Abstract
Background
The role of race and ethnicity in the outcomes of cardiac arrest (CA) complicating acute myocardial infarction (AMI) is incompletely understood.
Methods and Results
This was a retrospective cohort study of adult admissions with AMI‐CA from the National Inpatient Sample (2012–2017). Self‐reported race/ethnicity was classified as White, Black, and others (Hispanic, Asian or Pacific Islander, Native American, Other). Outcomes of interest included in‐hospital mortality, coronary angiography, percutaneous coronary intervention, palliative care consultation, do‐not‐resuscitate status use, hospitalization costs, hospital length of stay, and discharge disposition. Of the 3.5 million admissions with AMI, CA was noted in 182 750 (5.2%), with White, Black, and other races/ethnicities constituting 74.8%, 10.7%, and 14.5%, respectively. Black patients admitted with AMI‐CA were more likely to be female, with more comorbidities, higher rates of non–ST‐segment–elevation myocardial infarction, and higher neurological and renal failure. Admissions of patients of Black and other races/ethnicities underwent coronary angiography (61.9% versus 70.2% versus 73.1%) and percutaneous coronary intervention (44.6% versus 53.0% versus 58.1%) less frequently compared to patients of white race (p<0.001). Admissions of patients with AMI‐CA had significantly higher unadjusted mortality (47.4% and 47.4%) as compared with White patients admitted (40.9%). In adjusted analyses, Black race was associated with lower in‐hospital mortality (odds ratio [OR], 0.95; 95% CI, 0.91–0.99; P=0.007) whereas other races had higher in‐hospital mortality (OR, 1.11; 95% CI, 1.08–1.15; P<0.001) compared with White race. Admissions of Black patients with AMI‐CA had longer length of hospital stay, higher rates of palliative care consultation, less frequent do‐not‐resuscitate status use, and fewer discharges to home (all P<0.001).
Conclusions
Racial and ethnic minorities received less frequent guideline‐directed procedures and had higher in‐hospital mortality and worse outcomes in AMI‐CA.
Keywords: acute myocardial infarction, cardiac arrest, healthcare disparities, minorities, outcomes research, race
Subject Categories: Health Equity
Nonstandard Abbreviations and Acronyms
- CA
cardiac arrest
- HCUP
Healthcare Cost and Utilization Project
- IHCA
in‐hospital cardiac arrest
- NIS
National/Nationwide Inpatient Sample
- NRCPR
National Registry of Cardiopulmonary Resuscitation
- OOHCA
out‐of‐hospital cardiac arrest
Clinical Perspective
What Is New?
Racial and ethnic minorities with acute myocardial infarction and cardiac arrest had lower use of coronary angiography and percutaneous coronary interventions.
These differences in management together with greater comorbidity at presentation appear to influence outcomes associated with admissions for acute myocardial infarction and cardiac arrest.
Racial/ethnic minorities, specifically Hispanic and Asian/Pacific Islanders, experienced worse in‐hospital outcomes independent of patient‐ and hospital‐related factors.
What Are the Clinical Implications?
A greater emphasis on quantitative and qualitative research into the equitable care of racial/ethnic minorities with acute myocardial infarction and cardiac arrest is needed.
Further research into identifying the role of race/ethnicity at pathophysiological level would be beneficial.
Racial and ethnic disparities in treatment and outcomes of critical illness have been studied previously, including in patients presenting with acute cardiovascular conditions such as acute myocardial infarction (AMI), cardiac arrest (CA), and cardiogenic shock.1, 2, 3, 4 CA is of special interest as it has clearly defined treatment guidelines, and national registries such as the NRCPR (National Registry of Cardiopulmonary Resuscitation), have evolved into nationwide quality improvement initiatives, now known as GWTG‐R (Get with the Guidelines‐Resuscitation), to address gaps in care.5 Previous CA studies have shown clear racial/ethnic disparities, especially among Black patients, including an increased risk of fatal coronary artery disease, while receiving lower rates of percutaneous coronary intervention (PCI) and guideline‐directed medical therapies.4, 6, 7, 8, 9, 10, 11, 12 Patients of Hispanic and other ethnicitie, commonly grouped as “non‐White” or “other,” have also been found to have lower rates of coronary angiography and PCI, with increased morbidity and mortality from cardiovascular disease when compared with White patients.13, 14
Several theories have been posited to explain racial/ethnic disparities in outcomes of CA, many of which remain broadly debated. Discrepancies in out‐of‐hospital cardiac arrest (OOHCA) care, including lack of bystander cardiopulmonary resuscitation, lower rates of witnessed arrest, and less frequent shockable rhythms have been associated with increased mortality among racial/ethnic minorities.13, 14 Additionally, differences in care by hospital center may also contribute to outcomes of both OOHCA and in‐hospital cardiac arrest (IHCA), as Black and Hispanic patients are often cared for in hospitals that have been identified in national registries to have lower overall survival rates.5, 15 However, more recent work has shown that gaps in care are less pronounced, finding very little difference in prehospital care or outcomes by race/ethnicity in the past few years.16, 17, 18, 19
In light of these conflicting data, this study sought to assess the racial/ethnic differences in the management and outcomes of CA complicating AMI. We hypothesized that racial/ethnic minority patients would have worse outcomes with AMI‐CA as compared with White patients. We also sought to evaluate the racial/ethnic differences in demographics, clinical course, and management strategies of these cohorts to better inform clinical care for these patients.
Methods
Study Population, Variables, and Outcomes
The National (Nationwide) Inpatient Sample (NIS) is the largest all‐payer database of hospital inpatient stays in the United States. NIS contains discharge data from a 20% stratified sample of community hospitals and is a part of the Healthcare Quality and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality.20 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 24 secondary diagnoses, and procedural diagnoses. The HCUP‐NIS does not capture individual patients but captures all information for a given admission.20 Institutional review board approval was not required because of the publicly available nature of this de‐identified database. These data are available to other authors via the HCUP‐NIS database with the Agency for Healthcare Research and Quality.
