrombotic management with additional catheter‐directed thrombolysis and 58 (77%) patients receiving standard post‐thrombotic management only. Baseline characteristics were similar between both treatment groups. Table 1.
Figure 1. Trial profile.
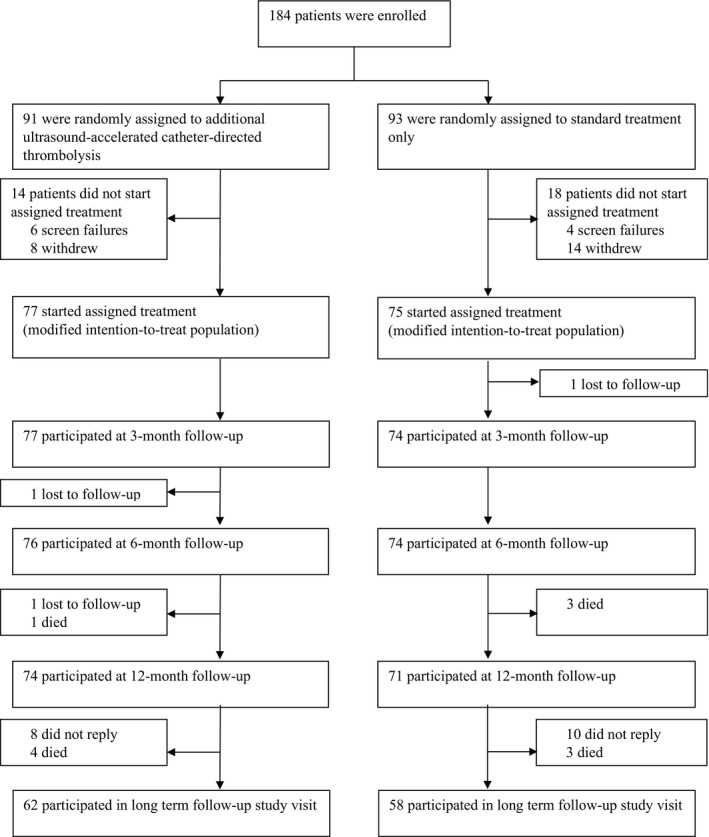
Table 1.
Baseline Characteristics: Long‐Term Follow‐Up
| Additional Thrombolysis (n=62) | Standard Treatment (n=58) | Total (N=120) | |
|---|---|---|---|
| Age, y | 46.5 (37.0–63.3) | 52.0 (37.8–64.0) | 49.0 (37.3–63.8) |
| Age, category | |||
| <40 y | 20 (32.3) | 16 (27.6) | 36 (30.0) |
| 40–65 y | 27 (43.5) | 31 (53.4) | 58 (48.3) |
| >65 y | 15 (24.2) | 11 (19.0) | 26 (21.7) |
| Sex | |||
| Women | 32 (51.6) | 31 (53.4) | 63 (52.5) |
| Men | 30 (48.4) | 27 (46.6) | 57 (47.5) |
| BMI* | 27.7±5.4 | 27.4±4.3 | 27.6±4.8 |
| BMI, category* | |||
| <25.0 | 18 (29.0) | 18 (31.0) | 36 (30.0) |
| 25.0–30.0 | 27 (43.5) | 24 (41.4) | 51 (42.5) |
| ≥30.0 | 14 (22.6) | 12 (20.7) | 26 (21.7) |
| Unknown | 3 (4.8) | 4 (6.9) | 7 (5.8) |
| Provoked DVT† | 29 (46.8) | 26 (44.8) | 55 (45.8) |
| No. of known risk factors | |||
| 1 | 24 (38.7) | 17 (29.3) | 41 (34.2) |
| >1 | 5 (8.1) | 9 (15.5) | 14 (11.7) |
| Surgery in the previous 2 mo | 6 (9.7) | 8 (13.8) | 14 (11.7) |
| Trauma in the previous 2 mo | 2 (3.2) | 3 (5.2) | 5 (4.2) |
| Pregnancy or childbirth in the previous 3 mo | 8 (12.9) | 4 (6.9) | 12 (10.0) |
| Hormone replacement therapy | 2 (3.2) | 0 | 2 (1.7) |
| Oral contraceptives | 8 (12.9) | 12 (20.7) | 20 (16.7) |
| Previous contralateral DVT | 6 (9.7) | 4 (6.9) | 10 (8.3) |
| Previous pulmonary embolism | 2 (3.2) | 4 (6.9) | 6 (5.0) |
| Active malignancy‡ | 1 (1.6) | 1 (1.7) | 2 (1.7) |
| Thrombus location | |||
| Left | 42 (67.7) | 44 (75.9) | 86 (71.7) |
| Right | 18 (29.0) | 12 (20.7) | 30 (25.0) |
| Bilateral§ | 2 (3.2) | 2 (3.4) | 4 (3.3) |
| Duration of symptoms at inclusion, d | 6.0 (3.0–11.0) | 6.5 (3.0–10.3) | 6.0 (3.0–11.0) |
| Anticoagulant therapy at inclusion | |||
| Vitamin K antagonistsǁ | 51 (82.3) | 50 (86.2) | 101 (84.2) |