Using the HCUP‐NIS data from January 1, 2012 through December 31, 2017, a cohort of adult admissions (>18 years) with AMI in the primary diagnosis field (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] 410.x and Tenth Revision, Clinical Modification [ICD‐10‐CM] I21.x‐22.x) were identified.21, 22, 23 A concomitant diagnosis of CA was identified using ICD‐9‐CM 427.5, 427.41, 99.60 and 99.63; ICD‐10‐CM I46.x, I49.01, I49.02; International Classification of Diseases, Tenth Revision, Procedure Coding System (ICD‐10‐PCS) 5A12012. The administrative codes for CA show a high positive predictive value for the presence of CA but poor discrimination between in‐hospital and out‐of‐hospital CA, and therefore all admissions with CA were grouped together.24, 25 The Deyo modification of the Charlson Comorbidity Index was used to identify the burden of comorbid diseases.26 Demographic characteristics including age, sex, race/ethnicity, hospital characteristics, acute organ failure, mechanical circulatory support, cardiac procedures, fibrinolytic use, tracheostomy, percutaneous endoscopic gastrostomy, and other noncardiac organ support use were identified for all admissions using previously used methodologies from our group (Table S1).21, 22, 23, 37 For the purposes of this analysis, race/ethnicity was classified as White, Black, and others (Hispanic, Asian or Pacific Islander, Native American, Others). Coding for race in NIS combines “race” and “ethnicity” provided by the data source into 1 data element (RACE). If both “race” and “ethnicity” were available, ethnicity was preferred over race in setting the HCUP value for “RACE.”20 Early coronary angiography was defined as performed on hospital day zero.28, 29 The hospital day of the performance of the procedure was used to time concomitant procedures.22, 27, 28, 29, 31
The primary outcome of interest was racial/ethnic disparities in in‐hospital mortality of admissions with AMI complicated by CA. The secondary outcomes included racial/ethnic disparities in coronary angiography, early coronary angiography, median time to angiography, PCI, mechanical circulatory support use, palliative care consultation, do‐not‐resuscitate status (DNR) use, hospitalization costs, hospital length of stay, and discharge disposition. Multiple subgroup analyses were performed to confirm the results of the primary analysis stratifying the population by sex (male/female), type of AMI (ST‐segment elevation myocardial infarction [STEMI] versus non–ST‐segment–elevation myocardial infarction [NSTEMI]), presence of cardiogenic shock, in‐hospital cardiac arrest, and receipt of PCI.
Statistical Analysis
In accordance with HCUP‐NIS recommendations, survey procedures using discharge weights provided with the HCUP‐NIS database were used to generate national estimates.38 Within the NIS, racial/ethnic classification was missing for ≈23% of the sample in 2000. Race coding improved in more recent years with 3.6% missing in the HCUP‐NIS 2017 data. The missing racial/ethnic data are unlikely to be missing completely at random. Certain states in the early years of the NIS are known to have withheld racial/ethnic classification.39 Admissions with missing race/ethnicity were excluded from the analysis. Chi‐square and t tests were used to compare categorical and continuous variables, respectively. Multivariable logistic regression was used to analyze trends over time (referent year 2012). Univariable analysis for trends and outcomes was performed and was represented as odds ratio (OR) with 95% CI. Multivariable logistic regression analysis incorporating age, sex, primary payer status, socioeconomic stratum, hospital characteristics, comorbidities, acute organ failure, AMI type, cardiac procedures, and noncardiac procedures was performed for assessing temporal trends of prevalence and in‐hospital mortality. For the sensitivity analyses, “low income” was defined as the 0 to 25th and 26th to 50th percentile of income quartiles, whereas “high income” was defined as >50th percentile for median income for zip code. Temporal trends in use of early coronary angiography, coronary angiography, and PCI were plotted stratified by type of AMI. For the multivariable modeling, regression analysis with purposeful selection of statistically (liberal threshold of P<0.20 in univariate analysis) and clinically relevant variables was conducted. Two‐tailed P<0.05 was considered statistically significant.
The inherent restrictions of the HCUP‐NIS database related to research design, data interpretation, and data analysis were reviewed and addressed.38 Pertinent considerations include not assessing individual hospital‐level volumes, treating each entry as an “admission” as opposed to individual patients, restricting the study details to inpatient factors because the HCUP‐NIS does not include outpatient data, and limiting administrative codes to those previously validated and used for similar studies. All statistical analyses were performed using SPSS v25.0 (IBM Corp, Armonk, NY).
Results
We identified 3 504 225 admissions for AMI between January 1, 2012 and December 31, 2017, of whom 182 750 (5.2%) were complicated by CA. Of these 182 750 admissions, White, Black, and other races/ethnicities comprised 74.8%, 10.7%, and 14.5%, respectively. White admissions with AMI‐CA were on average older, more likely to have Medicare insurance, and had higher rates of STEMI, atrial fibrillation/flutter, and shockable rhythms compared with admissions who were Black and other races/ethnicities. Black admissions with AMI‐CA were more likely to be female, belong to the lowest income quartile, have more comorbidities, and higher rates of NSTEMI compared with admissions who were White and other races/ethnicities (Table 1 and Table S2). Hospital characteristics of these admissions were relatively comparable during the study period (Table 1). During this 6‐year period, admissions with STEMI had a significantly higher prevalence of CA compared with admissions with NSTEMI (Figure 1A). Admissions of Black race had higher rates of concomitant CA in both STEMI and NSTEMI compared with other race/ethnicity categories (Figure 1A and 1B). Adjusted temporal trends revealed a declining trend in CA prevalence among both admissions with STEMI and NSTEMI in all races (Figure 1B).
Table 1.