| Direct oral anticoagulants# | 10 (16.1) | 5 (8.6) | 15 (12.5) |
Data are number (percentage), mean±SD, or median (interquartile range). Data represent the modified intention‐to‐treat population, which was included in this prespecified long‐term follow‐up analysis.
Body mass index (BMI) is defined as the patient’s weight in kilograms divided by the square of the patient’s height in meters (kg/m2).
Acute deep vein thrombosis (DVT) is considered unprovoked in the absence of the following risk factors: surgery in the previous 2 months, trauma in the previous 2 months, pregnancy or childbirth in the previous 3 months, use of hormone replacement therapy, use of oral contraceptives, and active malignancy.
Active malignancy is defined as a current metastatic or progressive cancer diagnosis or having received cancer treatment within the previous 6 months.
In the case of bilateral DVT, the leg with the most proximal localization was considered to be the index leg.
The vitamin K antagonists used during the study were acenocoumarol and phenprocoumon.
The direct oral anticoagulants used during the study were rivaroxaban, apixaban, and dabigatran.
At a median follow‐up of 39.0 months (interquartile range, 23.3–63.8) PTS occurred in 19 (30.6%) of 62 patients allocated additional thrombolysis compared with 26 (44.8%) of 58 patients from the standard treatment group (OR, 0.54; 95% CI, 0.26–1.15 [P=0.11]) (Table 2). The absolute difference was −14.2% (95% CI, −32.0% to 4.8%). The number of new diagnoses at the final follow‐up visit were 3 (4.8%) in the intervention group compared with 5 (8.6%) in the standard treatment group, respectively (P=0.64). PTS severity did not differ between the intervention group and the standard treatment group, classifying 5 (8.1%) versus 12 (20.7%) as mild (Villalta score 5–9, P=0.07), 13 (21.0%) versus 10 (17.2%) as moderate (Villalta score 10–14, P=0.60), and 1 (1.6%) versus 4 (6.9%) as severe (venous ulceration or Villalta score ≥15, P=0.20).
Table 2.