Baseline Characteristics of Admissions With AMI and CA Stratified by Race/Ethnicity
| Characteristic |
White (N=136 698) |
Black (N=19 468) |
Others* (N=26 584) |
P Value |
|---|---|---|---|---|
| Age, y | 66.8±12.9 | 63.9±13.5 | 64.9±13.4 | <0.001 |
| Female sex | 31.6 | 43.6 | 31.7 | <0.001 |
| Primary payer | ||||
| Medicare | 56.4 | 53.4 | 46.7 | <0.001 |
| Medicaid | 7.1 | 15.0 | 15.2 | |
| Private | 27.7 | 20.2 | 25.0 | |
| Others† | 8.8 | 11.4 | 13.1 | |
| Quartile of median household income for zip code | ||||
| 0–25th | 26.0 | 53.0 | 32.2 | <0.001 |
| 26th–50th | 28.0 | 22.3 | 22.6 | |
| 51st–75th | 25.3 | 15.1 | 23.6 | |
| 75th–100th | 20.7 | 9.5 | 21.6 | |
| Charlson Comorbidity Index | ||||
| 0–3 | 40.9 | 39.3 | 40.9 | <0.001 |
| 4–6 | 40.0 | 36.5 | 38.3 | |
| ≥7 | 19.1 | 24.2 | 20.8 | |
| Hospital teaching status and location | ||||
| Rural | 6.9 | 4.0 | 2.8 | <0.001 |
| Urban nonteaching | 32.2 | 22.5 | 32.6 | |
| Urban teaching | 60.9 | 73.5 | 64.6 | |
| Hospital bed‐size | ||||
| Small | 12.0 | 11.4 | 12.1 | <0.001 |
| Medium | 28.5 | 29.9 | 28.5 | |
| Large | 59.4 | 58.7 | 59.5 | |
| Hospital region | ||||
| Northeast | 17.6 | 13.9 | 16.1 | <0.001 |
| Midwest | 23.4 | 19.8 | 9.4 | |
| South | 40.9 | 56.9 | 37.1 | |
| West | 18.1 | 9.4 | 37.3 | |
| AMI type | ||||
| ST‐segment–elevation myocardial infarction | 62.7 | 51.7 | 60.1 | <0.001 |
| Non–ST‐segment–elevation myocardial infarction | 37.3 | 48.3 | 39.9 | <0.001 |
| Atrial fibrillation or flutter | 26.5 | 20.2 | 23.1 | <0.001 |
| Cardiac rhythm | ||||
| Shockable | 64.8 | 57.0 | 57.6 | <0.001 |
| Nonshockable | 35.2 | 43.0 | 42.4 | |
| Acute organ failure | ||||
| Multiorgan failure | 55.1 | 62.3 | 63.2 | <0.001 |
| Respiratory | 51.9 | 57.8 | 59.7 | <0.001 |
| Hepatic | 10.3 | 13.2 | 13.0 | <0.001 |
| Renal | 34.7 | 41.4 | 39.5 | <0.001 |
| Hematologic | 9.9 | 12.5 | 13.6 | <0.001 |
| Neurologic | 25.5 | 33.1 | 29.9 | <0.001 |
| Cardiogenic shock | 34.7 | 30.8 | 40.2 | <0.001 |
| Pulmonary artery catheterization | 2.4 | 2.4 | 3.2 | <0.001 |
| Invasive mechanical ventilation | 50.1 | 59.0 | 59.9 | <0.001 |
| Acute hemodialysis | 1.4 | 2.0 | 2.5 | <0.001 |
Represented as percentage or mean±SD; AMI indicates acute myocardial infarction; and CA, cardiac arrest.
Hispanic, Asian or Pacific Islander, Native American, Others.
Self‐Pay, No Charge, Others.
Figure 1. Trends in the prevalence and in‐hospital mortality of CA in admissions with AMI.
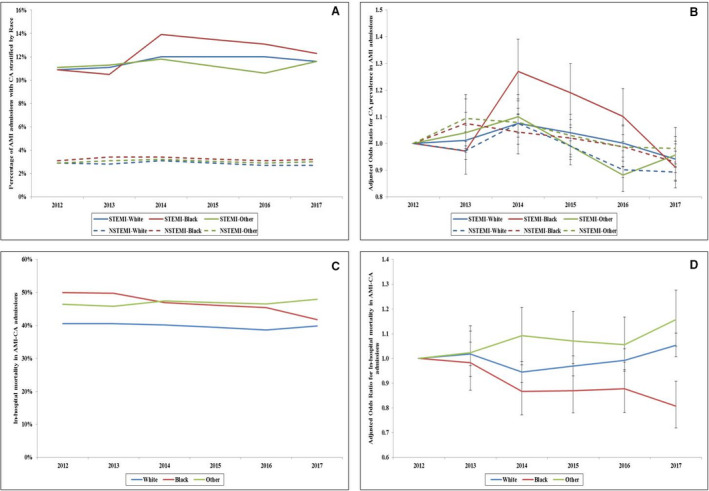
A, Unadjusted temporal trends of the proportion of admissions with AMI and CA stratified by race/ethnicity and type of AMI (P<0.001 for trend over time); B, Adjusted odds ratio for CA in STEMI and NSTEMI admissions by year stratified by race/ethnicity (with 2012 as the referent); adjusted for age, sex, comorbidity, primary payer, hospital region, hospital location and teaching status, and hospital bed size, atrial fibrillation/flutter, ventricular tachycardia/fibrillation, cardiogenic shock, coronary angiography, and percutaneous coronary intervention (P<0.001 for trend over time); C, Unadjusted in‐hospital mortality in admissions with AMI‐CA stratified by type of AMI and race/ethnicity (P<0.001 for trend over time); D, Adjusted odds ratio for in‐hospital mortality by year (with 2012 as the referent) in admissions with AMI‐CA stratified by type of AMI and race/ethnicity; adjusted for age, sex, comorbidity, primary payer, hospital region, hospital location and teaching status, hospital bed size, acute organ failure, cardiogenic shock, atrial fibrillation/flutter, ventricular tachycardia/fibrillation, coronary angiography, percutaneous coronary intervention, coronary artery bypass grafting, pulmonary artery catheterization, mechanical circulatory support, invasive mechanical ventilation, and acute hemodialysis (P<0.001 for trend over time). AMI indicates acute myocardial infarction; CA, cardiac arrest; NSTEMI, non–ST‐segment–elevation myocardial infarction; and STEMI, ST‐segment–elevation myocardial infarction.
Compared with White and Black admissions, other race/ethnicity admissions with AMI‐CA had higher rates of concomitant multiorgan failure, cardiogenic shock, and use of invasive mechanical ventilation. Black admissions had higher rates of concomitant neurological, renal, and hepatic organ failure compared with admissions who were White and other races/ethnicities (Table 1). Black and other race/ethnicity admissions with AMI‐CA received less frequent early coronary angiography (41.4% versus 50.2% versus 52.8%), coronary angiography (61.9% versus 70.2% versus 73.1%), PCI (44.6% versus 53.0% versus 58.1%), coronary artery bypass grafting, and mechanical circulatory support compared with White and other races/ethnicities (all P<0.001) (Table 2). The mean time to coronary angiography from admission was highest among Black patients (3.4±4.2 days) and lowest among White patients (3.0±3.7 days) (P<0.001) (Table 2 and Table S2). These disparities persisted over the 6‐year period with White admissions consistently receiving higher rates of early coronary angiography, coronary angiography, and PCI compared with admissions who were Black and other races/ethnicities among admissions with STEMI and NSTEMI and had shorter mean time to coronary angiography among admissions with NSTEMI (Figures 2A through 2D). However, a trend toward decrease in these disparities was noted across the study period. Admissions of other races/ethnicities had higher rates of acute organ failure, cardiogenic shock, mechanical circulatory support use, and pulmonary artery catheterization use.