Efficacy Outcomes: Long‐Term Follow‐Up
|
Additional Thrombolysis (n=62) |
Standard Treatment (n=58) |
Difference Between Treatment Groups, (95% CI) | OR (95% CI) | |
|---|---|---|---|---|
| Primary outcome | ||||
| PTS at final follow‐up visit assessed by Villalta criteria18 | 19 (30.6) | 26 (44.8) | −14.2% (−32.0% to 4.8%) | 0.54 (0.26 to 1.15) |
| PTS diagnosed at 12 mo | 8 (12.9) | 6 (10.3) | 2.6% (−9.8% to 13.5%) | 1.28 (0.42 to 3.96) |
| PTS diagnosed at final follow‐up visit | 3 (4.8) | 5 (8.6) | −3.8% (−11.0% to 5.8%) | 0.54 (0.12 to 2.36) |
| None (<5) | 43 (69.4) | 32 (55.2) | 14.2% (−4.8% to 32.0%) | 1.84 (0.87 to 3.88) |
| Mild (5–9) | 5 (8.1) | 12 (20.7) | −12.6% (−22.7% to 1.5%) | 0.34 (0.11 to 1.02) |
| Moderate (10–14) | 13 (21.0) | 10 (17.2) | 3.7% (−11.6% to 17.9%) | 1.27 (0.51 to 3.18) |
| Severe (≥15) | 1 (1.6) | 4 (6.9) | −5.3% (−8.4% to 3.0%) | 0.22 (0.02 to 2.04) |
| Moderate/severe (≥10) | 14 (22.6) | 14 (24.1) | −1.6% (−17.6% to 14.5%) | 0.92 (0.39 to 2.14) |
| Additional outcomes | ||||
| Mean Villalta score18 at final follow‐up visit, total | 4.19±3.90 | 4.40±3.03 | −0.20 (−1.47 to 1.07) | … |
| Mean score objective items | 1.40±1.76 | 1.28±1.36 | 0.13 (−0.44 to 0.70) | … |
| Mean score subjective items | 2.79±2.69 | 3.12±2.64 | −0.33 (−1.30 to 0.63) | … |
| PTS at final follow‐up visit according to the ISTH score17 | 29 (46.8) | 40 (69.0) | −22.2% (−39.8% to −2.8%) | 0.40 (0.19 to 0.84) |
| PTS diagnosed at 12 mo | 10 (16.1) | 10 (17.2) | −1.1% (−15.1% to 12.9%) | 0.92 (0.35 to 2.41) |
| PTS diagnosed at final follow‐up visit | 5 (8.1)‡ | 13 (22.4) | −14.3% (−24.4% to 0.1%) | 0.30 (0.10 to 0.92) |
| None (<5) | 33 (53.2)† | 18 (31.0) | 22.2% (2.8% to 39.8%) | 2.53 (1.20 to 5.34) |
| Mild (5–9) | 12 (19.4)† | 24 (41.4) | −22.0% (−37.5% to −4.0%) | 0.34 (0.15 to 0.77) |
| Moderate (10–14) | 15 (24.2) | 12 (20.7) | 3.5% (−12.7% to 18.8%) | 1.22 (0.52 to 2.89) |
| Severe (≥15) | 2 (3.2) | 4 (6.9) | −3.7% (−9.1% to 4.8%) | 0.45 (0.08 to 2.56) |
| Moderate/severe (≥10) | 17 (27.4) | 16 (27.6) | −0.2% (−17.3% to 16.8%) | 0.99 (0.45 to 2.21) |
| Mean venous clinical severity score19 at final follow‐up visit | 2.82±2.36 | 3.48±2.34 | −0.66 (−1.52 to 0.19) | … |
| Ulceration at any follow‐up assessment | 0 | 3 (5.2) | −5.1% (−6.8% to 2.3%) | 0.13 (0.01 to 2.51) |
Data are number (percentage) or mean±SD. In the case of bilateral deep vein thrombosis, the least favorable clinical scores were used. ISTH indicates International Society of Thrombosis and Haemostasis; OR, odds ratio; and PTS, post‐thrombotic syndrome.
P=0.01.
P=0.05.
Using the ISTH consensus scoring method, the proportion of patients with PTS at long‐term follow‐up was 29 (46.8%) of 62 patients in the intervention group compared with 40 (69.0%) of 58 patients in the standard treatment group (OR, 0.40; 95% CI, 0.19 to 0.84 [P=0.01]). The number of new diagnoses at the final follow‐up visit was significantly lower in patients with additional thrombolysis versus patients receiving standard treatment only: 5 (8.1%) versus 13 (22.4%), respectively (P=0.05). Only the number of patients with mild PTS differed significantly between groups: 12 (19.4%) in the thrombolysis group versus 24 (41.4%) in the standard treatment group (P=0.01). There were no differences in the distribution of PTS severity between the treatment groups according to the Villalta score or the VCSS (Table 2).