Table 2.
Clinical Outcomes of Admissions With AMI and CA Stratified by Race/Ethnicity
| Characteristic |
White (N=136 698) |
Black (N=19 468) |
Others* (N=26 584) |
P Value |
|---|---|---|---|---|
| In‐hospital mortality | 40.9 | 47.4 | 47.4 | <0.001 |
| Length of stay, d | 7.1±9.2 | 8.9±13.3 | 8.2±11.3 | <0.001 |
| Coronary angiography | 73.1 | 61.9 | 70.2 | <0.001 |
| Early coronary angiography | 52.8 | 41.4 | 50.2 | <0.001 |
| Mean time to angiography | 3.0±3.7 | 3.4±4.2 | 3.2±3.9 | <0.001 |
| Percutaneous coronary intervention | 58.1 | 44.6 | 53.0 | <0.001 |
| Mechanical circulatory support | 23.5 | 19.4 | 27.9 | <0.001 |
| Coronary artery bypass grafting | 9.3 | 7.3 | 10.5 | <0.001 |
| Palliative care consultation | 9.8 | 10.5 | 9.6 | 0.003 |
| Do‐not‐resuscitate status | 15.6 | 15.3 | 16.8 | <0.001 |
| Hospitalization costs (×1000 US dollars) | 146.8±186.9 | 151.5±183.1 | 198.9±248.6 | <0.001 |
| Discharge disposition | ||||
| Home | 55.2 | 47.3 | 51.6 | <0.001 |
| Transfer | 8.7 | 10.0 | 10.9 | |
| Skilled nursing facility | 23.1 | 28.3 | 23.1 | |
| Home with home health care | 12.2 | 13.5 | 13.6 | |
| Against medical advice | 0.8 | 0.9 | 0.7 | |
Represented as percentage or mean±SD; AMI indicates acute myocardial infarction; and CA, cardiac arrest.
Hispanic, Asian or Pacific Islander, Native American, Others.
Figure 2. Temporal trends in the use of cardiac procedures in admissions with AMI‐CA.
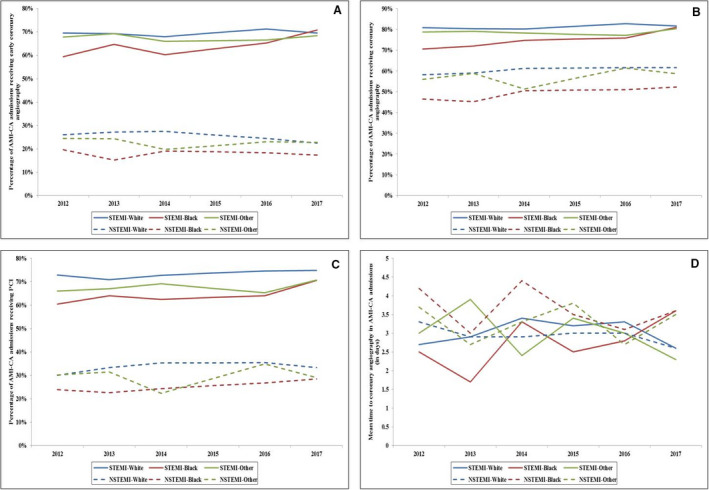
A, Temporal trends of the proportion of admissions with AMI‐CA receiving early coronary angiography; B, coronary angiography; C, PCI; and D, time to angiography stratified by type of AMI (P<0.001 for trend over time). AMI indicates acute myocardial infarction; CA, cardiac arrest; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Admissions of Black and other races/ethnicities with AMI‐CA had significantly higher unadjusted mortality (47.4% and 47.4%) as compared with White admissions (40.9%). In a multivariable logistic regression analysis with White race as referent, Black race was associated with lower in‐hospital mortality (OR, 0.95; 95% CI, 0.91–0.99; P=0.007) whereas other races/ethnicities had higher in‐hospital mortality (OR, 1.11; 95% CI, 1.08–1.15; P<0.001 compared with White race (Table S3). In‐hospital mortality remained relatively stable between 40% 50% across all race/ethnicity categories during this time period (Figure 1C and 1D).
Multiple subgroups analyses were performed to verify the primary outcome. In an analysis stratifying admissions as White versus non‐White, the non‐White cohort had higher adjusted in‐hospital mortality in the female, high‐income, STEMI presentation and PCI subgroups (Figure 3). Admissions of Black race with AMI‐CA had longer length of hospital stay, higher rates of palliative care consultation, less frequent DNR status use, and fewer discharges to home (Table 2). Admissions of other races/ethnicities had higher use of DNR status and higher hospitalization costs compared with White and Black admissions (Table 2).
Figure 3. Subgroup analyses for in‐hospital mortality in non‐White admissions with AMI‐CA compared with White race.
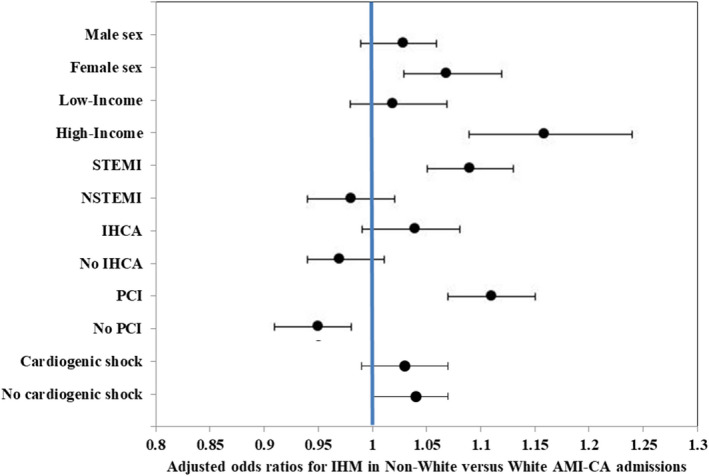
Multivariable adjusted odds ratios (ORs) (95% CIs) for in‐hospital mortality in non‐White admissions with AMI‐CA compared with White admissions with AMI‐CA. Each subgroup was adjusted for age, sex, race/ethnicity, comorbidity, primary payer, hospital region, hospital location and teaching status, hospital bed size, acute organ failure, cardiogenic shock, atrial fibrillation/flutter, ventricular tachycardia/fibrillation, coronary angiography, percutaneous coronary intervention, coronary artery bypass grafting, pulmonary artery catheterization, mechanical circulatory support, invasive mechanical ventilation, and acute hemodialysis. Adjusted ORs (95% CIs >1 signify a higher in‐hospital mortality. AMI indicates acute myocardial infarction; CA, cardiac arrest; IHCA, in‐hospital cardiac arrest; IHM, in‐hospital mortality; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
In a supplementary analysis, we expanded the other race/ethnicity category into Hispanic, Asian/Pacific Islander, Native American, and other groups to better understand the differences in these subgroups (Table S4). Compared with White admissions with AMI‐CA, all subgroups of other race/ethnicity category (Hispanic, Asian/Pacific Islander, Native American, and other groups) had higher rates of concomitant multiorgan failure and cardiogenic shock (Table S4). Use of coronary angiography was lower in Hispanic, Asian/Pacific Islander, and Native American groups with AMI‐CA with lower rates of PCI use seen in Hispanic and Asian/Pacific Islander in comparison to White admissions with AMI‐CA (Table S5). Mechanical circulatory support was used more often in Hispanic, Asian/Pacific Islander, Native American, and other groups compared with White admissions.
Similar to the primary analysis, compared with White admissions with AMI‐CA (40.9%), admissions belonging to Hispanic (49.3%), Asian/Pacific islander (48.7%), Native American (45.2%), and other (42.5%) groups had higher unadjusted in‐hospital mortality (Table S5). In adjusted analysis, with White race as referent, Black race was associated lower in‐hospital mortality (OR, 0.95; 95% CI, 0.91–0.99; P=0.008) whereas Hispanic (OR, 1.16; 95% CI, 1.11–1.21; P<0.001) and Asian/Pacific Islander (OR, 1.11; 95% CI, 1.03–1.19; P=0.003) groups were associated with higher adjusted in‐hospital mortality with Native American and Other ethnicity (OR, 1.03; 95% CI, 0.97–1.09; P=0.32) group having comparable in‐hospital mortality (Table S6).
Discussion
In this large contemporary study, evaluating for racial/ethnic differences in the management and outcomes of CA in admissions with AMI, we noted CA to complicate 5.2% of all admissions with AMI with a higher prevalence in the Black race. Admissions of Black and other races/ethnicities had consistently lower use of guideline‐directed therapies (such as coronary angiography and PCI), longer time to angiography, and greater use of palliative care and DNR status. Though Black race had higher unadjusted mortality, these differences were not noted when adjusted for in‐hospital factors and comorbid conditions. Admissions of other race/ethnicity categories had consistently higher in‐hospital mortality compared with White admissions.
Our sample had a heterogeneous race/ethnicity distribution with 74.8% of admissions with CA being White, and 10.7% and 14.5% of admissions of Black or other races/ethnicities respectively. Prior studies of IHCA include populations ranging from 73 to 88% White, and OOHCA populations range from 47 to 91% White depending on the geographic region represented.15, 40, 41, 42, 43, 44, 45, 46 Although our distribution is comparable to prior studies of both IHCA and OOHCA, it has been well documented that Black race is associated with higher incidence of CA.15, 40, 41, 42, 43, 44, 45, 46 The reasons behind this disparity in incidence of CA by race/ethnicity are likely multifactorial but raise significant concern for inequity in prearrest factors and resuscitation efforts.42, 43, 45
Prearrest factors contributing to disparities in CA include higher overall burden of comorbidities, poorly controlled comorbidities, and younger age in Black admissions with CA and AMI.10, 12 Insights from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?) initiative and Dynamic Registry reported higher rates of obesity, hypertension, diabetes mellitus, renal insufficiency, tobacco use, and history of heart failure or stroke.10, 12 Differences in rates of shockable rhythms in IHCA have also been proposed to account for a large degree of difference in outcomes.41, 43 However, when in‐hospital outcomes of pulseless electrical activity arrest and asystole have been compared between White and Black patients, Black patients still have lower survival to hospital discharge (OR, 0.85; 95% CI, 0.79–0.92) and lower rate of return of spontaneous circulation (OR, 0.88; 95% CI, 0.84–0.92) when compared with White patients.41 A study from the GWTG‐R database found that patient, event, and hospital characteristics could not fully explain this difference. Other potential explanations included the setting in which a patient arrests (telemetry versus nontelemetry unit) and/or control of chronic comorbidities, such as diabetes mellitus, hypertension, and chronic kidney disease. Another notable difference in admission characteristics is higher rate of CA in admissions with both STEMI and NSTEMI for Black patients, which is clearly correlated with increased mortality and may be reflective of lower rate of revascularization and use of primary/secondary prevention in this population.6 Finally, it is notable that Black admissions for AMI‐CA were more likely to be female. Prior studies have showed increased risk of fatal coronary artery disease events and increased need for multivessel PCI among Black women, highlighting a particularly vulnerable population.4, 14
Our study demonstrated lower rates of guideline‐directed therapies for Black and other racial/ethnic minority patients when compared with White patients admitted for AMI‐CA, which is consistent with other studies spanning several decades of investigation.6, 7, 9 Our data were consistent with studies by Ayanian et al and Peterson et al who found lower rates of coronary angiography and revascularization in Black patients across a variety of hospitals and found this trend to persist despite a large predicted benefit of an early invasive strategy.6, 9 Similarly, the California Cooperative Cardiovascular Project found that racial/ethnic minorities were less likely than White patients to have received cardiac catheterization and PCI. Asian patients have also been found to have lower rates of invasive cardiovascular procedures when compared with White patients.7