The HRs and 95% CIs stratified for center and adjusted for age, sex, clinical presentation of the thrombus, and extent of the thrombus for the intervention group versus the standard treatment group were 0.66 (95% CI, 0.36 to 1.23) using the original Villalta score and 0.75 (95% CI, 0.45 to 1.24) using the ISTH consensus scoring method.
No major bleedings or deaths occurred following the first year of follow‐up (Table 3). Recurrent venous thromboembolism occurred similarly in both groups. Patients from the additional thrombolysis group developed 3 (4.8%) pulmonary emboli and 3 (4.8%) recurrent nonstent DVT compared with 2 (3.4%) and 6 (10.3%) events, respectively, in patients from the standard treatment group. In‐stent thrombosis occurred in 2 (3.4%) patients from the intervention group, as well as in 1 (1.7%) patient allocated standard treatment only. Stent placement in this patient was performed after the 12‐month follow‐up visit and was indicated because of May‐Thurner syndrome.
Table 3.
Safety Outcomes: Long‐Term Follow‐Up
|
Additional Thrombolysis (n=62) |
Standard Treatment (n=58) |
Difference Between Treatment Groups, (95% CI) | OR (95% CI) | |
|---|---|---|---|---|
| Primary outcome | ||||
| Major bleeding20 | 0 | 0 | 0.0% | … |
| Secondary outcomes | ||||
| Pulmonary embolism | 3 (4.8) | 2 (3.4) | 1.4% (−5.7% to 6.8%) | 1.42 (0.23–8.84) |
| Recurrent (nonstent) DVT | 3 (4.8) | 6 (10.3) | −5.5% (−12.8% to 5.0%) | 0.44 (0.11–1.85) |
| In‐stent‐thrombosis | 2 (3.2) | 1 (1.7)* | 1.5% (−3.9% to 4.7%) | 1.90 (0.17–21.5) |
| Death | 0 | 0 | 0.0% | … |
Data are number (percentage). None of the comparisons in this table showed a statistically significant difference between groups. Reported results concern the occurrence of safety outcomes during long‐term follow‐up (>12 months after study inclusion).
One patient underwent venous stent placement after completing the 1‐year study follow‐up. Indication for treatment was the presence of May‐Thurner Syndrome. DVT indicates deep vein thrombosis; and OR, odds ratio.
There were no differences between groups regarding the characteristics of standard treatment. However, compared with follow‐up at 12 months the number of patients refraining from compression therapy increased: 34 (54.8%) compared with 11 (17.7%) of 62 patients in the intervention group (P<0.001) and 25 (43.1%) compared with 10 (17.2%) of 58 in the standard treatment group (P=0.002), there were no differences between groups. If compression therapy was used, adherence was high: 23 (82.1%) of 28 patients in the intervention group and 25 (75.8%) of 33 patients in the standard treatment group were adherent for >80% of days (P=0.77). At final follow‐up, anticoagulant therapy was used by 36 (58.1%) of 62 patients in the intervention group and 36 (62.1%) of 58 patients in the standard treatment group (P=0.65). The share of direct oral anticoagulants in anticoagulant treatment doubled compared with the 12‐month follow‐up in both groups: from 8 (25.0%) of 32 patients to 18 (50.0%) of 36 patients in the intervention group (P=0.03) and from 9 (24.3%) of 37 patients to 18 (50.0%) of 36 patients in the standard treatment group (P=0.02), and were similar between groups.
The quality‐of‐life data from the 12‐month follow‐up and the final follow‐up visit for both treatment groups are presented in Table 4. Change over time between the 12‐month and the long‐term follow‐up in general health‐related quality‐of‐life measures (SF‐36 and EQ5D) was only significantly different for SF‐36/Physical Health (P=0.05) favoring standard treatment. However, this difference was not clinically relevant based on the assumed minimal important difference as determined by the method of Norman et al.29 Disease‐specific health measures (VEINES‐QoL/Sym and intrinsic scores) were similar for both treatment groups.
Table 4.