We found differences in the rates of DNR status and palliative care consultation by race/ethnicity. The existing data surrounding this issue is mixed.17 We found Black patients were less likely to have a DNR status compared with White patients and other racial/ethnic minorities (15.3% versus 15.6% versus 16.8%). Some have suggested that the difference in outcomes of Black patients when compared with White patients after CA may be related to decreased use of DNR status in patients who would be unlikely to survive cardiopulmonary resuscitation, including those with dependency for activities of daily living, impaired renal function, advanced age, hypotension on admission, or admission with sepsis.17 This hypothesis is supported by our finding that Black patients and others admitted with AMI‐CA have both more comorbidities and higher rates of acute organ failure. We also found that Black patients were more likely to receive palliative care consultation when compared with White and other patients. Others have posited that the rise of palliative care is falsely improving survival in racial/ethnic minorities with more comorbidities who develop IHCA.47 Conversely, Woo et al found that Black and Hispanic patients received significantly lower rates of palliative care consultation and higher rates of aggressive care including renal replacement therapy, percutaneous gastrostomy, and tracheostomy.48 However, this was a cohort of patients presenting with OOHCA, suggesting that trends may be divergent depending on the setting in which a patient arrests.48
Finally, in our analysis we found unadjusted in‐hospital mortality was higher for admissions who were Black and other racial/ethnic minorities when compared with White admissions (47.4% and 47.4% versus 40.9%). This is consistent with several prior studies of both IHCA and OOHCA.5, 15, 17, 40, 41 Chan et al found Black patients less likely to survive to hospital discharge than White patients with IHCA (relative risk, 0.73; 95% CI, 0.67–0.79); however, adjustment for hospital center minimized much of this gap in the NRCPR registry suggesting that gaps in care were created by quality of hospital care instead of race/ethnicity alone.5 Merchant et al found a similar trend for OOHCA, with unadjusted survival favoring White patients and adjusted analysis showing the disparity was primarily accounted for by overall hospital survival.15 Furthermore, when hospitals were stratified by survival rate, Black patients actually fared better than White patients at “low survival” hospitals. Desai et al found that Hispanic and Asian patients had higher odds of in‐hospital mortality for multivessel PCI admissions (OR, 1.51 and 1.22 respectively).14 This population correlates in our study to other races/ethnicities, who were found to have higher adjusted mortality, the highest mean hospitalization cost, and overall longer length of stay. The category of other non‐White races/ethnicities captures people who are Hispanic, Asian or Pacific Islander, Native American, and others, who represent a growing proportion of the US population. Although it appears that gaps in care for AMI‐CA are improving in Black patients, it is widening in other non‐White populations, which warrants further investigation and intervention.16, 18
Limitations
Despite the HCUP‐NIS database’s attempts to mitigate potential errors by using internal and external quality control measures, this study has several limitations. Prior validation of administrative codes for AMI and CA reduces the inherent errors in the study.24, 25 The HCUP‐NIS database does not provide important information such as receipt of bystander cardiopulmonary resuscitation, quality of cardiopulmonary resuscitation, timing of multiorgan failure, timing of CA relative to AMI presentation, and extent of neurological injury. Echocardiographic data, angiographic variables, and hemodynamic parameters were unavailable in this database, which limits physiological assessments of disease severity. Despite best attempts at controlling for confounders by a multivariate analysis, it is possible that observed results could be because of residual confounding. Finally, our data are reflective of only in‐hospital outcomes and cannot comment on the long‐term outcomes of these admissions. Importantly, the NIS does not capture individual patients but identifies all information for each admission. Recurrent hospitalizations of the same individual will appear as distinct observations. Therefore, each encounter has been referred to as an “admission” as opposed to a “patient” in this analysis. Despite these limitations, this study addresses an important knowledge gap highlighting the racial/ethnic disparities in CA complicating AMI in a contemporary population.
Conclusions
Significant racial/ethnic disparities exist in in‐hospital mortality among admissions with AMI complicated with CA. The differences observed in in‐hospital management and comorbidity associated with race/ethnicity appears to have a role in associated outcomes. Racial/ethnic minorities continue to experience worse in‐hospital outcomes independent of patient and hospital‐related factors. Urgent quantitative and qualitative research into the equitable care of racial/ethnic minorities with AMI‐CA is needed to address this disparity.
Take Home Point
Significant racial/ethnic disparities observed in in‐hospital management and a higher proportion of comorbidities in racial/ethnic minorities appear to affect outcomes of admissions with AMI‐CA.
Sources of Funding
Dr Saraschandra Vallabhajosyula is supported by the Clinical and Translational Science Award (CTSA) Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Disclosures
None.
Supporting information
Tables S1–S6
Acknowledgments
Author contributions: Study design, literature review, statistical analysis: Subramaniam, Patlolla, Cheungpasitporn, Sundaragiri, Miller, Vallabhajosyula; Data management, data analysis, drafting manuscript: Subramaniam, Patlolla, Cheungpasitporn, Sundaragiri, Miller, Vallabhajosyula; Access to data: Subramaniam, Patlolla, Cheungpasitporn, Sundaragiri, Miller, Barsness, Bell, Holmes, Vallabhajosyula; Manuscript revision, intellectual revisions, mentorship: Barsness, Bell, Holmes, Vallabhajosyula; Final approval: Subramaniam, Patlolla, Cheungpasitporn, Sundaragiri, Miller, Barsness, Bell, Holmes, Vallabhajosyula.
(J Am Heart Assoc. 2021;10:e019907. DOI: 10.1161/JAHA.120.019907.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019907
For Sources of Funding and Disclosures, see page 10.