Quality of Life: Long‐Term Follow‐Up
| Additional Thrombolysis (n=62) | Standard Treatment (n=58) | |
|---|---|---|
| Generic Quality of Life | ||
| SF‐3621, Physical Health | No. | Δ From 12 mo* | SF‐36, Physical Health | No. | Δ From 12 mo* | |
|---|---|---|---|---|---|---|
| At 12 mo | 83.6±17.7 | 53 | … | 77.6±25.1 | 53 | … |
| Final follow‐up | 80.3±20.3 | 62 | −2.7 | 82.0±21.0 | 58 | +4.4 |
| P value for change (Δ) from 12 mo to final follow‐up visit within treatment groups: P=0.636 | ||||||
| P value for Δ from 12 mo to final follow‐up visit between treatment groups: P=0.048 | ||||||
| SF‐36, Mental Health | No. | Δ From 12 mo* | SF‐36, Mental Health | No. | Δ From 12 mo* | |
|---|---|---|---|---|---|---|
| At 12 mo | 86.1±39.1 | 52 | … | 81.6±14.7 | 53 | … |
| Final follow‐up | 82.4±15.4 | 60 | −3.1 | 84.8±15.6 | 58 | +2.7 |
| P value for Δ from 12 mo to final follow‐up visit within treatment groups: P=0.942 | ||||||
| P value for Δ from 12 mo to final follow‐up visit between treatment groups: P=0.292 | ||||||
| SF‐36, General Health | No. | Δ From 12 mo* | SF‐36, General Health | No. | Δ From 12 mo* | |
|---|---|---|---|---|---|---|
| At 12 mo | 66.3±17.3 | 52 | … | 66.4±22.9 | 53 | … |
| Final follow‐up | 66.1±17.5 | 60 | 0.0 | 69.3±24.5 | 58 | +2.8 |
| P value for Δ from 12 mo to final follow‐up visit within treatment groups: P=0.339 | ||||||
| P value for Δ from 12 mo to final follow‐up visit between treatment groups: P=0.339 | ||||||
| EQ5D22 | No. | Δ From 12 mo* | EQ5D | No. | Δ From 12 mo* | |
|---|---|---|---|---|---|---|
| At 12 mo | 86.8±13.8 | 53 | … | 83.4±20.0 | 53 | … |
| Final follow‐up | 84.5±15.9 | 60 | −1.6 | 86.2±18.4 | 58 | +2.8 |
| P value for Δ from 12 mo to final follow‐up visit within treatment groups: P=0.727 | ||||||
| P value for Δ from 12 mo to final follow‐up visit between treatment groups: P=0.214 | ||||||
| PDI23 | No. | Δ From 12 mo* | PDI | No. | Δ From 12 mo* | |
|---|---|---|---|---|---|---|
| At 12 mo | 8.7±11.9 | 48 | … | 12.8±15.8 | 52 | … |
| Final follow‐up | 11.8±14.6 | 53 | +1.22 | 9.6±13.6 | 57 | −2.7 |
| P value for Δ from 12 mo to final follow‐up visit within treatment groups: P=0.499 | ||||||
| P value for Δ from 12 mo to final follow‐up visit between treatment groups: P=0.072 | ||||||
| Disease‐Specific Quality of Life | ||||||
| VEINES‐QoL24, 25 | No. | Δ From 12 mo* | VEINES‐QoL | No. | Δ From 12 mo* | |
|---|---|---|---|---|---|---|
| At 12 mo | 50.0±11.1 | 43 | … | 50.2±8.8 | 47 | … |
| Final follow‐up | 49.9±8.7 | 61 | −1.2 | 50.1±11.4 | 58 | −0.1 |
| P value for Δ from 12 mo to final follow‐up visit within treatment groups: P=0.552 | ||||||
| P value for Δ from 12 mo to final follow‐up visit between treatment groups: P=0.623 | ||||||
| VEINES‐QoL Intrinsic26 | No. | Δ From 12 mo* | VEINES‐QoL Intrinsic | No. | Δ From 12 mo* | |
|---|---|---|---|---|---|---|
| At 12 mo | 70.8±17.5 | 53 | … | 68.9±17.8 | 53 | … |
| Final follow‐up | 70.6±14.9 | 61 | −0.5 | 72.2±16.9 | 58 | +2.6 |
| P value for Δ from 12 mo to final follow‐up visit within treatment groups: P=0.508 | ||||||
| P value for Δ from 12 mo to final follow‐up visit between treatment groups: P=0.322 | ||||||
Data are mean±SD. EQ5D indicates EuroQOL‐5D; PDI, Pain Disability Index; SF‐36, 36‐Item Short Form Health Survey; and VEINES‐QoL, Venous Insufficiency Epidemiological and Economic Study Quality of Life.
Δ from 12 mo represents the absolute difference from 12 mo to final follow‐up visit.
Discussion
In this long‐term follow‐up of patients from the CAVA trial, we found that differences in the prevalence of PTS between treatment groups did indeed increase over time.
At a median follow‐up of >3 years, the difference in absolute risk for the development of PTS according to the original definition of the Villalta score was nonsignificant, even though it had increased to −14.2%, from −6.1% at 1‐year follow‐up. Neither was there a difference in syndrome severity between groups. However, when the definition proposed by the ISTH17 was used for matters of comparability, as this was the definition used in both the CaVenT8, 11 and the ATTRACT trial,12, 14 a significant absolute difference of −22.2% in the proportion of PTS between groups favoring additional ultrasound‐accelerated catheter‐directed thrombolysis over standard treatment was observed. This difference was a result of a significantly higher number of new diagnoses of mild PTS at the final follow‐up visit in the standard treatment group. For neither definition of PTS, a clinically relevant change in any of the patient‐reported quality‐of‐life scores was found. This latter finding is in line with the 5‐year results as reported by the CaVenT trial investigators, who described an absolute risk reduction of 28% for PTS, which was also not associated with significant gains in any of the health‐related quality‐of‐life measures.11 This might be explained by the fact that in both trials, PTS was mild in the majority of cases. Discontinuation of compression stockings might have contributed to the increased number of newly diagnosed patients with mild PTS at the long‐term follow‐up compared with follow‐up at 12 months. This does not, however, explain why this would affect patients receiving standard treatment more than those following additional thrombolytic therapy.
The observed use of anticoagulants at long‐term follow‐up was similar with what would be expected according to current guidelines: the percentages of patients using anticoagulants at the final follow‐up visit matched the percentage of patients with unprovoked DVT registered at inclusion and therefore those eligible for long‐term anticoagulation.4 We observed an increased use of direct oral anticoagulants, which is also in compliance with current guidelines. However, a beneficial effect of direct oral anticoagulants on the development of PTS is not likely as both treatment groups were treated similarly. At long‐term follow‐up, the occurrence of in‐stent thrombosis was far less prevalent than in the acute phase. In contrast, the incidences for recurrent DVT and pulmonary embolism were not different from those in the first year of follow‐up, rendering the assumption that stenting might have a preventive effect on recurrent thrombosis less likely.
Our study has its limitations, the most important being the limited sample size of the main CAVA trial, which, as a result, also applies, maybe even more so, to this long‐term follow‐up study. Although treatment groups remained comparable without significant differences in baseline characteristics, differences in unobserved prognostic factors could have been introduced between groups. Furthermore, other limitations from the main analysis15 also apply to the present report including the lengthy recruitment period attributable to stringent inclusion criteria, which may affect the results’ generalizability, the high rate of withdrawals before start of allocated treatment, and the higher‐than‐expected number of post‐thrombotic diagnoses in the standard treatment group impacting the power. The strengths of the study are the high participation rate (78.9%) to the long‐term follow‐up with maintained comparability between treatment arms and the median follow‐up of >3 years, allowing adequate comparison with the long‐term results of the CaVenT trial.11
Conclusions
In this long‐term follow‐up of patients from the CAVA trial, we found that following additional ultrasound‐accelerated catheter‐directed thrombolysis differences in absolute risk for the development of PTS increased over time. Although this study was limited by its sample size, the overall findings indicate a reduction in the proportion of PTS at long‐term follow‐up limited to mild PTS and without associated gain in quality of life.