References
- 1.Institute of Medicine Committee on U, Eliminating R, Ethnic Disparities in Health C . In: Smedley BD, Stith AY, Nelson AR, ed. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington (DC): National Academies Press (US); Copyright 2002 by the National Academy of Sciences. All rights reserved; 2003. [PubMed] [Google Scholar]
- 2.Polsky D, Jha AK, Lave J, Pauly MV, Cen L, Klusaritz H, Chen Z, Volpp KG. Short‐ and long‐term mortality after an acute illness for elderly whites and blacks. Health Serv Res. 2008;43:1388–1402. DOI: 10.1111/j.1475-6773.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto GJ, Martin GS, Gong MN. Healthcare disparities in critical illness. Crit Care Med. 2013;41:2784–2793. DOI: 10.1097/CCM.0b013e3182a84a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. DOI: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, Rathore SS, Nallamothu BK. American Heart Association National Registry of Cardiopulmonary Resuscitation I. Racial differences in survival after in‐hospital cardiac arrest. JAMA. 2009;302:1195–1201. DOI: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayanian JZ, Udvarhelyi IS, Gatsonis CA, Pashos CL, Epstein AM. Racial‐differences in the use of revascularization procedures after coronary angiography. JAMA J Am Med Assoc. 1993;269:2642–2646. DOI: 10.1001/jama.1993.03500200056033. [DOI] [PubMed] [Google Scholar]
- 7.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. 2001;135:352–366. DOI: 10.7326/0003-4819-135-5-200109040-00012. [DOI] [PubMed] [Google Scholar]
- 8.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial‐infarction in the department‐of‐veterans‐affairs. JAMA: J Am Med Assoc. 1994;271:1175–1180. DOI: 10.1001/jama.1994.03510390045028. [DOI] [PubMed] [Google Scholar]
- 9.Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary‐revascularization procedures. Are the differences real? Do they matter? N Engl J Med. 1997;336:480–486. [DOI] [PubMed] [Google Scholar]
- 10.Slater J, Selzer F, Dorbala S, Tormey D, Vlachos HA, Wilensky RL, Jacobs AK, Laskey WK, Douglas JS, Williams DO, et al. Ethnic differences in the presentation, treatment strategy, and outcomes of percutaneous coronary intervention (a report from the national heart, lung, and blood institute dynamic registry). Am J Cardiol. 2003;92:773–778. DOI: 10.1016/S0002-9149(03)00881-6. [DOI] [PubMed] [Google Scholar]
- 11.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital‐level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005;43:308–319. DOI: 10.1097/01.mlr.0000156848.62086.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonel AF, Good CB, Mulgund J. Racial variations in treatment and outcomes of black and white patients with high‐risk non‐ST‐elevation acute coronary syndromes: insights from CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines?). ACC Current Journal Review. 2005;14:1–2. DOI: 10.1016/j.accreview.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Ford E, Newman J, Deosaransingh K. Racial and ethnic differences in the use of cardiovascular procedures: findings from the California cooperative cardiovascular project. Am J Public Health. 2000;90:1128–1134. DOI: 10.2105/ajph.90.7.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai R, Singh S, Fong HK, Goyal H, Gupta S, Zalavadia D, Doshi R, Savani S, Pancholy S, Sachdeva R, et al. Racial and sex disparities in resource utilization and outcomes of multi‐vessel percutaneous coronary interventions (a 5‐year nationwide evaluation in the United States). Cardiovasc Diagn Ther. 2019;9:18–29. DOI: 10.21037/cdt.2018.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merchant RM, Becker LB, Yang F, Groeneveld PW. Hospital racial composition: a neglected factor in cardiac arrest survival disparities. Am Heart J. 2011;161:705–711. DOI: 10.1016/j.ahj.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amen A, Karabon P, Bartram C, Irwin K, Dunne R, Wolff M, Daya MR, Vellano K, McNally B, Jacobsen RC, et al. Disparity in receipt and utilization of telecommunicator CPR instruction. Prehosp Emerg Care. 2020;24:544–549. DOI: 10.1080/10903127.2019.1680781. [DOI] [PubMed] [Google Scholar]
- 17.Ebell MH, Afonso AM. Pre‐arrest predictors of failure to survive after in‐hospital cardiopulmonary resuscitation: a meta‐analysis. Fam Pract. 2011;28:505–515. DOI: 10.1093/fampra/cmr023. [DOI] [PubMed] [Google Scholar]
- 18.Ghobrial J, Heckbert SR, Bartz TM, Lovasi G, Wallace E, Lemaitre RN, Mohanty AF, Rea TD, Siscovick DS, Yee J, et al. Ethnic differences in sudden cardiac arrest resuscitation. Heart. 2016;102:1363–1370. DOI: 10.1136/heartjnl-2015-308384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph L, Chan PS, Bradley SM, Zhou Y, Graham G, Jones PG, Vaughan‐Sarrazin M, Girotra S. American Heart Association get with the Guidelines‐Resuscitation I. Temporal changes in the racial gap in survival after in‐hospital cardiac arrest. JAMA Cardiol. 2017;2:976–984. DOI: 10.1001/jamacardio.2017.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HCUP . Introduction to hcup national inpatient sample (nis) 2012. 2012.