Sources of Funding
The CAVA trial was originally funded by a grant from ZonMw (The Netherlands Organisation for Health Research and Development, project number 171101001) and additional funding was provided by the board of the Maastricht University Medical Centre. The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosures
Dr Coppens reports grants and personal fees from Bayer Daiichi Sankyo, CSL Behring; grants from Boehringer Ingelheim, Pfizer, Bristol‐Meyers Squibb, and Sanquin Blood Supply; and personal fees from Sobi, NovoNordisk, and UniQure, outside the submitted work. Dr ten Cate reports personal fees and other from Bayer; other from Pfizer and Coagulation profile; and personal fees from LEO Pharma, Gideon Pharmaceuticals, and Alveron Pharma, outside the submitted work. Dr Wittens reports grants from BTG International Group during the conduct of the study. None of the above‐mentioned organizations had any involvement in the design, conduct, analysis, or report of the study data. The remaining authors have no disclosures to report.
Supporting information
(J Am Heart Assoc. 2021;10:e018973. DOI: 10.1161/JAHA.120.018973.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018973
For Sources of Funding and Disclosures, see page 10.
See Editorial by Obi and Barnes
REFERENCES
- 1.Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698–707. DOI: 10.7326/0003-4819-149-10-200811180-00004. [DOI] [PubMed] [Google Scholar]
- 2.Schulman S, Lindmarker P, Holmstrom M, Larfars G, Carlsson A, Nicol P, Svensson E, Ljungberg B, Viering S, Nordlander S, et al. Post‐thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006;4:734–742. DOI: 10.1111/j.1538-7836.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 3.Appelen D, van Loo E, Prins MH, Neumann MH, Kolbach DN. Compression therapy for prevention of post‐thrombotic syndrome. Cochrane Database Syst Rev. 2017;9:Cd004174. DOI: 10.1002/14651858.CD004174.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et al. Antithrombotic therapy for vte disease: chest guideline and expert panel report. Chest. 2016;149:315–352. DOI: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Tick LW, Doggen CJ, Rosendaal FR, Faber WR, Bousema MT, Mackaay AJ, van Balen P, Kramer MH. Predictors of the post‐thrombotic syndrome with non‐invasive venous examinations in patients 6 weeks after a first episode of deep vein thrombosis. J Thromb Haemost. 2010;8:2685–2692. DOI: 10.1111/j.1538-7836.2010.04065.x. [DOI] [PubMed] [Google Scholar]
- 6.Roumen‐Klappe EM, den Heijer M, Janssen MC, van der Vleuten C, Thien T, Wollersheim H. The post‐thrombotic syndrome: incidence and prognostic value of non‐invasive venous examinations in a six‐year follow‐up study. Thromb Haemost. 2005;94:825–830. DOI: 10.1160/TH05-03-0146. [DOI] [PubMed] [Google Scholar]
- 7.Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E, Tormene D, Mosena L, Pagnan A, Girolami A. Below‐knee elastic compression stockings to prevent the post‐thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249–256. DOI: 10.7326/0003-4819-141-4-200408170-00004. [DOI] [PubMed] [Google Scholar]
- 8.Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, et al. Long‐term outcome after additional catheter‐directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31–38. DOI: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 9.Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, et al. Determinants of health‐related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112. DOI: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 10.Ten Cate‐Hoek AJ, Toll DB, Büller HR, Hoes AW, Moons KG, Oudega R, Stoffers HE, van der Velde EF, van Weert HC, Prins MH, et al. Cost‐effectiveness of ruling out deep venous thrombosis in primary care versus care as usual. J Thromb Haemost. 2009;7:2042–2049. DOI: 10.1111/j.1538-7836.2009.03627.x. [DOI] [PubMed] [Google Scholar]
- 11.Haig Y, Enden T, Grøtta O, Kløw NE, Slagsvold CE, Ghanima W, Sandvik L, Hafsahl G, Holme PA, Holmen LO, et al. Post‐thrombotic syndrome after catheter‐directed thrombolysis for deep vein thrombosis (CaVenT): 5‐year follow‐up results of an open‐label, randomised controlled trial. Lancet Haematol. 2016;3:e64–e71. DOI: 10.1016/S2352-3026(15)00248-3. [DOI] [PubMed] [Google Scholar]
- 12.Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, Magnuson E, Razavi MK, Comerota AJ, Gornik HL, et al. Pharmacomechanical catheter‐directed thrombolysis for deep‐vein thrombosis. N Engl J Med. 2017;377:2240–2252. DOI: 10.1056/NEJMoa1615066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strijkers RH, Arnoldussen CW, Wittens CH. Validation of the let classification. Phlebology. 2015;30:14–19. DOI: 10.1177/0268355515569133. [DOI] [PubMed] [Google Scholar]
- 14.Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, Jaff MR, Razavi MK, Kindzelski AL, Bashir R, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139:1162–1173. DOI: 10.1161/CIRCULATIONAHA.118.037425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Notten P, Ten Cate‐Hoek AJ, Arnoldussen C, Strijkers RH, de Smet A, Tick LW, van de Poel MH, Wikkeling OR, Vleming LJ, Koster A, et al. Ultrasound‐accelerated catheter‐directed thrombolysis versus anticoagulation for the prevention of post‐thrombotic syndrome (CAVA): a single‐blind, multicentre, randomised trial. Lancet Haematol. 2020;7:e40–e49. doi: 10.1016/S2352-3026(19)30209-1. [DOI] [PubMed] [Google Scholar]
- 16.Notten P, Arnoldussen C, Brans R, de Smet AA, Tick LW, van de Poel MH, Wikkeling OR, Vleming LJ, Koster A, Jie KG, et al. Association of successful ultrasound‐accelerated catheter‐directed thrombolysis with post‐thrombotic syndrome: a post‐hoc analysis of the CAVA trial. Thromb Haemost. 2020;120:1188–1199. DOI: 10.1055/s-0040-1713171. [DOI] [PubMed] [Google Scholar]
- 17.Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C. Definition of post‐thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. 2009;7:879–883. DOI: 10.1111/j.1538-7836.2009.03294.x. [DOI] [PubMed] [Google Scholar]
- 18.Villalta S, Bagatella P, Piccioli A, Lensing AW, Prins MH, Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post‐thrombotic syndrome [abstract]. Haemostasis. 1994;24:158a. [Google Scholar]
- 19.Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–1312. DOI: 10.1067/mva.2000.107094. [DOI] [PubMed] [Google Scholar]
- 20.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. DOI: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson NK, Muller M, Cohen PD, Essink‐Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E. Translation, validation, and norming of the Dutch language version of the SF‐36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–1068. DOI: 10.1016/S0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 22.Brooks R. Euroqol: the current state of play. Health Policy. 1996;37:53–72. DOI: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 23.Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills. 1984;59:974. DOI: 10.2466/pms.1984.59.3.974. [DOI] [PubMed] [Google Scholar]
- 24.van der Velden SK, Biemans AA, Nijsten T, Sommer A. Translation and validation of the Dutch VEINES‐QOL/Sym in varicose vein patients. Phlebology. 2014;29:227–235. DOI: 10.1177/0268355513476279. [DOI] [PubMed] [Google Scholar]
- 25.Lamping DL, Schroter S, Kurz X, Kahn SR, Abenhaim L. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient‐reported measure of symptoms and quality of life. J Vasc Surg. 2003;37:410–419. DOI: 10.1067/mva.2003.152. [DOI] [PubMed] [Google Scholar]
- 26.Bland JM, Dumville JC, Ashby RL, Gabe R, Stubbs N, Adderley U, Kang'ombe AR, Cullum NA. Validation of the VEINES‐QOL quality of life instrument in venous leg ulcers: repeatability and validity study embedded in a randomised clinical trial. BMC Cardiovasc Disord. 2015;15:85. DOI: 10.1186/s12872-015-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnoldussen CW, Wittens CH. An imaging approach to deep vein thrombosis and the lower extremity thrombosis classification. Phlebology. 2012;27:143–148. DOI: 10.1258/phleb.2012.012s25. [DOI] [PubMed] [Google Scholar]
- 28.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Qual Life Res. 2005;14:1523–1532. DOI: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 29.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health‐related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. DOI: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 30.Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995;21:635–645. DOI: 10.1016/S0741-5214(95)70195-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