- 21.Vallabhajosyula S, Dunlay SM, Prasad A, Kashani K, Sakhuja A, Gersh BJ, Jaffe AS, Holmes DR Jr, Barsness GW. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73:1781–1791. DOI: 10.1016/j.jacc.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 22.Vallabhajosyula S, Kumar V, Vallabhajosyula S, Subramaniam AV, Patlolla SH, Verghese D, Ya'Qoub L, Stulak JM, Sandhu GS, Prasad A, et al. Acute myocardial infarction‐cardiogenic shock in patients with prior coronary artery bypass grafting: a 16‐year national cohort analysis of temporal trends, management and outcomes. Int J Cardiol. 2020;310:9–15. DOI: 10.1016/j.ijcard.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Vallabhajosyula S, Patlolla SH, Verghese D, Ya'Qoub L, Kumar V, Subramaniam AV, Cheungpasitporn W, Sundaragiri PR, Noseworthy PA, Mulpuru SK, et al. Burden of arrhythmias in acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2020;125:1774–1781. DOI: 10.1016/j.amjcard.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeZorzi C, Boyle B, Qazi A, Luthra K, Khera R, Chan PS, Girotra S. Administrative billing codes for identifying patients with cardiac arrest. J Am Coll Cardiol. 2019;73:1598–1600. DOI: 10.1016/j.jacc.2019.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallabhajosyula S, Jentzer JC, Zack CJ. Cardiac arrest definition using administrative codes and outcomes in acute myocardial infarction. Mayo Clin Proc. 2020;95:611–613. DOI: 10.1016/j.mayocp.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. DOI: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Vallabhajosyula S, Arora S, Lahewala S, Kumar V, Shantha GPS, Jentzer JC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, et al. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc. 2018;7:e010193. DOI: 10.1161/JAHA.118.010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR Jr, Prasad A. Hospital‐level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol. 2019;124:491–498. DOI: 10.1016/j.amjcard.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 29.Vallabhajosyula S, Dunlay SM, Kashani K, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Barsness GW. Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int J Cardiol. 2019;285:6–10. DOI: 10.1016/j.ijcard.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Vallabhajosyula S, Prasad A, Dunlay SM, Murphree DH Jr, Ingram C, Mueller PS, Gersh BJ, Holmes DR Jr, Barsness GW. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: a 15‐year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc. 2019;8:e011954. DOI: 10.1161/JAHA.119.011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallabhajosyula S, Prasad A, Sandhu GS, Bell MR, Gulati R, Eleid MF, Best PJM, Gersh BJ, Singh M, Lerman A, et al. Ten‐year trends, predictors and outcomes of mechanical circulatory support in percutaneous coronary intervention for acute myocardial infarction with cardiogenic shock. EuroIntervention. 2021;16:e1254–e1261. DOI: 10.4244/EIJ-D-19-00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallabhajosyula S, Shankar A, Patlolla SH, Prasad A, Bell MR, Jentzer JC, Arora S, Vallabhajosyula S, Gersh BJ, Jaffe AS, et al. Pulmonary artery catheter use in acute myocardial infarction‐cardiogenic shock. ESC Heart Failure. 2020;7:1234–1245. DOI: 10.1002/ehf2.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallabhajosyula S, Vallabhajosyula S, Burstein B, Ternus BW, Sundaragiri PR, White RD, Barsness GW, Jentzer JC. Epidemiology of in‐hospital cardiac arrest complicating non‐ST‐segment elevation myocardial infarction receiving early coronary angiography. Am Heart J. 2020;223:59–64. DOI: 10.1016/j.ahj.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Vallabhajosyula S, Ya'Qoub L, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Gersh BJ, Kashani K. Sex disparities in acute kidney injury complicating acute myocardial infarction with cardiogenic shock. ESC Heart Fail. 2019;6:874–877. DOI: 10.1002/ehf2.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aggarwal G, Patlolla SH, Aggarwal S, Cheungpasitporn W, Doshi R, Sundaragiri PR, Rabinstein AA, Jaffe AS, Barsness GW, Cohen M, et al. Temporal trends, predictors, and outcomes of acute ischemic stroke in acute myocardial infarction in the United States. J Am Heart Assoc. 2021;10:e017693. DOI: 10.1161/JAHA.120.017693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patlolla SH, Sundaragiri PR, Cheungpasitporn W, Doshi R, Barsness GW, Rabinstein AA, Jaffe AS, Vallabhajosyula S. Intracranial hemorrhage complicating acute myocardial infarction: an 18‐year national study of temporal trends, predictors, and outcomes. J Clin Med. 2020;9. DOI: 10.3390/jcm9092717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallabhajosyula S, Patlolla SH, Bell MR, Cheungpasitporn W, Stulak JM, Schears GJ, Barsness GW, Holmes DR. Same‐day versus non‐simultaneous extracorporeal membrane oxygenation support for in‐hospital cardiac arrest complicating acute myocardial infarction. J Clin Med. 2020;9. DOI: 10.3390/jcm9082613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318:2011–2018. DOI: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH, Fonarow GC. National differences in trends for heart failure hospitalizations by sex and race/ethnicity. Circ Cardiovasc Qual Outcomes. 2017;10:e003552. DOI: 10.1161/CIRCOUTCOMES.116.003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan PS, Nallamothu BK, Krumholz HM, Spertus JA, Li Y, Hammill BG, Curtis LH, American Heart Association Get with the Guidelines‐Resuscitation I . Long‐term outcomes in elderly survivors of in‐hospital cardiac arrest. N Engl J Med. 2013;368:1019–1026. DOI: 10.1056/NEJMoa1200657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razi RR, Churpek MM, Yuen TC, Peek ME, Fisher T, Edelson DP, American Heart Association's Get With The Guidelines‐Resuscitation I . Racial disparities in outcomes following PEA and asystole in‐hospital cardiac arrests. Resuscitation. 2015;87:69–74. DOI: 10.1016/j.resuscitation.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayegh AJ, Swor R, Chu KH, Jackson R, Gitlin J, Domeier RM, Basse E, Smith D, Fales W. Does race or socioeconomic status predict adverse outcome after out of hospital cardiac arrest: a multi‐center study. Resuscitation. 1999;40:141–146. DOI: 10.1016/S0300-9572(99)00026-X. [DOI] [PubMed] [Google Scholar]
- 43.Shah KS, Shah AS, Bhopal R. Systematic review and meta‐analysis of out‐of‐hospital cardiac arrest and race or ethnicity: black US populations fare worse. Eur J Prev Cardiol. 2014;21:619–638. DOI: 10.1177/2047487312451815. [DOI] [PubMed] [Google Scholar]
- 44.Wilde ET, Robbins LS, Pressley JC. Racial differences in out‐of‐hospital cardiac arrest survival and treatment. Emerg Med J. 2012;29:415–419. DOI: 10.1136/emj.2010.109736. [DOI] [PubMed] [Google Scholar]
- 45.York Cornwell E, Currit A. Racial and social disparities in bystander support during medical emergencies on US streets. Am J Public Health. 2016;106:1049–1051. DOI: 10.2105/AJPH.2016.303127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berg DD, Barnett CF, Kenigsberg BB, Papolos A, Alviar CL, Baird‐Zars VM, Barsness GW, Bohula EA, Brennan J, Burke JA, et al. Clinical practice patterns in temporary mechanical circulatory support for shock in the critical care cardiology trials network (CCCTN) registry. Circ Heart Fail. 2019;12:e006635. DOI: 10.1161/CIRCHEARTFAILURE.119.006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weisfeldt M, Becker L. Racial differences in in‐hospital cardiac arrest: Good news: Cautious optimism is welcome. JAMA Cardiol. 2017;2:984–985. DOI: 10.1001/jamacardio.2017.2432. [DOI] [PubMed] [Google Scholar]
- 48.Woo KK, Can A, Chang DW. Racial differences in the utilization of guideline‐recommended and life‐sustaining procedures during hospitalizations for out‐of‐hospital cardiac arrest. J Racial Ethn Health Disparities. 2020;7:403–412. DOI: 10.1007/s40615-019-00668-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6


